Abstract
OBJECTIVE:
Experimental studies on lung preservation have always been performed using animal models. We present ex vivo lung perfusion as a new model for the study of lung preservation. Using human lungs instead of animal models may bring the results of experimental studies closer to what could be expected in clinical practice.
METHOD:
Brain-dead donors whose lungs had been declined by transplantation teams were used. The cases were randomized into two groups. In Group 1, Perfadex® was used for pulmonary preservation, and in Group 2, LPDnac, a solution manufactured in Brazil, was used. An ex vivo lung perfusion system was used, and the lungs were ventilated and perfused after 10 hours of cold ischemia. The extent of ischemic-reperfusion injury was measured using functional and histological parameters.
RESULTS:
After reperfusion, the mean oxygenation capacity was 405.3 mmHg in Group 1 and 406.0 mmHg in Group 2 (p = 0.98). The mean pulmonary vascular resistance values were 697.6 and 378.3 dyn·s·cm-5, respectively (p = 0.035). The mean pulmonary compliance was 46.8 cm H2O in Group 1 and 49.3 ml/cm H2O in Group 2 (p = 0.816). The mean wet/dry weight ratios were 2.06 and 2.02, respectively (p = 0.87). The mean Lung Injury Scores for the biopsy performed after reperfusion were 4.37 and 4.37 in Groups 1 and 2, respectively (p = 1.0), and the apoptotic cell counts were 118.75/mm2 and 137.50/mm2, respectively (p = 0.71).
CONCLUSION:
The locally produced preservation solution proved to be as good as Perfadex®. The clinical use of LPDnac may reduce costs in our centers. Therefore, it is important to develop new models to study lung preservation.
Keywords: Lung Transplantation, Organ Preservation, Ischemia-Reperfusion Injury
INTRODUCTION
When an organ is removed from the human body, the organ is subjected to ischemic injury, which may result in temporary or permanent organ dysfunction after transplantation. The role of pulmonary preservation is to minimize ischemic effects by preserving the functional and morphological integrity of the lungs, thus improving function after transplantation. The preservation method adopted by most centers is pulmonary artery perfusion with a preservation solution at 4°C due to the technical simplicity and efficacy of this method (1). This technique is intended to cool the tissue evenly and to remove blood from the pulmonary vascular bed, preventing thrombosis and minimizing the cellular injury caused by macrophages and neutrophils. Several experimental studies have been conducted in the past two decades, demonstrating the superiority of extracellular solutions (Celsior, low-potassium dextran) over intracellular solutions (Euro-Collins, Wisconsin University) in lung transplantation (2-4).
Experimental research in the field of pulmonary preservation has always been performed with animal models. The species and model structures used vary widely, and the lack of a standardized model hampers the comparison of different studies.
Experimental models in medium and large animals are expensive and time consuming. Small animals are less expensive, and their use enables experiments to be performed in a shorter period of time. In vivo pulmonary transplantation models in rats are associated with higher mortality rates, except in centers where this technique is performed frequently. Cardiac and pulmonary vascular resistance measurements are difficult to perform in this model. Furthermore, anastomotic technical variability hampers the reproducibility of this model in different centers. Therefore, the isolated pulmonary perfusion model (ex vivo) has become popular in pulmonary preservation investigation centers. This model enables the assessment of the pulmonary function of lungs undergoing different episodes of cold ischemia without having to transplant them into other animals.
The improvement of cardiopulmonary bypass and the development of new perfusion solutions led to a revival of ex vivo human perfusion at the beginning of the 21st century (5-8). In this article, we present ex vivo lung perfusion (EVLP) as a new model for the study of pulmonary preservation (the comparison of different techniques and preservation solutions). EVLP combines the use of measurements from ex vivo animal models with the advantage of using human lungs, therefore bringing experimental research results closer to what is observed in clinical practice.
MATERIALS AND METHODS
This study was approved by the institutional ethics committee. We used human lungs from brain-dead donors that were rejected by pulmonary transplantation teams based on criteria previously defined by the International Society for Heart and Lung Transplantation (9).
