Summary
The binding site of the cyclic peptide antibiotics capreomycin and viomycin is located on the ribosomal subunit interface close to nucleotides C1409 in 16S rRNA and C1920 in 23S rRNA. In Mycobacterium tuberculosis, the 2′-hydroxyls of both nucleotides are methylated by the enzyme TlyA. Loss of these methylations through inactivation of TlyA confers resistance to capreomycin and viomycin. We report here that TlyA orthologs occur in diverse bacteria and fall into two distinct groups. One group, now termed TlyAI, has shorter N- and C-termini and methylates only C1920; the second group (now TlyAII) includes the mycobacterial enzyme, and these longer orthologs methylate at both C1409 and C1920. Ribosomal subunits are the preferred substrates for both groups of orthologs. Amino acid substitutions at the N-terminus of TlyAII reduce its ability to methylate these substrates. Growing pairs of recombinant TlyAII Escherichia coli strains in competition shows that even subtle changes in the level of rRNA methylation lead to significant differences in susceptibility to sub-inhibitory concentrations of capreomycin. The findings reveal that 2′-O-methyls at both C1409 and C1920 play a role in facilitating the inhibitory effects of capreomycin and viomycin on the bacterial ribosome.
Keywords: rRNA, 2′-O-methylation, rRNA methyltransferase, mRNA translation, ribosomal subunit interface, capreomycin/viomycin
Introduction
Post-transcriptional modifications of rRNAs facilitate ribosomal subunit assembly and conformation changes during protein synthesis (Chow et al., 2007, Grosjean, 2009, Lapeyre, 2005, Woodson, 2008). In bacteria, rRNA modifications are mainlyuridine isomerizations and methylations on the nucleobase or on the 2′-hydroxl of the ribose. In Escherichia coli, the most intensely studied bacterium, rRNAs modifications are generally added by enzymes that recognize single specific nucleotide targets (Purta et al., 2009). A subset of rRNA methylations confer resistance to many ribosome-targeting antibiotics (see reviews by Conn et al., 2009, Long and Vester, 2009, Poehlsgaard and Douthwaite, 2005, McCusker and Fujimori, 2012), and are often acquired by bacterial pathogens via an exogenous methyltransferase gene. There are, however, a few cases where drug resistance is conferred by loss of rRNA methylation after inactivation of an intrinsic methyltransferase gene (Helser et al., 1971, Okamoto et al., 2007). This latter type of mechanism has been observed in virulent strains of Mycobacterium tuberculosis where inactivation of the tlyA methyltransferase gene confers resistance to capreomycin and viomycin (Johansen et al., 2006, Maus et al., 2005b).
Capreomycin and viomycin are cyclic-peptide antimicrobial compounds, and capreomycin is particularly important in the treatment of tuberculosis caused by strains of M. tuberculosis that are resistant to first-line drugs (Almeida Da Silva and Palomino, 2011, Zhang and Yew, 2009). Capreomycin and viomycin both bind to the bacterial ribosome where interbridge B2a extends across the subunit interface and links the decoding region of 16S rRNA helix 44 with the highly conserved helix 69 of 23S rRNA (Johansen et al., 2006, Stanley et al., 2010). Within these helices, nucleotides C1409 of 16S rRNA and C1920 of 23S rRNA (E. coli rRNAs numbering throughout) are methylated at their 2′-hydroxyls by the M. tuberculosis methyltransferase TlyA (Johansen et al., 2006). Nucleotides C1409 and C1920 are brought into proximity upon association of the ribosomal subunits and formation of interbridge B2a (Fig. 1) (Selmer et al., 2006, Yusupov et al., 2001) and failure to methylate these nucleotides due to loss of TlyA activity results in resistance to capreomycin and viomycin (Johansen et al., 2006, Maus et al., 2005b).
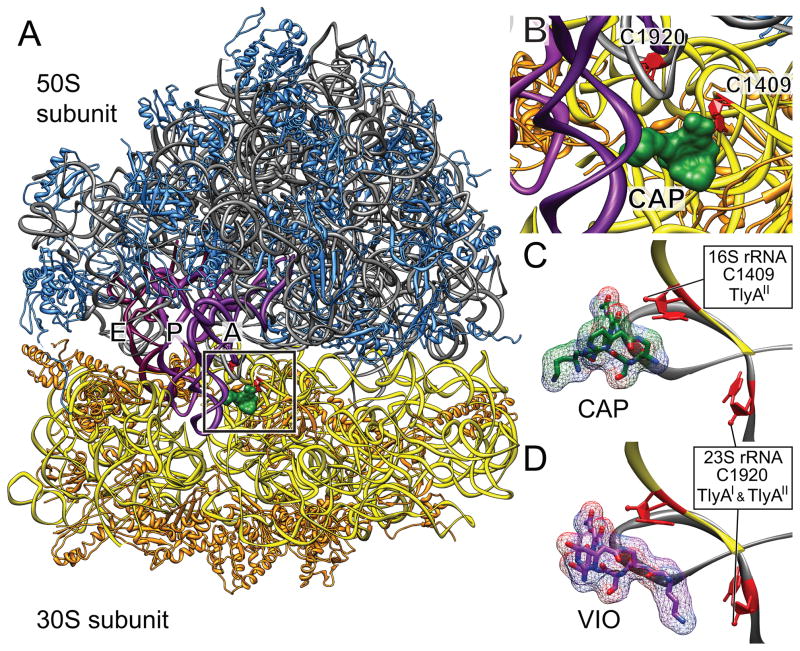
Fig. 1. Capreomycin and viomycin binding site on 70S ribosomes showing nucleotides C1409 and C1920 and methylation by TlyA orthologs.
A. Ribosome with capreomycin (green) bound showing interbridge B2a formed between 16S rRNA helix 44 and 23S rRNA helix 69 (boxed). The sites of methylation at nucleotides C1409 and C1920 are highlighted (red) within the box. This region is shown relative to the positions of the A-, P- and E-site tRNAs (purple); backbones of 16S rRNA (yellow), 30S r-proteins (orange), 23S rRNA (grey) and 50S r-proteins (blue) are shown. The ribosome is shown in the classical state (Stanley et al., 2010; Dunkle et al., 2011).
