Abstract
Protein kinase C (PKC) is the receptor for tumor promoting phorbol esters, which are potent activators of conventional and novel PKCs, but persistent treatment with phorbol esters leads to downregulation of these PKCs. However, PKCη, a novel PKC isozyme, resists downregulation by tumor-promoting phorbol esters, but little is known about how PKCη level is regulated. Phosphorylation and dephosphorylation play an important role in regulating activity and stability of PKCs. In the present study, we have investigated the molecular mechanism of PKCη regulation. Several PKC activators, including phorbol 12, 13-dibutyrate, 12-O-tetradecanoylphorbol-13-acetate and indolactam V caused upregulation of PKCη whereas the general PKC inhibitor Gö 6983, but not the conventional PKC inhibitor Gö 6976 led to the downregulation of PKCη. Upregulation of PKCη was associated with an increase in phosphorylation of PKCη. Silencing of phosphoinositide-dependent kinase-1, which phosphorylates PKCη at the activation loop, failed to prevent PKC activator-induced upregulation of PKCη. Knockdown of PKCε but not PKCα inhibited PKC activator-induced upregulation of PKCη. Thus, our results suggest that the regulation of PKCη is unique and PKCε is required for the PKC activator-induced upregulation of PKCη.
Keywords: PKCη, PKCε, tumor promoter, upregulation, phosphorylation
1. Introduction
Protein kinase C, a family of phospholipid-dependent serine/threonine kinases, plays a critical role in signal transduction and cell regulation [1,2]. On the basis of their structural features, the PKC family is categorized into three groups, conventional (α, βI, βII, and γ), novel (δ, ε, η, θ) and atypical (ζ, λ/ι). While conventional PKCs require Ca2+ and diacylglycerol (DAG) for their activities, novel PKCs are Ca2+-insensitive but DAG-dependent whereas atypical PKCs are insensitive to both Ca2+ and DAG [1]. PKC isozymes differ in biochemical properties, tissue-specific distribution and intracellular localization. Most cells express multiple PKC isozymes and they exhibit overlapping as well as distinct functions [3].
PKC serves as the receptor for tumor-promoting phorbol esters, which are potent activators of conventional and novel PKCs, and can substitute for the physiological activator DAG [3,4]. Sustained stimulation of PKCs by phorbol esters, such as TPA, has implicated the PKC isozymes in tumor promotion [2,5]. Prolonged treatment with tumor-promoting phorbol esters eventually leads to the downregulation of the phorbol ester-sensitive PKCs [6]. Both activation and downregulation of PKCs have been implicated in regulating cellular functions.
PKCs are not only subject to regulation by cofactors, but also via phosphorylation [3]. PKCs are phosphorylated at the conserved residues in the activation loop, turn motif and hydrophobic motif. The phosphorylation of PKCs primes them for activation and regulates their stability and subcellular localization [3,5,7,8]. PKCs are regulated by both autophosphorylation [9] and transphosphorylation [10]. It is generally believed that the priming phosphorylation of PKC occurs at the activation loop by phosphoinositide-dependent kinase-1 (PDK1) and is followed by autophosphorylation at the turn and the hydrophobic motifs [6]. Recent studies, however, suggest that PKCs may also be transphosphorylated by other members of the PKC family [3,6,7,11]. For example, PKCδ has been shown to be transphosphorylated by PKCε and vice versa [12]. This cross-regulation of PKCs may be an important way to integrate signals by various PKC isozymes.
PKCη is a member of the novel PKC isozymes that regulates cell proliferation, differentiation, secretion and apoptosis [13,14,15,16,17]. It is primarily expressed in epithelial cells and shares highest homology with PKCε [18]. PKCη is upregulated in breast cancer tissues [19] and overexpression of PKCη has been associated with resistance to chemotherapeutic agents [17,20,21,22,23,24]. Although PKCs have been implicated in tumor promotion, PKCη is the only phorbol ester-sensitive PKC isozyme that resists downregulation upon prolonged treatment with phorbol esters [20,25,26]. Little is known about the unique regulation of PKCη. In the present study, we have investigated the mechanism by which PKCη level is regulated. Our results indicate that in contrast to conventional and novel PKCs, which undergo downregulation following persistent treatment with PKC activators, PKCη is upregulated in response to PKC activators and is downregulated upon treatment with PKC inhibitors. We demonstrate for the first time that the PKC activator-induced upregulation of PKCη is regulated by PKCε, another member of the novel PKC family.
