Abstract
OBJECTIVE:
Celiac disease is a permanent enteropathy caused by the ingestion of gluten, which leads to an immune-mediated inflammation of the small intestine mucosa. The prevalence of celiac disease varies among different nations and ethnic backgrounds, and its diversity is determined by genetic and environmental factors. São Paulo city is one of the largest cities in the world, with a vast population and an important history of internal migratory flow from other Brazilian regions, as well as immigration from other, primarily European, countries, resulting in significant miscegenation. The aim of the present study was to estimate the prevalence of adults with undiagnosed celiac disease among blood donors of São Paulo by collecting information on the ancestry of the population studied.
METHODS:
The prevalence of celiac disease was assessed by screening for positive IgA transglutaminase and IgA endomysium antibodies in 4,000 donors (volunteers) in the Fundação Pró-Sangue Blood Center of São Paulo, São Paulo, Brazil. The antibody-positive subjects were asked to undergo a small bowel biopsy.
RESULTS:
Of the 4,000 subjects, twenty-four had positive tests, although both antibody tests were not always concordant. For example, ten subjects were positive for IgA tissue transglutaminase only. In twenty-one positive patients, duodenal biopsies were performed, and the diagnosis of celiac disease was confirmed in fourteen patients (Marsh criteria modified by Oberhuber). In this group, 67% claimed to have European ancestry, mainly from Italy, Portugal and Spain.
CONCLUSION:
The prevalence of celiac disease is at least 1:286 among supposedly healthy blood bank volunteers in São Paulo, Brazil.
Keywords: Celiac disease, Prevalence, Blood center, Anti-transglutaminase antibody, Anti-endomysium antibody, Human leukocyte antigen
INTRODUCTION
Celiac disease (CD) is characterized by chronic inflammatory lesions in the small intestine of genetically predisposed subjects. It is induced by gluten-rich food ingestion and is reversible upon withdrawal of gluten from the diet. Environmental, genetic and immunological factors are involved in the pathogenesis of CD (1-3).
During the last few decades, studies carried out in different countries have shown that the actual prevalence of CD is higher than previously thought (2,4,5). Surveys have been conducted among subjects from the general population and those belonging to risk groups using serological markers. These studies showed that the disease is underdiagnosed when heterogeneous clinical features are present, as CD may manifest in a variety of ways, ranging from typical signs and symptoms of malabsorption to even an absence of symptoms (5,6). Estimates based on serological epidemiological studies suggest that for each diagnosis made from typical clinical findings of CD, there are 3 to 7 oligo- or asymptomatic patients who are undiagnosed (3).
CD exhibits features of an autoimmune disease and may involve many organs other than the gastrointestinal tract (3,7). Furthermore, it shows variable morbidity, with several delayed complications when not treated early (2,8,9). This fact, coupled with the availability of noninvasive serological tests with high sensitivity and specificity, corroborates the importance of epidemiological studies in the general population and in specific risk groups to better understand the disease and to improve the diagnosis of atypical cases (2,5,7).
Most of the existing CD prevalence studies were conducted in apparently healthy blood bank donors who had not been previously selected for other studies. Several countries have reported a high prevalence of CD among donors: Israel, 1:157 (10); Iran, 1:166 (11); Tunisia, 1:355 (12); the United States of America, 1:125 (13); Denmark, 1:394; and Sweden 1:373 (14). In Brazil, screening studies carried out at blood banks indicated that the prevalence ranged from 1:681 (15) to 1:276 (16) donors.
São Paulo city, one of the largest metropolises in the world, is an urban center in Brazil that displays great ethnic diversity due to frequent European immigration (17). Apart from this, the city is an important center of internal migration, which, in turn, increases miscegenation even more. In addition to these facts, the amount of gluten-rich food normally ingested by the population is similar to that in European countries (18), where there is a high prevalence of the disease. As a possible triggering factor, there is also a high incidence of gastrointestinal infections during childhood, which may be involved in the pathogenesis of CD (19).