The en-bloc lung removal technique was the same as the one used in clinical practice by transplantation teams, including pulmonary artery trunk cannulation and perfusion with 50 ml/kg of preservation solution at 4°C.
This study was a non-inferiority study. The sample size required in this case is determined based on the goal of achieving sufficient power (type II error<0.1) to reject the null hypothesis when the magnitude of effect is very small and therefore clinically insignificant. This criterion would result in a very large sample, precluding the use of a resource-intensive method that involves the harvesting of human lungs. The sample size calculation was based on previously published experimental studies involving the comparison of lung preservation solutions in experimental models of lung transplantation in dogs and pigs (10-12).
From April 2009 to April 2010, 16 cases were randomized into two study groups. In Group 1 (eight cases), Perfadex® (Vitrolife, Göteborg, Sweden) was used as the preservation solution, and in Group 2 (eight cases), LPDnac (Farmoterápica, São Paulo, Brazil), a preservation solution manufactured in Brazil with a chemical composition identical to that of Perfadex®, was used.
After removal, the pulmonary block was transported and stored at 4°C for 10 hours. Then, reperfusion was started using the EVLP model as described below.
The pulmonary block was placed on a rigid, transparent support (XVIVO© Chamber; Vitrolife, Sweden). A cannula with a built-in catheter to enable continuous pulmonary artery pressure (PAP) monitoring was sutured into the trunk of the pulmonary artery. An orotracheal tube was introduced into the trachea and attached with cardiac tape (Figure 1). A membrane oxygenator was connected to a gas mixer, which was connected to two cylinders: one containing oxygen and the other containing a mixture of nitrogen (93%) and CO2 (7%). When this mixture was applied to the membrane oxygenator, it functioned as a “deoxygenator,” so that the prime at the inlet of the pulmonary artery had a gas concentration similar to that of venous blood.
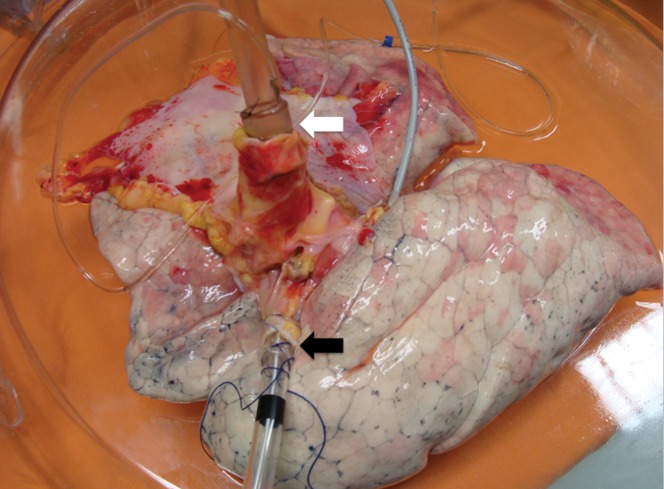
Figure 1.
The preparation of the lungs to start EVLP. A silastic cannula with a built-in pressure catheter was sewn into the pulmonary artery (the white arrow), and an endotracheal tube was inserted into the trachea (the black arrow).
The system, which was composed of tubes, a venous reservoir, a membrane oxygenator and a centrifuge pump, was filled with 1500 ml of Steen Solution® (Vitrolife, Sweden), an extracellular electrolyte solution containing albumin and dextran that was developed for EVLP.
The centrifuge pump was connected with low flow (100 ml/min), the air was removed from the system, and the circuit was connected to the pulmonary artery cannula. The pulmonary vein drainage flowed directly to an XVIVO© Chamber, which was connected to the circuit. The flow was gradually increased until it reached maximum flow, which was calculated as 40% of the estimated cardiac output (3x the body surface area). The PAP was monitored and kept below 20 mmHg throughout the process to minimize the development of edema. The temperature was slowly increased until normothermia (37°C) was achieved. When a temperature of 32°C was reached, ventilation was started under the following parameters: tidal volume of 8 ml/kg (donor weight), respiratory rate of 7/min, PEEP of 5 cm H2O and FiO2 of 100%.
