Abstract
Opioids can attenuate the peripheral chemoreceptor-mediated hypoxic ventilatory response (HVR) by acting on central μ-type opioid receptors. Since the medullary raphe region (MRRs) expresses abundant μ-receptors and participates in modulating HVR, we tested the role of μ-receptors within the caudal, medial, and rostral MRR (cMRR, mMRR, and rMRR) in modulating the HVR. We recorded cardiorespiratory activities and their responses to isocapnic hypoxia in anesthetized rats before and after local microinjection of DAMGO into the MRR, and intravenous administration of DAMGO (100 μg/kg) alone or coupled with a previous local injection of CTAP. Microinjecting DAMGO into the cMRR or mMRR but not the rMRR significantly attenuated the HVR. However, systemic DAMGO-induced HVR attenuation was not significantly affected by pretreating the cMRR and mMRR with CTAP. Our data suggest that cMRR and mMRR μ-receptors are capable of depressing the HVR, while their contribution to the attenuated HVR by systemic DAMGO is limited.
Keywords: brainstem, carotid body, breathing
1. Introduction
Opioids, the most frequently used drugs to relieve pain for almost 200 years, produce some side effects while acting as therapeutic agents. The most adverse among these side effects is the marked depression of breathing (Yeadon & Kitchen, 1989), mainly through stimulating central μ-receptors (Haji et al., 2003; Manzke et al., 2003). It is well documented that opioids depress the hypoxic ventilatory response (HVR) (Weil et al., 1975; Kryger et al., 1976; Santiago et al., 1979; Dahan et al., 1998; Colman & Miller, 2002; Romberg et al., 2003; Modalen et al., 2006) in both animals and humans. The HVR is predominately initiated by the stimulation of carotid body chemoreceptors (Bisgard & Neubauer, 1995). Early reports showed that intracarotid injection of opioids inhibited carotid sinus nerve activity via acting on δ-type opioid receptors in anaesthetized cats (McQueen & Ribeiro, 1980; Kirby & McQueen, 1986). In addition, recent studies point to a central inhibitory effect of opioids on the HVR in humans (Bailey et al., 2000; Modalen et al., 2006). To date, however, it remains unknown which central site(s) is/are responsible for the opioid-induced attenuation of the early HVR.
The medullary raphe region (MRR) is a critical structure involved in nociception because of a rich, local distribution of μ-receptors (Pan et al., 1990; Ding et al., 1996; Porreca et al., 2001; Pinto et al., 2003). The MRR plays an important role in controlling respiratory chemoreflexes. Although it is debatable, there are considerable studies showing that this region contains CO2-chemosensitive neurons and is important to hypercapnic ventilatory response (HCVR) (Bernard et al., 1996; Wang et al., 1998; Nattie & Li, 2001; Wang et al., 2001; Nattie et al., 2004; Taylor et al., 2005). It was reported that activating local neurons in the MRR augmented the ventilation via their projections to other respiratory-related nuclei (Zec & Kinney, 2003; Rosin et al., 2006; Mulkey et al., 2007). The MRR also potentially participates in the HVR. For example, local lesion or electrical/chemical stimulation have revealed that the MRR is able to modulate hypoxia-induced hyperventilation (Perez & Ruiz, 1995; Gargaglioni et al., 2003; Penatti et al., 2006; Taylor et al., 2006; Nucci et al., 2008). In agreement, a earlier study indicated a long-latency evoked potentials in the caudal MRR following stimulation of the carotid sinus nerve (Miura & Reis, 1969), suggesting a projection from the carotid sinus nerve to the MRR. These findings along with the recent results that activation of MRR μ-receptors inhibits baseline ventilation and its response to hypercapnia in anesthetized rats and conscious goats (Krause et al., 2006; Zhang et al., 2007), allow us to hypothesize that MRR μ-receptors are capable of modulating the HVR.
Our experiments were carried out in anesthetized and spontaneously breathing rats. The ventilatory responses to isocapnic hypoxia for 1 min were compared before and after administering DAMGO [(d-Ala2, N-Me-Phe4, Gly-ol)-Enkephalin], a selective μ-receptor agonist, locally into caudal, middle and rostral MRR (cMRR, mMRR and rMRR). Because cMRR and mMRR μ-receptors appeared to attenuate the HVR in our pilot studies, we also compared the effects of systemic DAMGO on the HVR before and after microinjection of CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2), a μ-receptor antagonist, into both the cMRR and mMRR.
2. Methods
Sprague-Dawley adult male rats were quarantined for 2 weeks, and food and water were provided ad libitum. The experimental protocols adhere to the American Physiological Society's Guiding Principles in the Care and Use of Animals and were approved by the Lovelace Respiratory Research Institute (LRRI) Institutional Animal Care and Use Committee. LRRI is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.1. General animal preparation
Rats (n = 45, 400 – 500 g) were anesthetized with urethane (1200 mg/kg, i.p.), with a supplemental dose (200 mg/kg, i.p.) administered if needed, to completely eliminate eye-blink and limb-withdrawal reflex throughout the experiment. The right femoral vein and artery were cannulated for drug administration and monitoring of arterial blood pressure (BP) and heart rate (HR), respectively. The trachea below the larynx was exposed through a midline incision, tracheotomized by blunt dissection, and cannulated. The tracheal cannula was connected to a pneumotachograph to record airflow. The pneumotachograph had a linear flow-pressure relationship in the range of 2–20 ml/s, a flow resistance of 0.046 cmH2O ml-1 s, and a dead space of 0.2 ml. Another end of the pneumotachograph was placed (~5 mm deep) in a plastic tube with a diameter five-fold greater than the pneumotachograph. A three-way stopcock was attached to the other side of the plastic tube and connected to a supplemental gases device through a gas mixing flow-meter (GF-3MP, Cameron Instrument Co., Port Aransas, TX). By turning the switch, the mixed gases to be inhaled from different gas tanks were controlled. During isocapnic hypoxia, CO2 was added to maintain the end tidal carbon dioxide pressure (PETCO2) within 2 mmHg deviated from the baseline value (Tatsumi et al., 1991). PETCO2 was measured via a carbon dioxide analyzer (MicroCapStar end-tidal carbon dioxide analyzer, Model 15-10000, CWE, Inc. USA) connected to a side-port of the tracheal cannula. Animals were placed into a rigid metal frame with the head fixed and centered in a stereotaxic apparatus (Model 1404, Kopf, Tujunga, CA). A hole (~10 mm in diameter) was drilled at the midline of the skull for microinjections into the MRR (detailed below). Their core temperature was monitored with a rectal probe and maintained at 36.5–37.5°C by a heat pad and radiant heat lamp. Oxygen-enriched room air (40% O2 balanced with nitrogen) was applied to serve as a baseline throughout the experiment.
