Abstract
Background and Objectives
Since statins and angiotensin receptor blockers are a frequently prescribed combination in patients with atherosclerotic cardiovascular diseases, we tested the interactive effects of simvastatin and losartan on atherosclerosis in apolipoprotein E (apoE)-/- mice.
Materials and Methods
Apolipoprotein E-/- mice were fed a high-fat, high-cholesterol (HFHC) diet for 12 weeks, with and without simvastatin (40 mg/kg) and/or losartan (20 mg/kg). The mice were divided into 5 groups and were fed as follows: regular chow (control diet, n=5), HFHC diet (n=6), HFHC diet with losartan (n=6), HFHC diet with simvastatin (n=6), and HFHC diet with both losartan and simvastatin (n=6).
Results
Losartan treatment in apoE-/- mice significantly decreased atherosclerotic lesion areas in whole aortic strips stained with Oil Red O. The plaque area measured at the aortic sinus level was reduced significantly by 17% (HFHC; 346830.9±52915.8 µm2 vs. HFHC plus losartan; 255965.3±74057.7 µm2, p<0.05) in the losartan-treated group. Simvastatin and simvastatin plus losartan treatments reduced macrophage infiltration into lesions by 33% (HFHC; 183575.6±43211.2 µm2 vs. HFHC plus simvastatin; 120556.0±39282.8 µm2, p<0.05) and 44% (HFHC; 183575.6±43211.2 µm2 vs. HFHC plus simvastatin and losartan; 103229.0±8473.3 µm2, p<0.001, respectively). In mice fed the HFHC diet alone, the smooth muscle cell layer in the aortic media was almost undetectable. In mice co-treated with losartan and simvastatin, the smooth muscle layer was more than 60% preserved (p<0.05). Given alone, losartan showed a slightly stronger effect than simvastatin; however, treatment with losartan plus simvastatin induced a greater inhibitory effect on atherosclerosis than either drug given alone. Serum lipid profiles did not differ significantly among the groups.
Conclusion
Losartan displayed anti-atherosclerotic effects in apoE-/- mice that were equivalent to or greater than the effects of simvastatin. Combined treatment with these drugs had greater effect than either drug alone.
Keywords: Atherosclerosis; Losartan; Simvastatin; Mice, knockout; Models, animal
Introduction
Hyperlipidemia is one of the major risk factors for atherosclerosis, and statins, which are potent inhibitors of hydroxyl-methylglutaryl-coenzyme A reductase, are given to suppress plasma lipid levels and to retard atherosclerosis. In patients with coronary artery disease, long-term treatment with statins may reduce plaque size and slow progression of coronary stenosis.1-4) In conjunction with lipid lowering effects, statins may reduce inflammation5) and improve endothelial function.6)
Hypertension is a major risk factor for stroke and ischemic heart disease.7) Angiotensin II (Ang II) promotes hypertension through vasoconstrictive and inflammatory activities, which also contribute to atherosclerosis.7) Therefore, angiotensin-converting enzyme (ACE) inhibitors8),9) and Ang II-receptor blockers (ARBs),10),11) mainly prescribed for the treatment of hypertension, may plausibly be used to treat patients with atherosclerotic coronary artery disease. Losartan is an Ang II type 1 receptor blocker used to reduce blood pressure. This drug may improve vascular functions as well as modulate inflammatory cytokine and chemokine expression, and formation of reactive oxygen and nitrogen species.7),12)
Both statins and ARBs display anti-oxidative, anti-proliferative, and anti-inflammatory effects that may retard or prevent atherosclerosis. A few groups have applied combined therapy with simvastatin and losartan in patients with hypercholesterolemia and hypertension13),14) and in rats;15) however, the mechanisms of the treatment effects observed have not been fully investigated.
In the present study, we investigated the potentially synergistic effects of simvastatin and losartan on atherosclerosis in high-fat high-cholesterol (HFHC)-fed apolipoprotein E (apoE)-/- mice by analyzing proteins, and proteases, related to atherosclerosis. We found that these drugs inhibited atherosclerosis, in part through preservation of the vascular smooth muscle cell (SMC) layer.
Materials and Methods
Animal model
All animal studies conformed to the guidelines of the Institutional Animal Care and Use Committee of Samsung Biomedical Research Institute. Seven-week-old apoE-/- mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and conditioned for one week at the Samsung Biomedical Research Institute under specific pathogen-free conditions.
