Abstract
OBJECTIVE:
This study sought to identify the relationship between fibroblast telomerase expression, myofibroblasts, and telomerase-mediated regulatory signals in idiopathic pulmonary fibrosis.
METHODS:
Thirty-four surgical lung biopsies, which had been obtained from patients with idiopathic pulmonary fibrosis and histologically classified as usual interstitial pneumonia, were examined. Immunohistochemistry was used to evaluate fibroblast telomerase expression, myofibroblast α-smooth muscle actin expression and the tissue expression of interleukin-4, transforming growth factor-β, and basic fibroblast growth factor. The point-counting technique was used to quantify the expression of these markers in unaffected, collapsed, mural fibrosis, and honeycombing areas. The results were correlated to patient survival.
RESULTS:
Fibroblast telomerase expression and basic fibroblast growth factor tissue expression were higher in collapsed areas, whereas myofibroblast expression and interleukine-4 tissue expression were higher in areas of mural fibrosis. Transforming growth factor-β expression was higher in collapsed, mural fibrosis and honeycombing areas in comparison to unaffected areas. Positive correlations were found between basic fibroblast growth factor tissue expression and fibroblast telomerase expression and between interleukin-4 tissue expression and myofibroblast α-smooth muscle actin expression. Negative correlations were observed between interleukin-4 expression and basic fibroblast growth factor tissue expression in areas of mural fibrosis. Myofibroblast α-smooth muscle actin expression and interleukin-4 tissue expression in areas of mural fibrosis were negatively associated with patient survival.
CONCLUSION:
Fibroblast telomerase expression is higher in areas of early remodeling in lung tissues demonstrating typical interstitial pneumonia, whereas myofibroblast α-smooth muscle actin expression predominates in areas of late remodeling. These events seem to be regulated by basic fibroblast growth factor and interleukin-4 tissue expression, respectively.
Keywords: Telomerase, Fibroblasts, α-smooth Muscle Actin, Interleukin-4, Idiopathic Pulmonary Fibrosis
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a devastating chronic fibrosing interstitial pneumonia of unknown etiology characterized by excessive collagen deposition and irreversible remodeling of the lung parenchyma (1,2). Histopathology typically reveals a pattern of usual interstitial pneumonia (UIP), which is typified by patchy areas of mural fibrosis interspersed with areas of honeycomb changes as well as collapsed and normal lung parenchyma (3). Although the etiology of IPF/UIP remains poorly understood, fibroblasts are believed to be key effectors cells (4,5) because they proliferate and form fibroblastic foci, which are thought to constitute the leading edge of fibrosis (6). Myofibroblasts, which represent differentiated fibroblasts, are located in those areas and are the primary cell type responsible for extracellular matrix (ECM) synthesis and tissue remodeling, which leads to the loss of alveolar function (5). A hallmark of myofibroblast differentiation is the expression of alpha-smooth muscle actin (α-SMA) (7).
The key factor in IPF pathogenesis may be the alveolar epithelium, which appears to trigger the fibrotic process due to an impaired healing capacity (8) that leads to alveolar epithelial cell apoptosis (9). Subsequent epithelial-mesenchymal crosstalk results in persistently activated myofibroblasts, which lead to an aberrant wound healing process (8). Our group previously demonstrated that the cause of this reduced alveolar epithelial regenerative capacity may be decreased telomerase expression in type 2 alveolar epithelial cells (10). In light of these (10) and other recent findings implicating telomerase in IPF pathogenesis (11-14), we sought to study fibroblast telomerase expression in IPF.
Telomerase is a specialized polymerase that adds telomere repeats to the ends of chromosomes, which compensates for the telomere loss that normally occurs with each cell division (15-16). Telomerase therefore increases a cell's life span and is essential for unlimited cellular proliferation (16). Bleomycin-induced lung injury and fibrosis are known to induce telomerase activity in rat lung fibroblasts, which results in greater numbers of these cells with increased proliferative capacity (17). Telomerase induction also regulates fibroblast differentiation; bleomycin injury-induced telomerase activity is localized primarily to non-myofibroblasts (17), and the loss of telomerase activity is associated with myofibroblast differentiation (18). Furthermore, bFGF (basic fibroblast growth factor) has been identified as the signal responsible for fibroblast telomerase activity induction, whereas TGF-β (transforming growth factor beta), and IL-4 (interleukin-4) are associated with reduced telomerase activity and the consequent increase in α-SMA expression leading to myofibroblast differentiation (19).
