Abstract
Purpose
Epigenetic aberrations have been reported in hepatocellular carcinoma (HCC). In this study of patients with unresectable HCC and chronic liver disease, epigenetic therapy with the histone deacetylase inhibitor belinostat was assessed. The objectives were to determine dose-limiting toxicity and maximum-tolerated dose (MTD), to assess pharmacokinetics in phase I, and to assess activity of and explore potential biomarkers for response in phase II.
Patients and Methods
Major eligibility criteria included histologically confirmed unresectable HCC, European Cooperative Oncology Group performance score ≤ 2, and adequate organ function. Phase I consisted of 18 patients; belinostat was given intravenously once per day on days 1 to 5 every 3 weeks; dose levels were 600 mg/m2 per day (level 1), 900 mg/m2 per day (level 2), 1,200 mg/m2 per day (level 3), and 1,400 mg/m2 per day (level 4). Phase II consisted of 42 patients. The primary end point was progression-free survival (PFS), and the main secondary end points were response according to Response Evaluation Criteria in Solid Tumors (RECIST) and overall survival (OS). Exploratory analysis was conducted on pretreatment tumor tissues to determine whether HR23B expression is a potential biomarker for response.
Results
Belinostat pharmacokinetics were linear from 600 to 1,400 mg/m2 without significant accumulation. The MTD was not reached at the maximum dose administered. Dose level 4 was used in phase II. The median number of cycles was two (range, one to 12). The partial response (PR) and stable disease (SD) rates were 2.4% and 45.2%, respectively. The median PFS and OS were 2.64 and 6.60 months, respectively. Exploratory analysis revealed that disease stabilization rate (complete response plus PR plus SD) in tumors having high and low HR23B histoscores were 58% and 14%, respectively (P = .036).
Conclusion
Epigenetic therapy with belinostat demonstrates tumor stabilization and is generally well-tolerated. HR23B expression was associated with disease stabilization.
INTRODUCTION
The outlook of patients with unresectable hepatocellular carcinoma (HCC) is poor, mainly because of advanced-stage disease at presentation and often the presence of concomitant chronic liver disease, which is mainly associated with chronic hepatitis B virus (HBV) infection in Asia. To date, the only systemic agent that has been shown to provide survival benefit over best supportive care is sorafenib.1,2 However, the overall prognosis of patients with HCC remains poor.
It has been well established that, in addition to numerous genetic abnormalities, epigenetic changes play an important role in gene expression and HCC pathogenesis.3–8 The process involves at least two interactive epigenetic modifications that result in transcriptional silencing of a variety of genes, including those implicated in the regulation of cell survival, proliferation, differentiation, and apoptosis. The first mechanism involves methylation of CpG islands located in the gene promoter regions of a series of tumor suppressor genes. The second involves histone acetylation that depends on the equilibrium between the activities of two groups of enzymes, histone acetyltransferases and histone deacetylases (HDACs).3
Reversal of these epigenetic processes and upregulation of genes are important to reverse the malignant phenotype and have become a potential therapeutic strategy in cancer treatment.8–12 Belinostat (PXD101; N-hydroxy-3-(phenylsulphamoylphenyl) acrylamide; molecular weight, 318) is a potent HDAC inhibitor (HDACI), which contains a zinc-chelating hydroxamic acid moiety.13 In HCC cell lines and xenografts, HDACIs, including belinostat, induce apoptosis and tumor regression but do not affect normal hepatocytes.8,13–17
We conducted a phase I/II study of belinostat in patients with unresectable HCC who had concomitant chronic liver disease. The objectives in the phase I part of the study were to determine dose-limiting toxicity (DLT) and maximum-tolerated dose (MTD) and to assess the pharmacokinetics of belinostat. Once the MTD was determined, the phase II portion of the study was conducted to determine the activity of epigenetic therapy with belinostat.
Although promising results have been shown with the use of HDACIs in a number of tumor types, data are limited on potential biomarkers that could enable appropriate selection of tumors that are likely to undergo a favorable clinical response. Recently, HR23B has been suggested to be a biomarker for clinical response to HDACIs.18 Thus, in this study, an exploratory analysis was also conducted to determine whether the expression of HR23B might be predictive for response to belinostat in HCC.
