Abstract
Male mosquitoes transfer seminal fluid proteins (hereafter ‘SFPs’) during mating. These proteins can have profound effects on female behavior in the yellow fever mosquito Aedes aegypti and the Asian tiger mosquito Ae. albopictus. SFPs are thought to be responsible for female refractoriness to mating in both species. However, only limited information is available about the duration of induced refractoriness or the quantity of SFPs required to be effective in Ae. albopictus. Here, we tested the duration of the effect of SFPs on female refractory behavior for both Aedes species. Additionally, we determined the lowest SFP dose required to induce female refractory behavior in Ae. aegypti. Virgin females were injected intra-thoracically with doses ranging from 0.25 to 0.008 equivalents of one male’s SFP amount. Our results demonstrate high sensitivity of female Ae. aegypti and Ae. albopictus to SFPs of their own species, with the majority of females becoming refractory at doses ≥ 0.031 male-equivalents after injection into the hemocoel. This effect was long-lasting in both species; none of the injected females were inseminated when presented with males of their own species 30 to 34 days post-injection, whereas most saline-injected control females mated at this time point. These results will aid future work to characterize individual SFPs involved in post-mating refractoriness in these two species. Moreover, they show that as is the situation in the mosquito Anopheles gambiae, and unlike Drosophila melanogaster, sperm are not required for the maintenance of a sexual refractoriness response in Ae. aegypti and Ae. albopictus.
Keywords: Aedes albopictus, Aedes aegypti, seminal fluid proteins, mating receptivity
1. Introduction
In addition to sperm, insects transfer a large number of proteins in their semen that can have profound effects on mated females (Avila et al., 2010; Gillott, 2003; Sirot et al., 2011). Seminal fluid proteins (hereafter ‘SFPs’) are produced in the male reproductive tract, including the accessory glands. In Drosophila melanogaster, where the majority of studies on insect seminal fluid have been conducted, 157 SFPs from distinct functional classes that are transferred to females during mating have been identified (Findlay et al., 2009; Findlay et al., 2008). These peptides and proteins induce a number of behaviors and responses in females such as refractoriness to mating (reviewed in Avila et al. (2010)). Some individual peptides have been associated with particular post-mating responses in Drosophila females. For example ‘sex peptide’ temporarily reduces female sexual receptivity after mating and stimulates oviposition (Chapman et al., 2003; Chen et al., 1988; Liu and Kubli, 2003), whereas ‘ovulin’ induces ovulation (Heifetz et al., 2000).
In other insects, including mosquito vectors of disease, SFPs have recently been described (Dottorini et al., 2007; Rogers et al., 2009; Sirot et al., 2011; Sirot et al., 2008). Characterization of the effects of seminal fluid substances on female behavior in these medically important species will support novel control efforts that focus on reproduction as the primary means of reducing mosquito populations (Helinski and Harrington, 2012; Knox and Scott, 2009; Scolari et al., 2011). In addition, improved knowledge of mosquito reproduction could lead to new targets to manipulate female behavior or reduce reproduction (Rogers et al., 2009; Sirot et al., 2008), and ultimately, reduce disease burden.
A number of early studies conducted in the last century indicated influences of male semen components on female behavior in the yellow fever mosquito Ae. aegypti (Craig, 1967; Fuchs et al., 1969; Fuchs et al., 1968; Fuchs and Hiss, 1970; Hiss and Fuchs, 1972). The majority of these studies concentrated on effects on sexual receptivity or oviposition behavior. They demonstrated that Ae. aegypti females injected with male homogenates (i.e., from whole body or reproductive organs), or females implanted with male reproductive glands were unreceptive to insemination (Craig, 1967; Fuchs et al., 1969; Fuchs and Hiss, 1970). Analogous effects were also observed in other mosquito species including Anopheles gambiae and An. stephensi (Shutt et al 2011), and Culex pipiens (Craig, 1967). Seminal proteins also stimulated oviposition behavior in Ae. aegypti and the Asian Tiger mosquito Ae. albopictus, such that virgin females that received male homogenate through injection or implantation laid batches of unfertilized eggs (Hiss and Fuchs, 1972; Leahy and Craig, 1965; Leahy, 1967). In addition, there was some evidence that SFPs are important for sperm function in Aedes mosquitoes; when Ae. aegypti females were mated to males whose accessory glands had been surgically removed, no fertile eggs were laid even though sperm was transferred (Adlakha and Pillai, 1975).