In light of these previous data from rat models of bleomycin-induced pulmonary fibrosis, which have suggested an interaction between fibroblast telomerase activity, bFGF, IL-4, TGF-β, and myofibroblast differentiation (17-19), the current study sought to evaluate the following aims in patients with IPF: (a) whether telomerase is over-expressed in fibroblasts in the areas of early and late remodeling in UIP; (b) the relationship between TGF-β, IL-4, and bFGF tissue expression, fibroblast telomerase and myofibroblast α-SMA expression; and (c) the impact of these factors on patient survival.
MATERIALS AND METHODS
Casuistic design
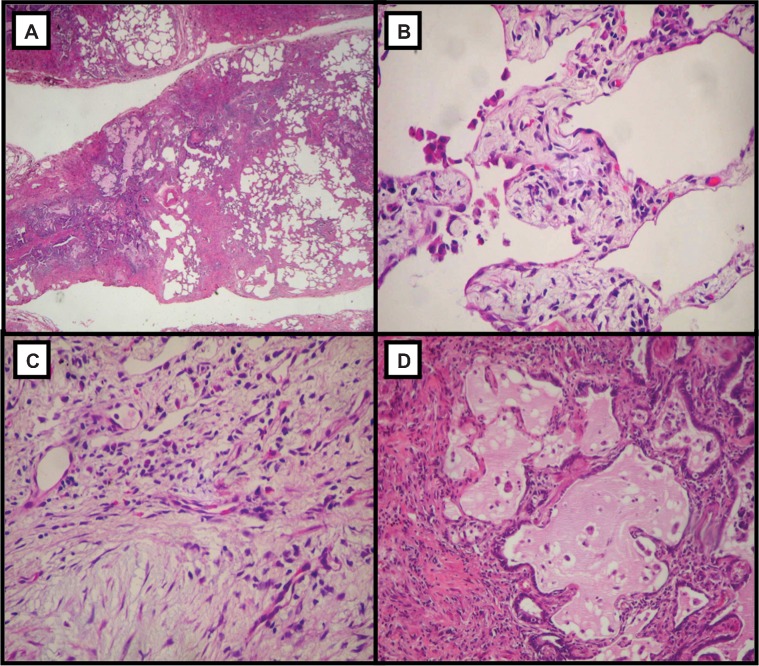
This study examined 34 open surgical lung biopsies, which had been obtained from patients with a clinical diagnosis of IPF (19 men and 15 women; median age: 69 years, range: 49-77 years) and histologically classified as UIP. The histological diagnosis of UIP was made in accordance with the criteria outlined in the American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias (20). UIP was characterized by the patchy subpleural and paraseptal distribution of parenchymal injury, with temporal heterogeneity observed at low magnification (Figure 1A). Areas of unaffected lung parenchyma with alveolar collapse and interstitial mononuclear infiltrates were characterized as collapsed areas (Figure 1B). Areas of septal fibro-myxoid tissue with fibroblastic foci were characterized as areas of mural fibrosis (Figure 1C). Irregular cystic air spaces between bands of fibrous connective tissue were characterized as honeycombing areas (Figure 1D).
Figure 1.
Panoramic view showing unaffected areas alternating with fibrous and cystic changes, which characterize the UIP histological pattern (A, ×40); collapsed area with alveolar collapse and interstitial mononuclear infiltrates (B, ×100); mural fibrosis area with septal fibromyxoid tissue and fibroblastic foci (C, ×100); and honeycombing area with irregular cystic air spaces between bands of fibrous connective tissue (D, ×100). Hematoxylin and eosin staining.
We retrospectively reviewed patients' charts to collect smoking history, current status, and pulmonary function test results. Overall survival was calculated as the time between the lung biopsy and the date of last contact or death. No patient was treated for IPF prior to lung biopsy.
Immunohistochemistry analysis
Immunohistochemical analysis was performed using commercially available kits to characterize TGF-β (polyclonal rabbit, cat Sc-146, 1:1500 dilution, Santa Cruz Biotechnology Inc., USA), IL-4 (polyclonal rabbit, cat 7919, 1:50 dilution, Santa Cruz Biotechnology Inc., USA), bFGF (monoclonal bovine anti-FGF- 2/basic FGF, clone bFM-2, cat 05-118, 1:100 dilution, Millipore Inc., USA) α-SMA (monoclonal mouse, clone 1A4, cat MO 851, 1:500 dilution, Sigma-Aldrich Inc., USA) and telomerase (polyclonal rabbit, cat 582005, 1:500 dilution, Calbiochem U.S. EMD Biosciences Inc., USA) expression.