PATIENTS AND METHODS
This multicenter phase I/II trial was initiated in October 2006. Centers that participated in the phase I portion of the study were selected from Cancer Therapeutics Research Group (CTRG) sites. Centers that participated in the phase II portion of the study were selected from the Mayo Phase 2 Consortium (P2C) sites, along with the CTRG sites. The institutional review boards of each participating center approved the study. In addition to obtaining consent to participate in the study, consent was also specifically sought from patients for the exploratory analysis, which involved using tissue obtained for diagnosis for the exploratory study. The study was sponsored by the Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). Registration of patients onto the phase I study was conducted at the Comprehensive Cancer Trials Unit of the Department of Clinical Oncology, Chinese University of Hong Kong (Hong Kong, China), and registration of patients in the phase II portion of the study was conducted by the Mayo P2C. The Comprehensive Cancer Trials Unit at the Chinese University of Hong Kong designed and coordinated the trial and was responsible for all aspects of data collection and analysis. This study has been registered at ClinicalTrials.gov.
Eligibility
Eligibility criteria included histologically/cytologically confirmed unresectable HCC, European Cooperative Oncology Group (ECOG) performance score ≤ 2, measurable disease (≥ one lesion with longest diameter > 10 mm on spiral computed tomography [CT]), life expectancy more than 12 weeks, absolute neutrophil count ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, serum creatinine ≤ 150 μmol/L, total bilirubin ≤ 30 μmol/L, albumin ≥ 28 g/L, ALT ≤ 5.0 × institutional upper limit of normal (UNL), alkaline phosphatase ≤ 6 × UNL, prothrombin time ≤ 4 seconds × ULN, and absence of clinical ascites.
The main exclusion criteria were Child's class C cirrhosis, other treatments less than 4 weeks before study entry or unrecovered adverse events due to agents received more than 4 weeks earlier, history of allergic reactions to compounds similar to belinostat, and significant cardiovascular disease, including marked prolongation of QT/QTc interval on ECG.
Pretreatment Evaluation
All patients underwent complete medical history and physical examination, blood profiles, ECG, and CT scan of abdomen and/or other disease sites.
Treatment Plan
Belinostat was added to 250 mL of 5% dextrose in water or 0.9% sodium chloride and administered intravenously over 30 minutes once per day on days 1 to 5, every 3 weeks. All patients received standard antiemetics of 5-HT3 antagonist with or without dexamethasone. Patients who were known to have chronic HBV were also given lamivudine before study treatment.
Phase I Study
For the phase I portion of the study, there were five dose levels of belinostat: 300 mg/m2 per day (level −1), 600 mg/m2 per day (level 1), 900 mg/m2 per day (level 2), 1,200 mg/m2 per day (level 3), and 1,400 mg/m2 per day (level 4). Level 1 was the starting dose level. DLT was defined during cycle 1 as any grade 4 hematologic toxicity; grade ≥ 3 nonhematologic toxicity (excluding alopecia); grade 3 nausea, vomiting, or diarrhea that did not respond to therapy; and treatment delay of more than 2 weeks.
Dose escalation was based on the modified Fibonacci method.19 The MTD was defined as the dose at which more than two of three or more than two of six patients experienced DLT. Three additional patients were entered onto the MTD portion of the trial to further define toxicity. The recommended phase II dose was defined as one dose below the MTD.
For each subsequent cycle, treatment was delayed if the absolute neutrophil count was less than 1.5 × 109/L or the platelet count was less than 100 × 109/mL on the scheduled day of drug administration. Patients who experienced grade 3 nonhematologic toxicity and any grade 4 hematologic toxicity continued to receive belinostat at the next lower dose level on resolution of all toxicities to grade 1. The drug was discontinued for grade 4 nonhematologic toxicity. For an individual, there could be a limit of two dose de-escalations for serious toxicity.
Treatment was continued, provided that toxicities were tolerable or until one of the following criteria applied: disease progression, intercurrent illness that prevented further treatment administration, unacceptable adverse events, patient's decision, or investigator's judgment.
Phase II Study
In the phase I part of the trial, since the MTD was not reached, the highest dose level (1,400 mg/m2 per day) was declared as the recommended phase II dose. The last six patients enrolled onto the phase I study were included in the analysis of the phase II study.
After cycle 1, dose modification for further treatment was similar to that for phase I patients except for grade 4 toxicity, in which belinostat treatment was not terminated but was decreased by one dose level.
Definitions of Response and Toxicity
CT assessment was performed after every two cycles and reviewed at individual sites. Tumor response assessment was according to the Response Evaluation Criteria in Solid Tumors (RECIST) Committee.20 Toxicity was graded according to National Cancer Institute Common Toxicity Criteria v3 (NCI-CTC v3). ECGs were reviewed at central as well as individual sites.