2.2. Microinjection into the MRR
For microinjection, a 0.5-μl microneedle (the tip OD = 0.25 mm, Hamilton, Reno, NV) prefilled with DAMGO (Sigma-Aldrich, St. Louis, MO) was inserted into the selected MRR region. DAMGO (0.35 μg/μl) was made in a solution of 0.9% saline containing 1% Chicago Sky Blue (Sigma Chemical, St. Louis, MO). According to the rat stereotaxic atlas of Paxinos and Watson (1998) and earlier studies (Zhang et al., 2007), the MRR, extending from 9–12 mm caudal to the bregma, was divided into three subregions – rMRR, mMRR, and cMRR – located at 9.0, 10.5, and 12.0 mm caudal to the bregma, respectively. The rMRR contained the magnus nucleus (RMg), the mMRR contained the RMg and its neighboring pallidus nucleus (RPa), and the cMRR contained the obscurus nucleus (ROb) and RPa. The central sites for the mMRR and rMRR were localized 9 mm ventral to the cerebellar surface, and each site received a 100-nl microinjection (35 ng DAMGO). Because the two nuclei in the cMMR were located separately and distantly, two injections (100 nl each) were given. The first injection was given when the needle was placed into the site 8.3 mm ventral to the cerebellar surface, corresponding to the center of the ROb. After this injection, the needle was advanced 1 mm deeper, corresponding to the RPa, for the second injection. Strategically, we chose the volume at which the microinjected DAMGO would act on a large enough number of cells within the raphe sites tested to evoke significant V̇e depression. According to the rat stereotaxic atlas of Paxinos and Watson (1998), each of the three subnuclei (RMg, RPa, ROb) covers a relatively large area (approximately 0.7– 1.0 mm3), centered at the middle. A volume of 100 nl was used because previous studies have calculated that microinjecting this volume into the brainstem could spread as far as ~1 mm3 (Lipski et al., 1988; Mitra et al., 1993). In fact, microinjecting this volume (100 nl) into the raphe (Nucci et al., 2008) and hypothalamus (Deolindo et al., 2008) of rats has been used by other investigators recently. DAMGO microinjections were made outside of the cMRR and mMRR in two rats to further confirm the site-dependence of the evoked responses to the DAMGO microinjection into the cMRR and mMRR. The microinjections were located at 12 mm caudal to bregma, 1 mm right to midline, and 8 mm and 9 mm ventral to the cerebellar surface, respectively, in one rat, and at 10.3 mm caudal to bregma, 1 mm right to midline, and 8.5 mm ventral to the cerebellar surface in another one. To block local μ-receptors, microinjections of CTAP (100 ng/100 nl containing 1% Chicago Sky Blue), a μ-receptor antagonist, were made in the cMRR and mMRR (100 nl for each injection).
2.3. Experimental protocols
Study Series I was designed to determine the influence of intra-MRR microinjection of DAMGO on the cardiorespiratory responses to hypoxia. After stabilization of the cardiorespiratory baseline values for at least 10 min, 10% O2 (balanced with nitrogen) for 1 min was given before and 5, 60, and/or 120 min after microinjection of DAMGO into the cMRR, mMRR or rMRR (n = 6 in each group).
Study Series II was performed to evaluate the contribution of MRR μ-receptors to the inhibition of the HVR by systemic administration of DAMGO. In our pilot experiment the activation of μ-receptors in the cMRR or mMRR but not the rMRR could attenuate the HVR, so this study series was carried out to test whether blockade of μ-receptors in both the cMRR and mMRR would diminish the systemic DAMGO-induced inhibition of the HVR. Six rats received the hypoxic challenge before and 5 min after systemic DAMGO (100 μg/kg), which is similar to our previous study (Zhang et al., 2007). Two hours later, the same protocols described above were repeated 3 min after CTAP (100 ng/100 nl) was microinjected into the cMRR and mMRR. Because systemic DAMGO has been reported to have an approximately 15-min half-life in mammals (Szeto et al., 2001; Zhang et al., 2007), this 2-h interval would ensure complete recovery from the first DAMGO injection before performing the second injection.
Study Series III was performed in 21 other rats divided into five groups. To determine whether the effect of microinjecting DAMGO on the HVR depression was uniquely mediated by μ-receptors (Group I, n = 4), CTAP was microinjected into the cMRR prior to local administration of DAMGO. In addition, the effects from microinjecting CTAP alone into the cMRR and mMRR on the baseline ventilation and the HVR were evaluated (Group II, n = 2). In order to clarify the specificity of DAMGO (Group III), the respiratory responses to intravenously administered and subsequently microinjected vehicle into the cMRR or mMRR were recorded (n = 5 each group). To test the site-dependency of the respiratory responses to local DAMGO on the cMRR and mMRR (Group IV, n = 2), DAMGO was microinjected outside of the cMRR and mMRR. The reproducibility of the HVR's response to systemic DAMGO was examined by repeating systemic DAMGO injection twice with an interval of 2 h (Group V, n = 3).
2.4. Identification of microinjection sites
After completing the experiment, the brainstem was removed and fixed by soaking in 4% paraformaldehyde (pH 7.4) for at least 36 h at 4°C and subsequently sectioned at a 40-μm thickness by a slicing machine (Leica, CM, 1850; Microsystems GMbH, Nussioch, Germany). Microinjections marked by Chicago Sky Blue were identified microscopically.