In brief, the sections were deparaffinized and rehydrated with Tris-buffered saline (0.0005 mol/L Tris and 0.15M NaCl, pH 7.6) for 10 minutes. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 minutes. Antigen unmasking was performed with 10 mM citrate buffer (pH 6.0); for IL-4, trypsin was also used. Next, the sections were washed in Tris-buffered saline and incubated with primary antibodies at the appropriate dilutions overnight at 4° to 8°C. The Novolink kit (code RE 7280-K, Max Polymer Detector System, Leica Biosystems Inc., UK) was used as a secondary antibody. The peroxidase reaction was developed using 3,3-diaminobenzidine tetrachloride (0.25 mg dissolved in 1 mL of 0.02% hydrogen peroxide) for 3 minutes.
Morphometry
TGF-β, IL-4 and bFGF tissue expression were assessed in 10 fields using the point-counting technique in unaffected, collapsed, mural fibrosis and honeycombing areas of the UIP biopsies (totaling 40 fields per case) using a 100-point grid with a known area (62500 μm2) attached to the ocular of the microscope (21). In each field, at 400× magnification, the lung tissue area was calculated according to the number of points within pulmonary tissue as a proportion of the total grid area. Subsequently, the number of positive cells was counted. The concentration of immunostained cells was determined as the overall sum of the number of positive cells divided by the sum of the respective area. The final result was expressed as a percentage of the marker expression in each area for each biopsy.
The same methodology was used to calculate myofibroblast α-SMA and fibroblast telomerase expression. However, immunostained cells were considered to be positive only when they were observed as morphologically similar to fibroblasts (i.e., thin cells with an elongated shape). Interobserver comparisons were performed in 20% of the slides by two observers (E.R.P. and V.L.C.). The coefficient of variation for the interobserver error in cell count was <5%.
Statistical analysis
The expression of each marker was individually compared in unaffected, collapsed, mural fibrosis and honeycombing area using paired sample t-tests. The Pearson test was used to determine the associations between the studied markers. Cox's regression model was used to analyze survival. All analyses were performed using SPSS statistical software (SPSS Inc., USA, 2009). The statistical significance was set at the level of p≤0.05.
The research protocol was approved by the Ethical and Scientific Committee at our institution Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo (CAPPesq 0731/07).
RESULTS
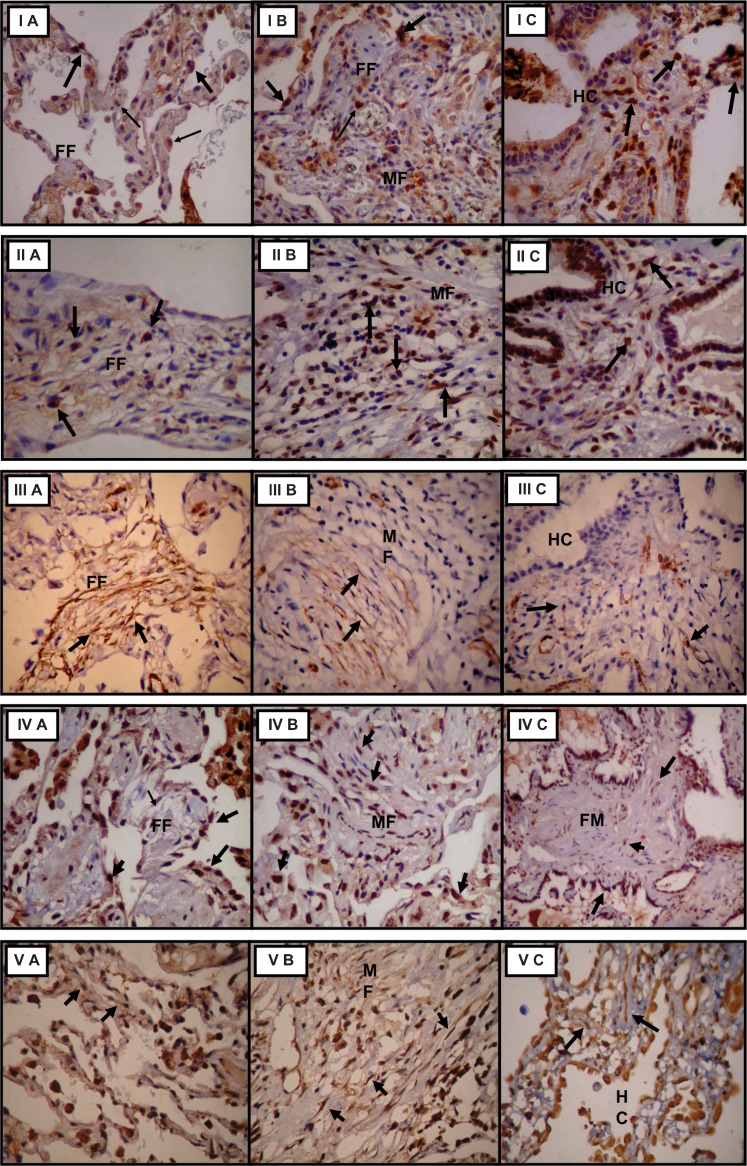