Protocol for Phase I Pharmacokinetic Studies and Methodology for HR23B Immunohistochemistry
Thirty-eight patients (seven in phase I and 31 in phase II) had pretreatment tissues available for this analysis. The details of the protocol for the phase I pharmacokinetic studies21 and the methodology for HR23B immunohistochemistry are provided in the Appendix (online only).
A semiquantitative evaluation of HR23B immunoreactivity was carried out by using a histoscore, which relates to the nuclear expression of HR23B in tumor cells. An intensity score of 0 to 3 was assigned for the intensity of tumor cells (0, none; 1, weak; 2, intermediate; 3, strong). A proportional score in percentage was given by the estimated proportion of positive tumor cells. To assess the average degree of staining within a tumor, multiple regions were analyzed. The formula for the histoscore is histoscore = Σ(I × Pi), where I is the intensity of nuclear staining and Pi is the percentage of stained tumor cells. The scoring was independently assessed by A.W.H.C. and J.H.M.T. who were blinded to the clinical outcomes.
Statistical Methods
For the phase II portion of the study, the primary end point was progression-free survival (PFS). The secondary end points were response according to RECIST, overall survival (OS), and toxicity. All patients were included in the analyses. The PFS was assessed from day 1 of treatment cycle 1 to the date when objective disease progression was observed. OS was calculated from day 1 of treatment cycle 1 to the date of death. Death was regarded as a progression event in those patients who died before disease progression. Patients without documented objective progression at the time of the final analysis were censored at the date of their last tumor assessment. Survival curves were constructed by using the Kaplan-Meier method.
Estimation of sample size for the phase II study was based on the following. For patients with HCC receiving a standard treatment, the median PFS would be approximately 1.4 months. We postulated that belinostat would achieve a median PFS of approximately 2.8 months and the corresponding lower limit of the 95% CI should exceed 1.4 months to conclude that the study drug had sufficient antitumor activity. Assuming the PFS follows an exponential distribution, we needed to observe at least 28 events to achieve this level of accuracy for the 95% CI with 90% power. To observe enough events for the study, we needed to enroll 42 patients and observe all patients for at least 3 months.
Exploratory analysis on HR23B expression was viewed as hypothesis generating. The optimal cutoff was determined by the receiver operating characteristic curve distribution analysis.22,23 Of a total histoscore of 300, the threshold for differentiating between positive and negative HR23B immunostaining was set at 100; tumors with histoscore less than 100 were categorized as “low histoscore”; those with scores ≥ 100 were categorized as “high histoscore.” Response rates in terms of disease stabilization (complete response [CR] plus partial response [PR] plus stable disease [SD]) for patients in association with HR23B histoscores (high or low) were compared by using Fisher's exact test and proportional hazard models, respectively.
RESULTS
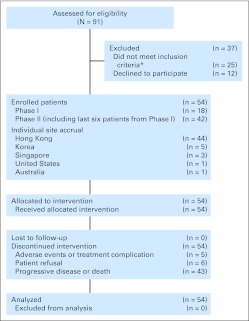
From October 2006 to December 2009, a total of 54 patients provided consent and were entered onto the study (Fig 1).
Fig 1.
Trial profile. (*) Reasons for not meeting inclusion criteria: poor liver functions (n = 14; including bilirubin > 30 μmol/L [n = 11], ALP > 600 IU/L [n = 1], ALT, 5 × upper limit of normal [n = 1], albumin < 20 g/dL [n = 1]), platelet count < 100 × 109/L (n = 5), poor performance status (n = 3), abnormal electrocardiogram (n = 1), active wound infection (n = 1), and liver biopsy unable to confirm hepatocellular carcinoma (n = 1).
Phase I Study
Eighteen patients were entered onto the study: three at level 1, three at level 2, six at level 3, and six at level 4. The median age was 57.9 years (range, 38 to 54 years). Sixteen (89%) were male, 14 (78%) had ECOG performance score of 0, all had Child's class A cirrhosis, 17 (94%) had chronic HBV, and one was negative for hepatitis B and C serology.
DLTs included one grade 3 increased ALT, one grade 3 diarrhea (subsequently resolved), one grade 3 abdominal distension (due to cirrhotic ascites), and one grade 3 anemia. At the maximum dose of 1,400 mg/m2 per day, MTD was not reached.