B. Enlargement of the boxed region.
C. Ribose targets for TlyAI and TlyAII methylation (red) relative to the binding site of capreomycin (drug carbons, green; oxygens, red; nitrogens, blue). In the classical state T. thermophilus (Stanley et al., 2010) and E. coli (Dunkle et al., 2011) ribosome structures, the distance between the 2′-O positions of C1409 and C1920 is approximately 21 Å, and this distance remains virtually unchanged in the intermediate translocation (hybrid)-state of the ribosome (Dunkle et al., 2011).
D. Same view with viomycin bound. Drug binding structures redrawn from the X-ray crystal PDB files 3KNH through 3KNO (Stanley et al., 2010).
Orthologs of the mycobacterial TlyA are found in numerous unrelated bacteria including Thermus thermophilus (Monshupanee et al., 2008). The T. thermophilus enzyme was shown to 2′-O-methylate the 23S rRNA nucleotide C1920 (Mengel-Jorgensen et al., 2006) without concomitant methylation at 16S rRNA nucleotide C1409 (Guymon et al., 2006), which indicated that the two methylation activities of TlyA could be differentiated (Monshupanee et al., 2008). Here, we investigate how the mycobacterial TlyA recognizes two distinct methylation targets on separate ribosomal subunits, and we evaluate the relative importance of the two methylations for inhibition by cyclic peptide drugs. The database sequences of TlyA show that the lengths of the N- and C-termini differ such that orthologs can be divided into two groups. Recombinant versions of TlyA orthologs from both groups were constructed and expressed in E. coli to determine their substrate specificities. Structural features of the orthologs that could be linked to substrate specificity were then altered by site-directed mutagenesis. Strains harbouring the mutant variants of TlyA were grown in competition in the presence of drug, and their growth was followed using a quantitative fluorescence PCR assay. The results demonstrate that even subtle differences in rRNA methylation levels produce measurable changes in susceptibility to capreomycin.
Results
Comparative analysis of TlyA sequences
Protein BLAST searches using M. tuberculosis TlyA as the queryagainst 2,011 bacterial genome sequences revealed 852 genomes that contain an ORF with better than 40% amino acid identity, and these were classed as TlyA orthologs. Sequences outside the Bacteria were observed with significant similarity to TlyA, although they generally fell below the threshold for inclusion as orthologs. These non-bacterial sequences comprised three insects (31% – 43% identity), eleven plants (36% – 41%), five algae (34% – 40%) and two Archaea (29% and 31%). Domains indicative of an RNA binding region, an S-adenosylmethionine (SAM) binding site (Martin and McMillan, 2002) and 2′-O-methyltransferase function (Aravind and Koonin, 1999, Feder et al., 2003, Maus et al., 2005b) are particularly well conserved in the ortholog sequences (Fig. 2).
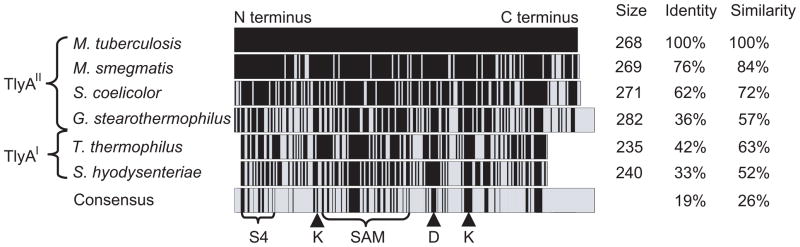
Fig. 2.
Comparison of TlyA sequences. The sequence of TlyA from M. tuberculosis H37Rv (NCBI accession number: CAB10951) is compared with orthologs from M. smegmatis MC2 155 (NCBI number YP_888048.1), S. coelicolor A3 (NC_003888), G. stearothermophilus (AB126617), T. thermophilus HB8 (AP008226) and S. hyodysenteriae (X61684). The total numbers of residues in the enzymes together with the percentages that are identical and functionally-similar to the M. tuberculosis sequence are listed on the right; these conserved regions are shown as black bars within the sequences. Based on their lengths, the sequences fall into the two distinct groups TlyAI and TlyAII. A consensus from these orthologs is shown highlighting the conserved S4-type RNA binding motif (S4), the S-adenosylmethionine (SAM) binding domain and three motifs indicative of 2′-O-methyltransferases (K, D and K) (Aravind and Koonin, 1999, Feder et al., 2003, Maus et al., 2005b).
Alignment of bacterial TlyA orthologs showed that they could be differentiated into two distinct groups on the basis of the lengths of their N- and C-termini (Fig. 2). The first group, now designated TlyAI, has the shorter termini. The second group, TlyAII, consists of enzymes that are at least four residues longer at the N-terminus with a highly conserved arginine or lysine at position 4; the C-termini of TlyAII proteins are up to fifty residues longer, but lack any obvious conservation.
TlyAorthologs methylate single or dual targets
The rRNA methyltransferase activities of five bacterial TlyAI and TlyAII orthologs (Fig. 2) were tested in vivo in recombinant strains of E. coli, a species that has no natural tlyA ortholog of its own. Methylation of rRNAs was analyzed by primer extension and MALDI-MS. Recombinant TlyAI enzymes from T. thermophilus and Serpulina hyodysenteriae methylated 23S rRNA nucleotide C1920, but not nucleotide C1409 in 16S rRNA; TlyAII enzymes derived from Mycobacterium smegmatis, Streptomyces coelicolor and Geobacillus stearothermophilus methylated at both C1409 and C1920 (Fig. 3). The MIC measurements showed that the susceptibility of the E. coli recombinants to capreomycin and viomycin increased between 2- and 8-fold upon expression of the different tlyA orthologs (Table 1). We noted considerable variation in the sequences, codon usage and expression levels of these orthologs, and concluded that a more uniform system was needed to ascertain whether tlyAI and tlyAII had significantly different effects on drug susceptibility.
