Abstract
OBJECTIVE:
Acute bronchiolitis is a leading cause of infant hospitalization and is most commonly caused by respiratory syncytial virus. Etiological tests are not required for its diagnosis, but the influence of viral screening on the therapeutic approach for acute bronchiolitis remains unclear.
METHODS:
A historical cohort was performed to assess the impact of viral screening on drug prescriptions. The study included infants up to one year of age who were hospitalized for bronchiolitis. Virus screening was performed using immunofluorescence assays in nasopharyngeal aspirates. The clinical data were obtained from the patients' medical records. Therapeutic changes were considered to be associated with viral screening when made within 24 hours of the release of the results.
RESULTS:
The frequency of prescriptions for beta agonists, corticosteroids and antibiotics was high at the time of admission and was similar among the 230 patients. The diagnosis of pneumonia and otitis was associated with the introduction of antibiotics but did not influence antibiotics maintenance after the results of the virus screening were obtained. Changes in the prescriptions were more frequent for the respiratory syncytial virus patients compared to patients who had negative viral screening results (p = 0.004), especially the discontinuation of antibiotics (p<0.001). The identification of respiratory syncytial virus was associated with the suspension of antibiotics (p = 0.003), even after adjusting for confounding variables (p = 0.004); however, it did not influence the suspension of beta-agonists or corticosteroids.
CONCLUSION:
The identification of respiratory syncytial virus in infants with bronchiolitis was independently associated with the discontinuation of antibiotics during hospitalization.
Keywords: Bronchiolitis, Respiratory Syncytial Virus, Therapeutics, Fluorescent Antibody Technique
INTRODUCTION
Acute bronchiolitis (AB) is a leading cause of hospitalization in children during their first year of life and is responsible for over 120,000 annual hospitalizations in developed countries (1). The morbidity for AB is even greater in developing countries (2). Ecological studies show that the hospitalization rates for AB have been increasing in recent decades (3). In Brazil, the prevalence of bronchiolitis is high, and hospitalization rates are increasing, according to an analysis of government data (4,5).
Respiratory syncytial virus (RSV) is the main etiological agent of AB and is responsible for up to 90% of cases during the RSV season. RSV can be identified in nasopharyngeal secretions using an immunofluorescence assay, culture, or molecular biology techniques. According to international clinical guidelines, these etiological tests are not required for the diagnosis of bronchiolitis, as the diagnosis can be made based on the clinical and epidemiological findings (6,7). Conversely, the influence of the etiological investigation on the therapeutic approach remains unclear in hospitalized patients with a clinical diagnosis of bronchiolitis. Although the recommended therapy consists of supportive measures, such as oxygen therapy for hypoxemic patients, hydration and maintaining an open airway, systemic antibiotics are often used in patients with AB, as are corticosteroids and bronchodilators (7,8). The request for etiological tests varies widely, and the influence of the results of such tests on drug prescription is rarely reported. A recent systematic review selected four studies that enrolled children from emergency departments. It suggested that viral test results may be associated with a reduction of antibiotic use, but the statistical power of the review was small because of the number of studies (9). Inpatient data are even scarcer. A retrospective study reported a reduced time of antibiotics use among those children who were found to be positive for RSV (10).
This study examines the hypothesis that an etiological diagnosis that is based on the results of an immunofluorescence assay (IFA), which is an accessible and affordable method, may help guide the treatment of AB, thereby reducing the use of systemic antibiotics. This study differs from previous investigations due to the analysis of the impact of viral testing results on the medical management of hospitalized infants.
PATIENTS AND METHODS
A historical cohort study was conducted on hospitalized infants in the Pediatric Clinic Division of the University Hospital at São Paulo University between January 2006 and December 2007. This service provides regionalized care to approximately 400,000 inhabitants of the western region of São Paulo. According to the hospital policy, infants diagnosed with a lower respiratory tract infection upon admission undergo nasopharyngeal aspirate (NPA) collection for respiratory virus screening. A commercial kit (Biotrin International Ltd., Dublin/Ireland) is used for the indirect IFA, which is standardized for the identification of seven respiratory viruses (RSV, adenovirus, influenza A and B, and parainfluenza 1, 2, and 3) and is used according to the manufacturer's instructions.
