Abstract
OBJECTIVES:
Pulmonary involvement in rheumatoid arthritis is directly responsible for 10% to 20% of all mortality. The best way to improve the prognosis is early detection and treatment. The forced oscillation technique is easy to perform and offers a detailed exam, which may be helpful in the early detection of respiratory changes. This study was undertaken to (1) evaluate the clinical potential of the forced oscillation technique in the detection of early respiratory alterations in rheumatoid arthritis patients with respiratory complaints and (2) to compare the sensitivity of forced oscillation technique and spirometric parameters.
METHODS:
A total of 40 individuals were analyzed: 20 healthy and 20 with rheumatoid arthritis (90% with respiratory complaints). The clinical usefulness of the parameters was evaluated by investigating the sensibility, the specificity and the area under the receiver operating characteristic curve. ClinicalTrials.gov: NCT01641705.
RESULTS:
The early adverse respiratory effects of rheumatoid arthritis were adequately detected by the forced oscillation technique parameters, and a high accuracy for clinical use was obtained (AUC>0.9, Se = 80%, Sp = 95%). The use of spirometric parameters did not obtain an appropriate accuracy for clinical use. The diagnostic performance of the forced oscillation technique parameters was significantly higher than that of spirometry.
CONCLUSIONS:
The results of the present study provide substantial evidence that the forced oscillation technique can contribute to the easy identification of initial respiratory abnormalities in rheumatoid arthritis patients that are not detectable by spirometric exams. Therefore, we believe that the forced oscillation technique can be used as a complementary exam that may help to improve the treatment of breathing disorders in rheumatoid arthritis patients.
Keywords: Rheumatoid Arthritis, Respiratory Diseases, Forced Oscillation Technique, Early Detection, Early Diagnosis
INTRODUCTION
Extra-articular manifestations occur in approximately 8-20% of Rheumatoid Arthritis (RA) cases (1). While the prevalence of other extra-articular manifestations has decreased, the prevalence of pulmonary involvement is increasing (2). Pulmonary involvement is a frequent extra-articular manifestation in RA and is directly responsible for 10% to 20% of all mortality (3,4). Recently, Hamblin and Horton (5) estimated that 50% of patients with RA will develop some form of respiratory abnormality during their lives. The best way to reduce joint damage and improve the prognosis of this disease is early treatment with disease-modifying drugs (6). Early treatment is able to improve not only the articular symptoms, but also respiratory abnormalities (7). However, the early diagnosis of respiratory changes is difficult due to the absence of clinical symptoms (1). Pulmonary involvement in RA usually occurs between 0-5 years after the onset of joint symptoms, and the identification of the respiratory changes in this group demands a sensitive method (8).
The Forced Oscillation Technique (FOT) offers a simple and detailed approach to investigate the mechanical properties of the respiratory system (9) and represents the current state-of-the art in the assessment of lung function (10). This method characterizes respiratory impedance and its two components: respiratory system resistance (Rrs) and respiratory system reactance (Xrs). The method is simple, requires only passive co-operation and does not entail any forced expiratory maneuvers. Another important advantage, particularly in pathophysiological research, is that the FOT can be used to provide information about the mechanical characteristics of the respiratory system that are complementary to that generated by spirometry (10,11). Recently, this technique has been successfully applied in the detection of early respiratory changes in smokers (12,13) and patients with sarcoidosis (14).
Therefore, the FOT has the potential to increase our knowledge of the pathophysiology of RA and to help in the clinical diagnosis of early respiratory alterations in this disease. However, to the best of the authors' knowledge, there are no data in the literature discussing the use of the FOT in RA patients with the exception of a conference report describing the early stages of the authors' work (15).
The purpose of the present study was two-fold: (1) to evaluate the clinical potential of the FOT in the early detection of alterations in the respiratory mechanics of RA patients and (2) to compare the diagnostic accuracy of the FOT and spirometric parameters in performing the cited task.
MATERIALS AND METHODS
Ethics considerations and groups selection
The institutional ethics committee of Pedro Ernesto University Hospital approved our study, which was registered in ClinicalTrials.gov: NCT01641705. The baseline data, including age, height, and weight, were obtained from each subject at the time of the procedures. Informed consent was obtained from all volunteers before inclusion in the study.
Cigarette smoking and disease activity/severity are consistent independent predictors of radiographic and physiologic abnormalities that suggest rheumatoid arthritis interstitial lung disease (16). Moreover, smoking is known to be associated with small airway disease in RA (17). To remove the effect of smoking, the present study involved a group of 20 RA patients and a control group of 20 volunteers; both groups were composed of never-smoking volunteers with normal spirometry. The control group was formed of students and employees of the State University of Rio de Janeiro and was composed of healthy subjects who presented with no history of pulmonary disease, cardiac disease or tobacco use. Patients with RA were from the Rheumatology Ambulatory unit of the HUPE. They were admitted to the Pneumology Department with respiratory complaints to perform pulmonary exams (18 from the 20 patients, 90%). This group showed a severity, as evaluated by the erythrocyte sedimentation rate index (18) of 31.6±18.7 mm, which can be considered above the normal range (19). This group was in moderate disease activity according to the Disease Activity Score (20), of 4.1±1.1 (19). The positive rheumatoid factor was found in 72% of these patients. All patients were in stable clinical condition and were classified as normal by the spirometric exams.
Spirometry
The forced expiratory volume in the first second (FEV1) (liter and %), forced vital capacity (FVC) (liter and %), FEV1/FVC ratio, the forced expiratory flow (FEF) between 25% and 75% of FVC (liter and %), and (FEF/FVC) ratio were measured for all subjects in a sitting position using a closed circuit spirometer (Vitatrace VT-139; Pró-médico, Rio de Janeiro, Brazil; and Collins/GS, Warren E. Collins, Inc., Massachusetts, USA). The measured values were related to the normal values reported by Pereira et al. (21). The forced expiratory maneuvers were repeated until three sequential measurements were obtained. The indexes studied were those obtained through the best curve, which was selected based on the highest values of FEV1 plus CVF. The quality control of the spirometry measurements was evaluated by the ATS criteria (22) with the software allowing the detection of non-acceptable maneuvers.
