Abstract
Interleukin (IL)-1β and IL-6 have been implicated in brain development, injury progression, and fetal/maternal immune interactions. We examined IL-1β and IL-6 protein expression in cerebral cortex (CC) and white matter (WM) from non-ischemic ovine fetuses at 87–90, 122–127, and 135–137 days of gestation, pregnant ewes at 87–90 and 135–137 days of gestation, and fetuses exposed to 48 or 72 h of reperfusion after ischemia. Protein expression was determined by Western immunoblot. In non-ischemic CC, IL-1β was higher (P<0.05) in adult sheep and fetuses at 135–137 than 87–90 and 122–127 days, and IL-6 higher at 122–127 than 87–90 days, and in adults than fetuses at 87–90, 122–127, and 135–137 days of gestation. In non-ischemic fetal WM, IL-6 was higher at 135–137 than 87–90 days, but IL-1β did not differ. In CC, IL-1β was higher in ewes at 135–137 than 87–90 days and IL-6 at 135–137 days and in non-pregnant adults than ewes at 87–90 days of gestation. In WM, IL-1β was higher in ewes at 135–137 than 87–90 days of gestation, but IL-6 did not differ. Forty-eight and 72 h after ischemia, CC IL-1β was higher than in non-ischemic fetuses. Seventy-two h after ischemia, IL-1β and IL-6 were higher in WM than CC. In conclusion, IL-1β and IL-6 exhibit developmental regulation in fetal brain, change during gestation in brains of pregnant ewes, show regional differences in normal brains of fetuses and ewes, demonstrate differential responses after ischemia in CC and WM, and IL-1β but not IL-6 increases after ischemia in CC.
Keywords: Cytokines, cerebral cortex, fetus, pregnancy, sheep, white matter
1. Introduction
Cytokines are low-molecular-weight proteins that mediate an array of functions in non-immune tissues including the central nervous system (CNS) (Deverman and Patterson, 2009; Lai et al., 2011; Pantoni et al., 1998; Thaxton and Sharma, 2010). IL-1β and IL-6 are two of the most widely studied pro-inflammatory cytokines that contribute to CNS damage (Denker et al., 2007; Dinarello, 2000). Although previous studies suggest that cytokines produced normally and pathologically during critical stages of fetal development affect normal CNS development, function, and behavior in later life, there is very little information regarding developmental changes in cytokine expression in different brain regions during gestation (Deverman and Patterson, 2009; Dziegielewska et al., 2000; Moro et al., 2008; Schmitz and Chew, 2008).
Cytokines are also critical in interactions between the maternal immune and reproductive systems and in establishing and maintaining pregnancy (Hanna et al., 2006; Hanna et al., 2000; Hill, 1992; Makhseed et al., 2000; Thaxton and Sharma, 2010; Wegmann et al., 1993). Both IL-1β and IL-6 synthesis has been documented in human placenta and placental cytokine expression changes in a gestational age-dependent manner (Hanna et al., 2006; Hanna et al., 2000; Hu et al., 1992; Kameda et al., 1990). However, maternal brain cytokine expression has not been examined, to the best of our knowledge, in any species during advancing gestation.
Hypoxic-ischemic brain injury is the most prevalent neurologic problem in the perinatal period (Volpe, 2001). Hypoxia-ischemia initiates an inflammatory response in the brain that is associated with the induction of cytokines including IL-1β and IL-6 (Hagberg et al., 1996; Szaflarski et al., 1995). Although increases in pro-inflammatory cytokines may exacerbate brain damage, IL-6 can mediate both pro- and/or anti-inflammatory effects after ischemia (Pantoni et al., 1998). Even though pro-inflammatory cytokines are elevated after ischemia, limited information is available regarding ischemia-related changes in cytokines in the fetal brain of a precocial species such as sheep.
