Abstract
CpG oligonucleotide 7909 (CpG 7909, PF-03512676), a synthetic 24mer single stranded agonist of TLR9 expressed by B cells and plasmacytoid dendritic cells, is immunomodulatory and can cause activation-induced death of chronic lymphocytic leukemia (CLL) cells. We report a phase I study of CpG 7909 in 41 patients with early relapsed CLL. A single intravenous dose of CpG 7909 was well tolerated with no clinical effects and no significant toxicity up to 1.05 mg/kg. Single dose subcutaneous CpG 7909 had a maximum tolerated dose (MTD) of 0.45 mg/kg with dose limiting toxicity of myalgia and constitutional effects. Multiple weekly subcutaneous doses at the MTD were well tolerated. CpG 7909 administration induced immunologic changes in CLL and non-malignant cells that were dose and route dependent. We conclude that multidose therapy with subcutaneous CpG 7909 (0.45 mg/kg) could be used in future phase II combination clinical trials for CLL.
Keywords: Chronic lymphocytic leukemia, CLL, CpG oligonucleotide 7909, PF-03512676, CpG 2006, TLR9
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent lymphoid malignancy in the US with a male predominance and median age of onset of 70 – 73 years[1]. Although there have been considerable improvements in the therapy for CLL over the past two decades, the disease remains incurable by conventional therapy and most patients with the disease will die of CLL or its complications[2]. Given this, there is clearly a need for more effective therapy.
The ability to achieve long term control of CLL by allogeneic hematopoietic stem cell transplantation has shown that effective therapeutic immune responses can be developed against CLL cells[3,4]. However, the autologous immune response mounted by patients against CLL cells is usually ineffective for reasons that are not well understood, but could include the low immunogenicity of the CLL cells and the altered immune function in patients with CLL[5,6]. Interventions that correct this immune deficiency could be useful for the treatment of CLL.
Toll-like receptors (TLR) are pattern receptors that bind selectively to unmethylated CpG dinucleotides which occur at high frequency in bacterial and viral DNA and at low frequency in human DNA[7,8]. TLR9 is expressed in humans predominantly by B cells (including CLL cells) and plasmacytoid dendritic cells (pDC)[5,7,8]. Among the most extensively studied TLR9 agonists is CpG 7909 (PF-03512676, CpG 2006), a synthetic 24mer single stranded oligonucleotide (ODN) (5'-TCGTCGTTTTGTCGTTTTGTCGTT-3') containing 4 unmethylated CpG motifs[8] with a phosphorothioate backbone resistant to degradation by DNAse (class B ODN)[7]. This molecule has undergone extensive in vitro examination of its effect on normal and malignant B lymphocytes including CLL and is reviewed briefly below.
CpG 7909 is endocytosed by B cells and pDC and then binds with TLR9 in the endosomal compartment to form a signaling complex[7]. In pDCs this results in type I IFN secretion which activates NK cells, monocytes, and other antigen presenting cells[7] and induces secretion of proinflammatory cytokines and chemokines[7,9]. We and others have found that in vitro treatment with CpG 7909 of a variety of malignant B lymphocytes, including CLL cells, induces rapid upregulation of CD20, CD40, CD80, CD86 and class II MHC which are all phenotypic changes consistent with a more competent antigen presenting cell[10,11] . These data provide important pre-clinical support for testing CpG 7909 as a therapy for lymphoid malignancies.
CpG 7909 is of additional interest in the treatment of CLL because CLL cells have higher levels of expression of TLR9 than normal B cells[12]. In the short term, culture of CLL cells with CpG ODN induces activation signaling (including through the NF-KB and JAK/STAT pathways) and proliferation. However, within a few days of subsequent culture, there is enhanced apoptosis of CLL cells consistent with activation-induced cell death[11,12]. These data suggest that treatment of patients with CLL with CpG 7909 could result in several beneficial effects including direct induction of CLL cell cytotoxicity, enhancement of CLL cell immunogenicity, and sensitization to the cytotoxicity of other drugs[5]. CpG 7909 could thus be of value in combination therapy of patients with CLL.