Despite efforts to extract from male reproductive tissues the causative agent that induced these female behaviors, no specific molecule was identified from Ae. aegypti (Fuchs et al., 1969; Fuchs et al., 1968). Those studies identified a semi-purified substance that was protein-based and was referred to as matrone. With the aid of current proteomics techniques, a large number of proteins transferred from males to females during mating have been identified in Ae. aegypti and An. gambiae (Dottorini et al., 2007; Rogers et al., 2009; Sirot et al., 2011; Sirot et al., 2008). To determine functions of individual SFPs, knock-out or knock-down insects can be created that lack one protein (i.e. by transgenesis or RNA interference) and the phenotype of those insects can be determined (Rogers et al., 2009; Thailayil et al., 2011). For such studies, detailed knowledge of the female behavior induced by SFPs in that species is required in order to obtain a baseline to which results can be compared. While the majority of past studies on sexual refractory mating focused on Ae. aegypti, the emerging vector status of Ae. albopictus (Benedict et al., 2007) requires the improved knowledge of the reproductive biology of this species. Even though both species have similar biology, a recent study showed that while Ae. albopictus SFPs induced female refractoriness behavior in Ae. aegypti, the reverse experiment failed to induce refractoriness in Ae. albopictus females (Tripet et al., 2011), emphasizing the need for species-specific experimentation. Here, we sought to determine if the effect of SFPs was long-lasting and dependent on dose for Ae. albopictus and Ae. aegypti.
In the majority of studies performed on sexual receptivity in mosquitoes, females were exposed to males two days post-injection or implantation for a duration of one to two days (Fuchs et al., 1969; Fuchs and Hiss, 1970; Gwadz et al., 1971). However, Craig (1967) showed that Ae. aegypti females implanted with male accessory glands were refractory to mating ten weeks post-treatment, although sample sizes were not reported. To determine the length of the refractory period, Ae. aegypti and Ae. albopictus females were injected with SFPs of their own species, and sexual receptivity was assessed for several weeks post-injection. To determine if long-lasting refractory behavior was dependent on dose, females were injected with a high or low dose of SFPs. These doses were determined based on results from a dose-response curve that we established for Ae. aegypti. The doses tested ranged from 0.25–0.008 male-equivalents, and were similar to the ranges used in previous studies with Ae. aegypti (Craig, 1967; Fuchs et al., 1969). We found high sensitivity of Ae. aegypti and Ae. albopictus females to SFPs of their own species after injection into the hemocoel, and the majority of females injected with doses of 0.031 or more male-equivalents were not inseminated when exposed to males. This effect was long-lasting; and none of the females injected with 0.031 male-equivalents were inseminated when presented with males up to 30 to 34 days post-injection for both Aedes species.
2. Material and Methods
2.1. Experimental procedures
2.1.1. Mosquitoes
Mosquitoes used in this study are important vectors of a large number of viral human pathogens including dengue and Chikungunya. Our Ae. aegypti mosquitoes came from a Mexican strain originally collected in Tapachula, Mexico and maintained in colony since 2006. This strain was supplemented in 2008 and 2009 with eggs from the field site. Our Ae. albopictus mosquitoes came from a New Jersey strain. This strain has been maintained in colony since 2005 and was supplemented in 2010 with new field material from New Jersey, USA. Medium body-size males and females were reared using methods described in Helinski and Harrington (2011). Pupae were sorted by size; large (mostly female) pupae were transferred to individual vials to ensure virginity, sexed after emergence, and placed in a cage. Small (mostly male) pupae were allowed to emerge in cages and sexed within 24 hrs after emergence, during which time the males should not have mated as male sexual maturity occurs 24 hrs post-eclosion (Clements, 1999). All adults were maintained in an environmental chamber with constant access to 10% sucrose at 25.9 ± 0.6 °C with 71.9 ± 9.5% RH, with a photoperiod of 10D:10L with a two hour simulated dusk and dawn period.
2.1.2. SFP homogenate preparation and injection
Accessory glands and seminal vesicles, including some cuticle, were dissected from 50 virgin males in 50 μl (1 male-equivalent/1 μl) of Aedes saline (Hayes, 1953). Tissues were homogenized, sonicated in a water bath for 15 sec, and then centrifuged at 12,000 rpm for 15 min at 0 °C. Supernatant was transferred to a fresh sterile tube and used for injections. Serial dilutions were made from this stock in Aedes saline (Table 1) according to procedures from previous studies (Craig, 1967; Fuchs et al., 1969). A dose equivalent to one-fourth (0.25) of a male’s SFPs was used as the highest dose. This dose was routinely used in previous studies and induced a complete refractory response to mating upon injection in virgin females (Craig, 1967; Fuchs and Hiss, 1970; Gwadz et al., 1971). All homogenates were stored at −20 °C until the start of experiments. For each experiment and replicate, new dissections were performed.
Table 1. Effect of SFPs dose on female sexual refractoriness in Ae. aegypti.