The ovine fetus has been extensively used to investigate many aspects of CNS development (Back et al., 2006; Gunn et al., 1997; Riddle et al., 2006; Stonestreet et al., 1999; Stonestreet et al., 2000). The neurodevelopment of the immature ovine brain is similar to that of premature infants with respect to completion of neurogenesis, onset of cerebral sulcation, and detection of evoked potentials (Back et al., 2006; Barlow, 1969; Bernhard et al., 1967; Cook et al., 1987). Full term in sheep pregnancy is 145–150 days of gestation. The preterm fetal sheep brain between 94 and 96 days of gestation is comparable to that of the preterm infant between 24 and 28 weeks of gestation, whereas fetal sheep at 135 days of gestation is similar to that of the full term human infant (Back et al., 2006). We selected fetal sheep over a wide range of gestational ages to have a broad developmental range, over which to examine developmental changes in the brain cytokines. We examined fetal sheep at 87–90, 122–127, and 135–137 days of gestation, which represents 60, 80 to 90% of the ovine gestation. These times in gestation are approximately similar to extremely preterm, late preterm and full term human infants, respectively (Back et al., 2006). Although rodents are frequently used to study brain development and injury, the rodent brain is immature at birth (Dobbing and Sands, 1979) and almost completely agyric. In contrast, similar to non-human primate and the human brain, the sheep brain develops prenatally and is gyrencephalic.
The rationale for our study was that, although IL-1β and IL-6 have been implicated in normal brain development, injury progression, and fetal/maternal immune interactions, information is currently not available for the temporal and spatial protein expression patterns during gestation in normal fetal and maternal brain or after ischemia in a precocial species such as sheep. Therefore, 1) we examined the effects of gestation on IL-1β and IL-6 protein expression in cerebral cortex and white matter of normal fetal sheep and pregnant ewes, and ischemia on IL-1β and IL-6 expression in cerebral cortex of fetal sheep, and 3) compared effects of ischemia on IL-1β and IL-6 expression between the cerebral cortex and white matter in the fetus.
2. Methods
2.1 Animal preparation and experimental design
The cerebral cortical and white matter samples used in present study were obtained from the placebo treated animals in our earlier work (Elitt et al., 2003; Petersson et al., 2002; Stonestreet et al., 1999; Stonestreet et al., 2000). The previous studies were conducted after approval by the Institutional Animal Care and Use Committees of The Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island and according to the National Institutes of Health Guidelines for the use of experimental animals (Elitt et al., 2003; Petersson et al., 2002; Stonestreet et al., 1999; Stonestreet et al., 2000). Details of the surgical preparation have been provided in previous publications (Elitt et al., 2003; Petersson et al., 2002; Stonestreet et al., 1999; Stonestreet et al., 2000). Briefly, surgery was performed under 1–2% halothane anesthesia on 45 mixed breed sheep. In the non-ischemic fetal groups, polyvinyl catheters were placed into a brachial veins and brachial arteries of the fetuses. In fetuses in the ischemic groups, surgery was performed as described above and, in addition, bilateral inflatable occluders (In Vivo Metric, Healdsburg, CA) and flow probes (Transonic Systems, Ithaca, NY) were placed around the carotid arteries (Elitt et al., 2003; Petersson et al., 2002). Two pairs of screws (Small Parts, Miami Lakes, FL) were placed onto the dura and connected to a recorder by insulated wires (Alpha Wire, Elizabeth, NJ) to measure the electrocorticogram (Elitt et al., 2003; Petersson et al., 2002). The electrocorticogram was measured to determine if the fetal cerebral cortical brain wave pattern became isoelectric during ischemia (Elitt et al., 2003; Petersson et al., 2002). In the ischemic groups, brain ischemia was induced for 30 minutes by inflating the carotid occluders as previously described (Elitt et al., 2003; Petersson et al., 2002). Then, the occluders were deflated and reperfusion was allowed to continue for 48 or 72 hours (Elitt et al., 2003; Petersson et al., 2002).