Phase I studies in healthy volunteers showed that of CpG 7909 was well tolerated at subcutaneous (SQ) doses up to 0.08 mg/kg and intravenous (IV) doses up to 0.32 mg/kg[13]. SQ administration caused transient changes in blood leukocyte levels consistent with an immune response and patients had transient injection site reactions and flu like symptoms, but there was no evidence of organ toxicity or autoimmunity[13]. There were minimal adverse events after IV administration[13]. Pharmacokinetic studies showed that IV administration resulted in rapid distribution of CpG 7909 to the liver, kidney and spleen while SQ injection resulted in high drug concentration in the injection site and draining nodes which were maintained for at least 2 weeks[13]. CpG 7909 has been tested as monotherapy and combination therapy for renal cell cancer, lung cancer, non-Hodgkins lymphoma (NHL), and cutaneous T cell lymphoma, and as an adjuvant for vaccine therapy for breast cancer and melanoma [7,10,12,14-17]. Monotherapy was well tolerated but had low efficacy with the best responses seen with direct injection of tumor tissue and SQ administration[7]. These data, together with the extensive pre-clinical information on the effects of CpG 7909 on CLL cells, provided a rationale for testing this drug in patients with CLL.
We report the results of a phase I trial of CpG 7909 in patients with early relapsed CLL that established the maximum tolerated single dose of SQ CpG 7909 and showed that multidose therapy with CpG 7909 is tolerable. There were no objective clinical responses to treatment but CpG 7909 did cause immunologic changes in both CLL and non-malignant cells that were dose and route dependant. These findings could be informative for future use of CpG 7909 in combination trials with chemotherapy and anti-CD20 monoclonal antibodies, or as an immunomodulating agent.
Materials and Methods
This clinical trial was performed at the Mayo Clinic Rochester and University of Iowa with the approval of both Institutional Review Boards according to the criteria of the Declaration of Helsinki and was registered at ClinicalTrials.gov (NCT00233506). The study was designed to assess the safety of IV and SQ CPG 7909 in patients with previously treated CLL over a range of doses. Patients with previously treated CLL diagnosed according to standard criteria were eligible for this study if they had absolute lymphocyte counts over 5 × 109/L but did not yet meet criteria for treatment of progressive disease[18] and were thus considered to have early relapse. All patients had to be off any prior treatment for CLL for 28 days prior to enrollment, and were not allowed to have treatment with fludarabine or alemtuzumab within the previous 42 days or with rituximab for the previous 120 days. Patients who had received CpG 7909 on dose levels 1-3 could be treated again on dose level 4. Enrollment was restricted to subjects > 18 years of age who had an ECOG performance status of 0 – 2, were not pregnant, had a negative direct antiglobulin test, had no history of autoimmune complications of CLL, and adequate hematopoietic (hemoglobin ≥ 10 g/dL, platelet ≥ 50 × 109/L, granulocytes > 1 × 109/L), hepatic, and renal function.
CpG 7909 (ProMune™) was provided by Pfizer (New York, NY) as a phosphate-buffered sterile saline solution without preservatives. All patients received allopurinol 300mg/d for seven days following the start of treatment and the first dose of CpG 7909 was given with 1L of IV fluid (dextrose saline/normal saline with bicarbonate). For the first 3 dose levels, patients were randomized to receive a single dose of CpG 7909 either IV (two hour infusion) or SQ. The starting dose was 0.15 mg/kg with a planned accrual of 6 subjects to each arm (SQ or IV) for a total of 12 patients per dose level. The second dose level was 0.45 mg/kg and the third dose level was 0.75 mg/kg. After the maximum tolerated dose (MTD) for SQ administration was determined to be 0.45 mg/kg, dose level 4 was modified to a single IV infusion of 1.05 mg/kg followed 28 days later by 0.45 mg/kg SQ weekly for 4-8 weeks for a single cohort of 6 patients. At the time of the 4th dose of SQ CpG 7909, patients were evaluated for efficacy and toxicity of treatment. In patients who were tolerating therapy and did not have evidence of progression of CLL, a decision to continue therapy for an additional 4 weeks could be made by the patient and treating physician.