Ae. aegypti females were injected with various doses of SFPs (i.e., doses are equivalent to one male’s SFPs amount). Saline-injected females and females that were not injected were used as controls. Subsets of females were exposed to males post-injection, and then assessed for insemination. Time post-injection that females were exposed to males, and the duration of the exposure period are shown.
| Day post-injection exposed to males | 1 | 2 | 2 | 4 | 2 | 4 |
|---|---|---|---|---|---|---|
| Duration of mating period (days) | 1 | 3 | 2 | 2 | 2 | 2 |
|
| ||||||
| Treatment (SFPs dose) | Percentage (%) of females inseminated (N) | |||||
| Rep 1 | Rep 2 | Rep 3 | ||||
| 0.250 | 0 (18) | 0 (7) | 0 (5) | 0 (9) | 0 (9) | 0 (9) |
| 0.063 | 0 (18) | 0 (8) | 0 (7) | 0 (2) | 0 (9) | 0 (9) |
| 0.042 | ND | ND | 0 (7) | 0 (7) | 0 (10) | 0 (9) |
| 0.031 | 59 (17) | 40 (5) | 33 (3) | 0 (9) | 22 (9) | 33 (9) |
| 0.016 | 59 (17) | 83 (6) | 89 (9) | 89 (9) | 100 (10) | 75 (8) |
| 0.008 | 41 (17) | 80 (5) | ND | ND | ND | ND |
| Saline I | 14 (14) | 100 (1) | 100 (8) | 100 (10) | 80 (10) | 83 (6) |
| Saline II | ND | ND | 75 (4) | 100 (1) | 75 (8) | 86 (7) |
| Control (no injection) | 100 (18) | ND | 100 (8) | 100 (9) | 100 (10) | 100 (10) |
The percentage of females that mated is indicated. (N) is the number of alive females dissected. Three replicates of the experiment were performed.
ND: not done.
To minimize the presence of sperm proteins in our samples, testes were not included among the extracted tissues. However, seminal vesicles in Aedes spp contain mature sperm as well as SFPs (Sirot et al. 2011). Similar to previous studies, only the supernatant was used for injection to ensure that the majority of sperm were removed.
Virgin females were chilled on ice for a maximum of 20 min, and injected (Picoinjector PLI-100, Harvard Apparatus, Hollison, MA) with a fine glass needle (pulled and beveled glass capillary tube) in the thorax with ~ 0.25 μl of homogenate. All solutions were kept at 4 °C during injections. Females injected with Aedes saline were used as a control treatment in all experiments. Prior to these experiments we verified that, similar to saline injections, the injection of gut extracts resulted in high levels of insemination (data not shown); indicating that the refractoriness response observed following SFPs injection was not caused by a non-specific reaction to tissue injection, and that saline-injection can thus be used as an appropriate control. Thirty females from each treatment were placed together in a recovery cage, consisting of a 0.5 L cardboard cup with a mesh lid. On top of the lid we placed a sugar wick and a damp paper towel to improve survival. Paper towels and wicks were refreshed every other day.
2.1.3. Receptivity assay
To assess if females were receptive to mating, individuals were transferred to a small cage (0.5 L) and 3 virgin males were added. Cages were provisioned with sugar and damp towels as described in 2.1.2. In addition to the saline-injected control females, non-injected females from the same batch were used as mating controls. Spermathecae were examined under a compound microscope for the presence of sperm (100 x). Thus, the term ‘refractory to mating’ used throughout this paper refers to the absence of sperm in the spermathecae of a female that had been exposed to a male. Only females that were able to fly and display normal behavior were selected for the receptivity assay.
2.2. Dose-response to sexual refractoriness in Ae. aegypti
We tested the lowest dose at which refractoriness to mating occurred for Ae. aegypti. Three replicates were performed using separately reared cohorts of mosquitoes. Age of males used for dissections was 3–8 days. Treatments consisted of a range of SFPs doses injected; from 0.25–0.008 male- equivalents (see Table 1). For each treatment, 3-day-old females (n= 20–25) were injected. Two groups of saline-injected controls were used in replicates 2 and 3.
To assess females’ mating receptivity, a subset of females from each treatment were exposed to males at 1–2 days post-injection for 1–2 days (Table 1). Males used as mates were between 4–9 days old. Females in Replicate 1 were only allowed to recover for one day post-injection. Consequently, few of the saline-injected control females mated (14%, Table 1). In subsequent replicates, therefore, females were given 2 days to recover from injections prior to placement in the mating assay. Females were removed, all live females were dissected (see 2.1.3), and new females were added to the cups (dead males were replaced). Two to three days later, their insemination status was determined (Table 1).