2. Materials and Methods
2.1. Materials
PDBu and TPA were purchased from Alexis Biochemicals (San Diego, CA). ILV was obtained from LC Laboratories (Woburn, MA) and Sigma (St. Louis, MO). Gö 6983 and Gö 6976 were purchased from Calbiochem (San Diego, CA). Polyclonal antibodies to PKCη, PKCδ and PKCε were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibody against PDK1 was purchased from Cell Signaling Technology, Inc. (Danvers, MA). Monoclonal antibody to PKCα was obtained from Upstate Biotechnology (Lake Placid, NY) and monoclonal antibody to PKCι was from BD Transduction Laboratories (San Jose, CA). Monoclonal antibody against actin was obtained from Sigma (St. Louis, MO). Horseradish-peroxidase-conjugated donkey anti-rabbit and goat anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). [32P]Orthophosphate was purchased from PerkinElmer, Inc. (Waltham, MA). Poly(vinylidenedifluoride) membrane was obtained from Millipore (Bedford, MA). Enhanced chemiluminescence detection kit was purchased from Amersham (Arlington Heights, IL).
2.2. Cell culture
Breast cancer cells were maintained in RPMI medium supplemented with 10% fetal bovine serum and 2 mM glutamine. Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco's modified minimal essential medium supplemented with 10% fetal bovine serum and 2 mM glutamine. Cells were kept in a humidified incubator at 37°C with 95% air and 5% CO2.
2.3. Transfection
Control non-targeting siRNA or SMARTpool siRNA against PKC isozymes, and PDK1 were introduced into MCF-7 or T47D cells using Lipofectamine 2000 or Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) and manufacturer's protocol. 48 h following siRNA transfection, cells were treated as indicated in the text and processed for Western blot analysis.
2.4. Reverse Transcriptase PCR
MCF-7 cells were treated with or without PDBu, ILV or Gö 6983 for 16 h. Total RNA was extracted using TRI Reagent from Molecular Research Center, Inc. (Cincinnati, OH). cDNA was synthesized using random primers and Improm II reverse transcriptase from Promega (Madison, WI). PCR amplification of cDNA was performed using Promega PCR Master Mix (Madison, WI), PKCη and β-actin primers. The sequences of forward and reverse PKCη primers were 5'-ATGCGGTGGAACTTGCCA-3' and 5'-CGTGACCACAGAGCATCTCATAGA-3' respectively. The sequences of the forward and reverse β-actin primers were 5'-ACCCAGCACAATGAAGATCA-3' and 5'-GCGCAAGTTAGGTTTTGTCA-3'. After PCR cycling, a 750 bp product for PKCη and 800-bp product for β-actin was detected by gel electrophoresis.
2.5. Immunoblot Analysis
Cells were lysed in extraction buffer containing 1 mM DTT, protease inhibitors and phosphatase inhibitors. Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on PVDF membranes. Western blot analysis was performed as described before [17].
2.6. Metabolic labeling
HEK293T cells were transiently transfected with either pcDNA3 or vector containing PKCη construct and radiolabeled with [32P]orthophosphate. Cells were treated with or without PDBu and immunoprecipated with either rabbit IgG or anti-PKCη antibody. Immunocomplexes were processed as described previously [27] and subjected to SDS-PAGE and autoradiography.