Pharmacokinetics Study
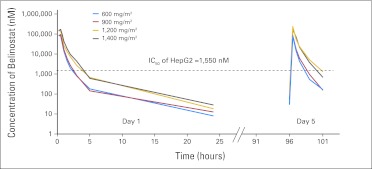
Pharmacokinetics of belinostat on day 1 were linear over the dose range of 600 to 1,400 mg/m2. The maximum plasma concentration (range, 28,052 to 58,234 ng/mL) and area under the plasma concentration-time curve (range, 19,499 to 47,503 hours·ng/mL) increased linearly with dose (Table 1). No drug accumulation was detected after 5 days of repeat dosing at these dose levels. Previously, our group reported that acetylated H3 expression increased significantly when the incubation concentration of belinostat on HepG2 cell lines reached 1 μmol/L and above.24 The estimated efficacious exposure concentration was defined as 1.55 μmol/L for the HepG2 cell line in preclinical models.16 Belinostat concentrations were above this for more than 2 hours at 600 and 900 mg/m2 and 4 hours at 1,200 and 1,400 mg/m2, respectively (Fig 2).
Table 1.
Pharmacokinetic Parameters for Belinostat After 30 Minutes of Intravenous Infusion
| Study Time | Parameter* | Dose Level (mg/m2) |
|||
|---|---|---|---|---|---|
| 600 | 900 | 1,200 | 1,400 | ||
| Day 1 | No. of patients | 3 | 3 | 6 | 22 |
| Cmax, ng/mL | 28,052 ± 2,710 | 31,308 ± 9,443 | 48,800 ± 16,024 | 58,234 ± 17,865 | |
| tmax, hours | 0.35 | 0.42 | 0.42 | 0.45 | |
| AUC0-5 hours, h · ng/mL | 18,929 ± 136 | 21,796 ± 5,490 | 36,887 ± 10,540 | 45,596 ± 13,910 | |
| AUC0-24 hours, h · ng/mL | 19,499 ± 370 | 22,267 ± 5,485 | 38,988 ± 11,781 | 47,503 ± 14,044 | |
| t1/2, hours | 3.54 ± 0.34 | 4.07 ± 0.39 | 4.14 ± 0.42 | 3.49 ± 0.73 | |
| Cl, L/h | 52.6 ± 3.75 | 70.5 ± 17.9 | 54.6 ± 15.8 | 54.1 ± 18.0 | |
| Vz, L | 268 ± 26.6 | 409 ± 76.7 | 305 ± 186 | 283 ± 151 | |
| Day 5 | AUC0-5 hours, h · ng/mL | 17,770 ± 1,323 | 20,485 ± 4,965 | 32,530 ± 8,054 | 52,555 ± 16,535 |
| Day 5/day 1† | AUC ratio | 0.94 | 0.94 | 0.88 | 1.15 |
Abbreviations: AUC, area under the serum concentration-time curve; AUC0-t, AUC from 0 to t hours; Cmax, maximum serum concentration; Cl, clearance; t1/2, terminal half-life; tmax, time to maximum serum concentration; Vz, volume of distribution.
Mean ± standard deviation except for tmax.
AUC ratio is calculated based on AUC0-5 hours on day 5 divided by AUC0-5 hours on day 1.
Fig 2.
Mean plasma disposition curve for belinostat at increasing dose levels. IC50, half maximal inhibitory concentration.
Phase II Study
The following analyses pertain to the 42 patients who were being enrolled onto the phase II study. The belinostat dose of 1,400 mg/m2 per day on days 1 to 5 every 3 weeks was used. Patient characteristics are provided in Table 2. The follow-up data were frozen on August 31, 2011. The median follow-up was 25.32 months (95% CI, 24.07 to 35.74 months). The median number of cycles was two (range, one to 12 cycles).
Table 2.
Baseline Characteristics of Patients in the Phase II Study
| Characteristic | No. of Patients | % |
|---|---|---|
| Total No. of patients | 42 | 100 |
| Sex | ||
| Male | 38 | 90 |
| Female | 4 | 10 |
| Age, years | ||
| Median | 57.5 | |
| Range | 35-76 | |
| ECOG performance status | ||
| 0 | 24 | 57 |
| 1 | 18 | 43 |
| Positive hepatitis status | ||
| Hepatitis B | 35 | 83 |
| Hepatitis C | 0 | 0 |
| Missing | 3 | 7 |
| Child-Pugh grading for cirrhosis | ||
| A | 41 | 98 |
| B | 1 | 2 |
| Baseline AFP > ULN | ||
| Yes | 34 | 81 |
| No | 8 | 19 |
| Tumor burden | ||
| Macroscopic vascular invasion | 5 | 12 |
| Extrahepatic disease | 31 | 74 |
| Prior therapy for HCC of any form | 34 | 81 |
| No. of prior systemic therapies | ||
| 0 | 26 | 62 |
| 1 | 11* | 26 |
| 2 | 4† | 10 |
| 3 | 1‡ | 2 |
| Prior local therapy with or without regional therapy | ||
| Surgery | 15 | 36 |
| Radiofrequency ablation | 3 | 7 |
| Transarterial therapy | 21 | 50 |
| Radiotherapy | 3 | 7 |
| Other prior therapy | 2§ | 5 |
Abbreviations: AFP, alpha fetoprotein; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; ULN, upper limit of normal.