2.3. The duration of sexual refractoriness
To determine if the sexual refractory period induced by SFPs was long-lasting, virgin female Ae. albopictus and Ae. aegypti (2–4 days) were injected with a high (0.25 male-equivalents) or low dose (0.031 male-equivalents) of SFPs of their own species. Age of males used for dissections was between 4–7 days. Two or three replicates of the experiment were performed for Ae. aegypti and Ae. albopictus, respectively. For each treatment, 60–70 females were injected. Females were held as described in 2.1.2.
Refractoriness to mating was assessed 2–3 (Ae. albopictus only), 7, 14, 21, 28 (replicates 1 and 3), and 32 (replicate 2) days post-injection. Females were exposed to males for 2 days before dissection. Too few Ae. albopictus females survived to 28 days to perform the last time point in replicate 1. The males used as mates were between 3–12 days of age with the exception of time point 21 in replicate 2 where males were 16–19 days of age. New batches of mosquitoes were reared to provide younger males for the later time points.
2.4. Statistical analysis
The numbers of females inseminated or not inseminated in the dose-response experiment were analyzed with logistic regression using SPSS v20 (SPSS, Chicago, IL). Replicate and treatment were included as variables in the model, and each treatment was compared against saline-injected control females. To allow for statistical testing for the doses 0.250, 0.063, and 0.042 for which all females were not inseminated, it was necessary to change the insemination status of one female to inseminated in order to run the analysis. A Bonferroni correction was applied to α= 0.05 to allow for multiple comparisons.
3. Results
3.1. Dose-response of refractoriness to SFP injection
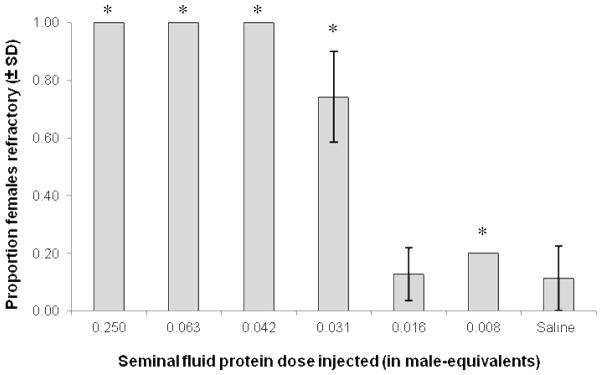
Ae. aegypti females responded to various amounts of injected SFPs. Females were injected with SFP doses ranging from 0.25 to 0.008 male-equivalents, and significant differences were observed in proportion of females refractory to mating (Logistic regression: χ2= 186.33, df= 7, P< 0.001; Fig. 1). Replicate was not a significant factor in the model (Wald= 0.2, P= 0.66). Females injected with doses 0.25, 0.063, or 0.042 male-equivalents were fully refractory to mating and no insemination was observed when these females were exposed to males up to 4 days post-injection (Table 1). In contrast, on average 89 ± 11 (SD)% of females injected with saline were inseminated. Significant differences in the proportion of females refractory to mating were observed between each treatment (i.e., 0.25, 0.063, 0.042) and saline-injected females (Wald= 43.3, 42.0, 31.0, respectively; P< 0.001; Fig. 1). Females injected with 0.031 male-equivalents did not show complete refractoriness to mating (Table 1). 74 ± 16 % of females were refractory, and a significant difference between saline-injected control females was observed (Wald= 25.1, P< 0.001; Fig. 1). Less than 20% of females injected with lower doses of 0.016 and 0.008 were refractory, and no significant difference was observed between dose 0.016 and saline-injected control females (Wald= 1.0, P= 0.32; Fig. 1). A significant difference was observed between dose 0.008 and saline-injected control females (Wald= 9.2, P= <0.01), probably as a result of the low proportion of females inseminated for the first mating in replicate 1 (i.e., 41%; Table 1). Females from replicate 1 that were exposed to males 1 day post-injection experienced low overall insemination (i.e., 14%, Table 1: column 2), which was likely the result of the short recovery time. In subsequent replicates, recovery time was increased to 2 days which resulted in high (i.e., 75–100 %) levels of insemination for saline-injected females (Table 1). Injections had only minor effects on female survival. Forty-eight hours after injection, female mortality was on average 2 ± 2 (SD)%, with the exception of one treatment in replicate 2 where 40% of females died.
Fig. 1.
Mean (± SD) proportion of virgin Ae. aegypti females refractory to mating when injected with a dose-range of seminal fluid proteins (SFPs). Data were averaged over three replicates, excluding females assayed 1 day post-injection in Replicate 1 due to short recovery time leading to low overall insemination (see section 2.2; Table 1: column 2). Saline-injected treatments I and II were combined. * indicate a significant difference compared to saline-injected control females (Logistic regression; Bonferroni corrections applied).
Ae. albopictus females were injected with a low (0.031) or a high (0.25) dose of SFPs. None of the females injected with the high dose were inseminated when exposed to males (Table 2A). The low dose of 0.031 prevented almost all females from becoming inseminated (Table 2A). These results are discussed in greater detail in section 3.2.