Only infants with the following criteria were included in this study: 12 months of age or younger, a diagnosis of bronchiolitis upon hospital discharge (according to the International Classification of Diseases-9 and regardless of other secondary diagnosis) and NPA collected within the first day of hospitalization. Infants who presented the following findings were excluded from the study: heart diseases, chronic pulmonary diseases, prematurity (less than 37 weeks of gestation), neuropathies, genetic syndromes, immunodeficiencies, congenital airway malformations and recurrent episodes of wheezing. The patients' medical records were analyzed by completing a standardized protocol, including the collection of demographic data, clinical findings, laboratory tests/chest X-ray on admission and during the hospitalization and drug prescriptions. The information on viral screening was collected from physicians' notes, which systematically included the test result, date, and time. We have considered the time of writing as the moment at which the physician became aware of the result. The other laboratory tests, clinical features, and diagnoses were also collected from the physicians' notes. The dates of hospitalization and discharge were obtained from the informatics system. Information regarding the date and time at which drug and oxygen therapy were started and stopped was collected from prescription records, and the prescriptions were considered to have been effectively administered according to the nurse validation. In accordance with standard procedures, changes to routine prescriptions were made after discussion with the medical staff during daily rounds. Changes in prescriptions that were made within 24 hours of the recording of the viral screening results were considered to be associated with these results.
This study was approved by the IRB of the University Hospital of the School of Medicine, University of São Paulo.
Statistical analysis
The categorical variables are described as proportions, the continuous parametric variables as means and standard deviations, and the nonparametric variables as medians and interquartile intervals. The chi-squared test was used to assess the associations between the categorical variables.
Given the study design (cohort) and the high prevalence rates of the outcomes, the effect size that was measured in this study was the hazard ratio, which is an expression of relative risk.
Two different regression models were built. In the first model, the outcome variable was “introduction of antibiotic therapy upon admission”, and the clinical and laboratorial characteristics were the explanatory variables. In the second model, the outcome variable was “suspension of antibiotic therapy after IFA result”, the main exposure variable was “etiological diagnosis of RSV” and the other clinical and laboratorial characteristics were the explanatory variables. A univariate Poisson regression was used to calculate the relative risk, and a multivariate model was built with the variables that presented a p<0.10 in the univariate analyses and other potential confounding variables, based on their clinical importance. The p-value was fixed at p = 0.05 for rejection of the null hypothesis. The statistical software STATA 10.0® (StataCorp LP 2005, College Station, TX, USA) was used for analysis.
The sample size was derived from convenience sampling of 126 infants on antibiotic therapy (main outcome). Assuming an increment from 10% to 50% in the rate of discontinuation of antibiotics, according to the test results and an alpha error probability of 5%, this sample size would imply a statistical power over 90%.
RESULTS
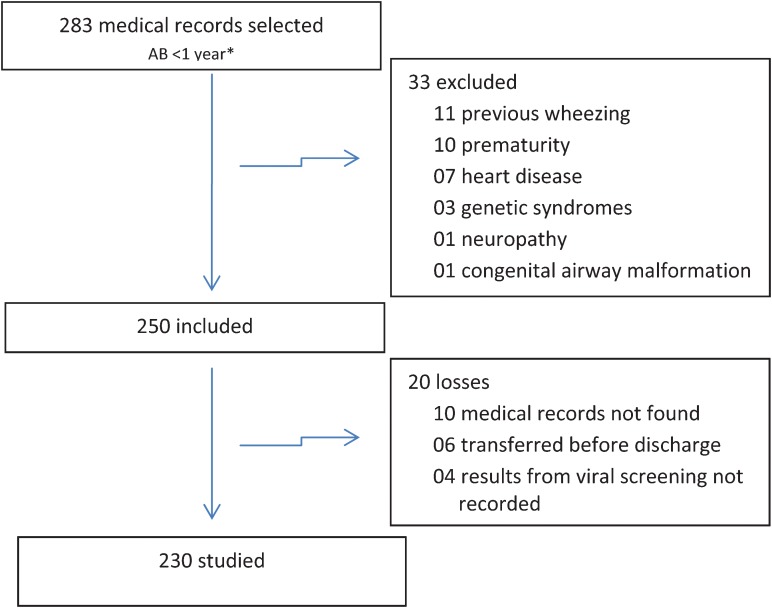
Among 1,199 infants admitted to our hospital, 852 (71%) were diagnosed with acute respiratory tract disease, and of these, 283 (33%) met the criteria required for inclusion in this study. Thirty-three infants were excluded according to the previously defined criteria. Twenty cases could not be analyzed due to incomplete data in their medical records. Therefore, 230 medical records were studied (Figure 1).