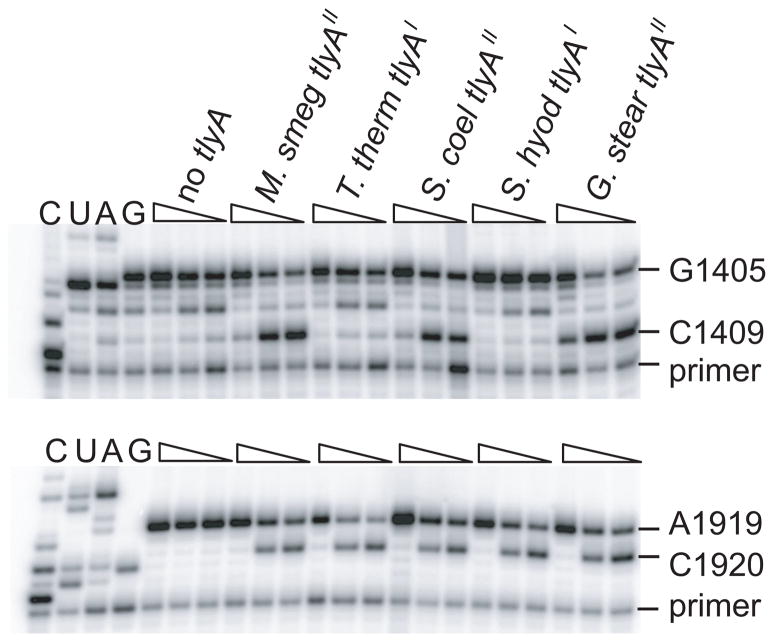
Fig. 3.
Gel autoradiograms of primer extensions showing 2′-O-methylations on E. coli rRNAs by recombinant TlyA orthologs. rRNAs were isolated from E. coli strains that contained the empty plasmid, pLJ102 (no tlyA), or a plasmid expressing recombinant tlyA from M. smegmatis (M. smeg), T. thermophilus (T. therm), S. coelicolor (S. coel), S. hyodysenteriae (S. hyod) and G. stearothermo-philus (G. stear). Decreasing dGTP concentration (wedges) intensifies reverse transcription termination at 2′-O-methylated C1409 in 16S rRNA (upper panel) and C1920 in 23S rRNA (lower panel). Run-through transcription terminated at G1405 and A1919 upon incorporation of ddCTP and ddTTP, respectively. Lanes C, U, A and G are dideoxy-sequencing reactions on unmodified E. coli rRNAs. Two additional, independent repetitions of the experiment yielded identical results.
Table 1.
Antibiotic susceptibilities of E. coli expressing recombinant tlyA orthologs. Each MIC (minimum inhibitory concentration) represents a minimum of three measurements, and the values were consistently the same under the conditions used.
| E. coli with plasmid-encoded tlyA orthologs | MIC (μg ml−1)
|
|
|---|---|---|
| capreomycin | viomycin | |
| Empty plasmid, no tlyA | 128 | 128 |
| T. thermophilus tlyAI | 32 | 32 |
| S. hyodysenteriae tlyAI | 32 | 32 |
| M. smegmatis tlyAII | 32 | 16 |
| S. coelicolor tlyAII | 64 | 64 |
| G. stearothermophilus tlyAII | 64 | 32 |
Ribosomal substrates of TlyA methyltransferases
The methylation substrates for TlyAI and TlyAII were identified in vitro using purified recombinant enzymes with a range of ribosomal substrates (16S and 23S rRNAs, 30S and 50S subunits and 70S ribosomes) isolated from E. coli and from the ΔtlyA strains of M. smegmatis and T. thermophilus. In the in vitro assays, TlyAI from T. thermophilus efficiently methylated C1920 in 50S subunits and, consistent with the results obtained in vivo, it failed to methylate C1409 in any of the substrates containing 16S rRNA (Fig. S1A, B, C). The 50S subunit was the preferred substrate for TlyAI, although a modest amount of methylation was seen with free 23S rRNA and 70S ribosomes.
The M. smegmatis TlyAII enzyme methylated efficiently at C1920 in 50S subunits in vitro, although weaker activity was also detected on 70S ribosome and 23S rRNA substrates (Fig. S1F). The M. smegmatis TlyAII modified C1409 in E. coli 30S subunits, and activity at this site on 16S rRNA and 70S ribosomes was negligible (Fig. S1D). Surprisingly, the M. smegmatis enzyme displayed only weak activity on its endogenous 30S subunits derived from the M. smegmatis ΔtlyA strain (Fig. S1E).
Residues essential for target recognition
The contribution of the N- and C- termini towards recognition of the two rRNA targets was investigated by mutagenizing the M. smegmatis TlyAII sequence. Deletion of four N-terminal residues (ΔA2-R3-R4-A5) abolished methylation at 16S rRNA nucleotide C1409, while methylation at 23S rRNA C1920 was retained, albeit with reduced efficiency (Fig. 4A). The same effect was obtained by substituting both Arg-3 and Arg-4 for alanines (R3A-R4A), however, methylation was unaltered when both of these basic residues were replaced by similarly charged lysines (R3K-R4K). The more important of the two arginines for 30S subunit recognition appears to be R4, as the single R4A substitution causes complete loss of C1409 methylation. Defective methylation by this latter mutant was partially rescued by substitution of R3 for lysine (R3K-R4A) (Fig. 4B and 4C), while substitution of R3 on its own (R3A) did not affect methylation. Deletion of the 27 C-terminal residues in TlyAII abolished methylation activity at both C1409 and C1920 (Fig. 4A), irrespective of whether the recombinant protein was constructed with or without a C-terminal His-tag.
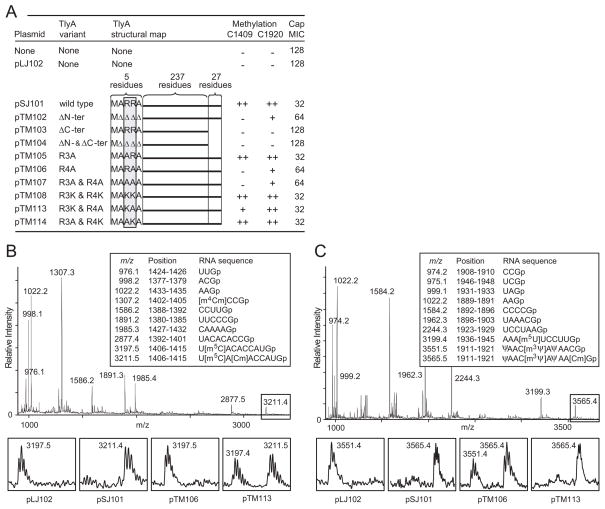
Fig. 4.