All patients were evaluated for dose limiting toxicity (DLT) using NCI Common Toxicity Criteria (CTCAE) version 3.0. Non hematologic DLT was defined as any grade 4 toxicity, any grade 3 toxicity present for 2 consecutive days (excluding nausea, vomiting, or fatigue), or any grade 2 cardiovascular toxicity present for 2 consecutive days. Hematologic DLT was defined as any grade 4 toxicity (excluding anemia) that persisted for 4 consecutive days. Although response assessment was not an endpoint of this phase I trial, responses in patients with measurable disease were recorded using standard criteria[18] by treating physicians. Patients in cohorts 1-3 were evaluated 2 months after treatment and patients in cohort 4 were evaluated 4 weeks after the single IV dose of CpG 7909 and 2 months after completion of SQ therapy. The absolute lymphocyte count was evaluated for changes at days 3, 7 and 28 after the first dose and then 2 months after completion of therapy for all patients. For patients on cohort 4, the absolute lymphocyte count was also measured every 2 weeks during SQ therapy and 1 week after completion of SQ therapy.
All adverse events, regardless of the relationship to the study drug, observed by the investigator or reported by the patient, were recorded with details of the duration and intensity of each episode, the action taken with respect to test drug and the patient outcome, on an appropriate source document at the clinical site. All adverse events were graded according to the NCI Common Toxicity Criteria (CTCAE) version 3.0.
Correlative Studies
Blood was collected in sodium heparin tubes from patient before and 3 and 7 days after the first dose of CpG 7909. 200μl of whole blood was stained for various cell surface markers with the appropriate antibodies, lysed using FACS Lysing Solution (BD Biosciences, San Jose, CA) and cells were then washed and fixed with 2% formaldehyde. Samples and compensation tubes were run within 72 hours on a LSR flow cytometer (BD Biosciences) and analyzed using CellQuest Pro software. The 3-color panels of fluorescent antibodies included combinations of PE, FITC, PerCP CD3; PerCP CD5; PE CD11C; FITC CD16; FITC, PerCP CD19; PE CD20; PE CD22; FITC CD27; PE CD32; PE CD38; FITC CD40; FITC CD52; PerCP CD56; FITC CD64; PerCP CD80; PE CD86; PE CD95; PerCP CD123; FITC CD154; FITC HLA-ABC; PE HLA-DR; FITC Lin1; PE TRAIL. The compensation tubes were stained with the appropriate color of anti-CD4. All antibodies were purchased from BD Biosciences except for anti-CD52, which was purchased from Caltag (Burlingame, CA).
Statistical Analysis
Adverse events were tabulated during the study by treatment arm, type, and grade. Descriptive statistics are reported here to summarize patient characteristics and baseline prognostic factors. Changes in clinical and correlative measures were quantified as the ratios of values at each of the follow-up days 3, 7, and 28 to corresponding pre-therapy baseline values. Linear mixed effects regression models were used to estimate mean ratios by treatment arm (IV and SQ), dosing cohort (0.15, 0.45, 0.75, 1.05 mg/kg), and follow-up day. Subject-specific random effects were included in the models to account for repeated follow-up measurements on each subject. Reported p-values shown here correspond to two-sided tests that mean ratios are not equal to 1, where a ratio of 1 indicates no baseline change.
Results
Forty one patients were enrolled between November 2004 and February 2010 (Table 1). For the SQ administration cohort in the dose level 1 group (0.15 mg/kg), only one patient experienced grade 4 neutropenia, which resolved in less than 4 days, and therefore did not meet the DLT criteria. At dose level 2 (0.45 mg/kg), no DLTs were recorded. Two grade 3 events were recorded (lymph node pain and elevated alanine transaminase level) which did not meet the DLT criteria. At dose level 3 (0.75 mg/kg), two patients experienced DLTs: one patient with grade 3 myalgia and another with grade 3 myalgia and constitutional symptoms. The MTD for the SQ therapy was thus 0.45 mg/kg. In contrast for the IV administration cohort no severe (grade 3+) adverse events or DLTs were recorded at any of the first three dose levels. Six patients were treated at the fourth dose level with 1.05 mg/kg IV followed 28 days later by 0.45 mg/kg SQ weekly for 4-8 weeks (cross-over cohort). One of these patients had previously received IV CpG 7909 at dose level 3. In the cross-over cohort, five patients received all nine doses of CpG 7909 and one stopped weekly subcutaneous therapy after four doses for personal reasons not related to toxicity or disease progression. There were no DLTs in the cross-over cohort with the only grade 3 toxicity being headache in one patient (Table 2). No patient achieved a clinical response to treatment based on the NCI-WG criteria of 1996[18]. However, there were significant but transient decreases in the mean absolute lymphocyte counts in patients receiving the SQ 0.15 mg/kg (day 3) and 0.45 mg/kg (day 3 and 7) doses of CpG 7909 (Figure 1).