Figure 1.
Flow chart of the patients selected for this study.
The mean age of the infants was four months (sd = 2.7). There was a slight predominance of males (n = 129; 56%). The highest incidence of AB and RSV identification occurred between February and July.
The main diagnosis on admission to the hospital was acute bronchiolitis (n = 65; 72%). Blood was collected for culture for aerobic and anaerobic bacteria in 149 (65%) cases, and bacterial growth was observed in three (2%) of them (DNase-negative Staphylococcus, Pseudomonas sp. and Streptococcus viridans). All of these patients were treated with antibiotics.
The medications most often prescribed upon hospital admission were beta-agonist inhalers, systemic corticosteroids and systemic antibiotics. The antibiotics most commonly prescribed were intravenous penicillin (n = 51; 40.5%) and oral amoxicillin (n = 29; 23.1%). Other antibiotics prescribed were macrolides (n = 17; 13.5%), cefuroxime (n = 08; 6.2%), ceftriaxone (n = 4; 3.1%), combination oxacillin and amikacin (n = 11; 8.7%), penicillin and amikacin (n = 4; 3.2%) ceftriaxone and macrolide (n = 1; 0.8%) and ceftriaxone and oxacillin (n = 1; 0.8%). No follow-up laboratory tests or chest X-rays were requested before changes in the prescriptions were made. The results of the laboratory evaluation and monitoring of patients during their hospitalization are shown in Table 1. RSV was identified as the sole agent in 64% of patients (RSV(+) group; n = 146); no viruses could be identified in 28% of patients (VIRUS(-) group). Other viruses were identified in 8% (19 patients: parainfluenza 3 (n = 8), parainfluenza 1 (n = 2), adenovirus (n = 3), influenza A (n = 1), influenza B (n = 1), co-detection (n = 4). Due to the limited number of cases and the heterogeneity of the viral agents identified, this group was not included in the comparative analysis. The average time for the physician to become aware of the viral test results was 40 hours after admission. No significant differences in the frequency of drug prescription upon admission were observed between the patients in the VIRUS(-) and RSV(+) groups. Following the results of the viral screening, changes in medical prescriptions for drugs were most frequent in the RSV(+) group. These changes consisted mainly of the discontinuation of antibiotic therapy (Table 2). The time of antibiotic discontinuation was, on average, 7.2 hours after the viral test result was recorded in the medical records.
Table 1.
Description of the therapies prescribed and laboratory exams performed upon hospital admission and the characteristics of the clinical courses of the 230 patients included in the study.
| THERAPY AND EXAMS UPON ADMISSION | Medical records with informationn (%) | |
| Beta-agonist inhalers - n (%)Systemic corticosteroids - n (%)Systemic antibiotics - n (%) | 185 (80%)119 (52%)126 (55%) | 230 (100)230 (100)230 (100) |
| Radiography with opacification - n (%)Positive blood cultures - n (%)*** | 118 (54%)03 (02%) | 217 (94)149 (65) |
| Pulse oximetry - median (p25–p75) | 89% (87–93) | 195 (85) |
| C-reactive protein*) - median (p25–p75)Leukocytes** - median (p25–p75) | 11 (5–27.25)11,100 (7,900–15,150) | 112 (49)149 (65) |
| Clinical course characteristics during hospitalization | ||
| ICU*** - n (%)Time in ICU*** - median (p25–p75)MV# - n (%)Time in MV# - median (p25–p75) | 39 (17%)4 days (3–6)16 (07%)3.5 days (2.25–5.75) | 230 (100)230 (100)230 (100)230 (100) |
| Total time in O2## - median (p25–p75) | 4 days (2–6) | 230 (100) |
| Hospitalization period - median (p25–p75) | 6 days (4–8) | 230 (100) |
Values in µg/dL; **peripheral leukocyte count in cells/mL; ***ICU = intensive care unit; #MV = mechanical ventilation; ##O2 = oxygen therapy.