After 60 minutes of EVLP, a sample of the perfusate from the pulmonary artery and another sample from the pulmonary vein were collected for blood gas analysis. The following variables were assessed: weight variation (ΔP = weight after EVLP–weight before EVLP), oxygenation capacity (ΔPO2 = PO2 of pulmonary veins-PO2 of pulmonary artery), pulmonary vascular resistance (PVR = [PAP/flow]x80) and pulmonary compliance (PC = tidal volume/[Pplateau-PEEP]). The left lung was isolated, weighed (wet weight) and placed in a chamber (60°C). After 24 hours, it was weighed once again (dry weight) to calculate the wet/dry weight ratio.
Mid-lobe biopsies were collected at three timepoints: during organ harvesting, after 10 hours of cold ischemia and after 1 hour of EVLP.
Lung fragments were fixed by immersion in 10% tamponated formalin. After 24 hours, paraffin blocks were produced, sectioned in 5 µm slices and stained with hematoxylin–eosin. Lab specimens were evaluated by light microscopy by a pathologist experienced in pleuropulmonary diseases. The following parameters were analyzed: interstitial edema, alveolar edema, arterial thickening, vascular thrombosis, interstitial fibrosis, alveolar fibrin, alveolar exudate, alveolar hemorrhage, interstitial hemorrhage, inflammatory interstitial infiltrate, pneumocystis, alveolar macrophages and necrosis. The pathologist ranked these changes according to their degree as 0, absent; 1, mild; 2, moderate; or 3, severe. The Lung Injury Score (LIS) was the sum of the values of all parameters.
Specimens of the above-described fragments were also prepared for immuno-histochemical analysis with the goal of detecting and quantifying apoptotic cells using the In situ Cell Death Detection Kit (Roche, Germany). DNA cleavage occurs during apoptosis, leading to the formation of breaks in the DNA and of DNA fragments (oligonucleosomes). These DNA strand breaks can be identified by adding nucleotides labeled with fluorescein to the free 3′-OH termini of DNA fragments through an enzymatic reaction. The TUNEL technique (TdT-mediated dUTP nick end labeling) is based on the capacity of the terminal deoxynucleotidyl transferase enzyme (TdT) to catalyze the addition of deoxyuridine triphosphate (dUTP) to the 3′-OH termini of DNA strands. Specimens were examined by fluorescence microscopy (Axioskop 2 Plus; Carl Zeiss, Germany) using a 590 nm filter. AxioVision digital image processing software (Carl Zeiss) was used to display images on a monitor with a 0.02 mm2 field, and apoptotic cells were visualized in a bright green color (Figure 2). The apoptotic cells were counted in five fields chosen at random (total area of 0.1 mm2; magnification of x400).
Figure 2.
Apoptotic cells photographed under a fluorescence microscope at high magnification (x400).
A descriptive analysis was performed in which the distributions of the variables were presented as the means and standard deviations or as the medians and interquartile intervals. Group comparisons of donor lung characteristics were conducted using the chi-square test (or Fisher's exact test) for categorical variables and Student's t-test for continuous variables. Statistical analysis of the outcome variables that were collected only once throughout the study was performed using Student's t-test or the Mann-Whitney test. For outcome variables collected at two different timepoints, repeated measures analysis of variance (ANOVA) was used. A type I error probability (α) of 0.05 was used for all inferential analyses. Descriptive and inferential statistical analyses were performed with the SPSS software, version 17.0.
RESULTS
The mean donor age was 45.6 (±20) years, and the donors included nine men and seven women. The lungs of 13 out of the 16 donors had been rejected due to unsatisfactory arterial blood gas values (PaO2 below 300 mmHg with FiO2 of 100% and PEEP of 5 cm H2O). Two donors, despite having satisfactory blood gas tests, were rejected due to pneumonia, and one donor was declined due to the lack of a compatible recipient on the waiting list. The most common causes of death were hemorrhagic stroke (seven donors) and subarachnoid hemorrhage (four donors). Table 1 shows that both study groups were similar with respect to the donor demographic characteristics. Histological analysis of the lungs suggested that both groups of lungs were morphologically similar (Table 2).