Considering the fundamental importance of the genetic component of CD and the marked miscegenation present in Brazil, the aim of this study was to estimate the prevalence of the disease in the adult population of São Paulo city and the correlation with racial/ethnic groups.
PATIENTS AND METHODS
PATIENTS
From September 2003 to August 2004, at the Fundação Pró-Sangue Blood Center of São Paulo, São Paulo, Brazil, 4,000 serum samples obtained from blood donors of both genders aged 18 to 65 years (median, 31 years), regardless of gender, were processed. The only inclusion criterion for the study was that donors had to have a fixed residence for at least two years in the city of São Paulo. Individuals with known CD were excluded from participation.
After signing the written consent and filling out a pre-established questionnaire, blood samples were drawn at the blood bank center. Serum samples were immediately centrifuged and stored at -20°C until serological tests were carried out.
The research protocol was approved by the Ethics Committee of the Hospital das Clínicas at the University of São Paulo School of Medicine and by the Ethics Committee of the Fundação Pró-Sangue Blood Center of São Paulo, São Paulo, Brazil.
STUDY DESIGN
This cross-sectional study evaluated the positivity of serological tests of 4,000 serum samples obtained at the blood bank during a 12-month period. All donors were asked to fill out a pre-established form to collect information about demographic data, including questions on self-reported ethnic categories based on the predetermined five-term system of the Brazilian Institute of Geography and Statistics (IBGE) (20): White, Mixed, Black, Yellow (meaning East Asians), and Amerindians. Ancestral roots were established by questions about the donors' ancestors' places of birth up to the third generation.
Subjects with positive tissue anti-transglutaminase (tTG) and/or anti-endomysial antibodies (AEA) were asked to answer a more detailed and extensive questionnaire about symptoms. These subjects were also requested to undergo an upper digestive endoscopy with a duodenal biopsy.
The chi-square and Fisher's exact tests were employed for analyzing nominal and proportion data sets obtained from both donor and CD patient groups.
SEROLOGICAL ASSAYS
Serological tests were carried out at the Laboratório de Gastroenterologia Clínica e Experimental - LIM 07, Hospital das Clínicas, University of São Paulo School of Medicine. Enzyme-linked immunosorbent assays (ELISAs) to detect IgA anti-guinea pig liver tTG antibodies and IgA AEA on human umbilical cords were performed for all samples.
Tissue anti-transglutaminase antibody (tTG) assay
Described originally by Dieterich et al. (21) and modified by Pereira et al. (22), this test employs guinea pig liver tissue transglutaminase (Product No. T-5398. Sigma-Aldrich Co., St. Louis, MO, USA) and goat anti-human IgA (α-chain specific) peroxidase conjugate (Product No. A-0295. Sigma-Aldrich Co.), diluted at 1:1,000. Quality control was assessed by utilizing two control serum samples in every assay. Intra- and inter-assay variations were 8.68% (n = 22) and 8.38% (n = 24), respectively.
Anti-endomysial antibody assay
This assay was performed according to the method described by Pereira et al. (22). Immunofluorescence tests for anti-endomysial antibodies were carried out using 2-μm cryosections of human umbilical cord, which were incubated with 1:5 pre-diluted serum in Tween-PBS. The samples were incubated with goat anti-human fluorescein-conjugated IgA (Product No. F-9637, Sigma-Aldrich Co., St. Louis, MO, USA) diluted 1:30. Samples were considered positive if there was a hexagonal pattern of fluorescence throughout the peritubular muscle layer of the human umbilical cord vessels, marking the extracellular connective tissue. Analyses were conducted by three observers using an immunofluorescence microscope (enhancement of 400x) to define both positivity and anti-endomysial antibody titration.