The mean levels of TGF-β, IL-4 and bFGF tissue expression as well as the mean levels of myofibroblast α-SMA and fibroblast telomerase expression are shown in Table 1. Significant associations between these markers are shown in Table 2. Briefly, TGF-β expression was higher in collapsed, mural fibrosis and honeycombing areas in comparison to unaffected areas (Figure 2 - I). IL-4 and myofibroblast α-SMA expression were higher in mural fibrosis areas (Figure 2 - II and IV), and bFGF and fibroblast telomerase expression were highest in collapsed areas (Figure 2 - III and V). No significant myofibroblast α-SMA expression, fibroblast telomerase expression or IL-4 tissue expression were found in unaffected areas. Positive correlations were observed between bFGF tissue expression and fibroblast telomerase expression and between IL-4 tissue expression and myofibroblast α-SMA expression. A negative correlation was observed between IL-4 and bFGF tissue expression in areas of mural fibrosis.
Table 1.
Mean levels of TGF-β, IL-4 and bFGF tissue expression and mean levels of myofibroblast α-SMA and fibroblast telomerase expression in each studied area. For tissue expression, all immunostained cells were considered to be positive. For fibroblasts expression, only immunostained cells with a morphological similarity to fibroblasts (thin cells with an elongated shape) were considered to be positive. Abbreviations: U, unaffected areas; C, collapsed areas; MF, mural fibrosis areas; and H, honeycombing areas.
| Marker | Unaffected (U) | Collapsed (C) | Mural Fibrosis (MF) | Honeycombing (H) | Statistical analysis |
| TGF-β | 9.19±2.16 | 19.63±5.70 | 16.48±4.11% | 17.08±4.52% | U × C: p = 0.01*U × MF: p = 0.01*U × H: p = 0.01*C × MF: p = 0.13C × H: p = 0.51MF × H: p = 0.22 |
| IL-4 | 0.0±0.0 | 3.27±5.48% | 14.03±6.02% | 7.91±4.04% | C × MF: p<0.01*C × H: p<0.01*MF × H: p<0.01* |
| bFGF | 10.57±6.24 | 15.50±4.99% | 11.62±3.62% | 12.21±4.68% | U × C: p = 0.01*U × MF: p = 0.72U × H: p = 0.31C × MF: p = 0.02*C × H: p = 0.03*MF × H: p = 0.65 |
| Myofibroblast α-SMA | 0.0±0.0 | 9.02±2.92% | 5.25±5.14% | 5.21±2.13% | C × MF: p<0.01*C × H: p<0.92MF × H: p<0.01* |
| FibroblastTelomerase | 0.0±0.0 | 6.76±1.31% | 5.26±1.13% | 5.01±1.14% | C × MF: p = 0.04*C × H: p = 0.03*MF × H: p = 0.19 |
Area (mean ± Standard Deviation).
Table 2.
Significant correlations between each marker for each studied area.
| Variables | Areas | Correlation Coefficient | p-value |
| bFGF and telomerase | Collapsed | 0.41 | 0.03 |
| Mural Fibrosis | 0.43 | 0.02 | |
| Honeycombing | 0.62 | 0.01 | |
| IL-4 and α-SMA | Collapsed | 0.39 | 0.05 |
| Mural Fibrosis | 0.60 | 0.01 | |
| Honeycombing | 0.62 | 0.01 | |
| BFGF and IL-4 | Mural Fibrosis | -0.63 | 0.01 |
Figure 2.