Table I.
Patient demographics and baseline clinicopathological measurements
| IV | SQ | Cross-Over | Total | |
|---|---|---|---|---|
| Number of Subjects | 19 | 17 | 6 | 41* |
| Age (years) | ||||
| Median | 65.0 | 58.0 | 64.0 | 63.5 |
| Range | (48.0-72.0) | (26.0-76.0) | (50.0-68.0) | (26.0-76.0) |
| Gender | ||||
| Females | 6 (31.6%) | 1 (5.9%) | 0 (0%) | 7 (16.7%) |
| Males | 13 (68.4%) | 16 (94.1%) | 6 (100%) | 35 (83.3%) |
| Number of Prior Therapies | ||||
| 1 | 11 (57.9%) | 14 (82.4%) | 5 (100%) | 30 (73.2%) |
| 2 | 2 (10.5%) | 2 (11.8%) | 0 (0%) | 4 (9.8%) |
| 3+ | 6 (31.6%) | 1 (5.9%) | 0 (0%) | 7 (17.1%) |
| Lymphocyte count (× 109/L) | ||||
| N | 17 | 16 | 5 | 38 |
| Median | 15.8 | 14.1 | 19.9 | 16.8 |
| Range | 4.0-204.2 | 8.9-75.4 | 6.4-268.2 | 4.04-268.2 |
| Baseline Cell Counts (× 109/L) | ||||
| N | 16 | 16 | 3 | 35 |
| TRAIL+CD19+ | ||||
| Median | 0.02 | 0.02 | 0.01 | 0.02 |
| Range | 0.01-0.37 | 0.01-0.08 | 0.01-8.18 | 0.01-8.18 |
| CD3-CD16+CD56+ | ||||
| Median | 0.19 | 0.20 | 0.25 | 0.20 |
| Range | 0.01-0.48 | 0.05-0.43 | 0.04-0.29 | 0.01-0.48 |
| CD3+CD80- | ||||
| Median | 1.26 | 1.06 | 2.61 | 1.09 |
| Range | 0.20-4.70 | 0.44-4.83 | 0.46-2.68 | 0.20-4.83 |
| CD3+CD38+ | ||||
| Median | 0.13 | 0.19 | 0.33 | 0.19 |
| Baseline MFI | ||||
| N | 17 | 17 | 4 | 38 |
| CD20 Median | 15.7 | 12.6 | 178.7 | 14.5 |
| Range | 3.8-70.5 | 5.6-64.4 | 12.5-1537.0 | 3.8-1537.0 |
| CD86 Median | 38.6 | 40.0 | 43.3 | 39.9 |
| Range | 33.1-74.4 | 32.2-54.0 | 29.1-65.1 | 29.1-74.4 |
One patient who participated in the single IV CpG dose level 3 cohort also participated in the cross over cohort.
Table II.
Adverse Events (grade 3-4) Attributable to CpG 7909 Therapy
| Dose Level | Dose (mg/kg) | Number of Grade 3-4 AEs | ||
|---|---|---|---|---|
| IV Arm | SQ Arm | Cross-Over Arm | ||
| 1 | 0.15 | 0 | 1a | - |
| 2 | 0.45 | 0 | 2b | - |
| 3 | 0.75 | 0 | 4c | - |
| 4 | IV 1.05 (×1) | - | - | 1d |
| SQ 0.45 (× 4-8) | ||||
Grade 4 neutropenia
One grade 3 cancer-related pain, one grade 3 SGPT (ALT)
Two grade 3 myalgia, one grade 3 fatigue, one grade 3 constitutional symptom
Grade 3 headache
Figure 1. Effect CpG 7909 on absolute lymphocyte counts.