HLA TYPING
HLA-DQ was performed in 21 blood donors with positive serological tests. The HLA-A, HLA-B and HLA-DQB1 Dynal RELI SSO typing kits (Product Nos. 830.01, 840.01, and 820.01; Dynal Biotech Ltd., Bromborough, Wirral, U.K.) were used to determine HLA. The gDNA was extracted using the ChargeSwitch® gDNA 50-100 µl blood kit (Product No. CS11000; Invitrogen Corporation, Carlsbad, CA, USA).
HLA-DQ typing was also performed in samples from 180 inhabitants of São Paulo city to verify the DQ2/DQ8 prevalence in the population.
UPPER DIGESTIVE ENDOSCOPY
An upper digestive endoscopy was performed to confirm the diagnosis in antibody-positive patients. Multiple duodenal biopsies were collected (two duodenal and six from the second section of the duodenum). The material obtained was placed on filter paper and fixed in 10% formalin for later processing.
HISTOLOGY
Formalin-fixed samples were stained with hematoxylin and eosin for histological study. The following features were evaluated: (1) crypt/villous ratio, (2) crypt regeneration, (3) characteristics of the inflammatory infiltrate in the section itself, and (4) type of atrophy. Two pathologists examined every slide to ensure standardization of the histological aspects of the samples using the histological classification of Marsh, modified by Oberhuber (23). This modified system establishes five lesion classes. In Marsh 0, there is normal architecture of the mucosa and less than 40 intraepithelial lymphocytes per 100 enterocytes in the villus epithelium. Marsh I is defined as normal architecture of the mucosa and more than 40 lymphocytes per 100 enterocytes in the villous epithelium. Marsh II involves crypt enlargement (hyperplasia), in which immature epithelial cells are produced in large numbers, and there is an influx of lymphocytes and plasmocytes. In this system, Marsh III has been reclassified and divided into three separate classes. In Marsh IIIa, there is partial villous atrophy combined with slight lymphocyte infiltration in epithelial cells and crypt hyperplasia. Marsh IIIb is marked by near total atrophy of the villi (villi still recognizable); crypt hyperplasia, in which immature epithelial cells are produced in greater proportions; and an influx of inflammatory cells. The final designation, Marsh IIIc, indicates total villous atrophy, hyperplasic crypts and infiltrative lesions. Samples with a preserved villous/crypt relationship, but with higher counts of intra-epithelial lymphocytes (IELs) in the hematoxylin and eosin staining, were also stained for immunohistochemistry to better determination of the IEL count (23).
RESULTS
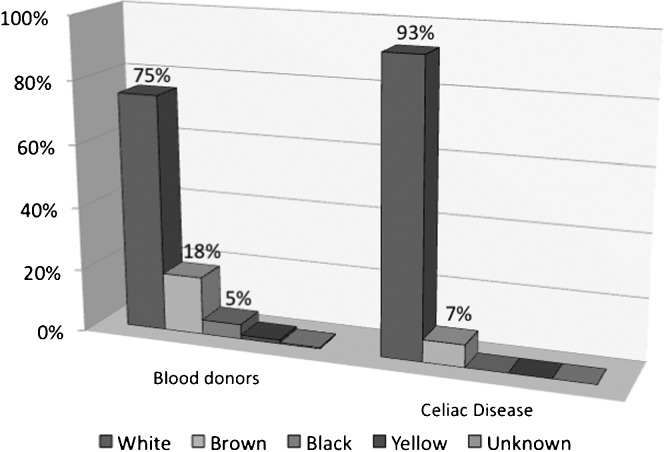
Blood bank population screening was carried out in 1,817 women (46.4%) and 2,183 men (54.6%) aged 18 to 65 years (median, 31 years). As shown in Table 1 and Figure 1, 75.3% of subjects were classified as White, 18.4% as Brown, 4.7% as Black, and 1.3% as Yellow. Sixteen subjects (0.4%) were considered of unknown ethnicity, as they declared not knowing their own ethnicity for unknown reasons.
Table 1.