Table 1.
Table 1 - The clinical and demographic characteristics of the donors.
| Group 1(n = 8) | Group 2(n = 8) | |||
| Donors | p-value | |||
| Gender (M:F) | 3:5 | 6:2 | 0.315 | |
| Age (years) | 50.2 (±19.4) | 40.9 (±20.8) | 0.367 | |
| BMI (kg/cm2) | 26.7 (±3.7) | 25.1 (±4.3) | 0.447 | |
| Smoking | 3 | 1 | 1.000 | |
| Pneumonia | 4 | 4 | 1.000 | |
| WBC count (/mm3) | 16,710 (±5244.6) | 14,685 (±3913.7) | 0.396 | |
| MV (days) | 5.9 (±3.4) | 7.8 (±5.8) | 0.439 | |
| PaO2 (mmHg)*) | 206.04 (±119.25) | 181.36 (±85.01) | 0.641 | |
The continuous variables are expressed as the means (±standard deviation), and the categorical variables are expressed as the absolute number.
Arterial blood gas analysis performed with FiO2 100% and PEEP 5 cm H2O.
BMI = body mass index, WBC = white blood cell, MV = mechanical ventilation.
Table 2.
Table 2 - The histological features of the donor lungs.
| Group 1(n = 8) | Group 2(n = 8) | ||
| p-value | |||
| LIS*) | 3.8 (±2.8) | 3.9 (±2.4) | 0.925 |
| Apoptotic cells (/mm2)#) | 175 (0-62.5) | 200 (75-562.5) | 0.561 |
Mean±standard deviation.
Median (interquartile range).
LIS = lung injury score.
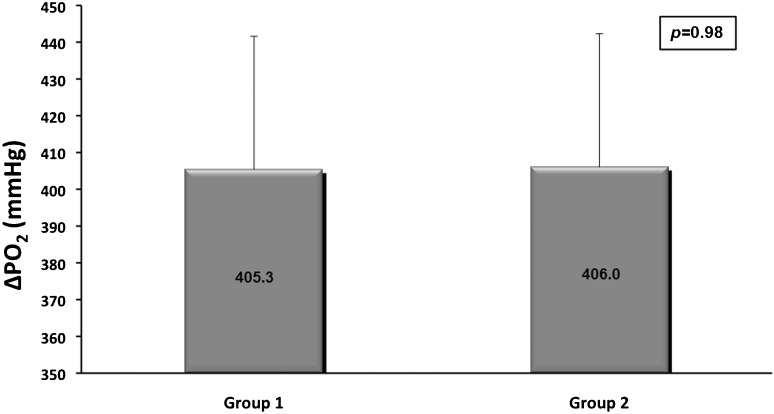
After 60 minutes of EVLP, there were mean weight gains of 68.0 g (±210.8 g) and 56.8 g (±98.9 g) in Groups I and II (p = 0.893), respectively. The mean ΔPO2 of Group I lungs was 405.3 mmHg (±52.4 mmHg), and the mean ΔPO2 of Group II lungs was 406.0 mmHg (±43.5 mmHg) [p = 0.98; Figure 3]. The mean PVR measured after lung reperfusion in Group I lungs was 697.6 dyn·s·cm-5 (493.0–1040.0 dyn·s·cm-5), whereas the mean PVR was 378.3 dyn·s·cm-5 (351.8–628.2 dina.s.cm-5) [p = 0.035] for Group II. The mean PC at the end of reperfusion in Group I was 46.8 cm H2O (±21.0 cm H2O), and the mean PC in Group II was 49.3 ml/cm H2O (±21.7 ml/cm H2O) [p = 0.816]. The mean wet weight-to-dry weight ratios were 2.06 (±0.28) and 2.02 (±0.70) in Groups I and II, respectively (p = 0.87).
Figure 3.