3. Results
3.1. Effect of PKC activators and inhibitors on PKCη levels
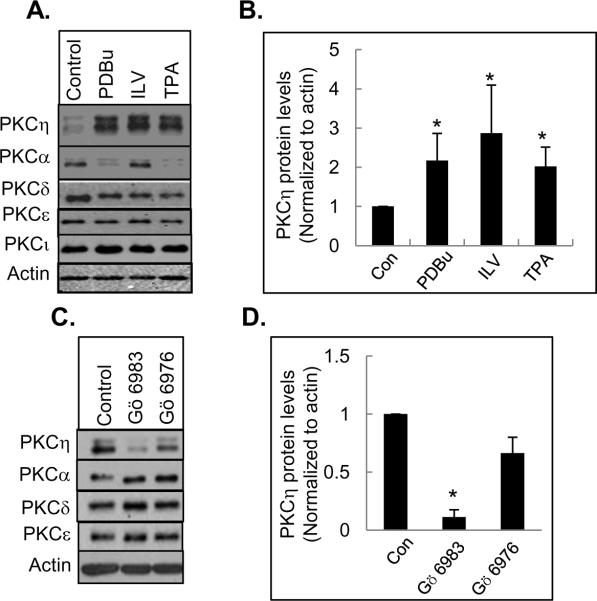
We have previously demonstrated that persistent treatment with phorbol 12, 13-dibutyrate (PDBu) caused upregulation of PKCη in MCF-7 breast cancer cells [20]. In the present study, we compared the effect of several structurally and functionally distinct PKC activators on PKCη level. While PDBu and TPA belong to the same class of compounds, indolactam V (ILV) is structurally distinct from phorbol esters. All three PKC activators caused substantial upregulation of PKCη (Fig. 1A and 1B). Based on the densitometric quantification of several independent experiments, PKC activators caused a significant increase in PKCη level (Fig. 1B). PKCη appeared as a doublet in the Western blot since it contains two major transcription initiation sites [29]. Prolonged treatment with PDBu and TPA caused downregulation of conventional PKCα and novel PKCδ although PKCε was less susceptible to PKC activator-induced downregulation (Fig. 1A). The level of phorbol ester-insensitive atypical PKCι remained unaltered, as expected (Fig. 1A). Consistent with our earlier reports, ILV had little effect on the downregulation of PKCα [28]. Thus, the regulation of PKCη is unique in comparison to other conventional and novel PKCs.
Fig. 1.
Effects of PKC activators and inhibitors on PKCη level. MCF-7 cells were treated with 1 μM PDBu, 10 μM ILV and 100 nM TPA for 15 h. (A) Western blot analysis was performed with total cell extract and probed with the indicated antibodies. Actin was used as a loading control. (B) Densitometric quantification of PKCη protein level from 3 separate experiments corrected for loading. Data represents the mean +/− s.e.m. The asterisk (*) indicates significant difference from control (P<0.05) using paired Student's t-test. (C) MCF-7 cells were treated with 1 μM Gö 6983 for 15 h. Total cell lysates were subjected to SDS-PAGE and Western blot analysis was performed using the indicated antibodies. (D) Densitometric quantification of PKCη protein expression from 3 separate experiments corrected for loading. Data represents the mean +/− s.e.m. The asterisk (*) indicates significant difference from the control (P<0.05) using paired Student's t-test.
Since PKC activators led to PKCη upregulation, we examined whether PKC inhibitors would induce downregulation of PKCη. We compared the effects of the general PKC inhibitor Gö 6983 and conventional PKC inhibitor Gö 6976. Gö 6983 but not Gö 6976 caused substantial downregulation of PKCη (Fig. 1C and 1D). The levels of PKC-α, -δ and -ε were not decreased by Gö 6983 treatment (Fig. 1C). The general PKC inhibitor bisindolylmaleimide also induced selective downregulation of PKCη (data not shown). Since atypical PKCs are phorbol ester-insensitive, these results suggest that the level of PKCη may be regulated by novel PKCs.
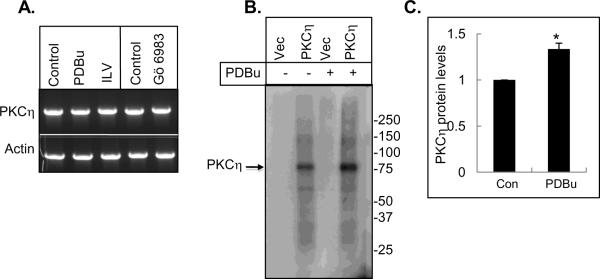
To determine if PKC activators and inhibitors alter PKCη expression at the mRNA level, we treated MCF-7 cells with PDBu, ILV or Gö 6983 and examined the mRNA expression by reverse-transcriptase PCR. As shown in Fig. 2A, the treatment of MCF-7 cells with PKC activators and inhibitors did not alter the mRNA expression of PKCη. Taken together, these results suggest that PKCη level is altered at the post-transcriptional level following treatment with PKC activators and inhibitors.