Eight patients had single-agent cytotoxic chemotherapy, one had combination cytotoxic chemotherapy, three had antiangiogenic agents, two had unknown type of systemic therapy.
Two patients had two lines of cytotoxic chemotherapy (one line of single-agent cytotoxic chemotherapy and one line of combination cytotoxic chemotherapy), one had one line of single-agent cytotoxic chemotherapy and one line of antiangiogenic agent, and one had one line of combination cytotoxic chemotherapy and one line of unknown agent.
One patient had two lines of cytotoxic chemotherapy (one line of single-agent cytotoxic chemotherapy and one line of combination cytotoxic chemotherapy) and another unknown type of systemic therapy.
Treatment was not specified in these two patients.
Response and Survival
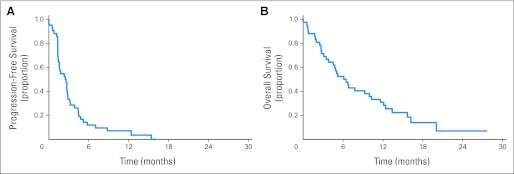
Five patients were not evaluated with CT assessments because of clinical deterioration; they were clinically assessed to have disease progression. Among the 42 patients, there was one patients with PR (2.4%), 19 with SD (45.2%), and 22 with PD (52.4%). For the patient who achieved PR, her disease was mainly confined to large peritoneal metastases; she received a total of 12 cycles of therapy, after which she developed disease progression; she has remained alive with disease 36 months after initiating belinostat therapy. Overall, the median PFS was 2.64 months (95% CI, 1.55 to 3.17 months; Appendix Fig A1A, online only). The median OS was 6.60 months (95% CI, 4.53 to 11.60; Appendix Fig A1B).
Toxicity
Toxicity was assessable in all 42 patients (Table 3). Grade ≥ 3 toxicity occurred in more than 5% of the patients and included abdominal pain, hyperbilirubinemia, increased ALT, anemia, and vomiting. Two patients had grade 4 anemia: one was associated with a bleeding peptic ulcer and the other was because of disease progression with hepatic rupture. No significant drug-related neutropenia or thrombocytopenia was observed. Mild fatigue and injection site reaction were common and occurred in approximately 50% of the patients. One patient developed sudden death; the patient went abroad and had no record of hospital admission before his death, which was determined to be the result of disease progression and was not likely to be the result of study medication.
Table 3.
Hematologic and Nonhematologic Toxicities According to National Cancer Institute Common Toxicity Criteria Version 3.0 (n = 42)
| Toxicity | Worst Grade |
|||||
|---|---|---|---|---|---|---|
| 1-2 |
3 |
4 |
||||
| No. | % | No. | % | No. | % | |
| Anemia | 0 | 1 | 2.4 | 2 | 4.8 | |
| Abdominal pain | 12 | 28.6 | 3 | 7.1 | 1 | 2.4 |
| ALT | 1 | 2.4 | 4 | 9.5 | 0 | |
| Hyperbilirubinemia | 0 | 4 | 9.5 | 0 | ||
| Vomiting | 14 | 33.3 | 3 | 7.1 | 0 | |
| Distension | 5 | 11.9 | 2 | 4.8 | 0 | |
| Prolonged QTc | 1 | 2.4 | 2 | 4.8 | 0 | |
| Dehydration | 0 | 2 | 4.8 | 0 | ||
| Hemorrhage, other | 0 | 2 | 4.8 | 0 | ||
| Anorexia | 14 | 33.3 | 1 | 2.4 | 0 | |
| Nausea | 14 | 33.3 | 1 | 2.4 | 0 | |
| Diarrhea | 8 | 19.0 | 1 | 2.4 | 0 | |
| Edema, limb | 7 | 16.7 | 1 | 2.4 | 0 | |
| Hiccoughs | 7 | 16.7 | 1 | 2.4 | 0 | |
| Allergic reaction | 3 | 7.1 | 1 | 2.4 | 0 | |
| Fatigue | 20 | 47.6 | 0 | 0 | ||
| Injection site reaction | 20 | 47.6 | 0 | 0 | ||
| Constipation | 14 | 33.3 | 0 | 0 | ||
| Insomnia | 12 | 28.6 | 0 | 0 | ||
| Dizziness | 9 | 21.4 | 0 | 0 | ||
| Mucositis, oral cavity | 5 | 11.9 | 0 | 0 | ||
| Cough | 4 | 9.5 | 0 | 0 | ||
| Dyspnea | 4 | 9.5 | 0 | 0 | ||
| Fever | 4 | 9.5 | 0 | 0 | ||
| Rash | 4 | 9.5 | 0 | 0 | ||
| Taste alteration | 4 | 9.5 | 0 | 0 | ||