Tissue TGF-β (I), IL-4 (II) and bFGF expression (III), myofibroblast α-SMA expression (IV), and fibroblast telomerase expression (V) in collapsed (A), mural fibrosis (B) and honeycomb (C) areas, 100X. FF, fibroblast foci; MF, mural fibrosis; HC, honeycomb.
The Cox proportional hazard model analysis of survival time identified four variables that were associated with survival (model likelihood ratio = 26.11; Chi-square = 13.74; p = 0.01): age greater than 68 years (Odds Ratio [OR] = 2.44, 95% Confidence Interval [CI]: 0.35 – 16.77, p = 0.03); full vital capacity (FVC) greater than 66% of predicted FVC (OR = 0.94, 95% CI:0.86–1.03, p = 0.02); myofibroblast α-SMA expression in mural fibrosis areas higher than 8.55% (OR = 4.84, 95% CI:0.57–41.03, p = 0.04); and IL-4 tissue expression in mural fibrosis areas higher than 13.9% (OR = 10.1, 95% CI:1.08–94.43, p = 0.04). Patients with myofibroblast α-SMA expression lower than 8.55% and those with IL-4 tissue expression lower than 13.9% in areas of mural fibrosis exhibited better survival (Figure 3).
Figure 3.
Cox multivariate analysis plots of survival probability versus follow-up time in months for patients >68 years with FVC<66%. Patients with myofibroblast α-SMA expression lower than 8.55% (A) or with IL-4 tissue expression lower than 13.9% (B) in areas of mural fibrosis appear as the top curve.
DISCUSSION
In the present study, we investigated myofibroblast α-SMA and fibroblast telomerase expression as well as related regulatory signals (TGF-β, IL-4, bFGF) involved in different stages of the fibrotic process of UIP/IPF using immunohistochemistry. Fibroblast telomerase expression was higher in areas of early remodeling and was associated with bFGF tissue expression, whereas myofibroblast α-SMA expression was higher in areas of late remodeling and was associated with IL-4 tissue expression. No significant association was found for TGF-β, which was highly expressed in collapsed, mural fibrosis and honeycombing areas. Noticeably, myofibroblast α-SMA expression and IL-4 tissue expression in mural fibrosis areas were associated with patient survival.
There is compelling evidence from both experimental and clinical studies to suggest the increased proliferative capacity of lung fibroblasts in cases of IPF. Jordana et al. demonstrated that primary lung fibroblasts derived from IPF patients proliferated more rapidly than did fibroblasts extracted from normal lungs (22), and Zhang and colleagues demonstrated similar results in fibroblasts isolated from bleomycin-induced pulmonary fibrosis (23). Telomerase expression may play a role in the mechanism responsible for these findings, as demonstrated by the study of Nozaki et al. (17), which showed that telomerase activity is induced in the affected lung tissue and isolated lung fibroblasts from rats with bleomycin-induced pulmonary fibrosis in a time-dependent manner. The activity was observed only during the period of active fibrosis, and its maximal values were coincident with the peak of fibrosis. Hence, increased levels of telomerase may contribute to the expansion of the fibroblast population by enhancing their proliferative capacity and extending their life span.
A growing body of evidence has demonstrated that lung fibroblasts in pulmonary fibrosis are heterogeneous with respect to phenotype and behavior (4,5). Myofibroblasts are a unique subpopulation of fibroblasts that share features with smooth cells, as they are contractile and express α-SMA. These large cells present a distinctive aggressive phenotype and are believed to be the main cell type responsible for collagen accumulation and tissue remodeling in IPF (5). However, myofibroblasts grow markedly more slowly than intermediate or small-sized fibroblast (24). This differing proliferative potential and phenotype may be due to telomerase activity, as this activity was detected primarily in non-myofibroblasts in the study from Nozaki et al. (17). Another study from the same group further clarified this potential link between telomerase expression and myofibroblast differentiation (18); using the same bleomycin-induced pulmonary fibrosis rat model, they demonstrated that the inhibition of fibroblast telomerase activity led to increased α-SMA expression, which represents a marker of myofibroblast differentiation, whereas the induction of telomerase activity inhibited α-SMA expression. These results suggest that the loss of telomerase activity is closely related to myofibroblast differentiation, which is suppressed by telomerase expression. In the present study, we found that fibroblast telomerase expression was higher in areas of early remodeling, whereas myofibroblast α-SMA expression was higher in areas of late remodeling. This finding may also provide evidence for the influence of telomerase expression on fibroblast differentiation. Interestingly, fibroblast differentiation is reportedly higher in cells obtained from early fibrosis as compared to cells from dense fibrosis (25), and these findings may also be explained by differences in telomerase expression.