Fig. 2.
Effects of PKC modulators on PKCη mRNA expression and phosphorylation. (A) MCF-7 cells were treated with 1 μM PDBu, 10 μM ILV or 1 μM Gö 6983 for 16 h. Total RNA was extracted and cDNA was synthesized by reverse transcriptase reaction. PKCη and β-actin cDNA were amplified by PCR and electrophoresed. Results are representative of at least 2 independent experiments. (B) HEK293T cells expressing empty vector or vector containing PKCη were radiolabeled with [32P]orthophosphate and immunoprecipitated with PKCη following treatment with or without PDBu. The arrow indicates PKCη. (C) Densitometric quantification of PKCη protein level from 3 separate experiments corrected for loading. Data represents the mean +/− s.e.m. The asterisk (*) indicates significant increase with PDBu treatment (P<0.05) using paired Student's t-test.
3.2. Effect of PKC activator and inhibitor on PKCη phosphorylation
Since persistent treatment with PKC activators cause activation of PKCs followed by dephosphorylation and downregulation of PKCs, we examined if upregulation of PKCη by PKC activators was associated with an increase in PKCη phosphorylation. We introduced PKCη in HEK293T cells, labeled with [32P]orthophosphate and immunoprecipitated PKCη following treatment with or without PDBu. We did not detect a phosphorylated band corresponding to PKCη in vector-transfected HEK293T cells (Fig. 2B). PKCη was constitutively phosphorylated in HEK293T cells expressing wild-type PKCη and PDBu further increased the level of phospho-PKCη (Fig. 2B). The densitometric scanning from three separate experiments indicated a significant increase in the phosphorylation status of PKCη in response to PDBu (Fig. 2C). These results suggest that upregulation of PKCη is associated with an increase in PKCη phosphorylation.
3.3. Regulation of PKCη level by transphosphorylation
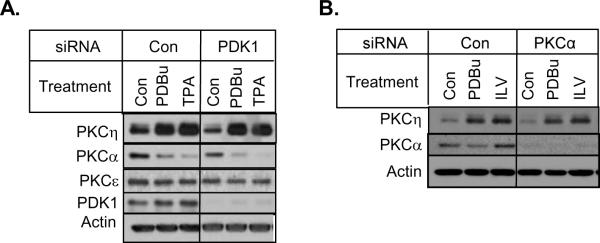
PDK1 is believed to phosphorylate the activation loop of AGC kinases, including PKC isozymes [7,8]. Recent evidence has also implicated PDK1 in phosphorylating PKCη at the activation loop [30]. We therefore examined whether PDK1 was involved in the activator-induced upregulation of PKCη. Fig. 3A shows that the silencing of PDK1 by siRNA decreased the basal level of PKCη but had little effect on the upregulation of PKCη by phorbol esters.
Fig 3.
Effect of PDK1 and PKCα knockdown on PKCη upregulation. (A) and (B) MCF-7 cells were transfected with the indicated siRNAs and then treated with or without 1μM PDBu, 100 nM TPA or 10 μM ILV for 16 h. Western blot analysis was performed with indicated antibodies. Actin was used as a loading control. Results are representative of 3 independent experiments.