Twenty-nine, 8-week-old apoE-/- mice were randomly divided into 5 groups, which were fed the normal diet only (n=5), a HFHC diet only (n=6), HFHC diet plus simvastatin (40 mg/kg/day, n=6), HFHC diet plus losartan (20 mg/kg/day, n=6), and HFHC diet plus losartan (20 mg/kg/day) plus simvastatin (40 mg/kg/day) (n=6). The HFHC diet contained 1.25% cholesterol, 6% fat, and 0.8% sodium chloride (CRF-1, Research Diets, Inc., New Brunswick, NJ, USA). Since the cholesterol concentration in the diet that we used here was too high to induce the atherosclerotic lesions by continuous feeding for 12 weeks, we developed the diet feeding scheme as follows: mice were fed the HFHC diets for one week and normal chow for 2 subsequent weeks to make one cycle; this cycle was repeated 4 times. After the final week of HFHC feeding (cycle four), all groups of mice were sacrificed.
The losartan and simvastatin were dissolved in pure water and administered to the mice three times weekly by tube feeding. HFHC-fed group were administered only HFHC and water and control group were fed only the normal chow and water. In all of the experiments, body weights were monitored throughout the treatment period. After 12 weeks, the mice were sacrificed by CO2 asphyxiation. Biochemical analysis, including total plasma cholesterol and triglyceride levels, were measured using an AU400 analyzer (Olympus, Tokyo, Japan) by Chemon Inc. (Yongin, Korea).
Histological examinations
The cross-sectional areas of atherosclerotic lesions were quantified by evaluating the lesion size in the aortic sinus. Briefly, at the time of sacrifice, the heart and aorta were perfused with phosphatebuffered saline (PBS) for 10 minutes and 4% paraformaldehyde for 5 minutes, and were then promptly removed. After fixation for one day in 10% buffered neutral formalin, aortic sinuses containing the heart and aortic root were embedded in frozen-section compound (Leica, Wetzlar, Germany), and kept at -70℃ until use. All samples were sectioned using a cryostat at -20℃, and 6 consecutive 8 µm sections were cut from the aorta where the valve cusps were visible. Plaques were stained with Oil Red O and counterstained with hematoxylin. The lesion area (µm2) of three sections was then quantified by computer-assisted morphometry (Image-Pro Plus, Silver Spring, MD, USA), and the average lesion size in each animal was calculated.
A treatment was considered 'effective' if there was a statistically significant difference between groups. A 'synergistic' treatment was one that did not show a difference or showed a difference, but without statistical significance, and however, co-treatment revealed a significant difference with statistical significance. When co-treatment demonstrated effects that had greater statistical significance compared to the effects of single treatment, it was defined as 'addictive'.
Immunofluorescence
Tissue sections were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with cold acetone for 15 minutes, blocked with 10% goat serum in PBS for 30 minutes, and reacted with primary antibody in PBS (1 : 100 to 1 : 2000 dilutions) overnight at 4℃. Primary antibodies used in the study were as follows: anti-MOMA2 (Cat#MCA519GT, AbD Serotec), anti-SMC actin (Cat#ab7817, Abcam), anti-metalloproteinase 9 (MMP9) (Cat#ab38898, Abcam), anti-tissue inhibitor of metalloproteinase 1 (TIMP1) (Cat#ab61224, Abcam), and anti-heat shock protein 27 (HSP27) (Cat#2442, Cell signaling) antibodies. After incubation, tissue sections were washed extensively with PBS, incubated with 1 : 100 Alexa 488- or Alexa 568-conjugated rabbit anti-mouse antibody (Molecular Probes, Eugene, OR, USA) in PBS for 30 minutes at room temperature, and washed 3 times with PBS. Nuclear staining was performed for 1 minute with DAPI (Molecular Probes) diluted 1 : 50000. Fluorescence images were obtained with a CSRL700 confocal microscope (ZEISS, Jena, Germany).
Statistical methods
Data were expressed as the mean±SD. Statistical significance was determined using unpaired Student's t-test, and a p less than 0.05 was considered significant.