| Urinary frequency | 4 | 9.5 | 0 | 0 | ||
Exploratory Analysis
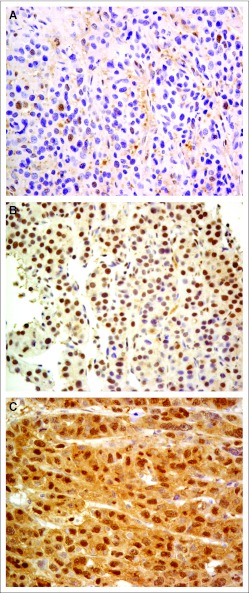
Of the 38 patients with pretreatment tumor tissues available for this analysis, there was one patient with PR, 18 with SD, and 19 with PD. The HR23B histoscores ranged from 30 to 265; Figure 3 illustrates tumors with different HR23B histoscores. Thirty-one patients had tumors with high HR23B histoscores; 18 (58%) achieved disease stabilization (PR plus SD) and 13 (32%) had PD. Seven patients had tumors with low HR23B histoscores; only one (14%) achieved disease stabilization and six (86%) had PD. The difference was statistically significant, with high HR23B histoscores associated with a higher rate of disease stabilization (P = .036).
Fig 3.
Immunohistochemical staining of HR23B of pretreatment tumor tissues: (A) tumor with histoscore 30/300 (low histoscore), (B) tumor with histoscore 150/300 (high histoscore), and (C) tumor with histoscore 255/300 (high histoscore).
DISCUSSION
To the best of our knowledge, this is the first study to report on epigenetic therapy for patients with advanced HCC. In two previous phase I studies among patients with solid tumors and advanced hematologic malignancies, the MTD of belinostat was determined to be 1,000 mg/m2 per day on days 1 to 5 every 3 weeks.25,26 In this study, in which the enrolled patients had advanced HCC and mild chronic liver impairment, the MTD was not reached at 1,400 mg/m2 per day. Pharmacokinetics showed that belinostat does not have saturable kinetics, despite liver impairment at the doses studied, and it undergoes rapid clearance, with modest interindividual variability. Since histone acetylation in blood has been shown to correlate with tumor acetylation and growth delay in a dose-dependent manner,13 and pharmacodynamics of belinostat have reported that the area under the plasma concentration-time curve for histone acetylation approaches a plateau at the MTD of 1,200 mg/m2,25 it is likely that the maximum biologically effective dose is approximately 1,200 mg/m2. As a result, although there was no DLT at 1,400 mg/m2, it was determined that further dose escalation was not warranted.
Administering belinostat at 1,400 mg/m2 per day on days 1 to 5 every 3 weeks resulted in grade 3 to 4 toxicities that occurred in 10% of the patients: abdominal pain, hyperbilirubinemia, and increased ALT. In contrast to the earlier phase I studies in which only one case of hepatoxicity was reported,25,26 hepatic impairment appeared to be more common in this study, possibly because of the presence of tumor within the liver as well as concomitant HBV-associated chronic liver disease. One of the concerns of using HDACIs is prolonged QTc associated with depsipeptide and LAQ82427,28; in this study, three patients had prolonged QTc (one grade 2 and two grade 3), but these were asymptomatic and no other significant cardiotoxicity was noted.
To date, efficacy of epigenetic therapy using HDACIs has been demonstrated mainly in advanced hematologic malignancies, in particular, cutaneous and peripheral T-cell lymphoma.29,30 This study demonstrates that HDAC inhibition may achieve disease stabilization in an HCC population for whom prior therapies have failed. The alpha-fetoprotein of the patient who achieved PR dropped from 8,258 to 2,756 μg/L, and another nine patients had stable alpha-fetoprotein during the study period, with seven of them documented to have SD on CT assessment. However, it is noted that the short PFS and the phase II design of this study preclude definitive assessment of antitumor activity of belinostat.