The oxygenation capacity after reperfusion (ΔPO2) in both groups (p = 0.98). The PaO2 is expressed in mmHg. The error bars represent the 95% CI.
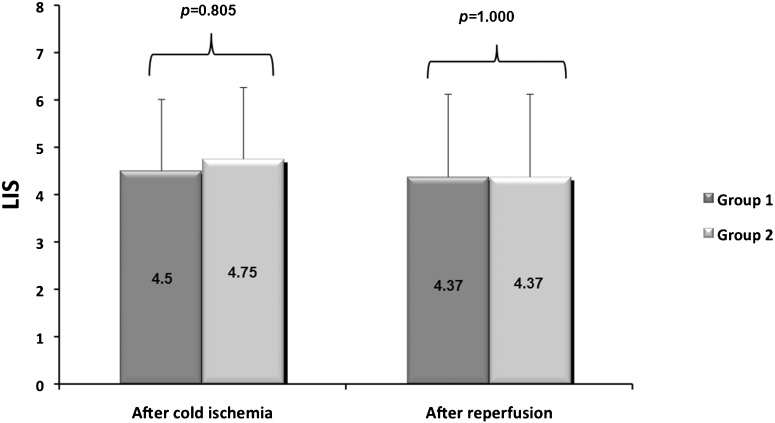
The mean LIS, determined based on the biopsy performed after 10 hours of cold ischemia, was 4.50 (±2.14) in Group I and 4.75 (±1.83) in Group II. For the biopsy performed after reperfusion using the ex vivo pulmonary assessment model, the mean LISs were 4.37 (±1.51) and 4.37 (±2.88), respectively (Figure 4). There was no statistically significant difference in the LIS between groups over time (p = 0.855).
Figure 4.
There was no statistically significant difference in the Lung Injury Score (LIS) between the groups over time (p = 0.855). The error bars represent the 95% CI.
The identification of apoptotic cells by the TUNEL assay in the pulmonary tissue harvested after the ischemic period showed an average of 168.75 (±173.08) apoptotic cells/mm2 in Group I and 200.00 (±160.36) apoptotic cells/mm2 in Group II. In the tissue harvested after reperfusion, mean numbers of 137.50 (±99.10) and 118.75 (±99.78) apoptotic cells/mm2 were observed, respectively. There were no statistically significant differences in the apoptotic cell counts between groups over time (p = 0.903).
DISCUSSION
The great success achieved by pulmonary transplantation in the past decades is partially due to the improvement in preservation techniques. The aim of these techniques is to minimize deleterious ischemic effects and reduce the incidence of graft failure after implantation. Several clinical and experimental studies have demonstrated that the most efficient strategy is the hypothermic perfusion of the lungs with a preservation solution. Nevertheless, primary graft dysfunction is still observed in 11% to 25% of recipients and is responsible for 30% of deaths in the first 30 days after transplantation, indicating that further investigation in the field of pulmonary preservation is required to improve both the techniques and the preservation solutions.
Research conducted in the area of pulmonary preservation has always used animal models. Experiments using medium and large animals are expensive and time consuming. In theory, bilateral pulmonary transplantation would be the method of choice to study pulmonary preservation; however, intraoperative mortality is very high. The need for a cardiopulmonary bypass machine and the technical difficulty of the procedure relative to other methods render bilateral transplantation inadequate for routine use in experimental research. The disadvantages of bilateral pulmonary transplantation are the reason why most pulmonary preservation studies using large animals involve unilateral transplantation. However, transplantation per se is not an acceptable method because the normal contralateral lung remains. The presence of the normal lung may lead to normal functional and hemodynamic results, even when the transplanted lung has not been preserved in a satisfactory manner. Therefore, it is necessary to perform a pneumonectomy or a contralateral pulmonary artery ligature so that the animal becomes dependent on the transplanted lung. However, these procedures are associated with cardiac overload, high hemodynamic instability and high mortality rates.