Results
Losartan more than simvastatin inhibits the lipid accumulation in the aortas of high-fat, high-cholesterol-fed apolipoprotien E-/- mice
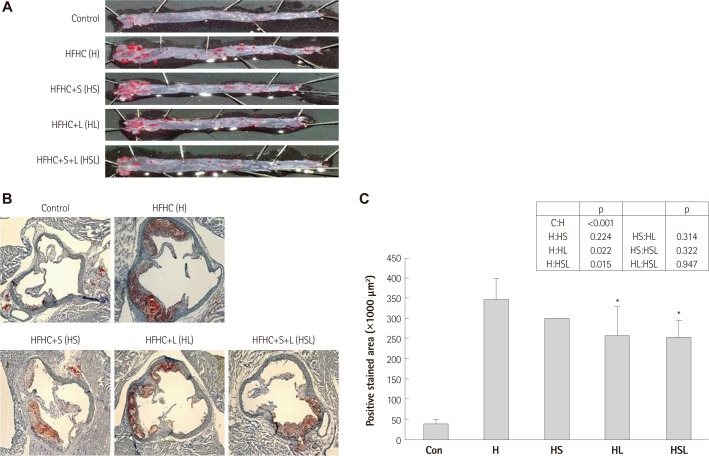
In the present study, we investigated the effects of simvastatin and/or losartan on atherogenesis in apoE-/- mice by quantifying lesion areas in the aortic sinuses of the mice fed a HFHC diet. Fig. 1A shows a longitudinal en face view of Oil Red O-stained lesions in the aorta. The mice fed HFHC for 12 weeks developed significantly larger atherosclerotic lesions in the aorta compared with the mice fed regular chow. The mice treated with simvastatin or losartan had significantly fewer atherosclerotic lesions compared with mice fed HFHC only. In particular, the treatment with simvastatin and losartan combined synergistically reduced the atherosclerotic lesion area (Fig. 1A).
Fig. 1.
Effects of simvastatin and/or losartan on lipid accumulation in aorta and aortic sinus in apoE-/- mice fed a high-fat, high-cholesterol (HFHC) diet. Mice were fed an HFHC diet for 12 weeks of HFHC with or without 40 mg/kg simvastatin and/or 20 mg/kg losartan twice weekly. A control group was fed regular chow for 12 weeks. A: representative en face Oil Red O-stained aorta from each group of apoE-/- mice is shown. B: representative Oil Red O-stained area in aortic sinus from each group of apoE-/- mice is shown. C: Oil Red O-positive areas of the aortic sinus were quantitatively analyzed by computer-assisted morphometery. All results are shown as the mean±SD. *p<0.05 compared with the HFHC group. S: simvastatin, L: losartan, S+L: simvastatin plus losartan, apoE-/-: apolipopretein E.
The atherosclerotic lesions in the cross-sections of the aortic roots from apoE-/- mice were also analyzed by Oil Red O staining. These sections revealed significantly higher lipid accumulation in mice fed HFHC than in mice fed regular chow (Fig. 1B). As shown in Fig. 1C, losartan treatment reduced lipid accumulation significantly by 29% compared to HFHC-fed mice (HFHC; 346830.9±52915.8 µm2 vs. HFHC plus losartan; 255965.3±74057.7 µm2, p<0.05). Simvastatin reduced lipid accumulation by 14% (299713.6±816.4 µm2), but the reduction was not significant (p=0.22). Mice co-treated with simvastatin and losartan showed a 30% reduction in lipid accumulation (253173.3±41893.2 µm2) compared with mice fed HFHC alone (p<0.05), Greater addictive decreasing effect was observed in the drugtreated groups (Fig. 1C, see p).
Total plasma cholesterol levels in HFHC-fed groups were not significantly influenced by simvastatin and/or losartan treatment (Table 1). The mean plasma triglyceride level was 101±43 mg/dL in the control group, 270±70 mg/dL in the HFHC-fed group, 220±99 mg/dL in the HFHC-fed simvastatin-treated group; 186±21 mg/dL in the HFHC-fed losartan-treated group and 172±98 mg/dL in the HFHC-fed simvastatin plus losartan-treated group. The simvastatin and/or losartan treatments did not significantly change the mean plasma low density lipoprotein-cholesterol (LDL-C) levels or mean plasma high density lipoprotein-cholesterol levels.
Table 1.