Forced Oscillation Technique
The instrumentation used for the evaluation of respiratory impedance by the FOT was developed in our laboratory and has been described previously (11). Briefly, a pseudorandom sinusoidal signal with a 2 cmH2O peak-to-peak of amplitude, containing all harmonics of 2 Hz between 4 and 32 Hz, was produced with a loudspeaker. The pressure input was measured with a Honeywell 176 PC pressure transducer (Microswitch, Boston, MA, USA), and the airway flows were measured with a screen pneumothacometer coupled to a similar transducer with a matched frequency response. The signals were digitized at a rate of 1024 Hz for periods of 16 s by a personal computer, and a fast Fourier transform was computed using blocks of 4,096 points with 50% overlap. To perform the FOT analysis, the volunteer remained in a sitting position, while keeping his/her head in a normal position and breathing at FRC through a mouthpiece. During the measurements, the subject firmly supported his/her cheeks and mouth floor using both hands, while a noseclip was worn. A minimal coherence function of 0.9 was considered adequate (11-15). Three measurements were made, and the final result of the test was calculated as the mean of these three measurements.
Data processing, presentation and statistical analysis
The impedance curves (resistance and reactance) were studied over the frequency range from 4 to 32 Hz. The analysis of linear regression in the resistive component of the impedance in the frequency range between 4 and 16 Hz was used to determine the intercept resistance (R0). The intercept is related to the total resistance of the respiratory system and is usually used as an index of airway obstruction (23). The mean resistance (Rm), which is related to the airway caliber (24), was also calculated for the frequency range from 4 to 16 Hz. The slope of the resistive component of the respiratory impedance (S) was also obtained and is associated with respiratory system homogeneity (25,26). The resonance frequency (fr) is defined as the frequency at which the rectance equals zero and is associated with the homogeneity of the respiratory system (27). The dynamic compliance of the respiratory system (Crs,dyn), which includes the airways, lung and chest wall, was estimated using the reactance (Xrs) at 4 Hz with the equation Crs,dyn = -1/(2πfXrs). The impedance module at 4 Hz (Zrs4) was also estimated. Physiologically, this parameter represents the total mechanical load of the respiratory system (28).
The sample size for this study was calculated using MedCalc version 8.2 (Medicalc Software, Mariakerke, Belgium). It was based on an anticipated comparison of the mean values of R0 obtained in preliminary studies (15) and an assumption of Type I and Type II errors of 1%. The minimal sample size required was 18 subjects in each group.
The commercial softwares MicrocalTM Origin® 6.0 (Microcal Software Inc., Northampton, USA) and STATISTICA for Windows, release 5.0 (StatSoft Inc., Tulsa, USA), were used to compare the differences between the groups. Because of the number of subjects of each group, a non-parametric Mann-Whitney U test was applied. A p-value of less than 0.05 was considered statistically significant.
The clinical potential of the FOT indexes in the early detection of respiratory alterations in RA was evaluated by means of receiver operating characteristic (ROC) analyses, which were conducted using MedCalc 8.2 (Medicalc Software, Mariakerke, Belgium).
Comparisons of the AUC among parameters obtained from the FOT and spirometry were conducted using MedCalc 8.2 following the theory described by Metz (29). The sensitivity, specificity, and AUC of spirometry and the FOT were obtained based on the optimal cut-off point, as determined from the ROC curve analysis (30).
RESULTS
Characteristics of the patients and controls
Table 1 compares the biometric and spirometric parameters of the control group and patients with RA. In the present study, there were 20 volunteers in each group. The biometric characteristics of the two studied groups were well matched, and there were no significant differences between the groups. The mean duration of the disease was 8.8 years. The spirometric parameters were not different between the groups.
Table 1.
Biometric and spirometric characteristics of the studied groups.
| Group AControln = 20 | Group BRAn = 20 | p-value | |
| Age (years) | 44.0±13.7 | 45.0±11.9 | 0.80 |
| Weight (Kg) | 63.0±9.8 | 68.2±8.0 | 0.76 |
| Height (cm) | 162.1±7.4 | 158.6±6.7 | 0.12 |
| Disease duration (years) | - | 8.8±4.6 | - |
| Gender (M/F) | 15/05 | 19/01 | - |
| FEV1 (L) | 3.1±0.8 | 2.9±0.5 | 0.27 |
| FEV1 (%) | 109.4±16.5 | 110.6±12.7 | 0.78 |
| FVC (L) | 3.7±0.9 | 3.4±0.6 | 0.35 |
| FVC (%) | 107.2±17.5 | 110.5±12.5 | 0.49 |
| FEV1/FVC | 85.3±3.6 | 83.6±4.8 | 0.22 |
| FEF25-75 (L) | 3.9±1.3 | 3.4±1.0 | 0.21 |
| FEF25-75 (%) | 120.9±32.4 | 116.1±31.5 | 0.63 |
| FEF/FVC | 105.6±23.1 | 99.7±22.5 | 0.42 |
RA: Rheumatoid Arthritis; n: number of subjects; ns: non-significant.