At the end of the studies, the cerebral cortex and white matter samples from the fetal and adult sheep were snap frozen in liquid nitrogen and stored at −80°C until analysis. Although some IL-1β and IL-6 protein degradation could have resulted from processing and freezing of the tissues, these procedures most likely had similar effects upon all of the samples from the different study groups, as the tissue samples were from studies performed over similar time periods (Elitt et al., 2003; Petersson et al., 2002; Stonestreet et al., 1999; Stonestreet et al., 2000). We did not have white matter available from the non-ischemic fetal sheep at 122–127 days of gestation, the non-pregnant adult sheep, or the fetal sheep exposed to ischemia and 48 h of reperfusion. The study subjects, age at which the brain samples were obtained, and numbers of sheep in each group, from which the cerebral cortical and white matter samples were obtained are summarized in table 1.
Table 1.
Study subjects, age at study, pre-study treatment, and numbers of animals.
| Subjects | Tissue Examined | N |
|---|---|---|
| Non-ischemic fetus at 87–90 days of gestation | Cerebral Cortex and White Matter | 6 |
| Non-ischemic fetus at 122–127 days of gestation | Cerebral Cortex | 6 |
| Non-ischemic fetus at 135–137 days of gestation | Cerebral Cortex and White Matter | 6 |
| Adult pregnant sheep at 87–90 days of gestation | Cerebral Cortex and White Matter | 7 |
| Adult pregnant sheep at 135–137 days of gestation | Cerebral Cortex and White Matter | 6 |
| Adult non pregnant sheep at 3 years of age | Cerebral Cortex | 3 |
| Fetal brain ischemia/48h reperfusion at 120–129 days of gestation | Cerebral Cortex | 5 |
| Fetal brain ischemia/72h reperfusion at 120–129 days of gestation | Cerebral Cortex and White Matter | 6 |
Values for age are range in days of gestation or years; Full term in sheep is 145–150 days of gestation
2.2 Protein extraction
Tissues from the cerebral cortex and white matter were extracted in buffer F (10 mM Tris pH 7.05, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 5 µM ZnCl2, 0.1 mM NaVO4, 1% Triton-X100) with 1% complete protease inhibitor cocktail (Sigma, St. Louis, MO). Total protein concentrations of the homogenates were determined with a bicinchoninic acid protein assay (BCA, Pierce, Rockford, IL). Aliquots of the extracted samples were stored at −80°C.
2.3 Western immunoblot detection and quantification of proteins
One hundred micrograms of total protein per well were fractionated by SDS-PAGE and transferred onto polyvinylidene diflouride membranes (0.2 µm, Bio-Rad Laboratories, Hercules, CA) using a semidry technique. Membranes were blocked with 10% non-fat milk and washed in Tris-buffered saline with 0.1% of Tween-20 (TBST) and then probed for IL-1β with primary rabbit polyclonal antibody (Lifespan Biosciences, Seattle, WA) at a dilution of 1:5,000 and for IL-6 with rabbit polyclonal antibody (Millipore Corp., Chicago, IL) at a dilution 1:1000. Immunoblots were incubated in primary antibody overnight at 4°C then incubated for 1 h at room temperature with goat anti-rabbit (Alpha Diagnostic, San Antonio, TX) horseradish peroxidase conjugated secondary antibodies at a dilution of 1:10,000. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL plus, Western Blotting Detection reagents, Amersham Pharmacia Biotech, Inc., Piscataway, NJ).
All experimental samples were normalized to the respective protein obtained from the cerebral cortex of non-study sheep. We extracted a large amount of non-study brain tissue, aliquoted and froze the tissue at −80°C. We used these aliquots from the same tissue extract on each immunoblot. For the purpose of this report, we refer to these samples as internal control samples. As we have previously described (Duncan et al., 2009; Kim et al., 2006; Malaeb et al., 2007; Ron et al., 2005; Sadowska et al., 2010; Sadowska et al., 2009) these samples served as an internal control for quality of loading, transfer of the samples, normalization of the cerebral cortical and white matter densitometric values and to permit accurate comparisons among the different immunoblots. The experimental protein autoradiographic densitometry values were expressed as a ratio to the internal control facilitating normalized comparisons among the groups. We have previously shown that this method correlates well with values that have been normalized as ratios to β-actin in newborn lambs (Kim et al., 2006). Moreover, we have also found that we could not use traditional housekeeping proteins such as actin, glyceraldehyde 3-phosphate dehydrogenase or beta-tubulin in our studies because they change during development in the sheep brain. Extracts from the 45 brain tissues were randomly placed on the different immunoblots because the maximum number of wells in each immunoblot was twenty-five. Therefore, for the purpose of illustration in the figures, we selected the immunoblot that most closely represented the mean values for each age group from the different immunoblots.