Methylation of rRNA by TlyA mutant enzymes in vivo.
A. Mutationsinthe N- and C-terminals of M. smegmatis TlyAII that affect the enzyme’s ability to methylate E. coli 16S and 23S rRNAs in vivo at nucleotides C1409 and C1920 (++, full; + partial; −, no methylation). Methylation levels were measured by both MALDI-MS and primer extension. Altered susceptibility of E. coli to capreomycin (Cap) is shown as MICs (μg ml−1); measurements in triplicate with identical results.Δ, deleted amino acid residue.
B. MALDI-MS analyses of 16S rRNA nucleotides 1377–1435 after RNase T1 digestion; rRNA from cells expressing wild-type TlyAII from pSJ101; boxes show the enlarged spectral region with nucleotide C1409 expressed from cells with different TlyA variants.
C. Corresponding MALDI-TOF MS analyses of 23S rRNA nucleotides 1889–1948 showing the enlarged peaks containing C1920. The measured m/z values (above the peaks) match the theoretical values (boxed) to within 0.1 Dalton.
In another set of recombinants, we extended the N- and C-termini of the T. thermophilus TlyAI enzyme in an attempt to expand its substrate range to include C1409. Recombinant versions of T. thermophilus TlyAI were constructed with the N- and/or C-termini from the M. smegmatis, S. coelicolor and G. stearothermophilus TlyAII sequences. The T. thermophilus TlyAI recombinants retained their ability to methylate at C1920 although none of them became capable of methylating C1409 (data not show).
Assessing the effects of rRNA methylation on capreomycin susceptibility
The varying degrees of modification at nucleotides C1409 and C1920 added by the different TlyAII mutants caused a 4-fold variation in drug sensitivity with MICs of 32 to 128 μg ml−1 (Fig. 4A). These MIC values were highly reproducible, but they were nevertheless insufficiently differentiated for the evaluation of subtle changes in drug sensitivity. We therefore opted for a growth competition assay over a large number of cell divisions, during which any difference in drug susceptibility would become apparent. For growth competition, pairs of E. coli strains expressing variants of the M. smegmatis tlyAII gene were grown together in the same medium for over fifty generations. Strains were identical except for substitutions of one or two residue at the N-terminal part of TlyAII. The substitutions affected rRNA methylation, which in turn was seen to influence growth rates in capreomycin even at concentrations well below the MICs. The proportion of each strain in the population was followed during the growth cycles using a fluorescence PCR assay that used the sequence differences in the tlyAII genes to distinguish the strains.
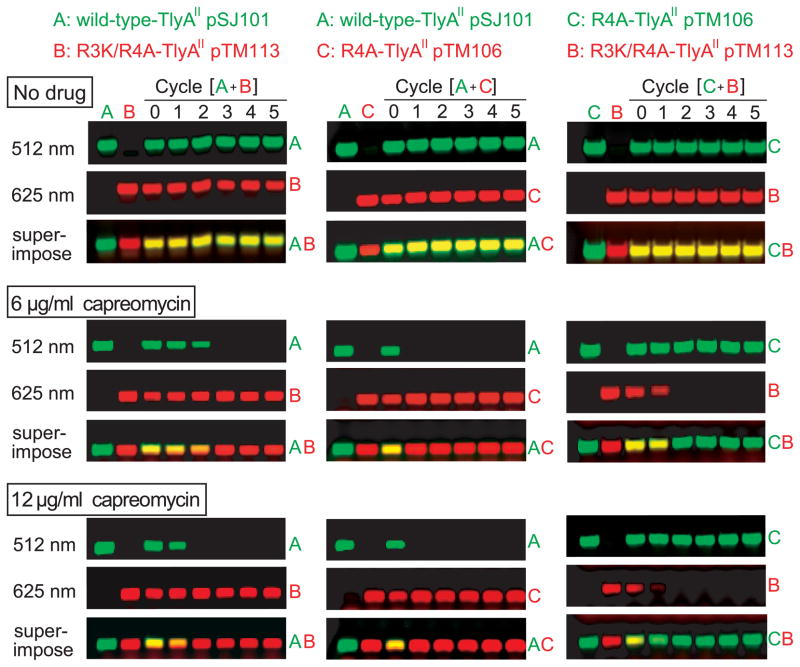
The E. coli strain harbouring wild-type TlyAII was rapidly out-competed by the R3K/R4A-TlyAII strain (Fig. 5), despite having been indistinguishable on the basis of MIC measurements (both 32 μg ml−1). At a sub-inhibitory level of capreomycin (6 μg ml−1) the proportion of cells with the wild-type TlyAII gradually declined and disappeared from the total population after two growth cycles corresponding to about 22 generations. Increasing the capreomycin concentration to 12 μg ml−1 accelerated the selection process and cells with wild-type TlyAII were out-competed already by the 11th generation of growth. When competing against strains such as R4A-TlyAII that are less effective at methylating their rRNAs, the strain with wild-type TlyAII was even more rapidly outgrown at subinhibitory drug levels (Fig. 5). In growth experiments with capreomycin, where both competing strains showed less than stoichiometric rRNA methylation patterns (shown here for the R4A versus the R3K/R4A mutant), the strain with the lower level of methylation invariably came to dominate the culture.
Fig. 5.
Cell growth competition assay. Pairs of E. coli strains with TlyA variants, differing in the degree of 2′-O-methylation at C1409 and C1920, were grown together through five cycles (with approximately eleven generations per cycle) in the presence of sub-inhibitory concentrations of capreomycin. The proportion of each strain was determined by PCR with fluorescently-labelled primers. Left column: wild-type strain expressing TlyAII from plasmid pSJ101 (A) grown in competition with the R3K/R4A-TlyAII strain (B, pTM113); the upstream PCR primers complementary to these tlyA sequences were respectively labelled with Cy3 (fluorescing green after excitation at 512 nm) and Cy5 (fluorescing red after excitation at 625 nm). Centre column: wild-type TlyAII strain grown in competition with the R4A-TlyAII strain (C, pTM106) and analyzed with Cy3 and Cy5 primers, respectively. Right column: R4A- and R3K/R4A-TlyAII strains grown in competition (note Cy3 is used here for the R4A strain). Upper row, no drugs; middle row, competition in 6 μg capreomycin ml−1; lower row, competition in 12 μg capreomycin ml−1. The superimpositions of the 170 bp PCR products appear yellow when the amounts of strains are approximately equal, and the red or green colour becomes predominant when one strain out-competes the other. Each data set is representative of a minimum of three growth assays.