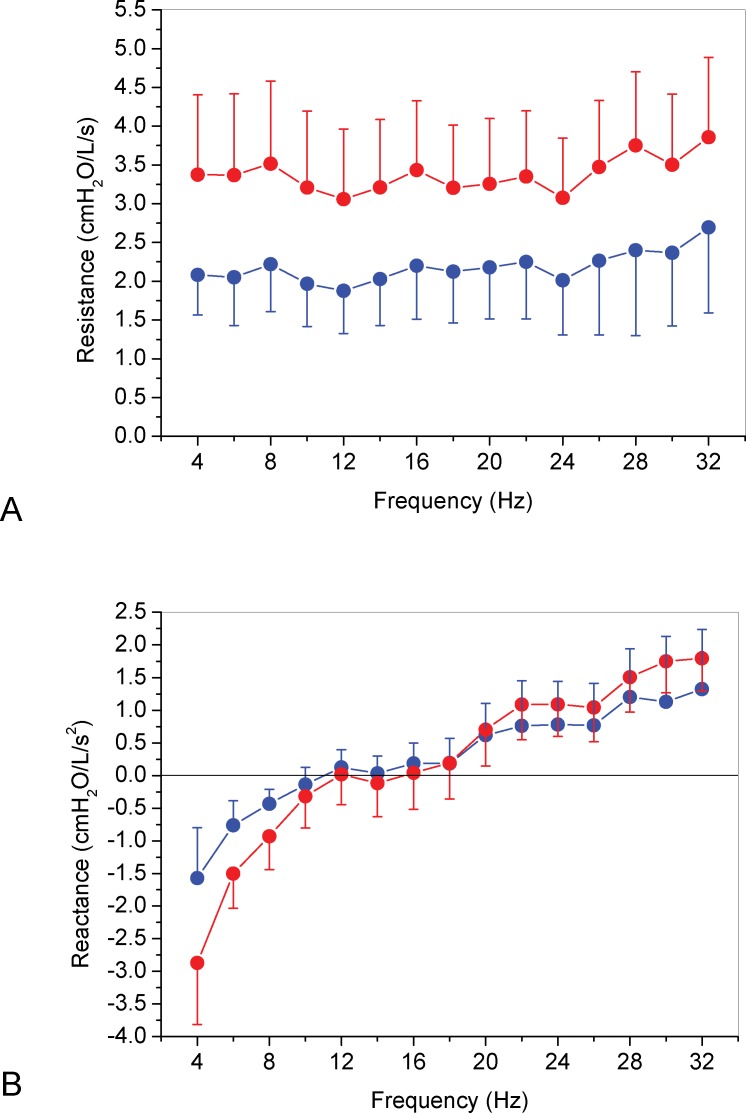
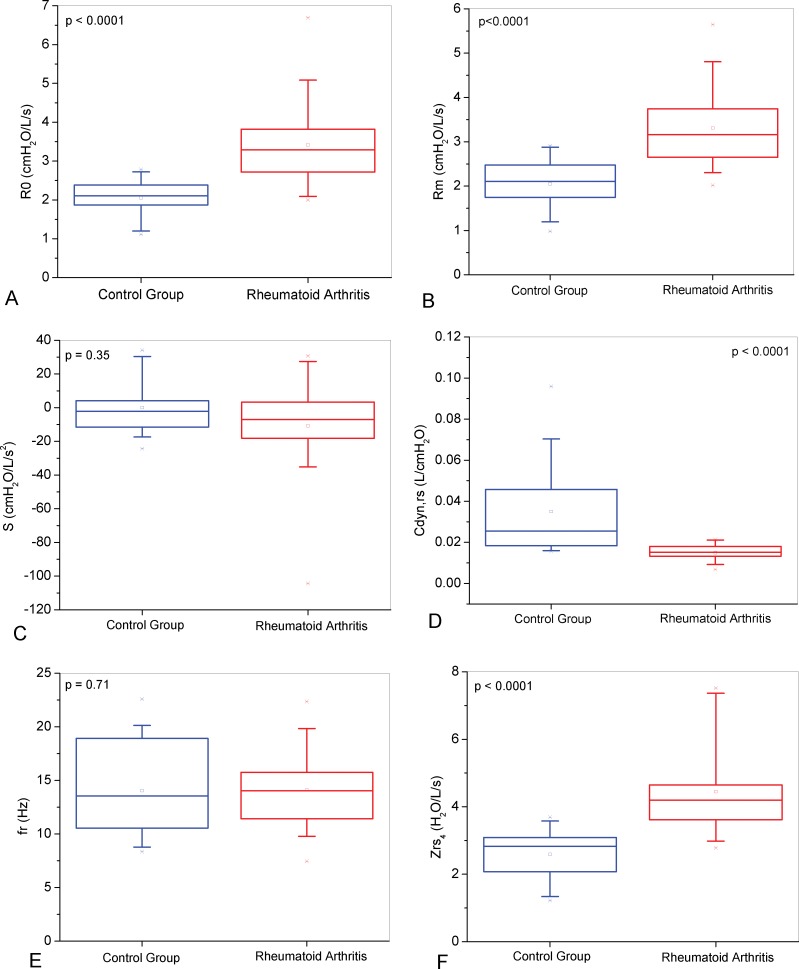
The mean curves of the Rrs and Xrs as a function of frequency, in normal and RA subjects, are presented in Figure 1. Respiratory resistance curve in RA were significantly different from the control curve (Figure 1A); p<0.0001), while respiratory reactance curves were not different between the groups (Figure 1B); p = ns). As described in Figure 2A, patients with RA presented with elevated R0 (p<0.0001) and Rm (Figure 2B); p<0.0001), but S was not significantly different (Figure 2C); p = 0.35). The presence of RA was associated with lower values of Crs,dyn (Figure 2D); p<0.0001), while the fr was not significantly different (Figure 2E); p = 0.71). In contrast, the Zrs4 was higher in RA patients (Figure 2F); p<0.0001).
Figure 1.
Mean curves of Rrs (A) and Xrs (B) as a function of frequency in normal (blue) and AR (red) groups.
Figure 2.
Comparisons among the total resistance (R0; Figure A), mean resistance (Rm; B), slope of the resistance values (S; C), dynamic compliance (Cdyn,rs; D), resonant frequency (fr; E) and impedance modulus (Zrs4; F) in the studied groups.
Table 2 shows the values of the sensitivity (Se), specificity (Sp), area under the curve (AUC) and cut-off point from the ROC analysis of the oscillometric and spirometric parameters.
Table 2.