Each immunoblot included samples from the treatment groups and three internal control samples. The internal control samples were included in three lanes, as the first, middle, and last samples on each immunoblot. We calculated a coefficient of variation for the internal control samples on each immunoblot. The values for the experimental samples were accepted as valid only if the coefficient of variation for the three internal control samples was <20% on the immunoblot. Molecular weight standards (Bio-Rad Laboratories) were included in each immunoblot. Uniformity in inter-lane loading was also established by Coomassie blue (Sigma, St. Louis, MO) staining of the polyacrylamide gels and uniformity of transfer to the polyvinylidene diflouride membranes was confirmed by Ponceau S staining (Sigma, St. Louis, MO) (Tseng et al., 2005).
2.4 Densitometric analysis
Band intensities were analyzed using Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). All experimental samples were normalized to the respective internal control samples. We used the average of the three internal control values on each immunoblot to normalize the experimental values. The final values represented an average of the densitometry values obtained from at least two different immunoblots and are presented as a ratio to the internal control sample.
2.5 Statistical analysis
Multivariate analysis of variance (MANOVA) was used to compare IL-1β and IL-6 protein expression in the cerebral cortex and white matter across the different age groups to assess the main effects. The factors were age groups (fetuses at 87–90, 122–127, 135–137 days of gestation, and adult sheep) in cerebral cortex and gestational age (fetuses at 87–90 and 135–137 days gestation) in the white matter (Fig. 1). Multivariate analysis of variance (MANOVA) was also used to compare IL-1β and IL-6 protein expression in the cerebral cortex and white matter of the pregnant ewes at 87–90 and 135–137 days of gestation and non-pregnant adult sheep (Fig. 2). One-way ANOVA was used to detect the effects of ischemia and the duration of reperfusion (48 and 72 hours) on cerebral cortical IL-1β and IL-6 expression in the fetuses at 122–127 gestation (Fig. 3). A within group Student’s T test was