Another study from Liu et al. examined the signals responsible for the induction of telomerase activity in the bleomycin-induced pulmonary fibrosis rat model (19). bFGF was shown to induce telomerase activity in fibroblasts from both control and bleomycin-treated lungs, but IL-4 and TGF-β inhibited telomerase activity and were accompanied by α-SMA expression. Similar correlations were observed in the present study; however, we analyzed IPF/UIP human cases. The only exception was TGF-β, which showed no correlation with fibroblast telomerase or myofibroblast α-SMA expression, even though it was highly expressed in all examined areas. This cytokine is reported to play a key role in IPF pathogenesis, as it is one of the more potent inducers of collagen deposition (26). A major role of TGF-β involves mediating the transition of quiescent fibroblasts into cells with an “activated” phenotype (27), which is consistent with fibroblast-to-myofibroblast differentiation (5). The fact that higher TGF-β expression was observed in the epithelium of collapsed areas may be evidence of epithelial-mesenchymal crosstalk that results in the persistent activation of fibroblasts. This scenario occurs in the context of recurrent injury to the alveolar epithelium, which leads to an inability to re-epithelialize the denuded basement membrane due to the impaired healing capacity of the alveolar epithelium (8,28).
To evaluate clinical impact and establish the relevancy of these findings to the evolution of the patients, telomerase activity and the levels of profibrotic cytokines and growth factors were evaluated as a function of patient survival. Multivariate analysis demonstrated a high risk of death for patients with IPF, low FVC, high myofibroblast α-SMA expression (OR = 4.84) or IL-4 tissue expression (OR = 10.1) in mural fibrosis areas. In fact, an increase in the number of fibroblast foci, which represent the primary loci of myofibroblasts expressing α-SMA, has already been shown to be associated with worsened prognosis (6,29). Interleukin-4 is a fibrotic cytokine that potently induces TGF-β production by pulmonary fibroblasts and directly stimulates these cells to produce collagen in vitro, which leads to increased ECM synthesis (30,31). IL-4 is generally produced by T lymphocytes, although it may be produced by different types of inflammatory cells, such as eosinophils and mast cells (32,33). It is worth noting that IL-4 promotor gene polymorphisms have been shown to have a pathogenic role in the etiology and pathogenesis of IPF (34). The predominance of IL-4 tissue expression in areas of late remodeling in IPF/UIP, as observed in our study, has also been described previously (35). Our results are therefore consistent with these studies.
As in bleomycin-induced lung fibrosis, the expansion of the lung fibroblast population occurs before the posterior emergence of myofibroblasts, and the fact that telomerase is selectively induced in fibroblasts indicates that telomerase participates in the pathogenesis of fibrosis at a stage prior to the differentiation of fibroblasts to myofibroblasts (19). Therefore, these telomerase-positive fibroblasts may represent an intermediate activated phenotype between quiescent fibroblasts and their differentiation to myofibroblasts (18). Similar assumptions for IPF/UIP can be made when analyzing our results. We observed a spatial relationship between fibroblast telomerase expression and bFGF tissue expression in areas of early remodeling and between myofibroblast α-SMA expression and IL-4 tissue expression in areas of late remodeling. Therefore, we hypothesize that the apoptosis of alveolar epithelial cells results in persistent activation of quiescent fibroblasts via bFGF production, which is characterized by increased fibroblast telomerase expression. This process would enhance fibroblast replicative capacity and increase the numbers of these cells, leading to the formation of fibroblast foci. The early stage of remodeling would therefore be primarily associated with the expansion of the fibroblast population. This phenomenon would then be followed by a decrease in fibroblast telomerase expression caused by IL-4 production, which would result in fibroblast differentiation into myofibroblasts. These cells exhibit a strong capacity for ECM synthesis and deposition and would characterize the late remodeling process and fibrosis establishment.