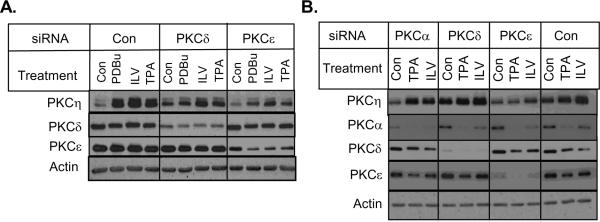
Since cross-regulation of PKC isozymes by other PKC family members has been suggested by several studies [11,12,31], we examined if the knockdown of a particular PKC isozyme affects PKCη level. As shown in Fig. 3B, the depletion of conventional PKCα had little effect on phorbol ester-induced upregulation of PKCη. While knockdown of PKCδ had a modest effect, the knockdown of novel PKCε substantially decreased the ability of PKC activators to enhance PKCη level in both MCF-7 (Fig. 4A) and T47D (Fig. 4B) cells. These results suggest that transphosphorylation of PKCη by novel PKCε may be responsible for PKCη upregulation.
Fig. 4.
Effects of novel PKC isozyme knockdown on PKCη upregulation. MCF-7 (A) and T47D (B) cells were transfected with indicated siRNAs. Cells were treated with or without 1 μM PDBu, 10 μM ILV or 100 nM TPA for 16 h. Western blot analyses were performed using indicated antibodies. Results are representative of 3 independent experiments.
4. Discussion
The results of our present study demonstrate that the regulation of PKCη is unique compared to other PKC isozymes. Although tumor-promoting phorbol esters are potent activators of conventional and novel PKCs [4], persistent treatment with phorbol esters leads to the downregulation of phorbol ester-sensitive PKCs causing termination of PKC signaling [32]. Downregulation of PKCs has important implications in regulating long-term cellular responses, such as cell proliferation, differentiation and tumor promotion [2,13]. We have shown that in contrast to other PKCs, prolonged treatment with PKC activators led to upregulation of PKCη whereas PKC-specific inhibitors triggered downregulation of PKCη. Furthermore, we made a novel observation that novel PKCs are involved in PKC activator-induced upregulation of PKCη.
It is generally believed that treatment with PKC activators leads to membrane translocation of PKCs followed by dephosphorylation [33]. The dephosphorylated PKCs are subject to downregulation by proteases [34]. However, fully phosphorylated PKCα was shown to be downregulated at the plasma membrane via the proteasome-mediated pathway [35]. In addition, the phosphorylated primed form of PKCε was downregulated by phorbol ester treatment independent of its intrinsic kinase activity [36]. It has been reported that active conformation of PKCη is necessary for its downregulation in baby hamster kidney (BHK) cells although TPA failed to downregulate PKCη in these cells [37]. Our results show that prolonged treatment with structurally distinct PKC activators, such as phorbol esters and ILV, caused an upregulation of PKCη in MCF-7 cells (Fig. 1A and 1B). The upregulation of PKCη by PKC activators was not unique to MCF-7 cells, and was observed in several cell types, including T47D, BT-20 and MCF-10CA1d cells (Fig. 4A, 4B and data not shown).
The activity, maturation, stability and localization of PKCs are regulated by phosphorylation and dephosphorylation events [7,8]. Our study suggests that PKCη level is regulated by phosphorylation. First, treatment with several PKC activators, such as TPA, PDBu and ILV induced upregulation of PKCη (Fig. 1A and 1B) but this upregulation was not associated with an increase in PKCη mRNA (Fig. 2A). Second, PKC-specific inhibitors Gö 6983 (Figs. 1C and 1D) and bisindolylmaleimide (data not shown) led to downregulation of PKCη. Third, upregulation of PKCη by PDBu was associated with an increase in PKCη phosphorylation (Fig. 2B and 2C).
Phosphorylation of PKCs is regulated by both autophosphorylation and transphosphorylation [3] and phosphorylation of PKCs at the activation loop is believed to prime them for activation [8]. Phosphoinositide-dependent kinase-1 (PDK1) has been shown to phosphorylate PKCs, at the activation loop and contributes to the stability of cPKCs and PKCε [32,33,38] PDK1 was also shown to phosphorylate PKCη at the activation loop [30]. However, knockdown of PDK1 did not prevent PKC activator-induced upregulation of PKCη (Fig. 3A).