2.5. Data acquisition and statistical analysis
Raw data of the airflow signal, BP, HR, PETCO2, and rectal temperature were digitized, monitored, and recorded using a PowerLab/8sp (model ML 785; ADInstruments Inc., Colorado Springs, CO) connected to a computer employing the PowerLab Chart 5 software. The airflow signals were integrated to generate tidal volume (VT), respiratory frequency (fR), and minute ventilation (V̇e). After stabilization, the cardiorespiratory baseline was determined by averaging the variables for 1 min immediately before administration of DAMGO. The cardiorespiratory responses to hypoxia were determined by measuring the variables at the last 10-s period of the hypoxic exposure and expressed by the percent change from baseline (Δ%). All data are presented as means ± SE. Two-way analysis of variance (ANOVA) for repeated measures was employed to compare the baseline and hypoxic cardiorespiratory variables before and after DAMGO administration, and one-way ANOVA was used to compare the effects of systemic DAMGO on the hypoxic responses (Δ% change from baseline) without or with CTAP pretreatment. A Student's t-test was used to compare the effects of microinjection of DAMGO into the MRR on the hypoxic responses. The Fisher's least significant difference test was used if the overall ANOVA had a P value less than 0.05. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was employed for statistical analysis. Difference is considered significant at a P value < 0.05.
3. Results
3.1. Effect of microinjections of DAMGO into the MRR on baseline cardiorespiratory activity and their responses to hypoxia
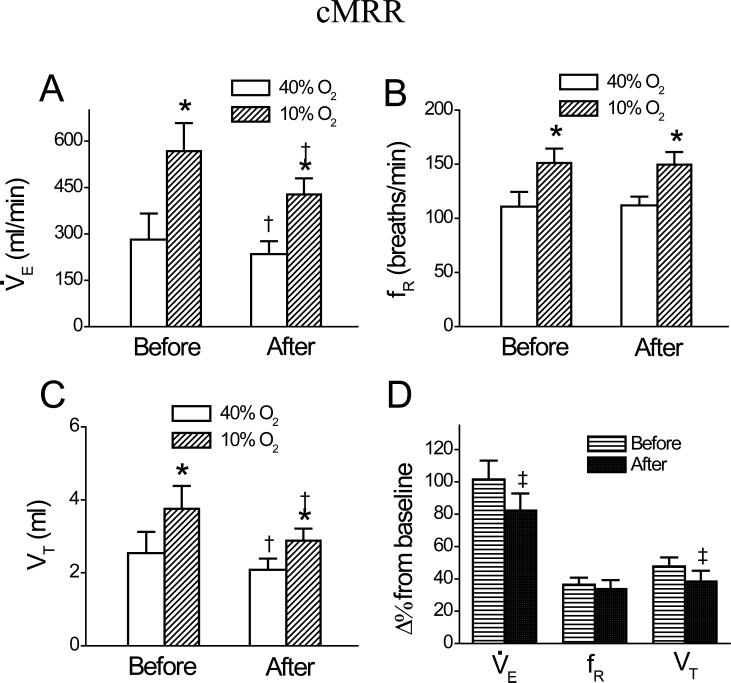
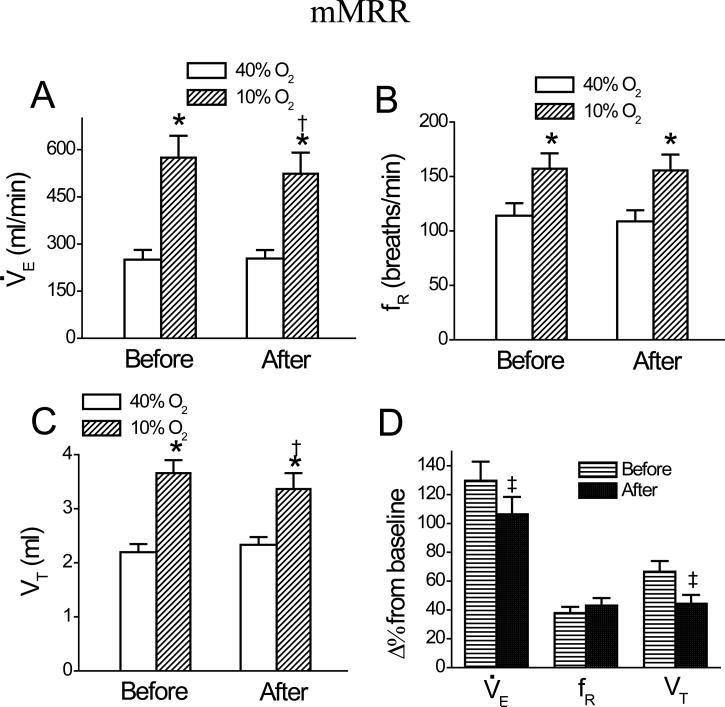
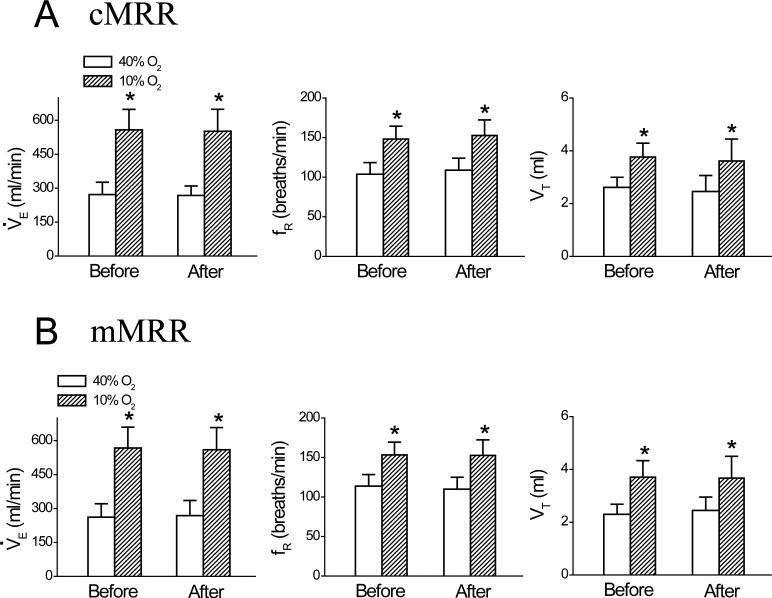
Microinjecting DAMGO into the cMRR significantly reduced the baseline V̇e by 17 ± 2%. A typical example and the corresponding group data (n = 6) are illustrated in Figs. 1 and 2, respectively. This microinjection also inhibited the HVR manifested by three measurements. First, as calculated from the values presented in Fig. 2A, DAMGO microinjection into the cMRR decreased the hypoxic V̇e by 25 ± 4%, which is significantly greater than the DAMGO-induced decrease in the baseline V̇e (17 ± 2%, P < 0.05). Second, hypoxia after intra-cMRR microinjection of DAMGO enhanced V̇e by 193 ± 21 ml/min, which was significantly smaller than that before this microinjection (286 ± 30 ml/min, P < 0.01). Third, the HVR (Δ% from baseline V̇e). after DAMGO (82 ± 10%) was significantly lower than that before DAMGO (103 ± 11%, P < 0.05, Fig. 2D). To test whether the depressant effect of DAMGO on the baseline V̇e and the HVR was uniquely mediated by opioid μ receptors, CTAP