We conclude that fibroblast telomerase expression is higher in the areas of early remodeling in UIP/IPF, whereas myofibroblast α-SMA expression predominates in areas of late remodeling, and these events seem to be regulated by bFGF and IL-4 tissue expression, respectively. Moreover, α-SMA and IL-4 expression in areas of mural fibrosis in UIP/IPF may represent surrogate markers for patient prognosis.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;16;345(7):517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Katzenstein AA, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathological classification. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1301–15. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 4.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24(5):591–8. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 5.Scotton C, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblasts in focus. Chest. 2007;132(4):1311–21. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164(6):1025–32. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148(2):527–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3(4):364–72. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 9.Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP) J Clin Pathol. 2001;54(2):132–8. doi: 10.1136/jcp.54.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waisberg DR, Barbas-Filho JV, Parra ER, Fernezlian S, Carvalho CR, Kairalla RA, et al. Abnormal expression of telomerase/apoptosis limits type II alveolar epithelial cell replication in the early remodeling of usual interstitial pneumonia/idiopathic pulmonary fibrosis. Human Pathology. 2010;41(3):385–91. doi: 10.1016/j.humpath.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Armanios MY, Chen JLLJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. N Engl J Med. 2007;356(13):1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 12.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104(18):7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–37. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–65. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 16.Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci U S A. 1998;95(1):90–2. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. American journal of respiratory cell and molecular biology. 2000;23(4):460–5. doi: 10.1165/ajrcmb.23.4.3958. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Hu B, Chung MJ, Ullenbruch M, Jin H, Phan SH. Telomerase regulation of myofibroblast differentiation. Am J Resp Cell Mol Biol. 2006;34(5):625–33. doi: 10.1165/rcmb.2005-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Nozaki Y, Phan SH. Regulation of Telomerase Activity in Rat Lung Fibroblasts. Am J Respir Cell Mol. Biol. 2002;26(5):534–40. doi: 10.1165/ajrcmb.26.5.4668. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society,European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 21.Gundersen HJG, Bendtesen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 22.Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, et al. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis. 1988;137(5):579–84. doi: 10.1164/ajrccm/137.3.579. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148(2):527–37. [PMC free article] [PubMed] [Google Scholar]
- 24.Uhal BD, Ramos C, Joshi I, Bifero A, Pardo A, Selman M. Cell size, cell cycle, and alpha-smooth muscle actin expression by primary human lung fibroblasts. Am J Physiol. 1998;275(5 Pt 1):L998–L1005. doi: 10.1152/ajplung.1998.275.5.L998. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Chen YY, Rusch V, Rabinivitch PS. Differential proliferation of fibroblast cultured and fibrotic human lungs. Am Rev Respir Dis. 1988;138(3):703–8. doi: 10.1164/ajrccm/138.3.703. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin A, Jenkins G. Role of integrin-mediated TGFβ activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37(Pt 4):849–54. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- 27.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-b1 induces a-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strieter RM. What differentiates normal lung repair and fibrosis. Proc Am Thorac Soc. 2008;5(3):305–10. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes AJ, Capone D, Mogami R, Lanzillotti RS, Melo PL, Jansen JM. Severity classification for idiopathic pulmonary fibrosis by using fuzzy logic. Clinics. 2011;66(6):1015–9. doi: 10.1590/S1807-59322011000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992;90(4):1479–85. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, et al. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163(4):2193–201. [PubMed] [Google Scholar]
- 32.Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101(3):436–41. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barata LT, Ying S, Meng Q, Barkans J, Rajakulasingam K, Durham SR, Kay AB. IL-4- and IL-5-positive T lymphocytes, eosinophils, and mast cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Allergy Clin Immunol. 1998;101(2Pt 1):222–30. doi: 10.1016/s0091-6749(98)70387-2. [DOI] [PubMed] [Google Scholar]
- 34.Vasakova M, Striz I, Slavcev A, Jandova S, Kolesar L, Sulc J. Th1/Th2 cytokine gene polymorphisms in patients with idiopathic pulmonary fibrosis. Tissue Antigens. 2006;67(3):229–32. doi: 10.1111/j.1399-0039.2006.00560.x. [DOI] [PubMed] [Google Scholar]
- 35.Ando M, Miyazaki E, Fukami T, Kumamoto T, Tsuda T. Interleukin-4-producing cells in idiopathic pulmonary fibrosis: an immunohistochemical study. Respirology. 1999;4(4):383–91. doi: 10.1046/j.1440-1843.1999.00209.x. [DOI] [PubMed] [Google Scholar]