PKCs can also undergo transphosphorylation by other members of the PKC family [3,12]. For example, PKCε rather than PDK1 was shown to phosphorylate PKCδ and PKCε at the activation loop whereas PKCδ induced autophosphorylation as well as transphosphorylation of PKCε at the hydrophobic motif [12]. We recently reported that depletion of PKCε enhanced PDBu-induced downregulation of PKCδ in HeLa cells [11]. Since the general PKC inhibitor Gö 6983 but not the conventional PKC inhibitor Gö 6976 induced PKCη downregulation (Fig. 1C and 1D) and atypical PKCs are phorbol ester insensitive, it is likely that PKCη is also regulated by novel PKC isozyme(s). Consistent with this notion, we found that depletion of cPKCα had little effect on PKC activator-induced upregulation of PKCη whereas knockdown of nPKCε attenuated PKCη upregulation (Fig. 4A and 4B).
The observation that PKCη is the only PKC isozyme upregulated by tumor-promoting phorbol esters suggests that PKCη may play an important role in tumorigenesis. Depending on the cellular context, PKCη may suppress tumorigenesis or promote malignant cell growth. For example, PKCη knockout mice were more susceptible to tumor promotion in two-stage skin carcinogenesis model [39]. In contrast, PKCη has also been implicated in breast cancer [19,20], glioblastoma [21], Hodgkin's lymphoma [40], lung cancer [22,41] and hepatocellular carcinoma [42]. This contrasting function of PKCη in different cell types is not unique to PKCη and has been noted with other novel PKCs, such as PKCδ [5] and PKCε [43]. PKCη is often overexpressed in breast cancer [19] and the level of PKCη is upregulated by estradiol in hormone-sensitive breast cancer cells [44]. Moreover, overexpression of PKCη confers resistance to chemotherapeutic drugs [17,20,21,22,23,24]. Thus, understanding the mechanism of PKCη upregulation has significant implications in cancer therapy.
Highlights
The regulation of PKCη is unique
PKCη is upregulated by PKC activators and downregulated by PKC inhibitors
PKC activator-induced upregulation of PKCη involves novel PKCε
Acknowledgements
This work was supported by the grant CA071727 from the National Cancer Institute. The authors wish to thank Ms Savitha Sridharan and Kirti Jain for critical reading of the manuscript.
The abbreviations used are
- DAG
diacylglycerol
- ILV
indolactam V
- PDBu
phorbol 12,13-dibutyrate
- PDK1
phosphoinositide-dependent protein kinase-1
- PKC
protein kinase C
- aPKC
atypical PKC
- cPKC
conventional PKC
- nPKC
novel PKC
- siRNA
short interfering RNA
- TPA
12-O-tetradecanoylphorbol 13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- [2].Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- [3].Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blumberg PM. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988;48:1–8. [PubMed] [Google Scholar]
- [5].Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. Scientific World Journal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Flint AJ, Paladini RD, Koshland DE., Jr. Autophosphorylation of protein kinase C at three separated regions of its primary sequence. Science. 1990;249:408–411. doi: 10.1126/science.2377895. [DOI] [PubMed] [Google Scholar]
- [10].Dutil EM, Keranen LM, DePaoli-Roach AA, Newton AC. In vivo regulation of protein kinase C by trans-phosphorylation followed by autophosphorylation. J Biol Chem. 1994;269:29359–29362. [PubMed] [Google Scholar]
- [11].Basu A, Sridharan S, Persaud S. Regulation of protein kinase C delta downregulation by protein kinase C epsilon and mammalian target of rapamycin complex 2. Cell Signal. 2009;21:1680–1685. doi: 10.1016/j.cellsig.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem. 2003;278:14555–14564. doi: 10.1074/jbc.M212644200. [DOI] [PubMed] [Google Scholar]