Values of area under the ROC curve (AUC), sensibility (Se), specificity (Sp) and respective cut points for the FOT and spirometric parameters.
| Se(%) | Sp(%) | AUC | Cut-off point | |
| R0 (cmH2O/L/s) | 70.0 | 100.0 | 0.89 | 2.78 |
| S (cmH2O/L/s2) | 70.0 | 65.0 | 0.63 | -4.18 |
| Rm (cmH2O/L/s) | 80.0 | 80.0 | 0.87 | 2.48 |
| Crs,dyn (L/cmH2O) | 80.0 | 80.0 | 0.88 | 0.018 |
| Fr (Hz) | 80.0 | 45.0 | 0.53 | 11.22 |
| Zrs4 (cmH2O/L/s) | 80.0 | 95.0 | 0.92 | 3.57 |
| FEV1 (L) | 70.0 | 50.0 | 0.59 | 3.11 |
| FEV1 (%) | 65.0 | 50.0 | 0.51 | 105.0 |
| FVC (L) | 75.0 | 55.0 | 0.58 | 3.75 |
| FVC (%) | 75.0 | 55.0 | 0.54 | 107.4 |
| FEV1/FVC | 70.0 | 55.0 | 0.64 | 85.7 |
| FEF25-75 (L) | 70.0 | 55.0 | 0.60 | 3.73 |
| FEF25-75 (%) | 60.0 | 65.0 | 0.55 | 110.9 |
| FEF/FVC | 50.0 | 60.0 | 0.56 | 95.6 |
Table 3 describes the comparative analysis among the AUCs of these parameters. The R0, Rm, Crs,dyn, and Zrs4 obtained by the FOT were characterized by significantly higher AUC values than those obtained by the spirometric parameters. In contrast, the AUC values for the S and fr were not significantly different from those obtained by spirometry.
Table 3.
Comparisons of AUC among oscillometric and spirometric parameters (AUCFOT - AUCspirometry). Positive values indicate higher area in FOT parameters.
| FEV1 | FEV1 | FVC | FVC | FEV1/FVC | FEF25-75 | FEF25-75 | FEF/FVC | |
| (L) | (%) | (L) | (%) | (L) | (%) | |||
| R0 | 0.31 | 0.39 | 0.31 | 0.35 | 0.26 | 0.29 | 0.34 | 0.26 |
| (cmH2O/L/s) | p<0.01 | p<0.001 | p<0.001 | p<0.01 | p<0.005 | p<0.001 | p<0.001 | p<0.005 |
| S | 0.05 | 0.13 | 0.05 | 0.09 | 0.002 | 0.03 | 0.08 | 0.07 |
| (cmH2O/L/s2) | p = 0.65 | p = 0.29 | p = 0.63 | p = 0.43 | p = 0.43 | p = 0.78 | p = 0.49 | p = 0.52 |
| Rm | 0.29 | 0.37 | 0.29 | 0.33 | 0.24 | 0.27 | 0.32 | 0.31 |
| (cmH2O/L/s) | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.008 | p<0.001 | p<0.001 | p<0.001 |
| Crs,dyn | 0.30 | 0.38 | 0.30 | 0.34 | 0.25 | 0.28 | 0.33 | 0.33 |
| (L/cmH2O) | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.004 | p<0.001 | p<0.001 | p<0.001 |
| Fr | 0.05 | 0.03 | 0.05 | 0.005 | 0.10 | 0.07 | 0.02 | 0.03 |
| (Hz) | p = 0.59 | p = 0.83 | p = 0.62 | p = 0.96 | p = 0.38 | p = 0.62 | p = 0.87 | p = 0.82 |
| Zrs4 | 0.33 | 0.41 | 0.33 | 0.38 | 0.28 | 0.31 | 0.36 | 0.36 |
| (cmH2O/L/s) | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
DISCUSSION
The main findings of the present study are that the FOT was able to clearly identify early respiratory alterations in RA patients and that such an identification was more accurate than that obtained using spirometric parameters. These results were obtained in a population of never-smoking RA subjects with respiratory complaints who presented normal spirometry.
It is important to emphasize that the majority of the RA patients studied in this work were referred to the pulmonology department due to respiratory complaints (90% of the patients, 18 of 20). However, all of the spirometric parameters for this group presented with non-significant decreases compared to the control and were in the normal range (Table 1). In agreement with these results, Laitnen et al. (31), when studying pulmonary function in patients with connective tissue diseases, showed normal results in RA patients. Other studies (32-39) also found spirometric values in the normal range in non-smoking RA patients.
In contrast, the FOT parameters were significantly different in the RA group compared to the control group. The R0 is related to total resistance of the respiratory system, including the Newtonian resistance of the airways and tissues as well as the delayed airway resistance resulting from gas redistribution. Thus, the significantly higher values for this parameter observed in RA patients (Figure 2A) may reflect an increase in airway resistance, as well as the impact of RA on pulmonary and chest wall tissues. In RA patients, the presence of bronquiectasis, bronchiolitis obliterans and diffuse alveolar damage is common; these conditions may explain the increase in R0. These results are consistent with those obtained by Perez et al. (32), who reported a high incidence of airway involvement in RA patients. The increase in the respiratory resistance at all frequencies in RA patients (Figure 1A) can also be explained by these findings.
The mean resistance was higher in the RA group compared to that of the control group, as described in Figure 1A and confirmed in Figure 2B (p<0.0001). In RA lung disease, patients may present airway alterations, such as obliterative bronchiolitis, bronchiectasis, and rheumatoid nodules (3,4), that can decrease the size of the airway and consequently increase the mean resistance. Faria et al. (14) also found higher values of R0 and Rm in a group of patients with sarcoidosis, another disease of the connective tissue that has autoimmunity characteristics like RA. Collins et al. (40) evaluated the maximal midexpiratory flow rates and suggested the presence of obstructive pulmonary disease in a small group of RA patients.
The RA group presented with a non-significant decrease in S (Figure 2C), which demonstrated that there was no change in respiratory homogeneity. This finding was different from the results of the previous study conducted with sarcoidosis patients (14). This discrepancy may be associated with the early stages of disease observed in the present study (FEV1(%) = 110.6±12.7; FEV1/FVC = 83.6±4.8) in comparison with the cited study (FEV1(%) = 98.2±18.1; FEV1/FVC = 81.1±4.2).