In addition to histone acetylation, DNA methylation by DNA methyltransferase is another common aberrant epigenetic mechanism in HCC,4–6 and it is involved the suppression of various genes, including RASSF1A, p16, p15, and p21(WAF1).4–6 The combination of HDACI with DNA methyltransferase inhibitor has shown enhanced antitumor effects in in vitro HCC studies.12,31 Although these agents have not been assessed in HCC, their efficacy has been reported in myelodysplastic syndrome and acute myelogenous leukemia.
There has been limited ability to identify biomarkers for appropriate use of epigenetic therapy. By using a genome-wide loss-of-function screen for HDACI-induced apoptosis,32 HR23B was identified to be a potential marker. In patients with cutaneous T-cell lymphoma, HR23B expression has been associated with response to HDACIs (suberoylanilide hydroxamic acid, belinostat, and depsipeptide).18 The findings from this study lend support to the suggestion that HR23B may be a generally useful biomarker for predicting clinical responses to HDACIs; although more clinical confirmation is required, its role in HCC could be further assessed by in vitro studies, for instance, by assessing changes in sensitivity to HDACI's effects with manipulation of HR23B levels in HCC cells.
Mechanistically, it has been shown that proteasomal activity is under aberrant control in tumor cells treated with HDACIs.33 In HR23B-expressing tumors, the combination of HDACI and proteasome inhibitor resulted in increased inhibitory effects on proteasome activity and has been suggested to have clinically additive effects.33,34
In summary, this study demonstrates that belinostat enables disease stabilization with a tolerable toxicity profile among patients with HCC. Although additional study would be required to confirm the usefulness of belinostat as a single agent, further improvement in clinical efficacy using epigenetic therapy for HCC will likely require combining HDACI with other novel compounds. HR23B as a potential biomarker needs confirmation and may enable better selection of appropriate patient population.
Appendix
Protocol for Phase I Pharmacokinetic Studies
Blood specimens were collected from patients on day 1 at predose, and also at 15 minutes, 30 minutes, 45 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 5 hours, and 24 hours after dosing of intravenous belinostat at doses between 600 and 1,400 mg/m2 per day began. On day 5, blood specimens were collected at the start of 30-minute intravenous infusion of belinostat and also at 30 minutes, 1 hour, 1.5 hours, 3 hours, and 5 hours. The concentrations of belinostat were quantified by a validated liquid chromatography/mass spectrometry assay.21 Noncompartmental analysis was used to calculate pharmacokinetic parameters with WinNonlin 5.3 (Scientific Consultant, Apex, NC).
Methodology for HR23B Immunohistochemistry
Five-micron sections were taken from formalin-fixed paraffin-embedded archive tissue blocks and deparaffinized, rehydrated, and rinsed in distilled water. Antigen retrieval was done by using a pressure cooker with 10 nmol/L citrate buffer (pH 6.0) for 10 min. The endogenous peroxidase activity was then blocked by incubating the slides in 3% hydrogen peroxide in methanol for 10 min. Imm unohistochemistry was performed by using monoclonal antibody against HR23B (1:400; Abcam ab88503; Abcam, Cambridge, United Kingdom). EnVision FLEX Visualization System (DAKO, Copenhagen, Denmark) was used for chromogen development. All slides were counterstained with hematoxylin.
Fig A1.
(A) Progression-free survival and (B) overall survival of 42 patients.
Footnotes
Supported by Grant No. N01-CM62205 from the Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD) and its collaborator Topotarget.