Ethnic distribution (20) of blood donors in São Paulo city from September 2003 to August 2004.
| ETHNICITY (20) | N | (%) |
| White | 3011 | (75.3) |
| Brown (mixed ethnicities) | 737 | (18.4) |
| Black | 186 | (4.7) |
| Yellow | 50 | (1.3) |
| Unknown | 16 | (0.4) |
| TOTAL | 4000 | (100.0) |
Figure 1.
Ethnic distribution in the study population with and without celiac disease.
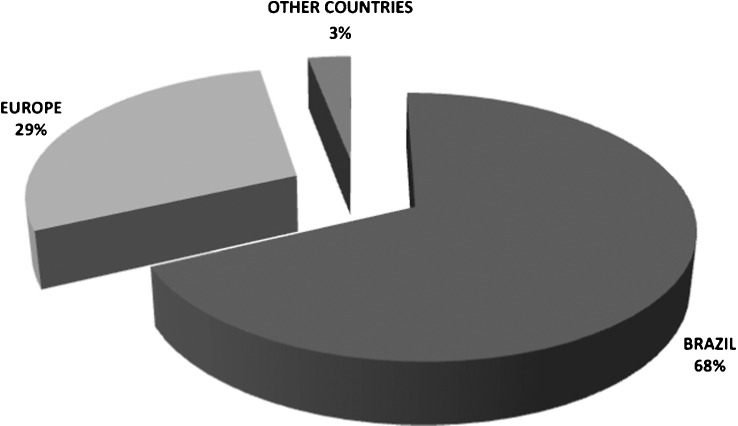
The vast majority of participants reported having been born in the southeast region of Brazil (77.8%), followed by the northeast region (17.7%). Based on the subjects' responses regarding relatives, 87.3 and 94.9% of the subjects' mothers and fathers, respectively, were Brazilians. With regard to first, second and third generations, most relatives not born in Brazil were born in European countries (Figure 2).
Figure 2.
Ancestrally distribution in the study population.
The 4,000 serum samples were examined using both tTG and AEA. The AEA positive predictive value was 100%, and the tTG positive predictive value was 85%. Test concordance was found in 11 cases, ten cases were only tTG+ and three cases were only AEA+.
Of the 24 donors with positive tests, 21 agreed to undergo an upper digestive endoscopy with duodenal biopsy; ten of them were tTG+/AEA+, three were tTG-/AEA+, and eight were tTG+/AEA-. Concerning duodenal endoscopic evaluation, only 29% displayed any changes; the most common finding was the mosaic appearance of the mucosa and, in one case, a decrease in duodenal folds.
Histological analysis, based on the Marsh criteria modified by Oberhuber (22), revealed two cases with an increase in intra-epithelial lymphocytes (>40 IELs/100 enterocytes), which were classified as type I; six cases with discreet villous atrophy were classified as type IIIa; three cases with marked villous atrophy were classified as type IIIb; and five cases with complete villous atrophy were classified as type IIIc (Figure 1). The remaining cases did not fit the criteria employed.
HLA typing was conducted in 21 patients, but only 14 exhibited HLA-DQ2 or -DQ8. Two of these patients were homozygous for HLA-DQB1*02 (DQ2), and one was homozygous for HLA-DQB1*0302 (DQ8). The HLA typing results in both cases of type I histological lesions did not confirm HLA DQ2/DQ8, nor did the results from four donors with nonspecific histological findings for CD.
In this study, 14 cases of CD were confirmed based on serological tests, HLA and atrophy observed on duodenal biopsy. The patients with CD were aged 18 to 51 years (median, 26 years), and 64.3% of them were women (M/F, 1:1.8). No patients mentioned a previous diagnosis of CD, and there were no complaints or symptoms of hepatic or thyroid disorders, small stature, infertility, osteoporosis or diabetes. No patients exhibited malabsorption, and two patients were asymptomatic. The majority of patients presented with a mild clinical picture, and the most common gastrointestinal symptoms were flatulence (78%), epigastralgia (28.6%), mouth ulcers (28.6%), and mild abdominal pain (21.4%). The most frequent extra-intestinal signs and symptoms were irritability (36%), anemia, fatigue (35.7%), and arthralgia (35%).