used to examine differences between cerebral cortical and white matter expression of IL-1β and IL-6 in the fetal sheep at 122–127 days of gestation after ischemia and 72 hours of reperfusion. If a significant difference was found by ANOVA, the Fischer least-significant-difference test was used to detect specific differences among the brain regions and study groups. Values were expressed as means ± SD. P values of <0.05 were considered statistically significant.
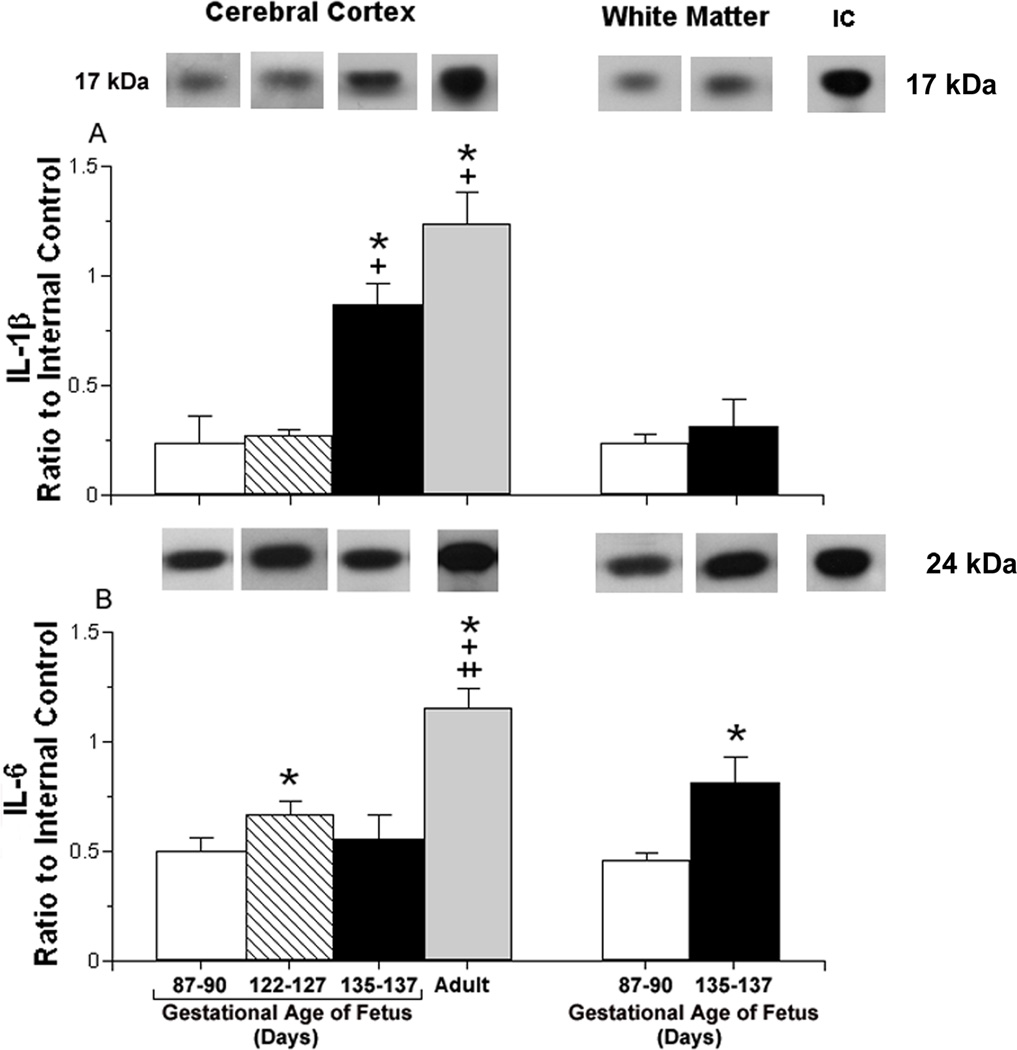
Fig. 1.
Constitutive interleukin protein expression plotted as a ratio to the internal control protein (IC) in the cerebral cortex and white matter of ovine fetuses.
Panel A shows: Representative Western immunoblots of IL-1β protein expression are shown for each study age group. The extracts from the brain tissues of the different age groups were placed on different immunoblots. Therefore, for the purpose of illustration in this and the subsequent figures, the most representative immunoblot for the mean value of each bar graph was presented for each age group.
IL-1β protein expression in the cerebral cortex of ovine fetuses at 87–90 (open bar, n=6), 122–127 (hatched bar, n=6), 135–137 (closed bar, n=6) days of gestation and in non-pregnant adult sheep (gray bar, n=3); [ANOVA, main effect for age group, F (3, 17) =72.8, P<0.001] and in white matter of ovine fetuses at 60 (open bar, n=6) and 135–137 (closed bar, n=6) days of gestation [ANOVA, main effect for age group, F (1,10) = 4.71, P<0.06]. IC is the internal control value.
Values are m±SD. *P<0.05 versus fetus at 87–90 days of gestation, +P<0.05 versus fetus at 122–127 days of gestation, ++P<0.05 versus fetus at 135–137 days of gestation.
Panel B shows: Representative Western immunoblots of IL-6 protein expression are shown for each study age group. IL-6 protein expression in the cerebral cortex of ovine fetuses at 87–90 (n=6), 122–127 (n=6), 135–137 (n=6) days of gestation and in non-pregnant adult sheep (n=3); [ANOVA, main effect for age group, F (3,17) = 11.9, P<0.0001] and for white matter of ovine fetuses at 87–90 (n=6) and 135–137 (n=6) days of gestation [ANOVA, main effect for age group, F(1,10) =35.8, P<0.001]. Symbol and statistical legends as for A.