In the absence of capreomycin, all E. coli tlyAII recombinant strains grew identically over 55 generations (Fig. 5), and extended culturing to over 100 generation revealed no growth advantage for any strain in the absence of drug (not shown).
Discussion
Capreomycin and viomycin inhibit protein synthesis on the bacterial ribosome by interacting across the interface of the ribosomal subunits (Johansen et al., 2006, Stanley et al., 2010, Yamada et al., 1978) and blocking the translocation of tRNA (Modolell and Vazquez, 1977). The crystal structure places the drugs between the A- and P-sites with the ribosome in the classical state for tRNA binding (Stanley et al., 2010), while functional studies indicate that the drugs are at the same site but with the ribosome in the pre-translocational hybrid state (Ermolenko et al., 2007, Johansen et al., 2006). Methylation of the rRNAs in both subunits by TlyA facilitates inhibition by capreomycin and viomycin, and this could be linked with the relative movement of the subunits between the different states during translocation. However, no adverse effect was seen on growth of the parent strains after inactivation of TlyA methylation (Maus et al., 2005b, Monshupanee et al., 2008). Furthermore, in the absence of drug, the competition growth assay revealed neither growth advantage nor biological cost in connection with TlyA-directed methylations (Fig. 5, upper row). Viewed together with the occurrence of TlyA orthologs in over 40 % of bacterial species, it seems likely that the methylations confer some advantage that has not yet become apparent under laboratory growth conditions.
Here, we show that TlyA orthologs segregate into two groups. The first group TlyAI is exemplified by the T. thermophilus ortholog and 2′-O-methylates nucleotide C1920 in 23S rRNA (Monshupanee et al., 2008), while the second group TlyAII includes the mycobacterial orthologs, and these enzymes methylate C1409 in 16S rRNA in addition to nucleotide C1920 (Johansen et al., 2006). The differences in substrate recognition and modification by TlyAI and TlyAII correlate with distinctive variations in the lengths of their N- and C-termini. The N-terminal domain corresponds to an S4-type RNA-binding motif commonly found in bacterial and eukaryotic RNA methyltransferases, pseudouridine synthases and ribosomal proteins (Aravind and Koonin, 1999, Arenas et al., 2011). In TlyAII this domain is preceded by several residues that have been modeled in silico as a finger-like projection with the conserved positively charged Lys/Arg at positions 3 and 4 on the surface (Arenas et al., 2011), and we show here that residue R4 plays an important role in broadening the range of RNA interaction to include 16S rRNA nucleotide C1409. However, this sequence on its own is not enough to direct the enzyme to nucleotide C1409 and, after extending the N-termini of TlyAI enzymes with the corresponding TlyAII sequences, recombinants still modified C1920 but without gaining the ability to methylate at C1409. Similar results were found for the C-termini of the enzymes. The longer C-terminus of TlyAII is clearly important for function (pTM103, Fig. 4), but its addition to TlyAI did not change enzyme specificity. The N- and C-terminal extensions in TlyAII appear to have evolved to be structurally and functionally integrated with the enzyme core, and thus merely grafting these sequences onto TlyAI does not extend its function.
Nucleotides C1409 and C1920 are located at the interface sides of their respective 30S (Schlünzen et al., 2000, Wimberly et al., 2000, Demirci et al., 2010) and 50S subunits (Tu et al., 2005, Schlünzen et al., 2003). Subunit association to form 70S ribosomes (Selmer et al., 2006, Yusupov et al., 2001, Bulkley et al., 2010, Dunkle et al., 2011) would prevent molecules as large as the TlyA methyltransferases from gaining access to nucleotides C1409 and C1920 (Fig. S3). Genetic evidence from a M. tuberculosis mutant with a 23S rRNA mutation (ΔA1916), which prevented methylation at C1920 but not at C1409, suggested that TlyA recognizes the two sites individually and presumably prior to subunit association (Johansen et al., 2006). This idea was verified here by in vitro methylation assays showing that both TlyAI and TlyAII prefer unassociated subunits as their substrates. The weak modification seen at C1920 with 70S ribosomes is consistent with partial dissociation to separate subunits during the assay. For C1409 methylation by TlyAII, the reaction kinetics is slower than at C1920, and no significant methylation was seen at C1409 when 70S ribosomes were used as the substrate. A moderate amount of modification was noted with free 23S rRNA, and C1920 is clearly accessibility in this substrate but presumably in a less than optimal conformation. It was noted that modification in vitro by the mycobacterial TlyAII on its own 30S subunits was relatively poor compared to E. coli 30S subunits, and contrasts with the stoichiometric modification observed in vivo (Johansen et al., 2006). This might suggest that modification at C1409 in mycobacteria occurs prior to completion of 30S subunit assembly.
Inhibition by capreomycin and viomycin is affected in a similar manner by the recombinant TlyA orthologs with MICs changing by up to 8-fold (Table 1). Such modest changes in MIC are sufficient to cause clinical resistance in virulent strains of M. tuberculosis that have lost tlyA function (Maus et al., 2005b, Maus et al., 2005a). Despite this, quantifying the relative effects on drug sensitivity of single and dual methylations by TlyAI and TlyAII proved difficult by MIC determination alone. The orthologs differ greatly in their gene and protein sequences (Fig. 2) and thus lacked uniformity in expression and enzymatic activities in the E. coli host. This obstacle was overcome by creating a series of strains that possessed different degrees of methylation at C1409 and C1920, and were genetically identical except for substitutions at N-terminal residues in recombinant M. smegmatis TlyAII.
We conclude from the data on these TlyAII strains that increases in the level of methylation at both the target nucleotides C1409 and C1920 result in heightened sensitivity to the cyclic peptide drugs. The importance of the C1409 is revealed by the strain with partial C1409/full C1920 methylation which, in capreomycin, out-competes the wild-type strain with full C1409/full C1920 methylation (Fig. 5). The role of C1920 methylation in increasing capreomycin sensitivity is established by the strains expressing TlyAI, where there is full C1920 methylation but no methylation at C1409 (Table 1).