Table 2.
Medications prescribed upon admission to the hospital and drug discontinuation after virus screening for patients in the VIRUS(-) and RSV(+) groups.
| Medications Prescribed Upon Admission | VIRUS(-) | RSV(+) | p-value | ||
| n = 65 | (100%) | n = 146 | (100%) | ||
| Antibiotics | 34 | (52%) | 75 | (52%) | 0.90 |
| Corticosteroids | 27 | (42%) | 81 | (55%) | 0.61 |
| Beta-Agonist | 48 | (74%) | 124 | (85%) | 0.06 |
| Changes in drug prescriptions | VIRUS(-) | RSV(+) | p-value | ||
| n = 65 | (100%) | n = 146 | (100%) | ||
| Any drug discontinuation# | 11 | (17%) | 54 | (37%) | 0.004 |
| Discontinuation of antibiotics | 06 | (09%) | 47 | (32%) | <0.001 |
| Discontinuation of corticosteroids | 06 | (09%) | 10 | (06%) | 0.546 |
| Discontinuation of beta-agonists | 00 | (00%) | 02 | (01%) | 0.343 |
Chi-squared test; #Any discontinuation in antibiotics and/or corticosteroids and/or beta agonists.
After the discontinuation of antibiotic therapy, two patients from the VIRUS(-) group required reintroduction of antibiotic therapy due to a worsening respiratory condition and radiological alterations. Two patients in the RSV(+) group also required the reintroduction of antibiotics (one due to the diagnosis of nosocomial pneumonia and the other due to acute otitis media). A sub-analysis was performed in which these four patients were included in the group with maintained antibiotic therapy, and the results revealed that the discontinuation of antibiotic therapy was also predominant in patients from the RSV(+) group (31.2% vs. 6.3%; p<0.001).
The introduction of antibiotic therapy upon admission to the hospital was associated with an age over 6 months, the presence of fever, thoracic radiography showing signs of opacification, diagnosis of acute otitis media (AOM) and pneumonia. After adjustment, according to the multivariate analysis, only the diagnoses of AOM and pneumonia were independent factors that predicted the introduction of antibiotic therapy (Table 3).
Table 3.
Univariate and multivariate analyses of the possible determinants of the introduction of antibiotic therapy upon admission of the 230 patients included in the study.
| Category | Antibiotic therapy not introducedn = 104(45.2%) | Antibiotic therapy introducedn = 126(54.8%) | Univariate Analyses | Multivariate Analyses | ||||
| RR | 95% CI | p-value | RR | 95% CI | p-value | |||
| RSV (+)* | 71 (48.6) | 75 (51.4) | 0.85 | 0.59-1.21 | 0.357 | - | - | |
| Age > 6 months | 08 (20.5) | 31 (79.5) | 1.60 | 1.07-2.40 | 0.023 | 0.95 | 0.61-1.48 | 0.808 |
| Male | 56 (43.4) | 73 (56.6) | 0.93 | 0.65-1.32 | 0.676 | - | - | |
| Fever on admission | 56 (37.3) | 94 (62.7) | 1.57 | 1.05-2.34 | 0.028 | 0.93 | 0.60-1.44 | 0.745 |
| Thoracic radiography with opacification | 18 (18.2) | 81 (81.8) | 2.41 | 1.65-3.52 | <0.001 | 1.48 | 0.94-2.32 | 0.091 |
| ICU | 14 (35.9) | 25 (64.1) | 1.21 | 0.78-1.88 | 0.389 | - | - | |
| MV | 02 (12.5) | 14 (87.5) | 1.67 | 0.96-2.91 | 0.070 | 1.30 | 0.74-2.28 | 0.370 |
| AOM | 00 (00.0) | 12 (100) | 1.91 | 1.05-3.47 | 0.033 | 4.66 | 2.28-9.51 | <0.001 |
| Pneumonia | 01 (1.1) | 94 (98.9) | 4.17 | 2.79-6.23 | <0.001 | 4.16 | 2.53-6.85 | <0.001 |
| Leukocyte count*<9,0009-13,000>13,000 | 15 (31.3)15 (32.6)16 (29.1) | 33 (68.7)31 (67.4)39 (70.9) | 1.02 | 0.81-1.28 | 0.890 | - | - | |
| Oximetry %mean (sd) | 91 (0.4) | 89 (0.4) | 0.96 | 0.92-1.01 | 0.101 | - | - | |
VIRUS(+) = etiological diagnosis of infection with any respiratory virus; RSV(+) = etiological diagnosis of infection with respiratory syncytial virus; *Result unavailable at the moment of introduction of antibiotic therapy; ICU = intensive care unit; MV = mechanical ventilation; AOM = diagnosis of acute otitis media; RR = relative risk; #Number of cells/ml; ##Oximetry was analyzed as a continuous variable, whereas the other variables were categorized.