Table 2. Duration of the effect of SFPs on female sexual refractoriness behavior in Ae. albopictus and Ae. aegypti.
Ae. albopictus (A) and Ae. aegypti (B) females were injected with a high (i.e., 0.25) or low (i.e., 0.031) dose of SFPs from their own species. Saline-injected females and females that were not injected were used as controls. Subsets of females were exposed to males at various days post-injection.
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Exposed to males post- injection (days) | Rep | Percentage (%) of females inseminated (N) | |||||||
| Ae. albopictus | Ae. aegypti | ||||||||
| 0.25 SFPs | 0.03 SFPs | Saline | Control (No Injection) | 0.25 SFPs | 0.03 SFPs | Saline | Control (No Injection) | ||
| 2–3 | 1 | 0 (7) | 14 (7) | 100 (7) | 100 (5) | ND | ND | ND | ND |
| 2 | 0 (9) | 40 (10) | 100 (10) | 100 (5) | |||||
| 3 | 0 (10) | 10 (10) | 100 (10) | 100 (5) | |||||
|
| |||||||||
| 7 | 1 | 0 (5) | 0 (9) | 100 (8) | 100 (5) | 0 (9) | 0 (9) | 89 (9) | 100 (5) |
| 2 | 0 (9) | 0 (8) | 100 (10) | 100 (5) | 0 (10) | 0 (10) | 89 (9) | 100 (5) | |
| 3 | 0 (9) | 0 (10) | 100 (10) | 100 (5) | ND | ND | ND | ND | |
|
| |||||||||
| 14 | 1 | 0 (6) | 0 (5) | 75 (6) | 100 (5) | 0 (9) | 0 (9) | 100 (10) | 100 (5) |
| 2 | 0 (9) | 0 (9) | 75 (8) | 100 (4) | 0 (9) | 0 (10) | 89 (9) | 100 (5) | |
| 3 | 0 (10) | 0 (9) | 100 (9) | 100 (5) | ND | ND | ND | ND | |
|
| |||||||||
| 21 | 1 | 0 (6) | 0 (6) | 70 (10) | 100 (5) | 0 (8) | 0 (6) | 71 (7) | 80 (5) |
| 2 | 0 (9) | 0 (7) | 80 (5) | 80 (5) | 0 (10) | 0 (10) | 100 (8) | 100 (5) | |
| 3 | 0 (5) | 0 (6) | 100 (5) | 80 (5) | ND | ND | ND | ND | |
|
| |||||||||
| 28–32 | 1 | ND | ND | ND | ND | 0 (11) | 0 (10) | 50 (2) | 80 (5) |
| 2 | 0 (6) | 0 (2) | 100 (4) | 100 (5)1 | 0 (8) | 0 (9) | 25 (4) | 50 (4) | |
| 3 | 0 (10) | 0 (11) | 67 (3) | 67 (6) | ND | ND | ND | ND | |
The percent of females that were inseminated is indicated. (N) is the number of alive females dissected. Two (Ae. aegypti) or three (Ae. albopictus) replicates of the experiment were performed.
ND: not done.
A different batch of females were used as non-injected control females.
3.2. Persistence of effects of SFP injection
To determine how long the effects of injected SFPs affected female refractoriness behavior, and whether this was dependent on dose, we assessed receptivity at up to 34 days post-injection for both Aedes species. Females were injected with a high (0.25) or a low (0.031) SFP dose. The effect of SFP injection on female refractoriness behavior was long-lasting in Ae. albopictus, and none of the females injected with a low or high dose of SFPs were inseminated when exposed to males 7–32 days post-injection for 2 days (Table 2A). In contrast, on average 88 ± 14% of saline-injected females were inseminated at these time points. Similar results were observed for Ae. aegypti, and none of the females injected with a high or low dose were inseminated when exposed to males 7–32 days post-injection for 2 days with males of their own species (Table 2B). Interestingly, a small proportion (i.e., 21 ± 16%) of Ae. albopictus females injected with the low dose of 0.031 male-equivalents were inseminated 2–3 days post-injection (Table 2A), while at later time points no insemination was observed.