- [13].Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993;59:257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- [14].Kashiwagi M, Ohba M, Chida K, Kuroki T. Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J Biochem. 2002;132:853–857. doi: 10.1093/oxfordjournals.jbchem.a003297. [DOI] [PubMed] [Google Scholar]
- [15].Fima E, Shtutman M, Libros P, Missel A, Shahaf G, Kahana G, Livneh E. PKCeta enhances cell cycle progression, the expression of G1 cyclins and p21 in MCF-7 cells. Oncogene. 2001;20:6794–6804. doi: 10.1038/sj.onc.1204885. [DOI] [PubMed] [Google Scholar]
- [16].Hussaini IM, Karns LR, Vinton G, Carpenter JE, Redpath GT, Sando JJ, VandenBerg SR. Phorbol 12-myristate 13-acetate induces protein kinase ceta-specific proliferative response in astrocytic tumor cells. J Biol Chem. 2000;275:22348–22354. doi: 10.1074/jbc.M003203200. [DOI] [PubMed] [Google Scholar]
- [17].Akkaraju GR, Basu A. Overexpression of protein kinase C-eta attenuates caspase activation and tumor necrosis factor-alpha-induced cell death. Biochem Biophys Res Commun. 2000;279:103–107. doi: 10.1006/bbrc.2000.3903. [DOI] [PubMed] [Google Scholar]
- [18].Hashimoto Y, Osada S, Ohno S, Kuroki T. A Ca(2+)-independent protein kinase C, nPKC eta: its structure, distribution and possible function. Tohoku J Exp Med. 1992;168:275–278. doi: 10.1620/tjem.168.275. [DOI] [PubMed] [Google Scholar]
- [19].Masso-Welch PA, Winston JS, Edge S, Darcy KM, Asch H, Vaughan MM, Ip MM. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res Treat. 2001;68:211–223. doi: 10.1023/a:1012265703669. [DOI] [PubMed] [Google Scholar]
- [20].Basu A. The involvement of novel protein kinase C isozymes in influencing sensitivity of breast cancer MCF-7 cells to tumor necrosis factor-alpha. Mol Pharmacol. 1998;53:105–111. doi: 10.1124/mol.53.1.105. [DOI] [PubMed] [Google Scholar]
- [21].Hussaini IM, Carpenter JE, Redpath GT, Sando JJ, Shaffrey ME, Vandenberg SR. Protein kinase C-eta regulates resistance to UV- and gamma-irradiation-induced apoptosis in glioblastoma cells by preventing caspase-9 activation. Neuro Oncol. 2002;4:9–21. doi: 10.1093/neuonc/4.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sonnemann J, Gekeler V, Ahlbrecht K, Brischwein K, Liu C, Bader P, Muller C, Niethammer D, Beck JF. Down-regulation of protein kinase Ceta by antisense oligonucleotides sensitises A549 lung cancer cells to vincristine and paclitaxel. Cancer Lett. 2004;209:177–185. doi: 10.1016/j.canlet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [23].Raveh-Amit H, Hai N, Rotem-Dai N, Shahaf G, Gopas J, Livneh E. Protein kinase Ceta activates NF-kappaB in response to camptothecin-induced DNA damage. Biochemical and biophysical research communications. 2011;412:313–317. doi: 10.1016/j.bbrc.2011.07.090. [DOI] [PubMed] [Google Scholar]
- [24].Sonnemann J, Gekeler V, Sagrauske A, Muller C, Hofmann HP, Beck JF. Down-regulation of protein kinase Ceta potentiates the cytotoxic effects of exogenous tumor necrosis factor-related apoptosis-inducing ligand in PC-3 prostate cancer cells. Mol Cancer Ther. 2004;3:773–781. [PubMed] [Google Scholar]
- [25].Chen CC, Wang JK, Chen WC. TPA induces translocation but not down-regulation of new PKC isoform eta in macrophages, MDCK cells and astrocytes. FEBS Lett. 1997;412:30–34. doi: 10.1016/s0014-5793(97)00697-2. [DOI] [PubMed] [Google Scholar]
- [26].Resnick MS, Luo X, Vinton EG, Sando JJ. Selective up-regulation of protein kinase C eta in phorbol ester-sensitive versus -resistant EL4 mouse thymoma cells. Cancer Res. 1997;57:2209–2215. [PubMed] [Google Scholar]