In the univariate analysis, the presence of RSV was the only variable associated with the discontinuation of antibiotic therapy. After adjustment for confounding factors, the etiological diagnosis of RSV remained the sole independent determinant of the discontinuation of antibiotic therapy (Table 4).
Table 4.
Univariate and multivariate analyses of the possible determinants of the discontinuation of antibiotics during the hospitalization of 109 patients from the VIRUS(-) and RSV(+) groups who received an initial prescription of antibiotic therapy.
| Univariate Analyses | Multivariate Analyses | ||||||||
| Category | Total number of cases | Antibiotic therapy discontinuedn (%) | Antibiotic therapy maintainedn (%) | RR | 95% CI | p-value | RR | 95% CI | p-value |
| RSV (+) | 75 | 47 (62.7) | 28(37.3) | 3.55 | 1.52-8.31 | 0.003 | 3.58 | 1.51-8.50 | 0.004 |
| Age > 6 months | 83 | 43 (51.8) | 40 (48.2) | 0.74 | 0.37-1.48 | 0.396 | 0.68 | 0.33-1.41 | 0.300 |
| Male | 63 | 29(46.0) | 34(54.0) | 1.13 | 0.66-1.95 | 0.650 | - | - | |
| Fever on admission | 80 | 39 (48.7) | 41(51.3) | 0.87 | 0.47-1.61 | 0.662 | - | - | |
| Fever for more than 24 h after admission | 47 | 22 (46.8) | 25 (53.2) | 0.97 | 0.74-1.27 | 0.813 | 0.94 | 0.71-1.26 | 0.697 |
| Thoracic radiography with opacification | 70 | 36(51.4) | 34(49.6) | 1.17 | 0.64-2.13 | 0.618 | - | - | |
| ICU | 23 | 11(47.8) | 12(52.2) | 0.98 | 0.50-1.90 | 0.951 | - | - | |
| MV | 12 | 5(41.7) | 7(58.3) | 0.84 | 0.34-2.12 | 0.714 | - | - | |
| AOM | 12 | 2(16.7) | 10(83.3) | 0.32 | 0.08-1.30 | 0.111 | 0.47 | 0.10-2.19 | 0.311 |
| Pneumonia | 82 | 43(52.4) | 39(47.6) | 1.42 | 0.71-2.82 | 0.322 | 1.12 | 0.53-2.39 | 0.764 |
| Leukocyte count*<9,0009-13,000>13,000 | 282830 | 14(50.0)13(46.4)14(46.7) | 14(50.0)15(53.6)16(53.3) | 0.97 | 0.67-1.40 | 0.856 | - | - | |
| Oximetry %mean (sd) | 93 | 89 (0.6) | 90 (0.6) | 0.98 | 0.91-1.05 | 0.522 | - | - | |
RSV(+) = etiological diagnosis of infection by the respiratory syncytial virus; ICU = intensive care unit; MV = mechanical ventilation; AOM = diagnosis of acute otitis media; *Number of cells/ml; RR = relative risk; #Risk reduction for each change in category.
DISCUSSION
The etiological diagnosis of RSV infection using IFA was an independent factor associated with the discontinuation of antibiotic therapy, which, based on clinical findings, was prescribed upon admission to hospitalized infants with bronchiolitis. The results of respiratory virus screening had no impact on the use of bronchodilators and corticosteroids. The results of this study suggest that an etiological diagnosis is an objective and useful parameter in clinical practice for bronchiolitis treatment.