The change in mean absolute lymphocyte counts (ALC) was measured prior to treatment and then at 3, 7, and 28 days after treatment with a single dose of CpG 7909 for patients on the IV (A) and SQ (B) arms and at days 3 and 28 for patients on the single IV dose arm of the crossover study. The estimated mean value of the ratios of ALC for each measurement compared to baseline are displayed along with standard error bars and * indicates p < 0.05.
Correlative Studies
Correlative studies were done on the peripheral blood of all trial subjects. Samples obtained at days 3 and 7 after administration of a single dose of CpG 7909 were compared to those drawn immediately before drug administration for each patient. For each subject, the ratio of the mean delta fluorescence intensity (dMFI) of the post treatment samples was compared to the dMFI of pretreatment samples. The change in absolute numbers of cells from baseline was also determined for select cell populations by multiplying the percentage of cells with the defined phenotype by the absolute lymphocyte count obtained from the complete blood cell count done that day. Data from all subjects treated via a given route (IV and SQ) irrespective of dose are presented, as are data for each dose cohort for each of the two routes of administration.
Changes in B cells were most notable in subjects that received the highest IV dose of CpG 7909 (1.05 mg/kg). These change included an increase in the expression of CD20 (Figure 2A), CD86 (Figure 2B) and TRAIL (Figure 2C) on CLL cells. However, these increases largely returned to baseline levels by day 7. No consistent changes in number of cells or expression of CD20, CD80 or TRAIL were seen in subjects treated via the SQ route (data not shown).
Figure 2. Effect of CpG 7909 on expression of CD20, CD86 and TRAIL.

Surface expression was measured by flow cytometry on peripheral blood cells collected 3 and 7 days after administration of a single dose of CpG 7909 and expressed as the delta mean fluorescence intensity (MFI) ratio (post-treatment/pre-treatment levels). The dark gray bars represent the estimated mean values for all measurements and the light gray bars the mean values for each time point. Error bars represent standard errors and * indicates p < 0.05. A: CD20 expression was significantly increased in CLL cells 3 days after treatment with 1.05 mg/kg of CpG 7909. B: CD86 expression was increased at 3 days after treatment with 0.75 mg/kg and 1.05 mg/kg CpG 7909. C: TRAIL expression was significantly increased at 3 days after treatment with 1.05 mg/kg and at 7 days after treatment with 0.75 mg/kg.
Changes in NK cell (CD3-CD16+CD56+) numbers determined by flow cytometry were seen in patients treated by the IV route with doses of 0.45 mg/kg and 0.75 mg/kg (Figure 3A). An increase in the number of T cells (CD3+CD80-) (Figure 4A) was seen in the IV arm. T cell activation, as indicated by the number of CD3+CD38+ cells, was seen in subjects who received higher doses of therapy via both routes (Figures 5A and 5B). This activation persisted for 7 days, particularly for the IV cohort. CD3+CD95+ cells were also increased in these subjects (data not shown).
Figure 3. Effect of CpG 7909 on NK cell counts.
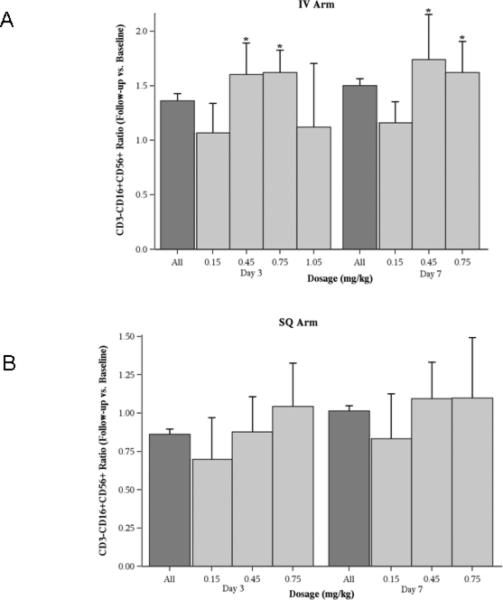
Absolute NK cell (CD3-CD16+CD56+) counts were measured before and after administration of a single dose of CpG 7909, and changes were expressed as a ratio of these measurements. The dark gray bars represent the mean value for all measurements and the light gray bars the estimated mean values for each time point. Error bars represent standard errors and * indicates p < 0.05. A: For IV administration NK cells were increased at 3 and 7 days for the 0.45 mg/kg and 0.75 mg/kg doses. B: SQ administration did not result in any significant changes in NK cell counts.