4. Discussion
Seminal fluid proteins (SFPs) are important modulators of female behavior and reproduction in a number of insects including mosquitoes (reviewed in Avila et al. (2010) and Gillott (2003)). Detailing the effects of SFP amount on female refractory behavior and duration will contribute to the functional characterizations of specific peptides and proteins. The main focus of this study was on the effects of dose and duration of SFPs on female refractoriness behavior in Ae. albopictus to advance our knowledge on the reproductive biology of this important emerging vector species (Benedict et al., 2007). Our results indicate that female Ae. albopictus, similar to Ae. aegypti, are highly sensitive to SFPs. Full refractory behavior to mating was observed at low quantities of SFPs injected into the hemocoel in both species. The lowest dose of SFPs that induced complete female refractoriness behavior in Ae. aegypti corresponded to 0.042 male-equivalents. A dose of 0.031 male-equivalents resulted in the large majority of females becoming refractory in both Aedes species. The effect of SFPs on female refractory behavior was long-lasting, and females injected with a low or a high dose of SFPs were not inseminated when exposed to males up to 34 days post-injection. The effect of SFPs on short-term Ae. aegypti refractoriness behavior following injection was characterized in previous studies; there, a dose of 0.03 male-equivalents induced full refractoriness (Craig, 1967; Fuchs et al., 1969). However, long-term effects were only reported in one study, in which an unknown number of females were stated to have remained unreceptive to insemination ten weeks after implantation of male accessory glands (Craig, 1967). Our results provide a more comprehensive and quantitative analysis of long-term refractory behavior in Ae. aegypti.
The effects of injected SFPs have been studied in other insects with varying results. For example, the injection of SFPs (i.e., corresponding to 0.125–0.25 male-equivalents) into Anopheles mosquitoes induced a strong refractoriness response to mating (Shutt et al., 2010). In D. melanogaster, the injection of 0.5 male-equivalents of methanol-extracted accessory gland homogenates reduced female receptivity by 80% (Chen et al., 1988). In the Tephritid fruit fly Anastrepha fraterculus, injection of 0.2 male SFP equivalents resulted in ~ 85% of laboratory reared females becoming refractory to mating compared to ~ 35% for saline-injected control females (Abraham et al., 2012). In another fruit fly, Bactrocera tryoni, injection of females with 0.5 male-equivalents resulted in ~ 65% of females refractory to a mate, compared to ~ 30% for saline-injected control females (Radhakrishnan and Taylor, 2007). A weaker response was also observed in two Callosobruchus seed beetles, where the injection of male reproductive gland homogenates resulted in a ~ 35–40% reduction of female receptivity when females were tested 2 days post-injection compared to water-injected control females (Yamane et al., 2008a; Yamane et al., 2008b). The strength of SFP-induced sexual refractoriness varied in the above studies, most likely due to experimental or species-dependent differences. Interestingly, sensitivity to SFPs corresponds with female mating frequency. Polyandrous species, like the fruit flies and the seed beetles, were less sensitive than the primarily monandrous Aedes and Anopheles mosquitoes.
The timing of onset of female refractoriness behavior after receiving SFPs in Aedes is unclear. Most studies, like ours, utilize a recovery period of one to two days post-injection to assure that females are able to fly and display normal behavior. When Ae. aegypti females were exposed to males immediately after the implantation of accessory glands, 26% of females were inseminated (Craig, 1967), suggesting that the effect is not immediate. However, it is unclear what effects the anesthesia and the invasive treatment themselves might have had on the timing of onset of refractoriness in those experiments. Interestingly, we observed that 21 ± 16% of Ae. albopictus females injected with the low dose were receptive to mating when tested 2–3 days post-injection, while at later time points none were inseminated. This suggests that there might be a delay in the refractory response at low SFP doses. Future studies should investigate the mechanisms for the apparent delayed induction of sexual refractoriness behavior at low doses of SFPs. A delay in refractory behavior induced by SFPs could account for some of the instances where polyandry has been observed for Ae. aegypti (Gwadz and Craig, 1970; Helinski et al., 2012).
In our study, the effect of SFPs on female receptivity was long-lasting, and females of both species were not inseminated when exposed to males for several weeks post-injection. The mechanisms that induce long-term female refractoriness in Aedes spp are unclear. In Drosophila, sex peptide bound to sperm tails is required to maintain long-term (i.e., ≥1 week) refractoriness responses to mating (Liu and Kubli, 2003; Peng et al., 2005). The strong long-term female refractoriness response we observed after intra-thoracic injection indicates that sperm are not needed to elicit this behavior in Aedes. In An. gambiae, it has also recently been reported that sperm are not needed to induce a refractory response to mating for at least 4 days following a mating with a spermless male (Thailayil et al., 2011). Additionally, thoracic SFP injections were sufficient to induce refractoriness in An. gambiae and An. stephensi (Shutt et al., 2010). These findings are not in agreement with a study that failed to induce refractoriness following intra-abdominal injections of male accessory gland substances (Klowden, 2001, 2006). However, Shutt et al. (2010) hypothesized that this discrepancy could have resulted from the location of injection and subsequent access to SFP receptors. The locations of specific receptors of SFPs in Aedes have not been identified, although one study in the mosquito Cx. tarsalis suggested the presence of SFPs receptors in the head (Young and Downe, 1987). In D. melanogaster, sex peptide enters the hemolymph, and sex peptide receptors have been found in the reproductive tract and central nervous system (Yapici et al., 2008). One consideration in future studies in Aedes will be the location of SFPs receptors after mating.