was microinjected into the cMRR prior to local administration of DAMGO (n = 4). We found that compared to the control (without drug treatment), intra-cMRR microinjection of DAMGO neither significantly altered the baseline respiration (V̇e , 271 ± 32 vs. 261 ± 31 ml/min; fR, 106 ± 7 vs. 103 ± 6 breaths/min; and VT, 2.5 ± 0.2 vs. 2.6 ± 0.2 ml) nor changed the hypoxic respiration (V̇e , 609 ± 51 vs. 600 ± 53 ml/min; fR, 158 ± 6 vs. 155 ± 7 breaths/min; and VT, 3.9 ± 0.2 vs. 3.9 ± 0.2 ml) after pretreating the cMRR with CTAP. Microinjection of DAMGO into the mMRR did not change baseline V̇e, fR, or VT, but it inhibited the HVR by 17% due to depressing the VT response (Fig. 3, n = 6). Usually, 60 min after administration, the DAMGO-induced ventilatory depression disappeared (Fig. 1,). Local vehicle microinjections into the cMRR and mMRR caused no significant changes in baseline or hypoxic V̇e , fR, or VT (Fig. 4, n = 5 each group).
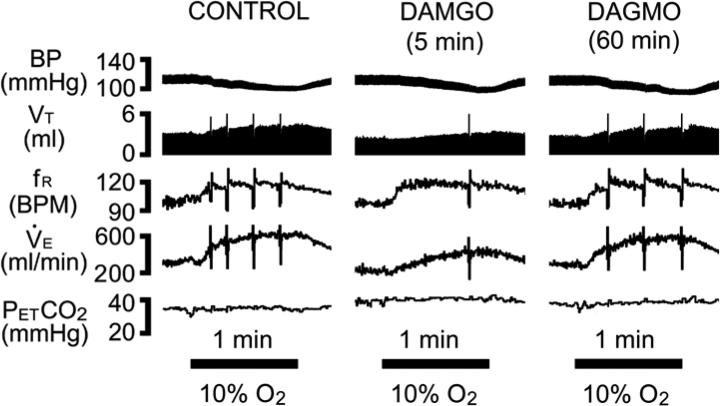
Fig. 1.
Experimental recordings of the cardiorespiratory responses to 10% O2 for 1 min before (left), 5 min (middle), and 60 min after (right) DAMGO was microinjected into the cMRR. Traces from top to bottom are arterial blood pressure (BP), tidal volume (VT), respiratory frequency (fR), minute ventilation (V̇e)., and end-tidal pressure of carbon dioxide (PETCO2). BPM= breaths/min. Sighs in VT, fR and V̇e traces are truncated to focus on the changes in these respiratory variables. The bars at the bottom reflect 1-min exposures to 10% O2.
Fig. 2.
Group data showing minute ventilation, V̇e (A); frequency, fR (B); and tidal volume, VT (C) during 40% O2 and 10% O2 and their responses to hypoxia (D) before and after intra-cMRR microinjection of DAMGO. Data are mean ± SE; n = 6; * P < 0.01 compared between 40% O2 and 10% O2; † P < 0.05 between before and after DAMGO under a given condition (40% O2 or 10% O2); ‡ P < 0.05 between the percent change (Δ%) change from baseline before and after DAMGO.
Fig. 3.
Group data showing minute ventilation, V̇e (A); frequency, fR (B); and tidal volume, VT(C) during 40% O2 and 10% O2 and their responses to hypoxia (D) before and after intramMRR microinjection of DAMGO. Data are mean ± SE; n = 6; * P < 0.01 compared between 40% O2 and 10% O2; † P < 0.05 between before and after DAMGO under a given condition (40% O2 or 10% O2); ‡ P < 0.05 between the percent change (Δ%) change from baseline before and after DAMGO.
Fig. 4.
Group data showing minute ventilation V̇e, frequency fR, and tidal volume VT during 40% O2 and 10% O2 before and after intra-cMRR (A) and intra-mMRR (B) microinjection of vehicle. Data are mean ± SE; n = 5 in each group; * P < 0.01 compared between 40% O2 and 10% O2.