Crs,dyn describes the elastic properties of the lung and bronchial wall compliances, the compliance of the chest wall/abdomen compartment, and the thoracic gas compression. The mean values of Crs,dyn in the RA patients were significantly smaller than in the normal subjects, which indicates the presence of restrictive alterations (Figure 2D). This finding is in agreement with the increase in the frequency dependence of dynamic compliance (FDC) observed by Hills et al. (37) in RA patients. The cited authors attributed this decrease in compliance to differing time constants throughout the lung. In RA disease, FDC could be due either to bronchiolar involvement that causes changes in resistance or to the uneven involvement of the alveoli and connective tissues as a result of the inflammatory or fibrotic changes of fibrosing alveolitis, leading to scattered areas of altered compliance (37). These changes in the connective tissues could potentially explain the differences in the reactance values at all frequencies in the RA patients (Figure 1B). In sarcoidosis patients, the Crs,dyn was also lower than that of the control group, which is consistent with the results of the present work (14).
The RA group showed values of fr that were non-significantly (p<0.71) different from those observed in the control group. This result, based on reactive properties, confirms the results obtained using the resistive properties (Figure 1B), in which the homogeneity is not altered in the initial phase of RA. A similar behavior was observed by Faria et al. (14) when studying patients with sarcoidosis.
The modifications in the resistive and elastic properties of RA patients resulted in a significantly higher Zrs4 compared to that of the control group (Figure 2F). As this parameter is related to the total mechanical load of the respiratory system, it may be associated with fatigue, breathlessness and a reduced quality of life. Therefore, the significant difference of this parameter is could explain the respiratory complaints of the studied RA patients (3,4). Faria et al. (14) also found increased values of Zrs4 in patients with sarcoidosis.
In RA, we can find a variety of lung disorders, such as usual interstitial pneumonia, rheumatoid nodules, non-specific interstitial pneumonia, bronchiolitis obliterans, and bronchiectasis. In addition, we may also find changes in the pleura, chest wall and pulmonary vasculature (1,4). These changes may introduce respiratory disorders with obstructive and restrictive natures. The results described in Figures 1 and 2 are consistent with the pathophysiological fundamentals involved in RA and provide a detailed description of these changes.
Sasson et al. (41), when comparing the small airway function of 19 RA nonsmokers with 47 healthy nonsmokers, did not find differences in the diffusing capacity, static lung compliance, and airway conductance at mid-to-low lung volumes. These results were different from that observed in the present work. These differences may be explained, at least in part, by the differences in the techniques used to evaluate the respiratory system.
ROC curves have been widely used in medicine to evaluate the performance of diagnostic tests (42). These graphics describe the probability of obtaining false negatives (specificity) relative to the probability of obtaining false positives (1 – specificity) for various criteria. Thus, the bigger the area under the curve (AUC) is, the more valid the test is. According to the literature, ROC curves with AUCs between 0.50 and 0.70 indicate low diagnostic accuracy; AUCs between 0.70 and 0.90, moderate accuracy; and AUCs between 0.90 and 1.00, high accuracy (42,43). An AUC of 0.80 is usually considered adequate for clinical use (42,43). Considering the oscillometric parameters, the measure of Zrs4 yielded high accuracy for clinical use (AUC>0.9), while the measures of R0, Rm and Crs,dyn obtained accuracy that was appropriate for clinical use (AUC>0.8). However, the measures of S and fr did not produce sufficiently accurate values of AUC. The spirometric parameters presented with low diagnostic accuracy (Table 2). Similar results were described in the detection of the early respiratory changes in smokers (12) and sarcoidosis patients (14). These results are in agreement with the findings of Metafratzi et al. (8), who evaluated the pulmonary involvement in patients with RA and no history of smoking using a sensible method (High Resolution Computer Tomography, HRCT). They concluded that HRCT abnormalities are common even when the X-ray and traditional pulmonary respiratory function are normal.
Mori et al. (44) studied small airway obstruction in patients with RA using HRCT. These authors observed by multiple regression analysis that the presence of respiratory symptoms (OR: 5.18, p = 0.002), smoking (OR: 2.78, p = 0.03) and a disease duration of more than 10 years (OR: 2.86, p = 0.012) were factors independently associated with obstructive changes in the small airways of the RA patients. These changes occurred without the presence of interstitial pneumonia or bronchiolitis. Our RA group presented with a similar disease duration (8.8 years), and, in agreement with the results described by Mori et al. (44), the FOT parameters associated with the small airways (R0, Rm, Crs,dyn, Zrs4) were able to adequately identify respiratory changes.
Bandos et al. (45) noted that the difference in AUC has become one of the most commonly used measures for comparing the performance of two diagnostic systems. According to Metz (29), when we have a number of ROC curves to compare, the AUC is usually the best discriminator. Table 3 shows that in never-smoking RA subjects, these analyses were clearly in favor of R0, Rm Crs,dyn and Zrs4. This suggests that these parameters could be more useful than spirometric parameters to detect early changes associated with RA.
The FOT measurements may be performed during normal tidal breathing, which makes this technique easy to conduct. These exams provide information that is applicable to resting conditions. Other tests require specific maneuvers that are not encountered during daily activities; thus, it may be unclear how these measurements can be extrapolated to describe patient symptoms. In addition, the FOT provides information that can be complementary to traditional measures of lung function and is less dependent on patient effort than spirometry (46). These characteristics, together with the results of the present study, indicate that the FOT may be useful in the routine clinical evaluation of the early signals of respiratory changes in RA patients.
There is a consensus in the literature that it is necessary to develop new, accurate, and non-invasive tests of lung function. Previous works suggested the FOT as a potentially useful alternative, whenever spirometry cannot be performed, and as an alternative clinical tool to non-invasively investigate the respiratory system (9,10,24,47,48). This technique was suggested by Crapo et al. (49) as an attractive alternative for diagnosing obstruction in COPD because it requires little patient effort and cooperation. King et al. (46) noted that FOT may be a sensitive measure of early airway disease. Postma et al. (50) consider the FOT to be a more sophisticated lung function test that may help further to delineate the characteristics of low lung function.