The growth competition assay provides a means of detecting relatively small amounts of compounds with the same inhibitory mechanism as the cyclic peptides capreomycin and viomycin. We note that the assay should in principle be capable of detecting other compounds such as thermorubin, which also binds across the subunit interface in the region of interbridge B2a but does so through direct interaction with the 2′-hydroxyl of C1409 (Bulkley et al., 2012). Methylation by TlyAII would thus be expected to confer resistance to thermorubin and, as a consequence of this, thermorubin-type and capreomycin-type compounds would have inverse effects upon competing pairs of TlyA strains.
Experimental procedures
Comparative analysis of TlyA sequences
Protein BLAST searches were carried out in the NCBI Genomic Blast database (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) using the M. tuberculosis TlyA as the query against the data available in April 2012. The cut-off was set at 40% identity, which has been reported to give clear discrimination between different protein families and domains (Gandhimathi et al., 2012). Data were processed using Cluster W (Larkin et al., 2007).
Bacterial strains, growth conditions and MIC determination
E. coli strain DH1 was grown at 37 °C in Lauria-Bertani (LB) medium containing ampicillin at 100 μg ml−1 to maintain plasmids, and IPTG at 1 mM for full induction of the lac promoter (Sambrook and Russell, 2001). Minimum inhibitory concentrations (MICs) of capreomycin and viomycin were determined as previously described (Johansen et al., 2006). Briefly, overnight cultures of E. coli were diluted to 10−5-fold and plated on to LB agar containing capreomycin or viomycin with the concentrations increasing in two-fold steps; measurements were made a minimum of three times. The MICs were defined as the lowest concentration at which no growth was observed after incubation for 48 h. The tlyA null-mutants M. smegmatis strain P2U (EZ::TN-kan tlyA nt.163) (Maus et al., 2005b) and T. thermophilus HB8 strain TM469 (ΔtlyA::htk1) (Monshupanee et al., 2008) were grown in Middle-brook 7 H9 medium (Remel and Difco) containing 0.2% (v/v) glycerol at 37 °C and in ATCC medium 1598 (Alfredsson et al., 1985) at 72 °C, respectively.
Cloning and mutagenesis of tlyA
Orthologs of tlyA were amplified from the genomic DNA of M. tuberculosis H37Rv, T. thermophilus HB8, Streptomyces coelicolor A3, Serpulina (Brachyspira) hyodysenteriae and Geobacillus stearothermophilus using complementary PCR primers (Table S1). Specific mutations were introduced into M. smegmatis tlyAII by PCR using mutagenic primers, and tlyA genes were inserted into the NdeI and BglII sites in plasmids pSJ101 or pLJ102 bringing them under control of the lac promoter (Johansen et al., 2006). Attention was paid to codon usage at the N-terminus that could affect expression (Gonzalez de Valdivia and Isaksson, 2004), such that the third codon in the wild-type M. smegmatis tlyAII sequence (CGG for arginine) was suitably matched in growth competition assays. E. coli strain DH1 was transformed with the recombinant plasmids (Table S2).
Protein expression and purification
E. coli cells were grown to an OD600 of 0.6 and TlyA expression was induced by adding IPTG to 1 mM. The cultures were grown for a further 4 h before harvesting the cells by centrifugation, cell pellets were resuspended and washing in 20 mM Tris-HCl, pH 7.5, 10 mM MgOAc2, 100 mM NH4Cl and then lyzed by sonication (Hansen et al., 2001). The TlyA proteins possessed a C-terminal histidine-tag were purified using Ni-NTA affinity agarose (Qiagen). The proteins were dialyzed in 20 mM Tris-HCl, pH 7.5, 10 mM MgOAc2, 250 mM NH4Cl, 6 mM 2-mercaptoethanol, 10% glycerol, using Amicon Ultra-4 Centrifugal Filter Devices (Milipore), and were analyzed by SDS gel electrophoresis.
Preparation of ribosomes and rRNAs
E. coli, M. smegmatis and T. thermophilus cells were harvested at an OD600 of 0.6 for preparation of ribosomal particles (Johansen et al., 2006, Monshupanee et al., 2008). Tight couple ribosomes, 50S and 30S subunits were isolated by sucrose gradient centrifugation, and fractions were pelleted by ultracentrifugation (Monshupanee et al., 2008). 16S and 23S rRNAs were isolated from their respective subunits by phenol extraction (Hansen et al., 2001).
In vitro methylation
In vitro methylation assays were carried out on 10 pmol substrate in 100 μl of 20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 100 mM NH4Cl, 6 mM 2-mercaptoethanol and 10% glycerol. RNA substrates were pre-incubated at 50 °C for 10 minutes and cooled to room temperature. Reactions were started by addition of 0.1 to 10 pmol recombinant TlyA enzyme and SAM to a final concentration of 1 mM. Reactions on E. coli and M. smegmatis ribosomal substrates were at 37 °C for 45 min, whereas T. thermophilus substrates were incubated at 65 °C. Reactions were terminated by extraction with phenol/chloroform; rRNAs were recovered by ethanol precipitation prior to analysis by primer extension and mass spectrometry.
Primer extension
DNA primers were 5′-labelled by polynucleotide kinase using 32P-γ-ATP (New England biolabs) and were hybridized to complementary rRNA regions (Stern et al., 1988). The 2′-O-ribose methylation on 16S rRNA nucleotide 1409 and 23S rRNA nucleotide 1920 were analyzed by varying dGTP concentrations (1 μM, 0.5 μM and 0.1 μM) in the presence of 40 μM (dATP, dCTP and ddTTP) or 40 μM (dATP, ddCTP and dTTP) and extending with AMV reverse transcriptase (Finnzymes) (Maden et al., 1995). Extension products were run on polyacrylamide-urea gels and visualized by phosphorimaging (Typhoon, Amersham Biosciences). Measuring 2′-O-methylation in this manner is non-quantitative, and therefore methylation levels were simply tabulated as ‘++’ (full), ‘+’ (partial) or ‘−’ (no methylation) after standardizing against rRNAs that had previously been defined as being fully methylated or without methylation (Johansen et al., 2006).