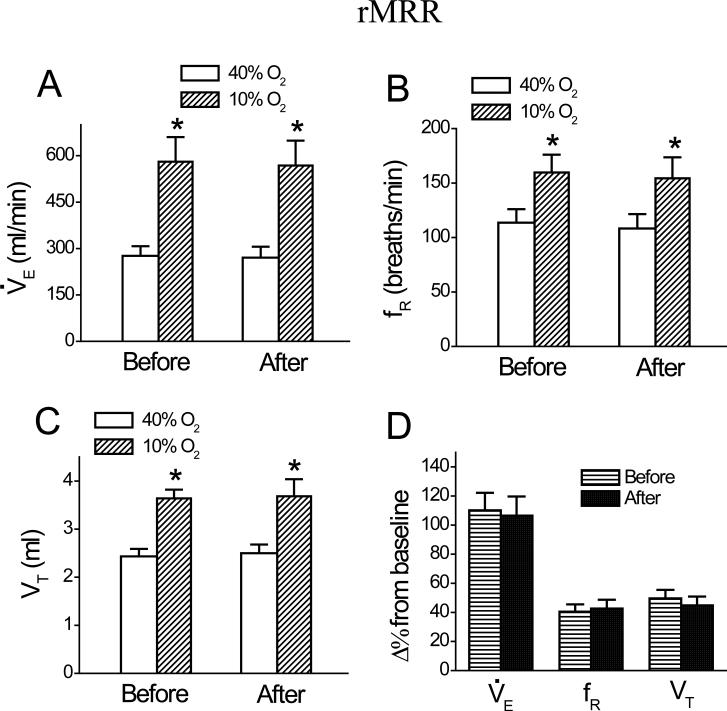
Microinjection of DAMGO into the rMRR did not change baseline or hypoxic V̇e , fR, or VT ( Fig. 5, n = 6). Similarly, microinjections of DAMGO 2 mm lateral to the raphe test sites in two other rats (Figs 6A, B) did not evoke significant changes in either baseline or hypoxic V̇e (271 ± 43 vs. 264 ± 51 ml/min; 554 ± 76 vs. 544 ± 79 ml/min, respectively), fR (113 ± 24 vs. 114 ± 26 breaths/min; 153 ± 25 vs. 156 ± 27 breaths/min, respectively), or VT (2.4 ± 0.6 vs. 2.3 ± 0.8 ml; 3.6 ± 0.6 vs. 3.5 ± 0.9 ml, respectively). The microinjection locations in the MRR and outside of the MRR are shown in Fig. 6. Generally, the spread of an injection marked by Chicago Sky Blue was approximately 1.0 mm (Fig. 6D). With respect to the cardiovascular activities, the baseline and hypoxic BP and HR were not markedly changed by microinjection of DAMGO into the cMRR, mMRR, or rMRR (data not shown).
Fig.5.
Group data showing minute ventilation, V̇e (A); frequency, fR (B); and tidal volume, VT (C) during 40% O2 and 10% O2 and their responses to hypoxia (D) before and after intra-rMRR microinjection of DAMGO. Data are mean ± SE; n = 6; * P < 0.01 compared between 40% O2 and 10% O2.
Fig. 6.
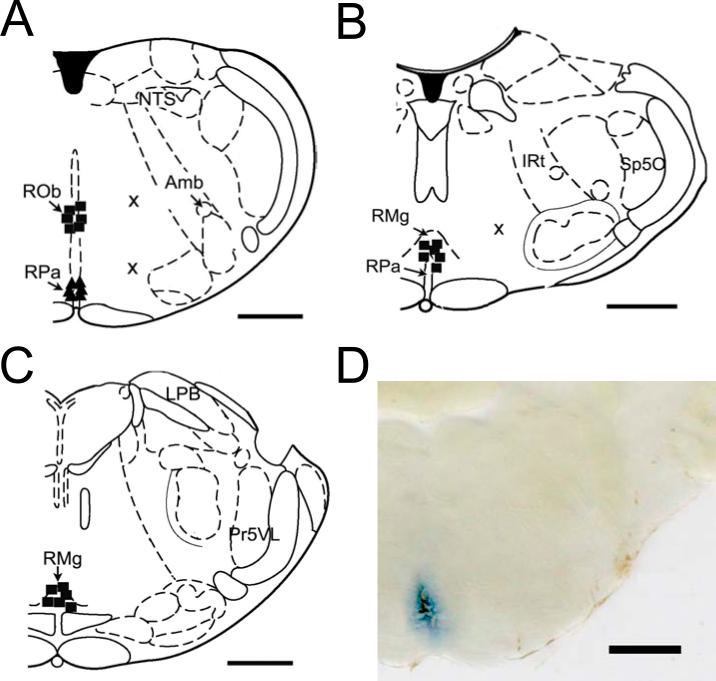
Diagram exhibiting the sites where the microinjections occurred within the caudal (cMRR), middle (mMRR), and rostral (rMRR) medullary raphe regions, respectively. The cMRR (A): the microinjections were made -11.6 to -12.3 mm caudal to bregma and either 8.3 mm (■) or 9.3 mm (▲) ventral to the cerebellar surface in six rats. Two other microinjections represented by “x” were made outside the cMRR in one rat. The mMRR (B): the microinjections (■) were confirmed at -10.04 to -10.52 mm caudal to the bregma and 9 mm ventral to the cerebellar surface in six rats with one microinjection outside the mMRR expressed by “x” in one rat. The rMRR (C): microinjections (■) were made in the rMRR -9.16 to -9.30 mm caudal to the bregma and 9 mm ventral to the cerebellar surface. Panel D displays a representative slice containing the mMRR, in which the microinjection was stained by Chicago Sky Blue. Amb, ambiguus nucleus; 7, facial nucleus; IRt, intermediate reticular; LPB, lateral parabrachial nucleus; NTS, nucleus of solitary tract; Pr5VL, ventrolateral part of principal sensory 5 nucleus; RMg, raphe magnus nucleus; RPa, raphe pallidus nucleus; ROb, raphe obscurus nucleus; RPa, raphe pallidus nucleus; Sp5O, oral part of spinal 5 nucleus.