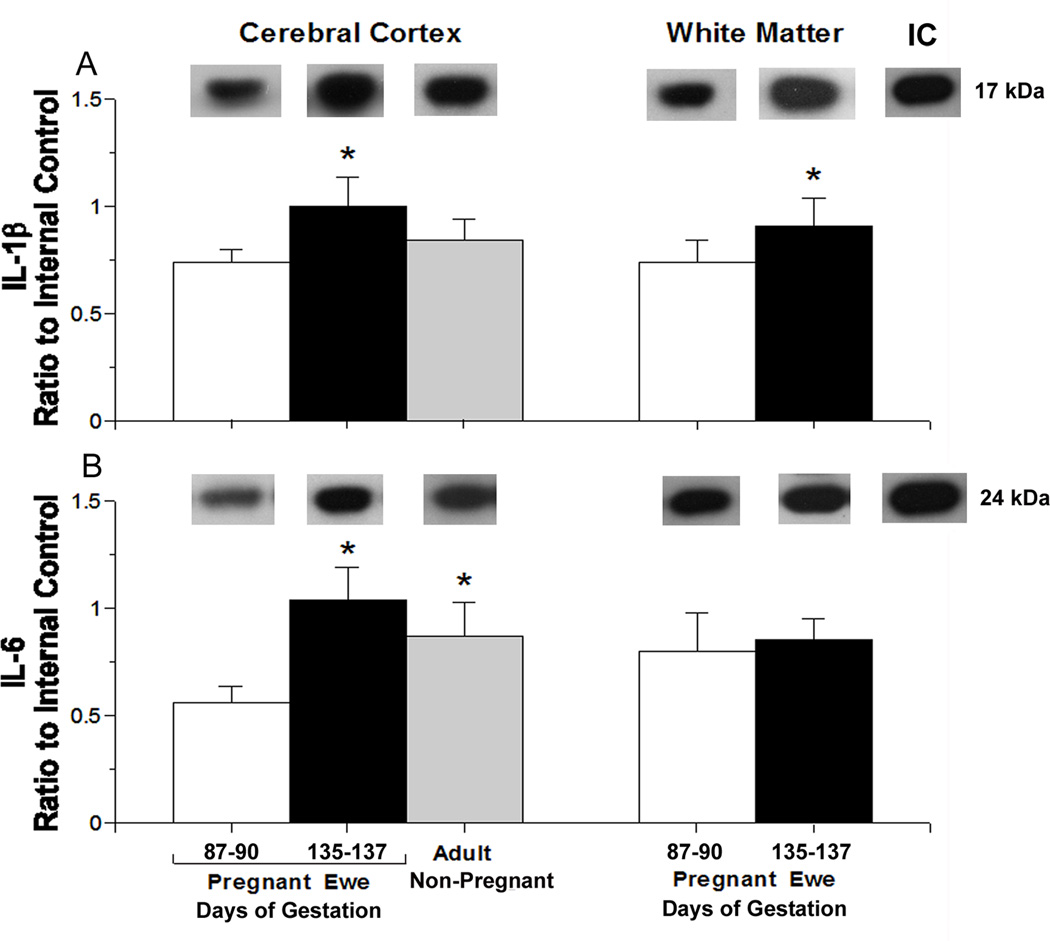
Fig. 2.
Constitutive interleukin protein expression plotted as a ratio to the internal control protein (IC) in the cerebral cortex and white matter of adult sheep.
Panel A shows: Representative Western immunoblots of IL-1β protein expression are shown for each study group. IL-1β protein expression in the cerebral cortex of pregnant adult sheep at 87–90 (n=6; open bar) and 135–137 (closed bar, n=6) days of gestation and non-pregnant adult sheep (gray bar, n=3) and in white matter of adult sheep at 87–90 (open bar, n=7) and 135–137 (closed bar, n=4) days of gestation; [ANOVA, main effect for group in cerebral cortex, F (2,12) =9.08, P<0.01 and in white matter [F (1,9) = 5.5, P<0.05 for white matter]. Values are m±SD, *P<0.05 versus pregnant ewe at 87–90 of gestation.