Figure 4. Effect of CpG 7909 on T cell counts.
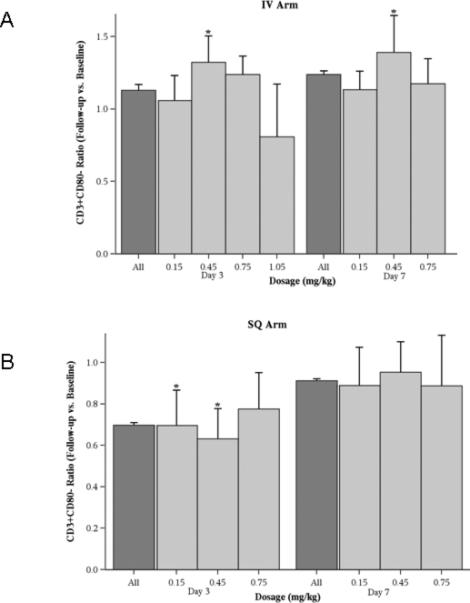
Absolute T cell (CD3+CD80-) counts were measured before and after administration of a single dose of CpG 7909, and changes were expressed as a ratio of these measurements. The dark gray bars represent the estimated mean values for all measurements and the light gray bars the mean values for each time point. Error bars represent standard errors and * indicates p < 0.05. A: For IV administration T cell counts were increased at 3 and 7 days for the 0.45 mg/kg dose. B: SQ administration resulted in decreases at 3 days with the 0.15 mg/kg and 0.45 mg/kg doses.
Figure 5. Effect of CpG 7909 on T cell activation.

Absolute activated T cell (CD3+CD38+) counts were measured before and after administration of a single dose of CpG 7909, and changes were expressed as a ratio of these measurements. The dark gray bars represent the estimated mean values for all measurements and the light gray bars the mean values for each time point. Error bars represent standard errors and * indicates p < 0.05. A: For IV administration activated T cells were increased at 3 days for the 0.75 mg/kg and 1.05 mg/kg doses and at 7 days for the 0.15 mg/kg, 0.45 mg/kg, and 0.75 mg/kg doses. B: For SQ administration activated T cells were increased at 3 and 7 days for the 0.75 mg/kg dose.
Discussion
We have determined that the single dose MTD of SQ CpG 7909 in patients with CLL is 0.45 mg/kg and have also found that this dose is tolerated when repeated weekly for up to 8 weeks. The MTD for single dose IV CpG 7909 was not reached at 1.05 mg/kg. These results are the first reported for patients with CLL and are similar to those reported for patients with other lymphoid malignancies[16,17]. A decrease in the number of circulating CLL cells was observed at day 3, but the pattern and transient nature of this decrease suggests it was likely due more to re-distribution of CLL cells than a significant antineoplastic effect. There was no evidence that CpG 7909 had a therapeutic clinical effect on CLL in this trial. However, immunologic changes were observed that are consistent with prior in vitro and clinical studies[10,11,13], and will help inform design of future studies of TLR9 agonists in the treatment of B cell malignancies. These changes included a measurable effect on the phenotype of both malignant and non-malignant cells. These effects were both dose and route dependent.
CLL cells had a transient but significant increase in the expression of CD20 in subjects who received the highest IV dose (1.05 mg/kg). Dose dependent changes on CLL expression of CD86 and TRAIL were also observed. These effects were not seen following SQ therapy, likely because peak CpG 7909 plasma levels were not sufficiently high to induce such changes.
CpG 7909 administration also resulted in increased NK and T cell counts and T cell activation in some IV cohorts which could be mediated by either direct or indirect effects of the drug. In contrast, SQ administration only resulted in an increase in activated T cells at the highest dose level. This was associated with local inflammation at the site of injection and anecdotally in draining lymph nodes as has been observed in other trials of CpG 7909[10]. Clinical symptoms consistent with cytokine production such as myalgia, malaise, and fevers were also seen at the higher SQ doses which were dose limiting.