The high frequencies of the prescription of antibiotics (55%), bronchodilators (80%) and corticosteroids (52%) for the initial treatment of infants with bronchiolitis indicate the difficulty in promoting a therapeutic approach based on scientific evidence. As in this study, the practice of prescribing these drugs has also been reported in other countries, despite current clinical guidelines (7). However, the use of ineffective or controversial therapies exposes the patient to adverse effects. In a retrospective study of more than 17,000 children under the age of one who were hospitalized with bronchiolitis, the use of antibiotics, bronchodilators and corticosteroids (45% of patients) was associated with longer hospital stays (9). Additionally, the inappropriate use of antibiotics may increase the risk of bacterial resistance and the cost of hospitalization (12).
In this study, despite the high rate of antibiotic use, viral screening contributed to the suspension of antibiotic therapy in most cases and served as a valuable tool for the adjustment of medical management. After adjusting for other possible causes for the suspension of antibiotic therapy, especially those that were relevant for the introduction of therapy, such as the diagnosis of otitis and pneumonia, the presence of RSV remained the sole independent factor that influenced this change in the therapeutic approach. We observed that other repeated laboratorial and radiological tests were not considered important for this clinical decision because none of them were requested before the changes in the prescriptions were performed. The test used for virus identification is highly sensitive for RSV identification and is economically viable and easily performed for hospitalized patients. Its predictive value for RSV varies according to the epidemiological scenario, and it is higher during peak seasonal periods for the agent (13). Although its sensitivity is not as high for other possible agents, such as adenovirus and influenza virus, this test is useful for AB cases, given that RSV is the predominant etiological agent. A recent study has shown that the use of molecular techniques improves the viral test sensitivity to other agents, such as human rhinovirus, but it does not influence the detection of RSV in cases of bronchiolitis (14).
The viral test results were added to medical records during the second day of hospitalization, and the discontinuation of antibiotics occurred within the next few hours. This finding confirms the association between the physician's awareness of the results and a change in the drug prescription during the next round and suggests that the viral test is a useful tool at the beginning of hospitalization.
Information regarding the impact of the etiological investigation on the medical management of hospitalized patients with AB is scarce, although in the in-hospital environment, virus screening may be useful to reduce the rates of nosocomial virus infections (6). Previous findings also indicate some benefit of the use of viral screening in therapeutic practice. Byington et al. analyzed the impact of a rapid diagnostic test for respiratory viruses on antibiotic use in a children's hospital during two annual winter seasons. In the first season, virus detection was associated with shorter durations of intravenous antibiotic therapy (mean 2.4 vs. 4.0 days) and oral antibiotic therapy (0.25 vs. 2.5 days) and fewer discharge prescriptions of oral antibiotics (37% vs. 52%) compared with virus-negative patients. During the second season, virus detection was associated with lower rates of intravenous antibiotic prescription than in the first season (26% vs. 44%), suggesting that the virus test contributed for a more accurate management of AB at that hospital (10). A systematic review concluded that the current evidence is insufficient but promising regarding the usefulness of viral screening in reducing the use of antibiotics in the pediatric emergency department (9).
According to our results, the factors associated with the introduction of antibiotic therapy upon admission to the hospital were those that might suggest bacterial etiology. The antibiotic therapy was most often prescribed for patients over six months of age and for those with fever, which may suggest that the pediatricians were more confident in their initial diagnosis of viral bronchiolitis in infants in their first six months of life without fever. However, the etiological diagnosis of RSV allowed for suspension of antibiotic therapy, even in older infants and in the presence of fever. Although RSV can frequently cause pneumonia and AOM, these diagnoses were strong indicators for the prescription of antibiotics (15). Nonetheless, these factors did not influence the suspension of antibiotic therapy following a positive screening result for RSV. In the population selected for this study, the inclusion criteria were selective for bronchiolitis, which explains the low rate of positivity of the blood cultures (2%). Similar to these results, other studies have suggested that the occurrence of bacterial infection in infants with bronchiolitis is not common (16).
This study has some limitations due to its retrospective nature. The influence of the experience and qualification of the physician responsible for the medical management could not be assessed. However, according to routine procedures at the hospital, decisions regarding the medical management of infants with bronchiolitis were made by the medical staff, which is homogenous as a result of the high frequency of this disease and the staff's clinical and research experience in this field of study (17-19).