Unlike the role for sex peptide in D. melanogaster receptivity, it is not known which specific protein or peptide is responsible for the induction of sexual refractoriness behavior in Aedes spp. Previous studies with Ae. aegypti identified a partially purified protein substance referred to as matrone that elicited the refractory response (Fuchs et al., 1969). More recent studies have made considerable progress in the identification of the suite of SFPs transferred in mosquitoes. A large number of SFPs were identified in Ae. aegypti that are transferred from males to females during mating (Sirot et al., 2011; Sirot et al., 2008), which will enable the functional characterization of proteins in this species. Rogers et al. (2009) identified a number of proteins in An. gambiae that are present in the mating plug. Functional characterization of the protein transglutaminase by reverse genetics revealed its importance in plug formation and subsequent sperm storage in Anopheles. Even though functional protein classes in ejaculates are usually conserved between species, many SFP sequences are rapidly evolving (reviewed in Swanson and Vacquier (2002)), and this also may be the case in mosquitoes. Recently, Tripet et al. (2011) demonstrated that Ae. albopictus SFPs induced female refractoriness behavior in Ae. aegypti, yet injection of Ae. aegypti SFPs did not prevent mating of Ae. albopictus females. Consequently, the identification and functional characterization of SFPs in Ae. albopictus will require de novo experimentation.
Even though we observed that low quantities of SFPs are sufficient to induce a long-term refractory response to mating upon injection into the hemocoel, the dose required for this response when SFPs are introduced into the female reproductive tract during copulation is likely to be higher since some of the SFPs may have to move into the hemolymph from the reproductive tract to reach their targets. Although this movement has not been demonstrated in Aedes species, in D. melanogaster, some SFPs enter the hemolymph following a mating while others remain confined within the female reproductive tract (Lung and Wolfner, 1999). For one of these SFPs, it was estimated that approximately 10% of the transferred amount entered the female circulatory system (Lung and Wolfner, 1999). If, in Aedes, SFPs have to enter the hemolymph to affect female refractoriness and if a similar percentage of SFPs moved into the hemolymph as in the case of the D. melanogaster SFP, then, based on our results, we could infer that refractoriness could be induced by insemination of females with around 0.31 of a male accessory gland equivalent. These results are in agreement with our finding that Ae. aegypti males are only able to inseminate 3–4 females consecutively before depletion of semen (i.e., proteins and/or sperm) is observed, indirectly, through a reduction in female fecundity (Helinski and Harrington, 2011). Besides receptivity, however, SFPs likely induce a wide range of other behaviors in mated female mosquitoes (reviewed in Sirot et al. (2008)), and the relative amounts of SFPs needed for these behaviors are unknown.
The data presented here on the effects of SFPs will provide a baseline to characterize individual SFPs involved in post-mating refractoriness in these two Aedes species. Elucidating the role of individual SFPs in mosquitoes will support novel vector control strategies, and could identify potential targets for control (Helinski and Harrington, 2012; Rogers et al., 2009; Sirot et al., 2008).
Highlights.
Female refractoriness to mating was determined for Ae. albopictus and Ae. aegypti
Females were injected in the hemocoel with seminal fluid proteins (SFPs)
Low doses of SFPs (≥ 0.042 male equivalents) induced complete refractory behavior
SFP effect was long-lasting and females were not inseminated 34 d post injection
First demonstration of long-lasting SFP induced refractoriness in Ae. albopictus
Acknowledgments
We thank Mark Brown for thoughtful discussions, Angela Douglas and her lab for generously allowing us the use of equipment, Sylvie Pitcher and other members of the Harrington and Wolfner labs for technical support, and two anonymous reviewers for their constructive comments. This study was supported by NIH/NIAID grant R01AI095491 and Hatch NYC 2011-12-219 to LCH and MFW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Prasit Deewatthanawong, Email: pd72@cornell.edu.
Laura K. Sirot, Email: lsirot@wooster.edu.
Mariana F. Wolfner, Email: mariana.wolfner@cornell.edu.
Laura C. Harrington, Email: lch27@cornell.edu.