Effect of simvastatin and/or losartan on lipid profiles in mice sera
ApoE-/- mice were fed with a high-fat high-cholesterol (HFHC) diet for 12 weeks with or without drug treatment. Effects of simvastatin and/or losartan on total cholesterol (T-CHO), triglyceride (TG), low density lipoprotein-cholesterol (LDL-C) and high density lipoprotein-cholesterol (HDL-C) in plasma of HFHC-fed apoE-/- mice were measured. S: simvastatin, L: losartan, S+L: simvastatin plus losartan, apoE-/-: apolipopretein E
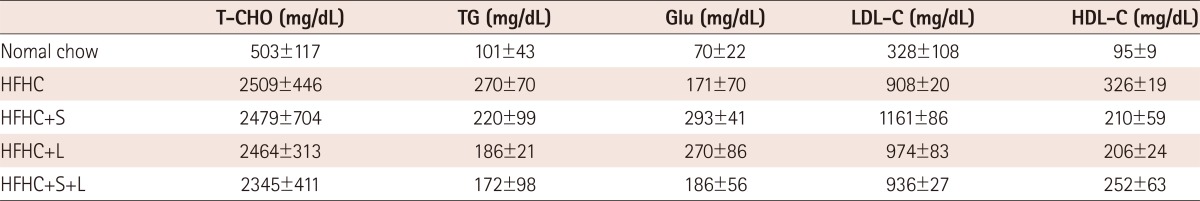
Simvastatin and losartan significantly affects macrophage infiltration into atherosclerotic lesions in the aortic sinuses of high-fat, high-cholesterol-fed apolipoprotien E-/- mice
Since monocytes/macrophages are the predominant cells found in atherosclerotic plaques, we examined the effects of the 2 drugs specifically on these cells. As shown in Fig. 2, the area of monocyte infiltration was 33% (120556.0±39282.8 µm2, p<0.05), 28% (138463.8±4018.4 µm2, p=0.106), and 44% (103229.0±8473.3 µm2, p<0.001) reduced in the groups treated with simvastatin, losartan, and the 2 drugs combined, respectively, compared with mice fed HFHC without drug treatment (183575.6±43211.2 µm2). The data indicate that groups treated with both simvastatin and combined drugs revealed a significant inhibitory effect on the inhibition of monocyte infiltration in atherosclerotic lesions. Simvastatin and losartan in combination had greater inhibitory effect than each drug treatment alone (Fig. 2B).
Fig. 2.
Effects of simvastatin and/or losartan on macrophage infiltration into atherosclerotic lesions in apoE-/- mice fed an HFHC diet. Mice were fed the HFHC diet for 12 weeks of HFHC with or without 40 mg/kg simvastatin and/or 20 mg/kg of losartan twice weekly. A control group was fed regular chow for 12 weeks. Tissue sections from the aortic sinuses of apoE-/- mice from each group were stained with rat monoclonal antibody to mouse macrophages (MOMA-2). A: representative MOMA-2-stained aortic sinuses from each group are shown. B: the MOMA-2-positive areas of the aortic sinus were quantitatively analyzed by computer-assisted morphometery. All results are shown as the mean±SD. *p<0.05, †p<0.01 compared with the HFHC group, respectively. S: simvastatin, L: losartan, S+L: simvastatin plus losartan, HFHC: high-fat, high-cholesterol, apoE-/-: apolipoprotein E.
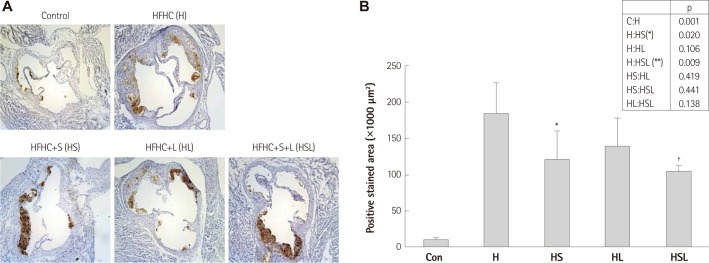
Simvastatin and losartan synergistically prevents smooth muscle layer destruction in the aortic media
The presence and localized pattern of SMCs in atherosclerotic lesions may potentially indicate plaque stability in disease progression. In aortic sinus sections from the HFHC-fed mice stained with alpha-SMC actin (α-SMC actin) antibody, the SMC layer in the arterial wall media showed much destruction. The preserved SMC area was significantly higher, 59.2% (p=0.266) and 60.1% (p=0.324), in mice treated with simvastatin and losartan, respectively, compared with mice fed HFHC without drug treatment (47.9%) (Fig. 3). The α-SMC actin-positive areas in HFHC-fed mice co-treated with simvastatin and losartan (n=6) were significantly greater, comprising 75.1% (p<0.05) of the total aortic sinus cross-sectional area as compared to 47.9% in the untreated HFHC-fed group (Fig. 3B). The co-administration of two drugs thus synergistically protected the SMC layer as compared to each single drug treatments (Fig. 3, p).