In this context, there is a considerable research effort to investigate the clinical potential of the FOT. Examples of recent research include the evaluation of lung function in children with repaired tracheo-oesophageal fistula (51), the detection of upper airway obstruction in patients with tracheal stenosis (52), the determination of airway hyperresponsiveness during mannitol challenge (53), and the analysis of patients with suspicion of acute lung rejection under circumstances when spirometry could not be correctly performed (54). Guo and collaborators (55) investigated the identification of DPOC in elderly subjects through the FOT and obtained maximum AUC values of 0.85, indicating an accurate detection. The FOT parameters were characterized by significantly better diagnostic accuracy than the spirometric parameters in the detection of the early adverse effects of smoking (12,13). This technique was also able to detect early respiratory changes in patients with sarcoidosis and normal espirometric exams (14).
Clinical pulmonary function tests are of prime importance for diagnosing, monitoring disease progression, and assessing the effectiveness of therapies, and they are central features in clinical practice guidelines for interstitial lung disease (56). In the particular case of RA, it has been suggested that early identification and intervention in patients with pulmonary involvement might improve quality of life and performance status. A practical, cost-effective way of identifying early pulmonary disease in patients with RA could yield significant benefits in patient outcomes (57,58). However, the cost of HRCT makes it impractical to screen all RA patients for lung affection (58). The present study was conducted as an effort to contribute in this direction and showed that R0, Rm, Crs,dyn and Zrs4 are very promising parameters for the non-invasive evaluation of early respiratory modifications due to RA.
We have attempted to investigate the value of respiratory impedance measurements in detecting early lung disease and in determining which patients are candidates for subsequent pulmonary investigation and imaging. One could argue that a gold-standard technique was not used in this work to define the early changes in respiratory mechanics. However, a gold-standard of very early lung damage is as yet impossible to obtain in human subjects (59). Because it was observed that respiratory complaints in RA patients were associated with respiratory abnormalities (58) and that respiratory symptoms were identified as predictors of lung disease, as determined by spirometry by Pappas et al. (57), we compared the performance of the FOT and spirometry in detecting these abnormalities in the same group of patients. Therefore, although we cannot precisely quantify the respiratory changes in the RA group, we are confident that these patients presented with clinically relevant early changes.
The FOT has practical limitations that need to be considered. The upper airway shunt may introduce underestimations in the resistance value (9,10), but providing firm support of the cheeks and jaw, as performed by the present subjects, effectively reduced the majority of errors due to movement in the upper airway wall. Because the resistive parameters (R0, Rm) presented with accurate diagnostic performance, this limitation is probably not a relevant problem in the present study. Although the FOT is very easy to carry out, caution must be taken to avoid the flexion of the neck, which may result in a narrowing of the hypopharynx and to make measurements at the level of the FRC. Because of interference with the subject's breathing signal, random and systematic errors may be introduced around the low frequency range (9,10). In the present work, the reliability of the results was guaranteed using a minimal coherence function of 0.9.
Another limitation in our study is that spirometry is not the gold standard for detecting respiratory disease (57). We chose to use spirometry rather than computed tomography scans as our marker of lung disease in this analysis because spirometry provides a common and low-risk diagnostic modality that often precedes radiographic evaluation in clinical practice (57).
The initial pathological respiratory repercussions of RA were adequately described by the FOT as higher values of R0, Rm and Zrs4 and lower values of Crs,dyn. These early changes were detected by the FOT parameters with high accuracy. In addition, the comparison of the diagnostic accuracy of the FOT and the spirometric parameters indicated that R0, Rm, Crs, dyn and Zrs4 were more sensitive than the spirometric indexes in diagnosing initial alterations due to RA.
ACKNOWLEDGMENTS
The authors would like to thank the Rheumatology Ambulatory unit of the Pedro Ernesto University Hospital for the referral of patients. This study was financially supported by the Brazilian Council for Scientific and Technological Development (CNPq) and the Rio de Janeiro State Research Supporting Foundation (FAPERJ).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Leonel D, Lucia C, A M, Martha-Alicia H, Blanca M. Pulmonary function test: its correlation with pulmonary high-resolution computed tomography in patients with rheumatoid arthritis. Rheumatol Int. 2011 doi: 10.1007/s00296-011-1933-8. [DOI] [PubMed] [Google Scholar]
- 2.Bartels CM, Bell CL, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of Rheumatoid Arthritis in United State veterans over 20 years. Rheumatology. (Oxford) 2010;49(9):1670–5. doi: 10.1093/rheumatology/keq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KK. Rheumatoid lung disease. Proceedings of the American Thoracic Society. 2007;4(5):443–8. doi: 10.1513/pats.200703-045MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amital A, Shitrit D, Adir Y. The lung in rheumatoid arthritis. Presse Med. 2011;40(1 Pt 2):e31–48. doi: 10.1016/j.lpm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hamblin MJ, Horton MR. Rheumatoid Arthritis-associated interstitial lung disease: Diagnostic dilemma. Pulmonary Medicine. 2011 doi: 10.1155/2011/872120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery P. Early Rheumatoid Arthritis: Therapeutic Strategies. Scandinavian Journal of Rheumatology. 1994;23(s100):3–7. doi: 10.3109/03009749409095196. [DOI] [PubMed] [Google Scholar]