Sixty-four percent of the subjects reported European ancestry, primarily from Italy, Portugal, and Spain. Approximately 7% of the subjects did not have any information about their ancestry.
The prevalence of HLA DQ2/DQ8 was estimated to be 36% (65 subjects) in the control group of 180 inhabitants of São Paulo city. Serological marker tests conducted in this sample group revealed one positive case of CD.
DISCUSSION
Based on the results of the present study, a CD prevalence of 1/286 was estimated among supposedly healthy blood bank volunteers aged 18 to 65 years in São Paulo, Brazil, which is 2.4 times lower than the prevalence reported in the USA (24) and is similar to that reported in Europe (25).
São Paulo city was an important migratory destination for European Caucasians from the end of the 19th century to the middle of the 20th century. As reported in the present study, HLA DQ2/DQ8 prevalence was estimated as 36% in the São Paulo population, which is close to that in European countries (30%) (26). These data confirm a genetic predisposition to develop CD.
São Paulo city is also the largest urban center in Brazil and an important internal migration area, favoring great miscegenation. The ethnic category distribution reported in the 2005 census in the southern region of Brazil was 80.7% White, 15.0% Brown (Multiracial), 3.6% Black, and 0.4% Yellow (27). As shown in Table 1, the present study revealed a very similar distribution, demonstrating indirectly that the population studied resembles that of the southern region of the country. In Brazil, the classification of Brown (Multiracial), namely Pardo in Portuguese, constitutes a mixture of all three colonial ethnicities (i.e., White, Black, and Amerindian), but people of this classification are currently of predominantly European ancestry (28).
Studies investigating the prevalence of CD in Curitiba (State of Paraná, Brazil), conducted in a population whose ancestry was 100% European, showed an estimated prevalence of 1:417 (22); however, studies in Ribeirão Preto (State of São Paulo, Brazil), where 54.5% of the population is of European ancestry, yielded an estimated prevalence of 1:273 (16), which is very close to that reported in the present study.
Furthermore, an increase in the ingestion of wheat in the country in recent decades may have favored the increase in CD in our society (18,22). This information suggests that if genetic and environmental factors remain unchanged (e.g., ingestion of gluten-rich food), the prevalence of CD in different geographic regions may be the same.
The biopsy-confirmed CD prevalence was at least 1:286 (3.5:1,000; 95% CI = 1.66-5.33) among healthy blood donors, indicating that there is a high CD prevalence in Brazil, which has been confirmed by other national studies.
The prevalence of CD may be even higher in the city of São Paulo, considering that the study was conducted at a blood bank with supposedly healthy subjects and excluded subjects with anemia (one of the most common extra-intestinal symptoms) (29), hepatitis C virus (HCV) (exogenously administered IFN-α may trigger CD in predisposed subjects by enhancing Th1 responses) (30,31), and type 1 diabetes mellitus (CD prevalence of 4% in T1DM) (32). Moreover, a measurement of the total IgA was not performed, and its deficiency is higher among celiac patients (3%) (33), which may have produced false-negative results in our study. Additionally, three subjects with positive serological tests refused to undergo a duodenal biopsy to confirm the presence of the disease.
On the other hand, to ensure a more accurate screening diagnosis of CD in blood bank settings, two serological tests were employed, namely tTG and AEA, both of which are considered to have good sensitivity and specificity (33,34). If only AEA had been evaluated, four tTG+ subjects would not have been included in the cohort, whereas three patients would not have been properly diagnosed if only tTG had been assessed. CD would be underdiagnosed if only one serological marker was employed in general population screening studies. This finding is corroborated by a study conducted in Israel suggesting that the use of only one serological test for screening the so-called healthy population is not enough to establish the true prevalence of CD (10).