3.2. Effect of systemic DAMGO on HVR
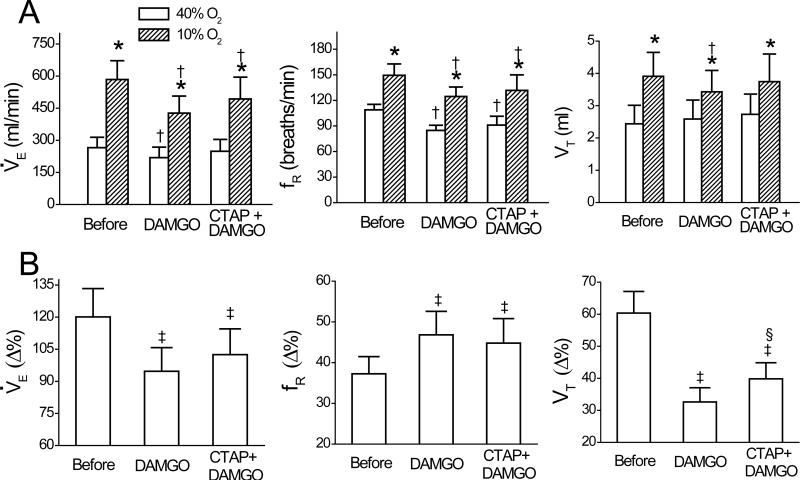
Intravenous administration of DAMGO significantly decreased baseline V̇e and fR but not VT, and it also reduced the V̇e, fR, and VT responses to hypoxia (Fig. 7A, n = 6). Systemic DAMGO lowered the hypoxic V̇e by 27 ± 4%, which was significantly greater than the DAMGO-induced decrease in the baseline V̇e (17 ± 2%, P < 0.05). Hypoxia after systemic DAMGO injection increased V̇e by 207 ± 31 ml/min, which was significantly smaller than that before DAMGO (319 ± 36 ml/min; P < 0.01). More importantly, the HVR (Δ% from baseline V̇e). after DAMGO (93% ± 11%) was significantly lower than that before DAMGO (120% ± 13%; P < 0.05) due to the depressed VT response. The hypotension response to hypoxia was not affected by systemic DAMGO (38 ± 4% vs. 36 ± 5%, P > 0.05). Intravenous injection of the vehicle (0.9% saline) produced no change in the baseline cardiorespiratory activities or their responses to acute hypoxia (not shown).
Fig. 7.
Comparison of the effects of systemic DAMGO and CTAP + DAMGO on respiratory variables before and during hypoxia (A) and their percent change (Δ%) response to hypoxia (B). CTAP + DAMGO represents microinjection of CATP into the caudal and middle medullary raphe region (cMRR and mMRR) prior to systemic DAMGO. Data are mean ± SE; n = 6; In panel A, * P < 0.01 compared between 40% O2 and 10% O2, † P < 0.05 between before and after DAMGO or CATP + DAMGO under a given condition (40% O2 or 10% O2). In panel B, ‡ P < 0.05 between the Δ% response to hypoxia before and after DAMGO or CATP + DAMGO; § P < 0.05 between the Δ% response to hypoxia in DAMGO and CTAP + DAMGO.
3.3. Influence of blocking MRR μ-receptors on the systemic DAMGO-induced attenuation of HVR
The baseline V̇e depression produced by systemic DAMGO injection alone disappeared after intra-cMRR and -mMRR microinjection of CTAP, a selective μ-receptor antagonist (Fig. 7A, n = 6). This pretreatment had a tendency to reduce systemic DAMGO-induced hypoxic ventilation depression by affecting the VT response, but this ventilatory change failed to reach significance (27% vs. 15%, P > 0.05, Fig. 7B). Systemic DAMGO significantly depressed baseline BP (124 ± 9 vs. 104 ± 11 mmHg, P < 0.05), and this depression was not changed by pretreatment with CTAP (119 ± 8 vs. 103 ± 9 mmHg, P < 0.05). As there was a 2-h interval between application of the first and second systemic DAMGO, we tested the reproducibility of the HVR over this period to rule out the effect of time-course (n = 3) and found that the effects of the first and second systemic DAMGO on the HVR were not remarkably different (-22 ± 3% vs. -20 ± 4% for V̇e; 25 ± 4% vs. 23 ± 4% for fR; -46 ± 5% vs. -45 ± 6% for VT). In addition, microinjection of CTAP alone into the cMRR and mMRR (n = 2) did not remarkably affect the baseline V̇e (275 ± 75 vs. 282 ± 81 ml/min), fR (105 ± 6 vs. 109 ± 10 breaths/min), or VT (2.6 ± 1 vs. 2.5 ± 1 ml); or the hypoxic V̇e (585 ± 94 vs. 591 ± 98 ml/min), fR (150 ± 13 vs. 152 ± 14 breaths/min), or VT (3.9 ± 1 vs. 3.8 ± 1 ml).
4. Discussion
4.1. Activation of μ-receptors in the cMRR and mMRR can depress HVR
Recent studies in humans point to a central inhibitory effect of opioids on the HVR. For example, intravenous administration of frakefamide, a peripherally acting μ-receptors agonist, failed to alter the HVR (Modalen et al., 2006). Intrathecal administration of morphine attenuated the early HVR (Bailey et al., 2000). Because morphine is hydrophilic and largely retained in the cerebrospinal fluid after intrathecal administration, the authors believed that a stronger depression of the HVR by intrathecal compared to intravenous administration suggested a central effect from opioids on the HVR. However, the central sites capable of exerting this opioid-induced inhibition of the HVR have not been fully identified. We found that microinjection of DAMGO into the cMRR or mMRR, but not the rMRR, attenuated the early HVR by ~20%, providing the evidence that among the MRR, cMRR, and mMRR μ-receptors are capable of attenuating the carotid body-mediated early HVR. It is worthy to note that this HVR attenuation is independent of the depressed baseline V̇e because intra-mMRR DAMGO microinjection did not depress the baseline V̇e, but it significantly attenuated the HVR. Moreover, the results from the three different measurements that subtracted or normalized the DAMGO-induced change in the baseline V̇e (see Results, Section 3.1) point to a depressed HVR by intra-cMRR administration of DAMGO.