Fig. 3.
Effects of simvastatin and/or losartan on smooth muscle cell destruction in HFHC-fed apoE-/- mice. Three sections of the aortic sinus from each animal were stained with alpha-SMC actin antibody. Mean staining values were expressed as a percentage of the total lesion area. A: representative examples of aortic sinus staining are shown for each group. B: the preserved medial layer was quantified by expressing the area positively stained with alpha-SMC actin antibody as a fraction (%) of the entire vessel wall area. All results are shown as the mean±SD. *p<0.01 compared with the HFHC group. S: simvastatin, L: losartan, S+L: simvastatin plus losartan, HFHC: high-fat, high-cholesterol, apoE-/-: apolipopretein E, SMC: smooth muscle cell.
Simvastatin and/or losartan differentially affect matrix metalloproteinase 9 and heat shock protein 27 expression in atherosclerotic lesions
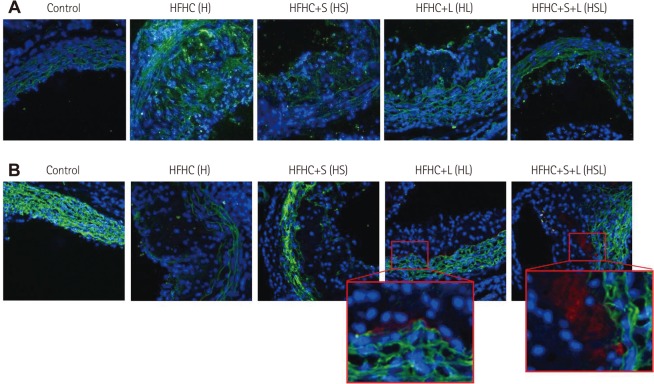
The extracellular matrix in normal vasculature was studied intensively to better understand plaque vulnerability or stability in disease progression. The significant protective effect that simvastatin and/or losartan exerted on the SMC layer in the vessel wall led us to investigate the well-known proteases, matrix MMP9 for clues to the action of these drugs. As expected, MMP9 expression in the aortic sinus of HFHC-fed apoE-/- mice was strongly induced; however, treatment with simvastatin and/or losartan remarkably suppressed this induction (Fig. 4A). Furthermore, losartan (but not simvastatin) treatment induced the expression of TIMP1 in the deep intimal area of HFHC-fed apoE-/- mice (Fig. 4B).
Fig. 4.
Effects of simvastatin and/or losartan on MMP9 and tissue inhibitor of metalloproteinase 1 (TIMP1) expression in the aortic sinus of HFHC-fed apoE-/- mice. Each section of the aortic sinus was stained with rabbit anti-mouse MMP9 or co-stained with goat anti-mouse SMC antibody and rabbit anti-mouse TIMP1 antibody followed by Alexa 488 donkey anti-goat IgG and Alexa 568 donkey anti-rabbit IgG. DAPI was used for nuclear staining. Immunofluorescence images were obtained by confocal microscopy (LSM700; ZEISS, Jena, Germany). A: MMP9. B: SMC & TIMP. S: simvastatin, L: losartn, S+L: simvastatin plus losartan. MMP9: metalloproteinase 9, HFHC: high-fat, high-cholesterol, apoE-/-: apolipopretein E, SMC: smooth muscle cell, DAPI: 4'-6-diamidino-2-phenylindole.