All celiac patients with biopsies indicating villous atrophy had confirmed HLA-DQ2 or DQ8 HLA typing results; two individuals with type I histological lesions did not have HLA DQ2/DQ8, ruling out the hypothesis of CD because the negative predictive value of this test is high (35).
In Brazil, three other population screening studies conducted at blood banks revealed the following prevalences of undiagnosed CD: 1:417 in Curitiba, 1:276 in Ribeirão Preto and 1:681 in Brasília (15,16,22).
Population screening carried out by our group in Curitiba showed that the prevalence of CD was lower than that in the city of São Paulo. This finding had not been anticipated, given the dominant European ancestry and lower internal migration in Curitiba, which suggests an underestimation of CD in this city. This is most likely due to the use of a single serological test for the initial screening, as the guinea pig anti-transglutaminase test shows a lower sensitivity compared with both the human anti-transglutaminase test and AEA (22).
The survey carried out in Ribeirão Preto, whose population of 500,000 inhabitants is approximately 5% of that of São Paulo city, employed an equivalent methodology concerning serological test sensitivity and showed a similar CD prevalence, providing evidence for the validity of the present study (16).
However, two population-screening studies conducted in Brasília yielded conflicting results. The first, demonstrating a prevalence of 1:681, was conducted in healthy blood donors and employed anti-gliadine as the initial test, which has a sensitivity and specificity between 52-100 and 71-100%, respectively (15). The second epidemiological survey was conducted in adult and pediatric patients at the University Hospital and used IgA AEA as the initial screening test, which showed a CD prevalence of 3.6 per 1,000 (approximately 1:278) (36). Such different results in the same city may be attributed to differences in the methodology and population between the two groups.
In the USA, the first population study of CD with blood donors, using IgA anti-gliadine (AGA) as an initial serological test, showed a CD prevalence of 1:250. However, the use of the human anti-transglutaminase test in the same sample showed a CD prevalence of 1:125, indicating that serological screening with AGA underestimated the CD prevalence in the USA (13).
When clinical presentation was evaluated, no patients showed typical manifestations of intestinal malabsorption. Flatulence, irritability, anemia, fatigue, and arthralgia were the most common unspecific symptoms/signs, which is evidence of the heterogeneous presentation of the disease (8,37).
Currently, the detection of atrophic alterations of the duodenal and jejunal mucosa, equivalent to type III Marsh lesions observed on biopsy, remains the gold standard in the diagnosis of CD (38). In the outpatient clinic at the University of São Paulo School of Medicine, six cases with positive serological tests, lacking either HLA DQ2/DQ8 haplotypes or villous atrophy, as well as one case with a positive serological test, HLA DQ2, and without villous atrophy, have been followed-up to evaluate the possibility of latent disease (23,34).
The present study suggests that the serological marker choice is vital to minimize the number of false-positive and -negative results, especially when the studied sample group is chosen from the general population and is therefore considered healthy. Combining two serological tests increased the sensitivity for the detection of CD and provided a better estimate of the prevalence of the disease in the city of São Paulo.
Although blood bank donors are generally highly selected groups that have been screened for risk factors and serological markers associated with a variety of infectious diseases, the present study demonstrated that the prevalence of CD in São Paulo city is similar to that in European countries. At the same time, the findings confirmed the importance of undiagnosed atypical cases in the population of São Paulo city.
It is reasonable to assume that the main factors contributing to the obtained prevalence were the high ingestion of wheat, as in other countries with a high incidence of the disease, and the population ancestry, as most citizens come from European countries with a high prevalence of CD.