Our new finding that cMRR and mMRR μ-receptors are capable of attenuating the carotid body-mediated early HVR is indirectly supported by previous studies. The neurons in the MRR reportedly express abundant μ-receptors (Pan et al., 1990; Ding et al., 1996; Porreca et al., 2001; Pinto et al., 2003) and participate in modulating the HVR (Perez & Ruiz, 1995; Gargaglioni et al., 2003; Penatti et al., 2006; Taylor et al., 2006; Nucci et al., 2008). Activation of cMRR and mMRR μ-receptors have been shown to depress the HCVR (Krause et al., 2006; Zhang et al., 2007). Moreover, studies indicated a pathway between the MRR and the nucleus tractus solitarius (Humphrey, 1967; Miura & Reis, 1969) where the peripheral chemoreceptor afferents make their first synapses. Our results show that μ-receptors in the cMRR and mMRR, but not in the rMRR, are capable of attenuating the HVR. Because the nuclei within the three MRR regions are reportedly involved in the HVR (Lalley, 1986; Gargaglioni et al., 2003) and the rMRR also expresses μ-receptors in the MRR (Ding et al., 1996; Kalyuzhny & Wessendorf, 1997), it is questionable why microinjecting DAMGO into the rMRR fails to alter the baseline V̇e and the HVR. Our interpretation is that μ-receptors in the rMRR may be involved mainly in nociception (Pan et al., 1990; Ding et al., 1996; Porreca et al., 2001; Pinto et al., 2003) with a limited effect on modulating respiration as compared to those in the cMRR and mMRR. Actually the different responses in the caudal-rostral plane was also observed in other study in which focal acidosis leads to a larger ventilatory response when applied to the caudal nucleus tractus solitarius (NTS) than the rostral NTS (Nattie & Li, 2002).
4.2. The contribution of cMRR and mMRR μ-receptors to the inhibition of HVR by systemic DAMGO is limited
In the present study, DAMGO administered systemically and microinjected locally into the MRR (cMRR and/or mMRR) results in a nearly identical reduction of both baseline V̇e (17% vs. 17%) and hypoxic V̇e (27% vs. 25%). Therefore, one might reason that the entire effect of systemically injected DAMGO is mediated by the cMRR and mMRR. To verify this assumption, we evaluated the role of cMRR and mMRR μ-receptors in the respiratory responses to systemically injected DAMGO. Surprisingly, pretreating the cMRR and mMRR with CTAP prevented the baseline V̇e depression after systemic DAMGO administration, but it failed to significantly alter the systemic DAMGO-induced HVR depression. This result raised two interesting questions. First, why is the systemic DAMGO-induced HVR depression not significantly affected by blocking the local μ-receptors although local DAMGO microinjection inhibits the HVR? To address this issue, we tested whether DAMGO exerts its inhibitory effects via acting on other types of opioid receptors instead of μ-receptor. As a result, DAMGO microinjected into the cMRR did not significantly alter either baseline V̇e or the HVR after blockade of local μ-receptors, indicating that the DAMGO-induced respiratory inhibitions are mediated by μ-receptors. An alternative explanation is that different from local microinjection, the DAMGO concentration in the MRR generated from systemic administration is not sufficient to significantly depress the HVR. In other words, other respiratory-related nuclei that are more crucial to the HVR and more sensitive to systemic DAMGO may dominantly contribute to the systemic DAMGO-induced HVR depression. Indeed, the nucleus tractus solitarius, known to be critical to the HVR (Housley & Sinclair, 1988), expresses higher μ-receptors compared to the MRR (Ding et al., 1996; Porreca et al., 2001). More importantly, we recently observed that the V̇e response to pure N2 for 10 s was completely blocked by microinjecting DAMGO into the nucleus tractus solitarius at a volume much smaller than that used in this study (Zhang et al., 2009). Second, why does local blockade of μ-receptors prevent the eupneic V̇e depression caused by systemic DAMGO administration with little effect on the HVR depression? It is well-established that eupneic breathing is maintained predominantly by CO2 rather than O2, and the MRR is critical in controlling the HCVR with relatively less importance placed on the HVR (Schlaefke, 1981; Taylor et al., 2005). Thus, it is reasonable to assume that activating MRR μ-receptors with systemic DAMGO administration significantly contributes to inhibiting the eupneic breathing and HCVR, with limited impact on the HVR. In fact, we have reported that the inhibitory effect on the HCVR from systemic DAMGO administration was diminished by 53% by microinjecting the same volume of CTAP into the cMRR (Zhang et al., 2007).
In the present study, systemic DAMGO administration depressed fR during eupneic breathing, and inhibited both VT and fR during hypoxia. This inconsistency may be related to the fact that the pool of neurons involved in maintaining eupneic breathing and in the evoked HVR are not the same. For example, CO2-chemosensitive neurons are mainly responsible for maintaining eupneic breathing with limited effect on the HVR. Therefore, the possibility that μ-receptors on a different pool of neurons differently impact the breathing pattern may contribute to the result that systemic DAMGO administration inhibits fR at rest, and both VT and fR during hypoxia.
4.3. Methodological Consideration
Due to technical difficulty, we were unable to determine the precise diffusion of the DAMGO microinjections into the MRR. Howerer, based on the diffusion of Chicago Sky Blue, the injection spread appears to be not larger than 1.0 mm (see Fig. 6D). Again, our data showing that DAMGO microinjections into the regions 1.0 mm outside the cMRR or the mMRR had no remarkable effect on V̇e support the conclusion that the spread was considerably limited. Consistent with our study, several investigators have previously calculated that microinjection of a volume of 100 nl into the brainstem could spread as far as 1.0 mm (Lipski et al., 1988; Mitra et al., 1993). However, we still cannot rule out the possible involvement of the vicinity of the MRR in the DAMGO-induced depression of the HVR.
4.4. Conclusion
In summary, our results show that the HVR was significantly attenuated by DAMGO administered systemically and microinjected locally into the cMRR and mMRR, respectively, in anesthetized rats. However, blockade of cMRR μ-receptors did eliminate the local DAMGO-induced HVR depression, but this blockade coupled with blocking mMRR μ-receptors did not significantly alter the systemically administered DAMGO-induced HVR attenuation. We conclude that cMRR and mMRR μ-receptors are capable of attenuating the HVR, but their role in systemically administered DAMGO-induced depression of the HVR seems to be limited.
Acknowledgements
The authors thank Jianguo Zhuang, M.D., Ph.D. (Associate research scientist), Pathophysiology Program, Lovelace Respiratory Research Institute, Albuquerque, New Mexico, for his assistance in the experiment setup and statistical analysis.
Funding: This study is supported by the National Heart, Lung, and Blood Institute Grant HL074183, Bethesda, MD and American Lung Association Grant (RT-83131-N), New York, NY.