Even though IFA enables the diagnosis of other respiratory viruses, a statistical analysis of these cases could not be performed due to their low frequencies, the heterogeneity of the viral agents that we found and the fact that the medical management for other viral infections not caused by RSV could differ. Further studies with an appropriate casuistic are necessary to perform such an analysis.
We excluded patients with chronic diseases and prematurity to avoid distortions in the analysis because these comorbidities themselves influence antibiotic use. The number of such cases in a general hospital would not allow us to analyze them separately. Therefore, our results cannot be generalized to these patients. The obtained results also may not be extrapolated to outpatients. However, a systematic review suggested that viral screening as part of the emergency service for children with respiratory infections may be promising (9).
The results of this study suggest that the etiological diagnosis of RSV infection in hospitalized infants with bronchiolitis was independently associated with the discontinuation of antibiotic therapy. Affordable laboratory methods, such as the IFA, for the identification of viruses may help decrease inappropriate prescription of antibiotics for these infants. This may contribute to a more cost-effective approach and to a reduction in the inappropriate use of antibiotics for inpatients. Future prospective studies are needed to minimize the impact of bias and to confirm these results.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Robinson RF. Impact of respiratory syncytial virus in the United States. Am J Health Syst Pharm. 2008;65(23 Suppl 8):S3–6. doi: 10.2146/ajhp080438. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;292(12):1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Available at: http://www.tabnet.datasus.gov.br. Accessed June 28, 2011
- 5.Vieira SE, Stewien KE, Queiroz DA, Durigon EL, Török TJ, Anderson LJ, et al. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalization in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2001;43(3):125–31. doi: 10.1590/s0036-46652001000300002. [DOI] [PubMed] [Google Scholar]
- 6.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(2):342–9. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 8.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115(4):878–84. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 9.Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the emergency department. Cochrane Database Syst Rev. 2009;7(4)4:CD006452. doi: 10.1002/14651858.CD006452.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Byington CL, Castillo H, Gerber K, Daly JA, Brimley LA, Adams S, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230–4. doi: 10.1001/archpedi.156.12.1230. [DOI] [PubMed] [Google Scholar]
- 11.King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Arch Pediatr Adolesc Med. 2004;158(2):127–37. doi: 10.1001/archpedi.158.2.127. [DOI] [PubMed] [Google Scholar]
- 12.Wang EE, Einarson TR, Kellner JD, Conly JM. Antibiotic prescribing for Canadian preschool children: evidence of overprescribing for viral respiratory infections. Clin Infect Dis. 1999;29(1):155–160. doi: 10.1086/520145. [DOI] [PubMed] [Google Scholar]
- 13.Henrickson KJ, Hall CB. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26(11 Suppl):S36–40. doi: 10.1097/INF.0b013e318157da6f. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento MS, Souza AV, Ferreira AV, Rodrigues JC, Abramovici S, Silva Filho LV. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics. 2010;65(11):1133–7. doi: 10.1590/S1807-59322010001100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel JA, Nguyen DT, Revai K, Chonmaitree T. Role of respiratory syncytial virus in acute otitis media: implications for vaccine development. Vaccine. 2007;25(9):1683–9. doi: 10.1016/j.vaccine.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duttweiler L, Nadal D, Frey B. Pulmonary and systemic bacterial co-infections in severe RSV bronchiolitis. Arch Dis Child. 2004;89(12):1155–7. doi: 10.1136/adc.2004.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botosso VF, Zanotto PM, Ueda M, Arruda E, Gilio AE, Vieira SE, et al. Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog. 2009;5(1):e1000254. doi: 10.1371/journal.ppat.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.do Nascimento CA, Leal AL, Souza TS, de Moraes CT, Comone P, Tenório EC, et al. One-step reverse transcriptase polymerase chain reaction for the diagnosis of respiratory syncytial virus in children. J Virol Methods. 2008;148(1-2):115–9. doi: 10.1016/j.jviromet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 19.De Paulis M, Gilio AE, Ferraro AA, Ferronato AE, do Sacramento PR, Botosso VF, et al. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J) 2011;87(4):307–13. doi: 10.2223/JPED.2100. [DOI] [PubMed] [Google Scholar]