- 7.Vassallo R, Matteson E, Charles Clinical response of rheumatoid arthritis-associated pulmonary fibrosis to tumor necrosis factor-alpha inhibition. Chest. 2002;122(3):1093–6. doi: 10.1378/chest.122.3.1093. [DOI] [PubMed] [Google Scholar]
- 8.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scand J Rheumatol. 2007;36(5):338–44. doi: 10.1080/03009740701393957. [DOI] [PubMed] [Google Scholar]
- 9.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, et al. The Forced Oscillation Technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–41. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 10.Kaczka DW, Dellacá RL. Oscillation mechanics of the respiratory system: applications to lung disease. Crit Rev Biomed Eng. 2011;39(4):337–59. doi: 10.1615/critrevbiomedeng.v39.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo PL, Werneck MM, Gianella-Neto A. New impedance spectrometer for scientific and clinical studies on the respiratory system. Rev Sci Instrum. 2000;71(7):2867–72. [Google Scholar]
- 12.Faria ACD, Lopes AJ, Jansen JM, Melo PL. Evaluating the forced oscillation technique in the detection of early smoking-induced respiratory changes. Biomed Eng Online. 2009;25:8–22. doi: 10.1186/1475-925X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faria AC, Costa AA, Lopes AJ, Jansen JM, Melo PL. Forced oscillation technique in the detection of smoking-induced respiratory alterations: diagnostic accuracy and comparison with spirometry. Clinics. 2010;65(12):1295–304. doi: 10.1590/S1807-59322010001200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faria ACD, Lopes AJ, Jansen JM, Melo PL. Assessment of respiratory mechanics in patients with sarcoidosis using forced oscillation: correlations with spirometric and volumetric measurements and diagnostic accuracy. Respiration. 2009;78(1):93–104. doi: 10.1159/000213756. [DOI] [PubMed] [Google Scholar]
- 15.Faria AD, Lopes AJ, Jansen JM, Pinheiro GC, Melo PL. Diagnostic performance of the Forced Oscillation Technique in the detection of early respiratory changes in rheumatoid arthritis. Conf Proc IEEE Eng Med Biol Soc. 2010:6034–7. doi: 10.1109/IEMBS.2010.5627605. [DOI] [PubMed] [Google Scholar]
- 16.Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid Arthritis Lung Disease – Determinants of Radiographic and Physiologic Abnormalities. Arthritis Rheum. 1996;39(10):1711–9. doi: 10.1002/art.1780391014. [DOI] [PubMed] [Google Scholar]
- 17.Mountz JD, Turner RA, Collins RL, Gallup KR, Jr, Semble EL. Rheumatoid arthritis and small airways function. Effects of disease activity, smoking, and alpha 1-antitrypsin deficiency. Arthritis Rheum. 1984;27(7):728–36. doi: 10.1002/art.1780270702. [DOI] [PubMed] [Google Scholar]
- 18.Ranganath VK, Elashoff DA, Khanna D, Park G, Peter JB, Paulus HE. Age adjustment corrects for apparent differences in erythrocyte sedimentation rate and C-reactive protein values at the onset of seropositive rheumatoid arthritis in younger and older patients. J Rheumatol. 2005;32(6):1040–2. [PubMed] [Google Scholar]
- 19.Consenso Brasileiro de Doenças Reumáticas: Atualização do Consenso Brasileiro no Diagnóstico e Tratamento da Artrite Reumatoide. Temas de Reumatologia Clínica. 2009;10(1):6–14. [Google Scholar]
- 20.Fransen J, van Riel PLCM. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;3(Suppl. 39):S93–S9. [PubMed] [Google Scholar]
- 21.Pereira CAC, Barreto SP, Simões JG, Pereira FWL, Gerstler JG, Nakatani J. Valores de referência para espirometria em uma amostra da população brasileira adulta. J Pneumol. 1992;18:10–22. [Google Scholar]
- 22.American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis. 1987;136(50):1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 23.Lorino AM, Zerah F, Mariette A, Harf A, Lorino H. Respiratory resistive impedance in obstructive patients: linear regression analysis vs. Viscoelastic modeling. Eur Respir J. 1997;10(1):150–5. doi: 10.1183/09031936.97.10010150. [DOI] [PubMed] [Google Scholar]
- 24.MacLeod D, Birch M. Respiratory input impedance measurement: forced oscillation methods. Medical & biological engineering & computing. 2001;39(5):505–16. doi: 10.1007/BF02345140. [DOI] [PubMed] [Google Scholar]
- 25.Peslin R, Hannhart B, Pino J. [Mechanical impedance of the chest in smokers and non-smokers (author's transl)] Bulletin europeen de physiopathologie respiratoire. 1981;17(1):93–105. [PubMed] [Google Scholar]
- 26.Pride NB. Forced oscillation techniques for measuring mechanical properties of the respiratory system. Thorax. 1992;47(4):317–20. doi: 10.1136/thx.47.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying Y, Peslin R, Duvivier C, Gallina C, Felicio da Silva J. Respiratory input and transfer mechanical impedances in patients with chronic obstructive pulmonary disease. Eur Respir J. 1990;3(10):1186–92. [PubMed] [Google Scholar]
- 28.Nguyen YT, Demoulin B, Schweitzer C, Bonabel-Chone C, Marchal F. Identification of bronchodilator responsiveness by forced oscillation admittance in children. Pediatric research. 2007;62(3):348–52. doi: 10.1203/PDR.0b013e3180db2933. [DOI] [PubMed] [Google Scholar]
- 29.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 30.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–93. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 31.Laitinen O, Salorinne V, Poppius H. Respiratory function in systemic lupus erythematosus, scleroderma, and rheumatoid arthritis. Ann Rheum Dis. 1973;32(6):531–5. doi: 10.1136/ard.32.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez T, Rémy-Jardin M, Cortet B. Airways involvment in rheumatoid arthritis – Clinical, functional, and HRCT findings. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1658–65. doi: 10.1164/ajrccm.157.5.9710018. [DOI] [PubMed] [Google Scholar]