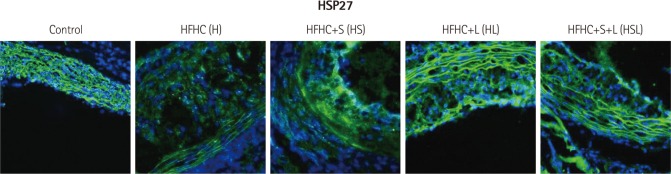
Currently, the small HSP27 is used as a biomarker for atherosclerotic progression. While HSP27 expression decreased in the aortic sinus from the HFHC-fed apoE-/- mice in this study, simvastatin and losartan clearly suppressed this decrease (Fig. 5). Interestingly, the effects of simvastatin and losartan on HSP27 expression were mainly restricted to the neointimal layer in case of simvastatin and the whole area in case of losartan in tissue sections of the aortic sinus, respectively (Fig. 5). These results indicate that the 2 drugs influence differentially on MMP9, TIMP1, and HSP27 expression in atherosclerotic lesions of HFHC-fed apoE-/- mice.
Fig. 5.
Effect of simvastatin and/or losartan on heat shock protein 27 (HSP27) expression in the aortic sinuses of HFHC-fed apoE-/- mice. Tissue sections were stained with rabbit anti-mouse HSP27 followed by Alexa-488 goat anti-rabbit IgG. Nuclei were stained with DAPI. Immunofluorescence images were obtained by confocal microscopy (LSM700; ZEISS, Jena, Germany). S: simvastatin, L: losartan, S+L: simvastatin plus losartan, HFHC: high-fat, high-cholesterol, apoE-/-: apolipopretein E, DAPI: 4'-6-diamidino-2-phenylindole.
Discussion
The statins are widely acknowledged to reduce blood levels of LDL-cholesterol, a lipid involved in the initiation and progression of atherosclerosis. The scope of clinical effects that statins influence continues to expand.16-19) Their roles include improving endothelial function, atherosclerotic plaque stabilization, oxidative stress reduction, anti-inflammatory effect, and inhibition of thrombogenesis.19),20) ACE inhibitors8),9) and ARBs10),11) are reported to improve endothelial function, presumably by inhibiting LDL oxidation, in addition to lowering blood pressure.21) ACE inhibitors and ARBs are mainly prescribed for the treatment of hypertension and heart failure, however they do not have much of a purpose in the prevention and treatment of atherosclerosis.
While drugs with similar mechanisms may induce additive effects, the combined effects of drugs with different mechanisms are less easily predictable. In the present study, we assessed the potentially addictive and synergistic effects of simvastatin and losartan in combination on selected hallmarks of atherosclerosis. Using HFHC-fed apoE-/- mice, we found that losartan, more than simvastatin, significantly reduced lipid accumulation in the aortas and aortic sinuses in HFHC-fed apoE-/- mice (Fig. 1). Simvastatin did not induce significant changes in lipid or LDL levels (Table 1). Co-treatment of simvastatin with losartan showed greater addictive effects (Fig. 1C). Koh et al.13) found that losartan reduced blood pressure, while simvastatin alone significantly improved the lipid profile; and they observed comparable beneficial effects on flow mediated vasodilation and inflammatory cytokines in hypercholesterolemic and hypertensive patients. ApoE-/- mice may have inherent problems as a model to study drug effects on atherosclerosis because of lack of apoE. The resultant lipid metabolism may be quite different from patients with atherosclerosis.
We previously found that simvastatin changes the lipid composition in atherosclerotic plaques, in the absence of significant effects on serum lipids of apoE-/- mice.22) Simvastatin, more than losartan significantly inhibited macrophage infiltration in the aorta and in plaques of the aortic sinus in HFHC-fed apoE-/- mice (Fig. 2). In both humans and mice, statins suppress inflammation by inhibiting proinflammatory chemokine release by cells within the artery wall.17),19),20),23) Losartan, on the other hand, may regulate vascular SMC proliferation by inhibiting deoxyribonucleic acid synthesis and modulating other functions such as Ang II-induced secretion of tissue-type plasminogen activator, MMPs, and TIMP.24),25) These distinctions in mechanism may explain why co-treatment with simvastatin and losartan, but not either drug alone, addictively and synergistically protected the media layer in aortic sinus from destruction in HFHC-fed apoE-/- mice (Fig. 3).