References
- Abraham S, Cladera J, Goane L, Teresa Vera M. Factors affecting Anastrepha fraterculus female receptivity modulation by accessory gland products. Journal of Insect Physiology. 2012;58:1–6. doi: 10.1016/j.jinsphys.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Adlakha V, Pillai MK. Involvement of male accessory gland substance in the fertility of mosquitoes. Journal of Insect Physiology. 1975;21:1453–1455. doi: 10.1016/0022-1910(75)90207-3. [DOI] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annual Review of Entomology. 2010;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne and Zoonotic Diseases. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. CABI Publishing; Wallingford: 1999. [Google Scholar]
- Craig GB. Mosquitoes: Female monogamy induced by male accessory gland substance. Science. 1967;156:1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biology. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Research. 2009;19:886–896. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs MS, Craig GB, Jr, Hiss EA. The biochemical basis of female monogamy in mosquitoes. I. Extraction of the active principle from Aedes aegypti. Life Sciences. 1968;7:835–839. doi: 10.1016/0024-3205(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Craig GB, Despommier DD. The protein nature of the substance inducing female monogramy in Aedes aegypti. Journal of Insect Physiology. 1969;15:701–709. [Google Scholar]
- Fuchs MS, Hiss EA. The partial purification and separation of the protein components of matrone from Aedes aegypti. Journal of Insect Physiology. 1970;16:931–939. doi: 10.1016/0022-1910(70)90223-4. [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annual Review of Entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gwadz RW, Craig GB., Jr Female polygamy due to inadequate semen transfer in Aedes aegypti. Mosquito News. 1970;30:355–360. [Google Scholar]
- Gwadz RW, Craig GB, Jr, Hickey WA. Female sexual behavior as the mechanism rendering Aedes aegypti refractory to insemination. Biological Bulletin. 1971;140:201–214. doi: 10.2307/1540069. [DOI] [PubMed] [Google Scholar]
- Hayes RO. Determination of a physiological saline for Aedes aegypti (L.) Journal of Economic Entomology. 1953;46:624–627. [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Jr, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Current Biology. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Helinski MEH, Harrington LC. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. Journal of Medical Entomology. 2011;48:202–211. doi: 10.1603/me10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski MEH, Harrington LC. Considerations for male fitness in successful genetic vector control programs. Springer; Dordrecht: 2012. In Press. [Google Scholar]
- Helinski MEH, Valerio L, Facchinelli L, Scott TW, Ramsey J, Harrington LC. Evidence of polyandry for Aedes aegypti in semifield enclosures. American Journal of Tropical Medicine and Hygiene. 2012;86:635–641. doi: 10.4269/ajtmh.2012.11-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss EA, Fuchs MS. The effect of matrone on oviposition in the mosquito, Aedes aegypti. Journal of Insect Physiology. 1972;18:2217–2227. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: absence of control by male accessory gland substances. Journal of Insect Physiology. 2001;47:661–666. doi: 10.1016/s0022-1910(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. Journal of Insect Physiology. 2006;52:679–684. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Knox TB, Scott TW. Vector control for prevention of dengue- current status and future strategies, Advances in Human Vector Control, ACS Symposium Series. American Chemical Society. 2009:39–57. [Google Scholar]
- Leahy M, Craig G. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and Ae. albopictus. Mosquito News. 1965;25:448–452. [Google Scholar]
- Leahy MG. Non-specificity of the male factor enhancing egg-laying in Diptera. Journal of Insect Physiology. 1967;13:1283–1292. [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochemistry and Molecular Biology. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, Taylor PW. Seminal fluids mediate sexual inhibition and short copula duration in mated female Queensland fruit flies. Journal of Insect Physiology. 2007;53:741–745. doi: 10.1016/j.jinsphys.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari F, Siciliano P, Gabrieli P, Gomulski LM, Bonomi A, Gasperi G, Malacrida AR. Safe and fit genetically modified insects for pest control: from lab to field applications. Genetica. 2011;139:41–52. doi: 10.1007/s10709-010-9483-7. [DOI] [PubMed] [Google Scholar]
- Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Medical and Veterinary Entomology. 2010;24:91–94. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Poulson RL, McKenna MC, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochemistry and Molecular Biology. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski MEH, Kimura M, Deewathanawong P, Wolfner MF, Harrington LC. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. Plos Neglected Tropical Diseases. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nature Review Genetics. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Thailayil J, Magnusson K, Godfray HC, Crisanti A, Catteruccia F. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1104738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. American Journal of Tropical Medicine and Hygiene. 2011;85:265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T, Kimura Y, Katsuhara M, Miyatake T. Female mating receptivity inhibited by injection of male-derived extracts in Callosobruchus chinensis. Journal of Insect Physiology. 2008a;54:501–507. doi: 10.1016/j.jinsphys.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Yamane T, Miyatake T, Kimura Y. Female mating receptivity after injection of male-derived extracts in Callosobruchus maculatus. Journal of Insect Physiology. 2008b;54:1522–1527. doi: 10.1016/j.jinsphys.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Young ADM, Downe AE. Male accessory-gland substances and the control of sexual receptivity in female Culex tarsalis. Physiological Entomology. 1987;12:233–239. [Google Scholar]