Experiments using small animals cost less, and a larger number of experiments can be performed within a shorter period of time. Isolated pulmonary perfusion models have become popular due to the technical difficulties associated with pulmonary transplantation in rats. This model enables the evaluation of lung function without the need for transplantation into other animals. The technique consists of en-bloc ex vivo pulmonary perfusion using venous blood. Physiological parameters (such as oxygenation, compliance and pulmonary vascular resistance) can be measured, allowing the comparison of different techniques and preservation solutions.
The current EVLP model in humans was developed by Steen et al. in Sweden to evaluate and recondition lungs removed from “marginal” donors or non-heart-beating donors (5-7). The method was improved by other groups and is currently used in several centers for the ex vivo assessment and recovery of lungs rejected for transplantation (13,14).
In this study, we demonstrated the feasibility of the EVLP model for comparing preservation solutions in human lungs, providing results closer to those obtained in clinical practice without putting real-life patients at risk.
With the aim of verifying the quality of a new preservation solution for clinical purposes – LPDnac – that was manufactured by a national lab, we decided to test this solution in our pulmonary perfusion model in rats (15). After this first stage, before using it in patients, we decided to test it in human lungs, using the EVLP model. This model has offered very consistent and reliable results.
Perfadex®, a preservation solution that is considered to be the gold standard for pulmonary preservation, was used as a control. Both analyzed groups consisted of lungs donated and rejected for transplantation. The groups were similar with respect to functional and histological characteristics, and therefore, the differences found after reperfusion could be attributed to the quality of the pulmonary preservation.
The degree of pulmonary edema is inversely proportional to the preservation quality. Therefore, variables indicating edema formation, such as the variation in the wet/dry weight ratio, are present in most pulmonary preservation studies. In our study, these variables show similar values in both groups. Oxygenation capacity is the most important parameter for functional assessment because, physiologically, the primary role of the lungs is gas exchange. Our results demonstrated that the oxygenation capacity was similar in both groups, showing that the quality of pulmonary preservation obtained with LPDnac is equivalent to that obtained with Perfadex®. This result is similar to what was found in a study by Soares PRO (15). In ischemia-reperfusion injury, there is a reduction in pulmonary compliance due to the development of alveolar and interstitial edema. In our study, the results for this variable are consistent with previously described results: there were no differences in compliance between the groups. One of the variables analyzed was discrepant. We noticed a smaller PVR in the group of lungs preserved with LPDnac. Because the two groups were similar and because the procedures were performed by the same team, we could not attribute this difference to technical aspects. Because the p-value was borderline, it is possible that this difference occurred by chance and may disappear if the sample size were greater.
Pulmonary ischemia is associated with a series of histological alterations, including alveolar edema, the rupture and thickening of the alveolar-capillary membrane and focal hemorrhage. We used a score based on the semi-quantitative analysis of changes observed by conventional light microscopy. Both groups had the same degree of tissue injury after cold ischemia and after reperfusion.
There was a small number of apoptotic cells in the lungs assessed in our study, possibly due to the short period of ischemia of less than 12 hours and a reperfusion period of only one hour. When the two groups were compared, the numbers of apoptotic cells were equivalent for both the ischemia and reperfusion periods.
One of the limitations of our study is the use of an acellular solution instead of blood, which minimized the effects of reperfusion injury. Blood perfusion would allow the simulation of the interaction between circulating cells and the pulmonary endothelium with all of the associated consequences: leucocyte activation and migration, platelet activation, thrombosis and the production of cytokines and free oxygen radicals. The use of an acellular solution explains why the mean LIS values for both groups were relatively low. We chose not to use human blood, which would lead to ethical issues in this type of research in addition to significantly increasing the costs. Another limitation of the study was the short reperfusion period. However, these limitations do not weaken our conclusions because the results are consistent and clinically significant.
The two preservation solutions were similar, as demonstrated by the fact that the quality of lung preservation obtained with LPDnac was the same as that obtained with Perfadex. The EVLP system was shown to be a good tool to perform pulmonary preservation studies for transplantation.