Presented in part at the 2011 European Cancer Organization meeting, Stockholm, Sweden, September 23-27, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00321594.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Winnie Yeo, Qian Tao, Brigette B. Ma
Administrative support: Winnie Yeo, Frankie K.F. Mo, Ka F. To, Charles Erlichman
Provision of study materials or patients: Winnie Yeo, Hyun C. Chung, Joel Picus, Michael Boyer, Jane Koh, Sun Y. Rha, Edwin P. Hui, Hei C. Jeung, Jae K. Roh, Ka F. To, Anthony W.H. Chan, Joanna H.M. Tong, Boon C. Goh
Collection and assembly of data: Winnie Yeo, Hyun C. Chung, Stephen L. Chan, Ling Z. Wang, Robert Lim, Joel Picus, Michael Boyer, Frankie K.F. Mo, Jane Koh, Sun Y. Rha, Edwin P. Hui, Hei C. Jeung, Jae K. Roh, Simon C.H. Yu, Ka F. To, Joanna H.M. Tong, Charles Erlichman, Anthony T.C. Chan, Boon C. Goh
Data analysis and interpretation: Winnie Yeo, Qian Tao, Anthony W.H. Chan, Boon C. Goh
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer. 2006;94:179–183. doi: 10.1038/sj.bjc.6602918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B, Guo M, Herman JG, et al. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003;163:1101–1107. doi: 10.1016/S0002-9440(10)63469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong S, Yeo W, Tang MW, et al. Intensive hypermethylation of the CpG island of Ras association domain family 1A in hepatitis B virus-associated hepatocellular carcinomas. Clin Cancer Res. 2003;9:3376–3382. [PubMed] [Google Scholar]
- 6.Zhong S, Tang MW, Yeo W, et al. Silencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomas. Clin Cancer Res. 2002;8:1087–1092. [PubMed] [Google Scholar]
- 7.Coradini D, Speranza A. Histone deacetylase inhibitors for treatment of hepatocellular carcinoma. Acta Pharmacol Sin. 2005;26:1025–1033. doi: 10.1111/j.1745-7254.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiba T, Yokosuka O, Arai M, et al. Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. J Hepatol. 2004;41:436–445. doi: 10.1016/j.jhep.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Herold C, Ganslmayer M, Ocker M, et al. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J Hepatol. 2002;36:233–240. doi: 10.1016/s0168-8278(01)00257-4. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Shimada M, Harimoto N, et al. Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int J Cancer. 2003;103:572–576. doi: 10.1002/ijc.10699. [DOI] [PubMed] [Google Scholar]
- 11.Svechnikova I, Gray SG, Kundrotiene J, et al. Apoptosis and tumor remission in liver tumor xenografts by 4-phenylbutyrate. Int J Oncol. 2003;22:579–588. [PubMed] [Google Scholar]
- 12.Armeanu S, Pathil A, Venturelli S, et al. Apoptosis on hepatoma cells but not on primary hepatocytes by histone deacetylase inhibitors valproate and ITF2357. J Hepatol. 2005;42:210–217. doi: 10.1016/j.jhep.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Plumb JA, Finn PW, Williams RJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003;2:721–728. [PubMed] [Google Scholar]
- 14.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: The hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 15.Pathil A, Armeanu S, Venturelli S, et al. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology. 2006;43:425–434. doi: 10.1002/hep.21054. [DOI] [PubMed] [Google Scholar]
- 16.Ma BB, Sung F, Tao Q, et al. The preclinical activity of the histone deacetylase inhibitor PXD101 (belinostat) in hepatocellular carcinoma cell lines. Invest New Drugs. 2010;28:107–114. doi: 10.1007/s10637-009-9219-7. [DOI] [PubMed] [Google Scholar]
- 17.Venturelli S, Armeanu S, Pathil A, et al. Epigenetic combination therapy as a tumor-selective treatment approach for hepatocellular carcinoma. Cancer. 2007;109:2132–2141. doi: 10.1002/cncr.22652. [DOI] [PubMed] [Google Scholar]
- 18.Khan O, Fotheringham S, Wood V, et al. HR23B is a biomarker for tumor sensitivity to HDAC inhibitor-based therapy. Proc Natl Acad Sci U S A. 2010;107:6532–6537. doi: 10.1073/pnas.0913912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Wang LZ, Chan D, Yeo W, et al. A sensitive and specific liquid chromatography-tandem mass spectrometric method for determination of belinostat in plasma from liver cancer patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2409–2414. doi: 10.1016/j.jchromb.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 23.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 24.Wang L, Goh BC, Lwin TW, et al. Phase I pharmacokinetics and metabolic pathway of belinostat in patients with hepatocellular carcinoma. J Clin Oncol. 2010;28(suppl; abstr 2585):225s. [Google Scholar]
- 25.Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 26.Gimsing P, Hansen M, Knudsen LM, et al. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 2008;81:170–176. doi: 10.1111/j.1600-0609.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 27.Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 28.Molife R, Fong P, Scurr M, et al. HDAC inhibitors and cardiac safety. Clin Cancer Res. 2007;13:1068–1069. doi: 10.1158/1078-0432.CCR-06-1715. author reply. [DOI] [PubMed] [Google Scholar]
- 29.Piekarz RL, Bates SE. Epigenetic modifiers: Basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 31.Steele N, Finn P, Brown R, et al. Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer. 2009;100:758–763. doi: 10.1038/sj.bjc.6604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fotheringham S, Epping MT, Stimson L, et al. Genome-wide loss-of-function screen reveals an important role for the proteasome in HDAC inhibitor-induced apoptosis. Cancer Cell. 2009;15:57–66. doi: 10.1016/j.ccr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuele S, Lauricella M, Carlisi D, et al. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis. 2007;12:1327–1338. doi: 10.1007/s10495-007-0063-y. [DOI] [PubMed] [Google Scholar]