- [27].Lu D, Huang J, Basu A. Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem. 2006;281:22799–22807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- [28].Basu A, Kozikowski AP, Lazo JS. Structural requirements of lyngbyatoxin A for activation and downregulation of protein kinase C. Biochemistry. 1992;31:3824–3830. doi: 10.1021/bi00130a013. [DOI] [PubMed] [Google Scholar]
- [29].Bacher N, Zisman Y, Berent E, Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Molecular and cellular biology. 1991;11:126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lachmann S, Bar S, Rommelaere J, Nuesch JP. Parvovirus interference with intracellular signalling: mechanism of PKCeta activation in MVM-infected A9 fibroblasts. Cell Microbiol. 2008;10:755–769. doi: 10.1111/j.1462-5822.2007.01082.x. [DOI] [PubMed] [Google Scholar]
- [31].Ziegler WH, Parekh DB, Le Good JA, Whelan RD, Kelly JJ, Frech M, Hemmings BA, Parker PJ. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr Biol. 1999;9:522–529. doi: 10.1016/s0960-9822(99)80236-x. [DOI] [PubMed] [Google Scholar]
- [32].Hansra G, Garcia-Paramio P, Prevostel C, Whelan RD, Bornancin F, Parker PJ. Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem J. 1999;342(Pt 2):337–344. [PMC free article] [PubMed] [Google Scholar]
- [33].Lee HW, Smith L, Pettit GR, Smith JB. Bryostatin 1 and phorbol ester down-modulate protein kinase C-alpha and -epsilon via the ubiquitin/proteasome pathway in human fibroblasts. Mol Pharmacol. 1997;51:439–447. [PubMed] [Google Scholar]
- [34].Prevostel C, Alice V, Joubert D, Parker PJ. Protein kinase C(alpha) actively downregulates through caveolae-dependent traffic to an endosomal compartment. J Cell Sci. 2000;113(Pt 14):2575–2584. doi: 10.1242/jcs.113.14.2575. [DOI] [PubMed] [Google Scholar]
- [35].Leontieva OV, Black JD. Identification of two distinct pathways of protein kinase Calpha down-regulation in intestinal epithelial cells. J Biol Chem. 2004;279:5788–5801. doi: 10.1074/jbc.M308375200. [DOI] [PubMed] [Google Scholar]
- [36].Cameron AJ, Escribano C, Saurin AT, Kostelecky B, Parker PJ. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat Struct Mol Biol. 2009;16:624–630. doi: 10.1038/nsmb.1606. [DOI] [PubMed] [Google Scholar]
- [37].Kang BS, French OG, Sando JJ, Hahn CS. Activation-dependent degradation of protein kinase C eta. Oncogene. 2000;19:4263–4272. doi: 10.1038/sj.onc.1203779. [DOI] [PubMed] [Google Scholar]
- [38].Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- [39].Chida K, Hara T, Hirai T, Konishi C, Nakamura K, Nakao K, Aiba A, Katsuki M, Kuroki T. Disruption of protein kinase Ceta results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 2003;63:2404–2408. [PubMed] [Google Scholar]
- [40].Abu-Ghanem S, Oberkovitz G, Benharroch D, Gopas J, Livneh E. PKCeta expression contributes to the resistance of Hodgkin's lymphoma cell lines to apoptosis. Cancer Biol Ther. 2007;6:1375–1380. doi: 10.4161/cbt.6.9.4527. [DOI] [PubMed] [Google Scholar]
- [41].Krasnitsky E, Baumfeld Y, Freedman J, Sion-Vardy N, Ariad S, Novack V, Livneh E. PKCeta is a novel prognostic marker in non-small cell lung cancer. Anticancer research. 2012;32:1507–1513. [PubMed] [Google Scholar]
- [42].Lu HC, Chou FP, Yeh KT, Chang YS, Hsu NC, Chang JG. Analysing the expression of protein kinase C eta in human hepatocellular carcinoma. Pathology. 2009;41:626–629. doi: 10.3109/00313020903273076. [DOI] [PubMed] [Google Scholar]
- [43].Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Karp G, Maissel A, Livneh E. Hormonal regulation of PKC: estrogen up-regulates PKCeta expression in estrogen-responsive breast cancer cells. Cancer Lett. 2007;246:173–181. doi: 10.1016/j.canlet.2006.02.012. [DOI] [PubMed] [Google Scholar]