This prevalence study is important because it was conducted in the largest urban center in Brazil, which represents 6% of the country's population and is representative of the population of the southern region of Brazil, which is the region with the highest European ancestry. Nevertheless, it is crucial that CD prevalence be mapped in other cities to assess regional population differences, as the central-western, northern and northeastern states of the country have greater ethnic differences, mainly due to miscegenation with people of African and Amerindian (indigenous) ethnicity.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1. National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28-30, 2004 Gastroenterology 2005. April;128(4 Suppl 1)S1–S9. [DOI] [PubMed] [Google Scholar]
- 2.Abdulkarim AS, Murray JA. Review article: The diagnosis of coeliac disease. Aliment Pharmacol Ther. 2003 Apr;17(8):987–95. doi: 10.1046/j.1365-2036.2003.01442.x. [DOI] [PubMed] [Google Scholar]
- 3.Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease. Gastroenterology. 2005;128(4 Suppl 1):S47–S51. doi: 10.1053/j.gastro.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Schuppan D, Dennis MD, Kelly CP. Celiac disease: epidemiology, pathogenesis, diagnosis, and nutritional management. Nutr Clin Care. 2005;8(2):54–69. [PubMed] [Google Scholar]
- 5.Mulder CJ, Cellier C. Coeliac disease: changing views. Best Pract Res Clin Gastroenterol. 2005;19(3):313–21. doi: 10.1016/j.bpg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40(1):1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology. 2005;128(4 Suppl 1):68–73. doi: 10.1053/j.gastro.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346(3):180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 9.Queiroz MS, Nery M, Cancado EL, Giannella-Neto D, Liberman B. Prevalence of celiac disease in Brazilian children of short stature. Braz J Med Biol Res. 2004;37(1):55–60. doi: 10.1590/s0100-879x2004000100008. [DOI] [PubMed] [Google Scholar]
- 10.Shamir R, Lerner A, Shinar E, Lahat N, Sobel E, Bar-or R, et al. The use of a single serological marker underestimates the prevalence of celiac disease in Israel: a study of blood donors. Am J Gastroenterol. 2002;97(10):2589–94. doi: 10.1111/j.1572-0241.2002.06028.x. [DOI] [PubMed] [Google Scholar]
- 11.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Moghadam KF, Farhadi M, Ansari R, et al. High prevalence of coeliac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15(5):475–8. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 12.Mankai A, Landolsi H, Chahed A, Gueddah L, Limem M, Ben Abdessalem M, et al. Celiac disease in Tunisia: serological screening in healthy blood donors. Pathol Biol (Paris) 2006;54(1):10–3. doi: 10.1016/j.patbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Neri E, Not T, Horvath K, Kryszak D, Drago S, Di Pierro MR, et al. Human tissue transglutaminase ELISA and an old study: a revision of the blood donor screening study for coeliac disease in the USA. Scand J Gastroenterol. 2004;39(2):195–7. doi: 10.1080/00365520310007530. [DOI] [PubMed] [Google Scholar]
- 14.Weile I, Grodzinsky E, Skogh T, Jordal R, Cavell B, Krasilnikoff PA. High prevalence rates of adult silent coeliac disease, as seen in Sweden, must be expected in Denmark. Apmis. 2001;109(11):745–50. doi: 10.1034/j.1600-0463.2001.d01-141.x. [DOI] [PubMed] [Google Scholar]
- 15.Gandolfi L, Pratesi R, Cordoba JC, Tauil PL, Gasparin M, Catassi C. Prevalence of celiac disease among blood donors in Brazil. Am J Gastroenterol. 2000;95(3):689–92. doi: 10.1111/j.1572-0241.2000.01847.x. [DOI] [PubMed] [Google Scholar]
- 16.Melo SB, Fernandes MI, Peres LC, Troncon LE, Galvao LC. Prevalence and Demographic Characteristics of Celiac Disease Among Blood Donors in Ribeirao Preto, State of Sao Paulo, Brazil. Dig Dis Sci. 2006;51(5):1020–1025. doi: 10.1007/s10620-006-9340-9. [DOI] [PubMed] [Google Scholar]
- 17.Sao Paulo (Estado) Secretaria da Agricultura Departamento de Imigração e Colonização. Estatística dos trabalhos executados pelo Departamento de Imigração e colonização durante o ano de 1961 Sao Paulo, 1962, p. 44. 1962 [Google Scholar]
- 18.de Freitas IN, Sipahi AM, Damiao AO, de Brito T, Cancado EL, Leser PG, et al. Celiac disease in Brazilian adults. J Clin Gastroenterol. 2002;34(4):430–4. doi: 10.1097/00004836-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Trabulsi LR, Toledo MR, Kitagawa SM, Candeias JA. Diarrheal disease in children in Sao Paulo. Kansenshogaku Zasshi. 1988;62 Suppl:97-104 [PubMed] [Google Scholar]
- 20.Special Edition of Monthly Employment Survey about Color and Race. Instituto Brasileiro de Geografia e Estatística. Noticias. Brasilia, November 11, 2006. http://www.ibge.gov.br/english/presidencia/noticias/noticia_visualiza.php?id_noticia=737&id_pagina;=1. Accessed on May 2011.