MALDI-TOF mass spectrometry
The fragments consisting of 16S rRNA nucleotides 1377 to 1435 and 23S rRNA nucleotides 1889 to 1948 were isolated by hybridization to complementary oligodeoxynucleotides (Table S1) (Johansen et al., 2006). The isolated RNA sequences were digested with RNase T1 and cyclic phosphates were hydrolyzed with HCl. The RNA digested products were analyzed on a PerSeptive Voyager-DE STR MALDI mass spectrometer (Applied Biosystems) in positive ion mode (Andersen et al., 2004, Kirpekar et al., 2000). Spectra were calibrated using m/z software (Proteometrics Inc).
Competition growth assay and florescence PCR
Approximate equal numbers of two E. coli strains habouring different tlyA variants were mixed in LB medium with ampicillin, IPTG and capreomycin at 0 to 32 μg ml−1. After 24 h incubation (approximate eleven generations), the stationary phase cells were diluted 2000-fold and the cycle was repeated. Aliquots of cells were harvested by centrifugation at the end of each cycle; the cell pellets were resuspended in 10 vol of deionized water, boiled for 2 min, and centrifuged. The mixture of plasmid-encoded tlyA genes in the supernatant was assessed by Taq DNA Polymerase (Promega) PCR using one non-labeled reverse primer and two forward primers that were 5′-fluorescence-labeled with either cyanine 3 (Cy3, green) or cyanine 5 (Cy5, red) and complementary to a specific tlyA variant (Table S1). Reactions were carried out such that the amount of PCR product was proportional to amount of the original template: 50 μl PCR reactions with 2 μl of the supernatant template, 1.25 U Taq DNA Polymerase (goTaq, Promega), 2.5 nmol of each dNTP, 50 pmol of each primer. PCR was over 20–22 cycles with denaturation at 98 °C for 2 min in the initial step and subsequently for 15 s; annealing at 65 °C for 30 s; and extension at 72 °C for 20 s. The PCR products of 170 base pairs were run on 3% agarose gels and were detected using an Amersham Biosciences Typhoon calibrated for Cy3 at 512 nm and Cy5 at 625 nm. The two fluorescence scans were superimposed using the Image Quant (Amersham Biosciences) program to reveal the relative proportions of tlyA variants in each sample.
Supplementary Material
Acknowledgments
We thank Märit Pringle for providing S. hyodysenteriae DNA, Gilles van Wezel for providing the S. coelicolor strain and Claus Asger Lykkebo for the graphics in Figs. 1 and S3. We gratefully acknowledge support from the Danish Research Agency (FNU-rammebevillinger 09-064292/10-084554) (S.D.), the Nucleic Acid Center of the Danish Grundforskningsfond (S.D.), the US National Institutes of Health (GM19756) (A.D.) and the A1B1 project of the Faculty of Science, Chulalongkorn University (T.M.). This manuscript is dedicated to the memory of a dear friend and colleague, the late Shanna K. Johansen.
Abbreviations used
- SAM
S-adenosyl-methionine
- MALDI-MS
matrix assisted laser desorption/ionization mass spectrometry
- MIC
minimum inhibitory concentration
References
- Alfredsson G, Baldursson S, Krjstjansson JK. Nutritional diversity among Thermus spp. isolated from Icelandic hot spring. Syst Appl Microbiol. 1985;6:308–311. [Google Scholar]
- Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011;66:1417–1430. doi: 10.1093/jac/dkr173. [DOI] [PubMed] [Google Scholar]
- Andersen TE, Porse BT, Kirpekar F. A novel partial modification at C2501 in Escherichia coli 23S ribosomal RNA. RNA. 2004;10:907–913. doi: 10.1261/rna.5259404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. Novel predicted RNA-binding domains associated with the translation machinery. J Mol Evol. 1999;48:291–302. doi: 10.1007/pl00006472. [DOI] [PubMed] [Google Scholar]
- Arenas NE, Salazar LM, Soto CY, Vizcaino C, Patarroyo ME, Patarroyo MA, Gomez A. Molecular modeling and in silico characterization of Mycobacterium tuberculosis TlyA: possible misannotation of this tubercle bacilli-hemolysin. BMC Struct Biol. 2011;11:16. doi: 10.1186/1472-6807-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkley D, Innis CA, Blaha G, Steitz TA. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc Natl Acad Sci USA. 2010;107:17158–17163. doi: 10.1073/pnas.1008685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkley D, Johnson F, Steitz TA. The antibiotic thermorubin inhibits protein synthesis by binding to inter-subunit bridge B2a of the ribosome. J Mol Biol. 2012;416:571–578. doi: 10.1016/j.jmb.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CS, Lamichhane TN, Mahto SK. Expanding the nucleotide repertoire of the ribosome with post-transcriptional modifications. ACS Chem Biol. 2007;2:610–619. doi: 10.1021/cb7001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn GL, Savic M, Macmaster R. Antibiotic resistance in bacteria caused through modification of nucleosides in 16S ribosomal RNA. In: Grosjean H, editor. DNA and RNA Modification Enzymes: Structure, mechanism, function and evolution. Austin, Texas: Landes BioScience; 2009. pp. 525–536. [Google Scholar]
- Demirci H, Murphy F, Belardinelli R, Kelley AC, Ramakrishnan V, Gregory ST, Dahlberg AE, Jogl G. Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function. RNA. 2010;16:2319–2324. doi: 10.1261/rna.2357210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle JA, Wang L, Feldman MB, Pulk A, Chen VB, Kapral GJ, Noeske J, Richardson JS, Blanchard SC, Cate JH. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science. 2011;332:981–984. doi: 10.1126/science.1202692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolenko DN, Spiegel PC, Majumdar ZK, Hickerson RP, Clegg RM, Noller HF. The antibiotic viomycin traps the ribosome in an intermediate state of translocation. Nat Struct Mol Biol. 2007;14:493–497. doi: 10.1038/nsmb1243. [DOI] [PubMed] [Google Scholar]
- Feder M, Pas J, Wyrwicz LS, Bujnicki JM. Molecular phylogenetics of the RrmJ/fibrillarin superfamily of ribose 2′-O-methyltransferases. Gene. 2003;302:129–138. doi: 10.1016/s0378-1119(02)01097-1. [DOI] [PubMed] [Google Scholar]