Further investigations should include the evaluation of new preservation strategies, such as perfluorocarbon ventilation, surfactant administration and ex vivo perfusion with antibiotics, thrombolytic agents and other drugs. The inclusion of additives such as antioxidants in preservation solutions may lead to the development of even better preservation solutions.
ACKNOWLEDGMENTS
This work was supported by grants from FAPESP (Foundation for Research Support of São Paulo), Vitrolife (Göteborg, Sweden), Braile Biomédica (São José do Rio Preto, Brazil) and Farmoterápica (São Paulo, Brazil). The authors have no relevant financial relationships or conflicts of interest to disclose.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.de Perrot M, Keshavjee S. Lung preservation. Chest Surg Clin N Am. 2003;13(3):443–62. doi: 10.1016/s1052-3359(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 2.Maccherini M, Keshafjee SH, Slutsky AS, Patterson GA, Edelson JD. The effect of low-potassium dextran versus Euro-Collins solution for preservation of isolated type II pneumocytes. Transplantation. 1991;52(4):621–6. doi: 10.1097/00007890-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Bins OAR, DeLima NF, Buchanan SA, Cope JT, King RC, Marek CA, et al. Both blood and crystalloid-based extracellular solutions are superior to intracellular solutions for lung preservation. J Thorac Cardiovasc Surg. 1996;112(6):1515–21. doi: 10.1016/S0022-5223(96)70010-7. [DOI] [PubMed] [Google Scholar]
- 4.Strüber M, Hohlfeld JM, Fraund S, Kim P, Warnecke G, Haverich A. Low-potassium-dextran solution ameliorates reperfusion injury of the lung and protects surfactant function. J Thorac Cardiovasc Surg. 2000;120(3):566–72. doi: 10.1067/mtc.2000.107831. [DOI] [PubMed] [Google Scholar]
- 5.Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357(9259):825–9. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 6.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–52; discussion 252. doi: 10.1016/s0003-4975(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 7.Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, et al. First human transplantation of a nonaccep Table donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191–4. doi: 10.1016/j.athoracsur.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Pego-Fernandes PM, Medeiros IL, Mariani AW, Fernandes FG, Unterpertinger FV, Samano MN, et al. Ex vivo lung perfusion: early report of brazilian experience. Transplant Proc. 2010;42(2):440–3. doi: 10.1016/j.transproceed.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Aigner C, Seebacher G, Klepetko W. Donor selection. Chest Surg Clin N Am. 2003;13(3):429–42. doi: 10.1016/s1052-3359(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 10.Keshafjee SH, Yamazaki F, Cardoso PF, McRitchie DI, Patterson GA, Cooper JD. A method for safe twelve-hour pulmonary preservation. J Thorac Cardiovasc Surg. 1989;98(4):529–34. [PubMed] [Google Scholar]
- 11.Steen S, Kimblad PO, Sjöberg T, Lindberg L, Ingemansson R, Massa G. Safe lung preservation for twenty-four hours with Perfadex. Ann Thorac Surg. 1994;57(2):450–7. doi: 10.1016/0003-4975(94)91016-2. [DOI] [PubMed] [Google Scholar]
- 12.Sommer SP, Warnecke G, Hohlfeld JM, Gohrbandt B, Niedermeyer J, Kofidis T, et al. Pulmonary preservation with LPD and Celsior solution in porcine lung transplantation after 24 h of cold ischemia. Eur J Cardiothorac Surg. 2004;26(1):151–7. doi: 10.1016/j.ejcts.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, Haithcock JA, Nicotra WA, Koukoulis G, Inokawa H, Sevala M, et al. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81(4):1205–13. doi: 10.1016/j.athoracsur.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9(10):2262–9. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 15.Soares PRO, Braga KAO, Nepomuceno NA, Pazetti R, Correia AT, Cardoso PFG, et al. Comparison between Perfadex and locally manufactured low-potassium dextran solution for pulmonary preservation in an ex vivo isolated lung perfusion model. Transplant Proc. 2011;43(1):84–8. doi: 10.1016/j.transproceed.2010.12.005. [DOI] [PubMed] [Google Scholar]