REFERENCES
- Bailey PL, Lu JK, Pace NL, Orr JA, White JL, Hamber EA, Slawson MH, Crouch DJ, Rollins DE. Effects of intrathecal morphine on the ventilatory response to hypoxia. N Engl J Med. 2000;343:1228–1234. doi: 10.1056/NEJM200010263431705. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol. 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AL, editors. Regulation of Breathing. Marcel Dekker Inc.; 1995. p. 617. [Google Scholar]
- Colman AS, Miller JH. Lack of involvement of mu(1) opioid receptors in dermorphin-induced inhibition of hypoxic and hypercapnic ventilation in rat pups. Respir Physiol Neurobiol. 2002;131:199–212. doi: 10.1016/s1569-9048(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998;88:903–913. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Deolindo M, Pelosi GG, Tavares RF, Aguiar Correa FM. The ventrolateral periaqueductal gray is involved in the cardiovascular response evoked by l-glutamate microinjection into the lateral hypothalamus of anesthetized rats. Neuroscience letters. 2008;430:124–129. doi: 10.1016/j.neulet.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Coimbra NC, Branco LG. The nucleus raphe magnus modulates hypoxia-induced hyperventilation but not anapyrexia in rats. Neurosci Lett. 2003;347:121–125. doi: 10.1016/s0304-3940(03)00671-2. [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DR. Neuronal activity in the medulla oblongata of the cat evoked by stimulation of the carotid sinus nerve. In: Kezdi P, editor. Baroreceptors and Hypertension. Pergamon Press; Toronto: 1967. pp. 131–168. [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. CNS GABA neurons express the mu-opioid receptor: immunocytochemical studies. Neuroreport. 1997;8:3367–3372. doi: 10.1097/00001756-199710200-00035. [DOI] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. British journal of pharmacology. 1986;88:889–898. doi: 10.1111/j.1476-5381.1986.tb16263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Pan LG, Davis S, Opansky C, Qian B. DAMGO increases CO2 sensitivity and disrupts respiratory rhythm and pattern when injected into the pre-Botzinger Complex (pre-Botz C) of awake goats. FASEB J Abstr. 2006;20:A370. [Google Scholar]
- Kryger MH, Yacoub O, Dosman J, Macklem PT, Anthonisen NR. Effect of meperidine on occlusion pressure responses to hypercapnia and hypoxia with and without external inspiratory resistance. Am Rev Respir Dis. 1976;114:333–340. doi: 10.1164/arrd.1976.114.2.333. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol. 1986;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. Journal of neuroscience methods. 1988;26:169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science (New York, NY. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. British journal of pharmacology. 1980;71:297–305. doi: 10.1111/j.1476-5381.1980.tb10939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra J, Dev NB, Trivedi R, Amini S, Ernsberger P, Cherniack NS. Intramedullary sodium cyanide injection on respiratory and vasomotor responses in cats. Respiration physiology. 1993;93:71–82. doi: 10.1016/0034-5687(93)90069-m. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. Termination and secondary projections of carotid sinus nerve in the cat brain stem. The American journal of physiology. 1969;217:142–153. doi: 10.1152/ajplegacy.1969.217.1.142. [DOI] [PubMed] [Google Scholar]
- Modalen AO, Quiding H, Frey J, Westman L, Lindahl S. A novel molecule with peripheral opioid properties: the effects on hypercarbic and hypoxic ventilation at steady-state compared with morphine and placebo. Anesth Analg. 2006;102:104–109. doi: 10.1213/01.ANE.0000184254.85567.80. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci TB, Branco LG, Gargaglioni LH. 5-HT1A, but not 5-HT2 and 5-HT7, receptors in the nucleus raphe magnus modulate hypoxia-induced hyperpnoea. Acta physiologica (Oxford, England) 2008;193:403–414. doi: 10.1111/j.1748-1716.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006;101:1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S. Medullary responses to chemoreceptor activation are inhibited by locus coeruleus and nucleus raphe magnus. Neuroreport. 1995;6:1373–1376. doi: 10.1097/00001756-199507100-00003. [DOI] [PubMed] [Google Scholar]
- Pinto M, Lima D, Castro-Lopes J, Tavares I. Noxious-evoked c-fos expression in brainstem neurons immunoreactive for GABAB, mu-opioid and NK-1 receptors. Eur J Neurosci. 2003;17:1393–1402. doi: 10.1046/j.1460-9568.2003.02586.x. [DOI] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr., Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg R, Olofsen E, Sarton E, Teppema L, Dahan A. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003;99:788–798. doi: 10.1097/00000542-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Santiago TV, Johnson J, Riley DJ, Edelman NH. Effects of morphine on ventilatory response to exercise. J Appl Physiol. 1979;47:112–118. doi: 10.1152/jappl.1979.47.1.112. [DOI] [PubMed] [Google Scholar]
- Schlaefke ME. Central chemosensitivity: a respiratory drive. Reviews of physiology, biochemistry and pharmacology. 1981;90:171–244. doi: 10.1007/BFb0034080. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, Desiderio DM, Schiller PW. In vivo pharmacokinetics of selective mu-opioid peptide agonists. The Journal of pharmacology and experimental therapeutics. 2001;298:57–61. [PubMed] [Google Scholar]
- Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Influences of gender and sex hormones on hypoxic ventilatory response in cats. J Appl Physiol. 1991;71:1746–1751. doi: 10.1152/jappl.1991.71.5.1746. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511(Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med. 1975;292:1103–1106. doi: 10.1056/NEJM197505222922106. [DOI] [PubMed] [Google Scholar]
- Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol. 1989;33:1–16. doi: 10.1016/0301-0082(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus of the solitary tract in the medulla oblongata: a DiI labeling study. Auton Neurosci. 2003;105:131–144. doi: 10.1016/S1566-0702(03)00027-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology. 2007;107:288–297. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang C, Xu F. Stimulating opioid mu-receptors in the commissural subdivision of the nucleus tractus solitarius (cNTS) abolishes the carotid body-mediated ventilatory response in anesthetized rats. Experimental Biology Abstrct, Program # 62120. 2009.