- 21.Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115(6):1317–21. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 22.Pereira MA, Ortiz-Agostinho CL, Nishitokukado I, Sato MN, Damiao AO, Alencar ML, et al. Prevalence of celiac disease in an urban area of Brazil with predominantly European ancestry. World J Gastroenterol. 2006;12(40):6546–50. doi: 10.3748/wjg.v12.i40.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11(10):1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Katz KD, Rashtak S, Lahr BD, Melton LJ, 3rd, Krause PK, Maggi K, et al. Screening for Celiac Disease in a North American Population: Sequential Serology and Gastrointestinal Symptoms. Am J Gastroenterol. 2011;106(7):1333–9. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004;36(7):492–8. doi: 10.1016/j.dld.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Kapitany A, Toth L, Tumpek J, Csipo I, Sipos E, Woolley N, et al. Diagnostic significance of HLA-DQ typing in patients with previous coeliac disease diagnosis based on histology alone. Aliment Pharmacol Ther. 2006;24(9):1395–402. doi: 10.1111/j.1365-2036.2006.03133.x. [DOI] [PubMed] [Google Scholar]
- 27.http://www.sidra.ibge.gov.br/pnad/pnadpb.asp?o=3&i=P (accessed on May 2011)
- 28.Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy FSG, et al. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6(2):e17063. doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnam US, Felder LR, Raskin JB. Prevalence of occult celiac disease in patients with iron-deficiency anemia: a prospective study. South Med J. 2004;97(1):30–4. doi: 10.1097/01.SMJ.0000051059.23259.56. [DOI] [PubMed] [Google Scholar]
- 30.Plot L, Amital H. Infectious associations of Celiac disease. Autoimmun Rev. 2009;8(4):316–9. doi: 10.1016/j.autrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Durante-Mangoni E, Iardino P, Resse M, Cesaro G, Sica A, Farzati B, et al. Silent celiac disease in chronic hepatitis C: impact of interferon treatment on the disease onset and clinical outcome. J Clin Gastroenterol. 2004;38(10):901–5. doi: 10.1097/00004836-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Cronin CC, Feighery A, Ferriss JB, Liddy C, Shanahan F, Feighery C. High prevalence of celiac disease among patients with insulin-dependent (type I) diabetes mellitus. Am J Gastroenterol. 1997;92(12):2210–2. [PubMed] [Google Scholar]
- 33.Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128(4 Suppl 1):38–46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease. Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;128(4 Suppl 1):25–32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Kaukinen K, Partanen J, Maki M, Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97(3):695–9. doi: 10.1111/j.1572-0241.2002.05471.x. [DOI] [PubMed] [Google Scholar]
- 36.Pratesi R, Gandolfi L, Garcia SG, Modelli IC, Lopes de AP, Bocca AL, et al. Prevalence of coeliac disease: unexplained age-related variation in the same population. Scand J Gastroenterol. 2003;38(7):747–50. doi: 10.1080/00365520310003255. [DOI] [PubMed] [Google Scholar]
- 37.Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128(4 Suppl 1):19–24. doi: 10.1053/j.gastro.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992;102(1):330–54. [PubMed] [Google Scholar]