As we report here, MMP9 was markedly reduced, but TIMP and HSP27 were increased by simvastatin and/or losartan treatment in the aorta from HFHC-fed apoE-/- mice (Figs. 4 and 5). As we have shown, HSP27 is a potentially useful biomarker of progression of atherosclerosis.26),27) Specifically, the protein is diminished in carotids from patients with an atherosclerotic coronary artery and it is likely to have protective function in SMC death under hypoxic conditions (unpublished data). Our study demonstrated that simvastatin and losartan independently overcome the reduction of HSP27 by HFHC-feeding in mice. Interestingly, HSP27 induction by simvastatin was mainly restricted to the neointimal layer, while the induction by losartan covers the whole lesion area in tissue sections of the aortic sinus (Fig. 5). These differential effects of the 2 drugs on HSP27 expression may provide some clues at least to prevent SMC preservation during the development of atherosclerotic lesions. Currently, we are investigating the effects of simvastatin and/or losartan on HSP27 expression in SMCs and infiltrating immune cells, and whether the cells undergo apoptosis when HSP27 expression is reduced or knocked-down.
In conclusion, by distinct mechanisms, simvastatin and losartan favorably modified lipid accumulation, macrophage infiltration, and HSP27 and TIMP expression in atherosclerotic plaques in HFHC-fed apoE-/- mice. These effects on key components of atherosclerosis suggest that combination therapy with simvastatin and losartan may be beneficial for patients with atherosclerotic cardiovascular diseases.
Acknowledgments
This study was supported by grants from the Korean Society of Cardiology (2006) and the Korea Research Council of Fundamental Science and Technology (KRCF) through Basic Research Project managed by the Korea Research Institute of Standards and Science (KRISS).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Oliver MF, de Feyter PJ, Lubsen J, et al. Effect of simvastatin on coronary atheroma: the Multicentre Anti-Atheroma Study (MAAS) Lancet. 1994;344:633–638. [PubMed] [Google Scholar]
- 3.Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106:2884–2887. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 4.Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001;104:387–392. doi: 10.1161/hc2901.093188. [DOI] [PubMed] [Google Scholar]
- 5.Albert MA, Danielson E, Rifai N, Ridker PM PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, et al. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Reserarch Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 10.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 11.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 12.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 13.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Koh KK, Quon MJ, Lee Y, Shin EK. The effects of simvastatin, losartan, and combined therapy on soluble CD40 ligand in hypercholesterolemic, hypertensive patients. Atherosclerosis. 2007;190:205–211. doi: 10.1016/j.atherosclerosis.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Bayorh MA, Layas MF, Mann G, Feuerstein GZ, Eatman D. The effect of diet on simvastatin and losartan enhancement of endothelial function. Clin Exp Hypertens. 2007;29:311–325. doi: 10.1080/10641960701500463. [DOI] [PubMed] [Google Scholar]
- 16.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 17.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–657. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? a meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 19.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparrow CP, Burton CA, Hernandez M, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 21.Hayek T, Attias J, Coleman R, et al. The angiotensin-converting enzyme inhibitor, fosinopril, and the angiotensin II receptor antagonist, losartan, inhibit LDL oxidation and attenuate atherosclerosis independent of lowering blood pressure in apolipoprotein E deficient mice. Cardiovasc Res. 1999;44:579–587. doi: 10.1016/s0008-6363(99)00239-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Lee ES, Lee JY, et al. Multiplex coherent anti-stokes Raman spectroscopy images intact atheromatous lesions and concomitantly identifies distinct chemical profiles of atherosclerotic lipids. Circ Res. 2010;106:1332–1341. doi: 10.1161/CIRCRESAHA.109.208678. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie-Majd A, Maca T, Bucek RA, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 24.Papakonstantinou E, Roth M, Kokkas B, Papadopoulos C, Karakiulakis G. Losartan inhibits the angiotensin II-induced modifications on fibrinolysis and matrix deposition by primary human vascular smooth muscle cells. J Cardiovasc Pharmacol. 2001;38:715–728. doi: 10.1097/00005344-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Li D, Mehta JL. Modulation of matrix metalloproteinase-1, its tissue inhibitor, and nuclear factor-kappa B by losartan in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2002;39:332–339. doi: 10.1097/00005344-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Park HK, Park EC, Bae SW, et al. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation. 2006;114:886–893. doi: 10.1161/CIRCULATIONAHA.105.541219. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Ventura JL, Duran MC, Blanco-Colio LM, et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]