Taken together, these results suggest the choice of the IV or SQ routes of administration for future studies should be based on the design of the study and the rational for including CpG 7909. If the goal of CpG 7909 as a therapy is to induce direct changes on CLL cells, repeated higher doses of IV therapy will likely be required. Hypothetically, if such an approach is tolerable, it could increase sensitivity of the CLL cells to death signals via TRAIL, enhance sensitivity of CLL cells to anti-CD20 therapy, or increase the immunogenicity of the CLL cells via increased expression of costimulatory molecules.
Local toxicity as well as systemic effects seen in this study and others with higher doses of SQ therapy suggests that the effects of CpG 7909 on immune effector cells after SQ injection are secondary to production of cytokines produced at the site of SQ injection by local pDCs. Thus, if the goal of CpG 7909 therapy is activation of immune effector cells, the SQ route would seem attractive.
We conclude that administration of single dose CpG 7909 has a measurable effect on the level of expression of selected markers on both CLL cells and non-malignant cells. These effects were dose and route dependant. Higher dose IV therapy resulted in transient phenotypic changes in the CLL cells including upregulation of CD20, CD86 and TRAIL. Both IV and SQ therapy resulted in changes in NK cells and T cells consistent with systemic immune activation and cytokine-induced immune activation. The MTD for the IV arm was not reached. The MTD for the SQ route was reached at 0.45 mg/kg. No therapeutic response was observed in either cohort. However, these studies do provide important insights into the route and dosing of CpG 7909 and related TLR9 agonists for future studies. The IV route has a greater direct effect on the CLL cells, and either route results in systemic immune activation.
Acknowledgements
This study was funded by the University of Iowa/Mayo Clinic Lymphoma SPORE CA097274.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Zent CS, Kay NE. Management of chronic lymphocytic leukemia patients with a high risk of adverse outcome: The Mayo Clinic approach. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.568654. Epub Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, Agura E, Chauncey TR, Pulsipher MA, McSweeney PA. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–20. doi: 10.1200/JCO.2007.15.4757. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S, Bunjes D, Cohen S, Schubert J, Hegenbart U. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–47. doi: 10.1182/blood-2010-03-275420. others. [DOI] [PubMed] [Google Scholar]
- 5.Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2007;21:53–60. doi: 10.1038/sj.leu.2404456. [DOI] [PubMed] [Google Scholar]
- 6.Zent CS. Cell-mediated immunity in chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:1775–6. doi: 10.3109/10428194.2010.514083. [DOI] [PubMed] [Google Scholar]
- 7.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahrsdorfer B, Muhlenhoff L, Blackwell SE, Wagner M, Poeck H, Hartmann E, Jox R, Giese T, Emmerich B, Endres S. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11:1490–9. doi: 10.1158/1078-0432.CCR-04-1890. others. [DOI] [PubMed] [Google Scholar]
- 9.Decker T, Schneller F, Sparwasser T, Tretter T, Lipford GB, Wagner H, Peschel C. Immunostimulatory CpG-oligonucleotides cause proliferation, cytokine production, and an immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood. 2000;95:999–1006. [PubMed] [Google Scholar]
- 10.Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter-Brown L, Rook AH. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J Am Acad Dermatol. 2010;63:975–83. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Jahrsdorfer B, Wooldridge JE, Blackwell SE, Taylor CM, Griffith TS, Link BK, Weiner GJ. Immunostimulatory oligodeoxynucleotides induce apoptosis of B cell chronic lymphocytic leukemia cells. J Leukoc Biol. 2005;77:378–387. doi: 10.1189/jlb.0604373. [DOI] [PubMed] [Google Scholar]
- 12.Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, Chen W. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115:5041–52. doi: 10.1182/blood-2009-03-213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–71. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Nakao M, Fukuyama C, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Ohe Y, Ohki E, Hashimoto J. Phase I study of TLR9 agonist PF-3512676 in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Sci. 2010;101:188–95. doi: 10.1111/j.1349-7006.2009.01361.x. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, Al-Adhami M, Readett D, Krieg AM, Leichman CG. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–86. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 16.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, Rasmussen WL, Krieg AM, Weiner GJ. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 17.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J, Hainsworth J, Sparano JA, Tsai DE, Horning S. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res. 2007;13:6168–74. doi: 10.1158/1078-0432.CCR-07-0815. others. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]