- 33.Vergnenegre A, Pugnere N, Antonini MT, Arnaud M, Melloni B, Treves R, et al. Airway obstruction and rheumatoid arthritis. Eur Respir J. 1997;10(5):1072–8. doi: 10.1183/09031936.97.10051072. [DOI] [PubMed] [Google Scholar]
- 34.Terasaki H, Fujimoto K, Hayabuchi N, Ogoh Y, Fukuda T, Muller NL. Respiratory symptoms in rheumatoid arthritis relation between high resolution CT findigs and functional impairment. Radiat Med. 2004;22(3):179–85. [PubMed] [Google Scholar]
- 35.Davidson C, Brooks AGF, Bacon PA. Lung function in rheumatic arthrits – A clinic survey. Ann Rheum Dis. 1974;33(4):293–7. doi: 10.1136/ard.33.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whorwell PJ, Wojtolewjki JA, Lacey BW. Respiratory function in rheumatoid arthritis – Short reports. Br Med J. 1975;2(5964):175. doi: 10.1136/bmj.2.5964.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hills EA, Davies S, Geary M. Frequency-Dependence of Dynamic Compliance in Patients with Rheumatoid-Arthritis. Thorax. 1979;34(6):755–61. doi: 10.1136/thx.34.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geddes DM, Webley M, Emerson PA. Airway obstruction in rheumatoid arthritis. Ann Rheum Dis. 1979;38(3):222–5. doi: 10.1136/ard.38.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortet B, Perez T, Roux N, Flipo RM, Duquesnoy B, Delcambre B, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56(10):596–600. doi: 10.1136/ard.56.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins RL, Turner RA, Johnson AM, Whitley NO, McLean RL. Obstructive pulmonary disease in rheumatoid arthritis. Arthritis Rheum. 1976;19(3):623–8. doi: 10.1002/art.1780190316. [DOI] [PubMed] [Google Scholar]
- 41.Sasson CS, McAlpine SW, Tashkin DP, Baydur A, Quismorio FP, Morgan ES. Small airways function in nonsmokers with rheumatoid arthritis. Arthritis Rheum. 1984;27(11):1218–26. doi: 10.1002/art.1780271103. [DOI] [PubMed] [Google Scholar]
- 42.Zweig MH, Campbel G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–77. [PubMed] [Google Scholar]
- 43.Golpe R, Jiménez A, Carpizo R, Cifrian JM. Utility of home oximetry as a screening test for patients with moderate and severe symptoms of obstructive sleep apnea. Sleep. 1999;22(7):932–7. [PubMed] [Google Scholar]
- 44.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with Rheumatoid Arthritis. Mod Rheumatol. 2011;21(2):164–73. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandos AI, Rockette HE, Gur D. A permutation test sensitive to differences in areas for comparing ROC curves from a paired design. Statistics in medicine. 2005;24(18):2873–93. doi: 10.1002/sim.2149. [DOI] [PubMed] [Google Scholar]
- 46.King GG. Cutting edge technologies in respiratory research: lung function testing. Respirology. 2011;16(6):883–90. doi: 10.1111/j.1440-1843.2011.02013.x. [DOI] [PubMed] [Google Scholar]
- 47.LaPrad AS, Lutchen KR. Respiratory impedance measurements for assessment of lung mechanics: Focus on asthma. Respir Physiol Neurobiol. 2008;163(1-3):64–73. doi: 10.1016/j.resp.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navajas D, Farré R. Forced oscillation technique: from theory to clinical applications. Monaldi Arch Chest Dis. 2001;56(6):555–62. [PubMed] [Google Scholar]
- 49.Crapo RO, Jensen RL, Hargreave FE. Airway inflammation in COPD: physiological outcome measures and induced sputum. The European respiratory journal Supplement. 2003;41:19s–28s. doi: 10.1183/09031936.03.00077902. [DOI] [PubMed] [Google Scholar]
- 50.Postma DS, Brusselle G, Bush A, Holloway JW. I have taken my umbrella, so of course it does not rain. Thorax. 2011;67(1):88–9. doi: 10.1136/thoraxjnl-2011-200758. [DOI] [PubMed] [Google Scholar]
- 51.Harrison J, Martin J, Crameri J, Robertson CF, Ranganathan SC. Lung function in children with repaired tracheo-oesophageal fistula using the forced oscillation technique. Pediatr Pulmonol. 2010;45(11):1057–63. doi: 10.1002/ppul.21282. [DOI] [PubMed] [Google Scholar]
- 52.Verbanck S, de Keukeleire T, Schuermans D, Meysman M, Vincken W, Thompson B. Detecting upper airway obstruction in patients with tracheal stenosis. J Appl Physiol. 2010;109(1):47–52. doi: 10.1152/japplphysiol.01103.2009. [DOI] [PubMed] [Google Scholar]
- 53.McClean MA, Htun C, King GG, Berend N, Salome CM. Cut-points for response to mannitol challenges using the forced oscillation technique. Respir Med. 2011;105(4):533–40. doi: 10.1016/j.rmed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Hamakawa H, Sakai H, Takahashi A, Zhang J, Okamoto T, Satoda N, et al. Forced oscillation technique as a non-invasive assessment for lung transplant recipients. Adv Exp Med Biol. 2010;662:293–8. doi: 10.1007/978-1-4419-1241-1_42. [DOI] [PubMed] [Google Scholar]
- 55.Guo YF, Sun TY, Hermann F, Janssens JP. Comparison or airway resistance measurements by the forced oscillation technique and the interrupter technique for detecting chronic obstructive pulmonary disease in elderly patients. Chin Med J. 2005;118(22):1921–4. [PubMed] [Google Scholar]
- 56.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 57.Pappas DA, Giles JT, Connors G, Lechtzin N, Bathon JM, Danoff SK. Respiratory symptoms and disease characteristics as predictors of pulmonary function abnormalities in patients with rheumatoid arthritis: an observational cohort study. Arthritis Res Ther. 2010;12(3):R104. doi: 10.1186/ar3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youssef AA, Machaly SA, El-Dosoky ME, El-Maghraby NM. Respiratory symptoms in rheumatoid arthritis: relation to pulmonary abnormalities detected by high-resolution CT and pulmonary functional testing. Rheumatol Int. 2012;32(7):1985–95. doi: 10.1007/s00296-011-1905-z. [DOI] [PubMed] [Google Scholar]
- 59.Verbanck S, Schuermans D, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers: the small airways revisited. Am J Respir Crit Care Med. 2004;170(4):414–9. doi: 10.1164/rccm.200401-037OC. [DOI] [PubMed] [Google Scholar]