- Gandhimathi A, Nair AG, Sowdhamini R. PASS2 version 4: an update to the database of structure-based sequence alignments of structural domain superfamilies. Nucleic Acids Res. 2012;40:D531–534. doi: 10.1093/nar/gkr1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de Valdivia EI, Isaksson LA. A codon window in mRNA downstream of the initiation codon where NGG codons give strongly reduced gene expression in Escherichia coli. Nucleic Acids Res. 2004;32:5198–5205. doi: 10.1093/nar/gkh857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H. DNA and RNA modification enzymes: Structure, mechanism, function and evolution. Austin Texas: Landes Biosciences; 2009. [Google Scholar]
- Guymon R, Pomerantz SC, Crain PF, McCloskey JA. Influence of phylogeny on posttranscriptional modification of rRNA in thermophilic prokaryotes: the complete modification map of 16S rRNA of Thermus thermophilus. Biochemistry. 2006;45:4888–4899. doi: 10.1021/bi052579p. [DOI] [PubMed] [Google Scholar]
- Hansen LH, Kirpekar F, Douthwaite S. Recognition of nucleotide G745 in 23S ribosomal RNA by the RrmA methyltransferase. J Mol Biol. 2001;310:1001–1010. doi: 10.1006/jmbi.2001.4836. [DOI] [PubMed] [Google Scholar]
- Helser TL, Davies JE, Dahlberg JE. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971;233:12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Johansen SK, Maus CE, Plikaytis BB, Douthwaite S. Capreomycin binds across the ribosomal subunit interface using tlyA-encoded 2′-O-methylations in 16S and 23S rRNAs. Mol Cell. 2006;23:173–182. doi: 10.1016/j.molcel.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Kirpekar F, Douthwaite S, Roepstorff P. Mapping posttranscriptional modifications in 5S ribosomal RNA by MALDI mass spectrometry. RNA. 2000;6:296–306. doi: 10.1017/s1355838200992148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B. Conserved ribosomal RNA modifications and their putative roles in ribosome biogenesis and translation. In: Grosjean H, editor. Fine-tuning of RNA Functions by Modification and Editing. New York: Springer Verlag; 2005. pp. 263–284. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Long KS, Vester B. Antibiotics resistance in bacteria caused by modified nucleosides in 23S ribosomal RNA. In: Grosjean H, editor. DNA and RNA Modification Enzymes: Structure, mechanism, function and evolution. Austin, Texas: Landes BioScience; 2009. pp. 537–549. [Google Scholar]
- Maden BE, Corbett ME, Heeney PA, Pugh K, Ajuh PM. Classical and novel approaches to the detection and localization of the numerous modified nucleotides in eukaryotic ribosomal RNA. Biochimie. 1995;77:22–29. doi: 10.1016/0300-9084(96)88100-4. [DOI] [PubMed] [Google Scholar]
- Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Current Opinion in Structural Biology. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- Maus CE, Plikaytis BB, Shinnick TM. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005a;49:3192–3197. doi: 10.1128/AAC.49.8.3192-3197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus CE, Plikaytis BB, Shinnick TM. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005b;49:571–577. doi: 10.1128/AAC.49.2.571-577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker KP, Fujimori DG. The chemistry of peptidyltransferase center-targeted antibiotics: enzymatic resistance and approaches to countering resistance. ACS Chem Biol. 2012;7:64–72. doi: 10.1021/cb200418f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel-Jorgensen J, Jensen SS, Rasmussen A, Poehlsgaard J, Iversen JJ, Kirpekar F. Modifications in Thermus thermophilus 23S ribosomal RNA are centered in regions of RNA-RNA contact. J Biol Chem. 2006;281:22108–22117. doi: 10.1074/jbc.M600377200. [DOI] [PubMed] [Google Scholar]
- Modolell J, Vazquez D. The inhibition of ribosomal translocation by viomycin. Eur J Biochem. 1977;81:491–497. doi: 10.1111/j.1432-1033.1977.tb11974.x. [DOI] [PubMed] [Google Scholar]
- Monshupanee T, Gregory ST, Douthwaite S, Chungjatupornchai W, Dahlberg AE. Mutations in conserved helix 69 of 23S rRNA of Thermus thermophilus that affect capreomycin resistance but not posttranscriptional modifications. J Bacteriol. 2008;190:7754–7761. doi: 10.1128/JB.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Tamaru A, Nakajima C, Nishimura K, Tanaka Y, Tokuyama S, Suzuki Y, Ochi K. Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria. Mol Microbiol. 2007;63:1096–1106. doi: 10.1111/j.1365-2958.2006.05585.x. [DOI] [PubMed] [Google Scholar]
- Poehlsgaard J, Douthwaite S. The bacterial ribosome as a target for antibiotics. Nat Rev Microbiol. 2005;3:870–881. doi: 10.1038/nrmicro1265. [DOI] [PubMed] [Google Scholar]
- Purta E, O’Connor M, Bujnicki JM, Douthwaite S. YgdE is the 2′-O-ribose methyltransferase RlmM specific for nucleotide C2498 in bacterial 23S rRNA. Mol Microbiol. 2009;72:1147–1158. doi: 10.1111/j.1365-2958.2009.06709.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, New York: 2001. [Google Scholar]
- Schlünzen F, Harms JM, Franceschi F, Hansen HA, Bartels H, Zarivach R, Yonath A. Structural basis for the antibiotic activity of ketolides and azalides. Structure (Camb) 2003;11:329–338. doi: 10.1016/s0969-2126(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Schlünzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FV, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Stanley RE, Blaha G, Grodzicki RL, Strickler MD, Steitz TA. The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome. Nat Struct Mol Biol. 2010;17:289–293. doi: 10.1038/nsmb.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Moazed D, Noller HF. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- Tu D, Blaha G, Moore PB, Steitz TA. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell. 2005;121:257–270. doi: 10.1016/j.cell.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Wimberly BT, Brodersen DE, Clemons WMJ, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- Woodson SA. RNA folding and ribosome assembly. Curr Opin Chem Biol. 2008;12:667–673. doi: 10.1016/j.cbpa.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Mizugichi Y, Nierhaus KH, Wittmann HG. Resistance to viomycin conferred by RNA of either ribosomal subunit. Nature. 1978;275:460–461. doi: 10.1038/275460a0. [DOI] [PubMed] [Google Scholar]
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 Å resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1320–1330. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.