Panel B shows: Representative Western immunoblots of IL-6 protein expression are shown for each study group. IL-6 protein expression in the cerebral cortex of pregnant adult sheep at 87–90 (n=6) and 135–137 (n=6) days of gestation and non-pregnant adult sheep (n=3) and in white matter of pregnant adult sheep at 87–90 (n=7) and 135–137 (n=4) of days gestation; [ANOVA, main effect for group, F= (2,12) 21.8, P<0.001 in cerebral cortex and F(1,9) = 0.30, P=0.6 in white matter]. Symbol and statistical legends as for A.
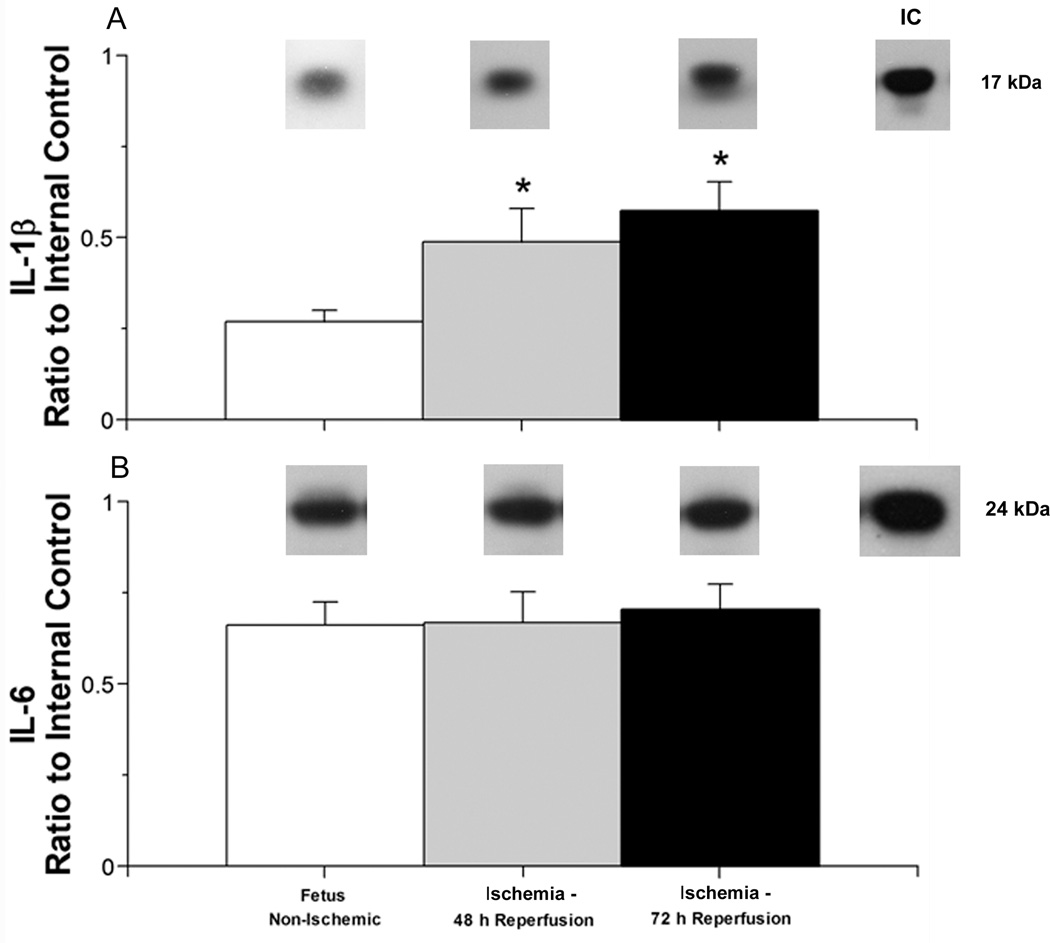
Fig. 3.
Interleukin protein expression plotted as a ratio to the internal control protein (IC) in the cerebral cortex of ovine fetuses at 122–127 days of gestation exposed the ischemia and reperfusion insult.
Panel A shows: Representative Western immunoblots of IL-1β protein expression are shown for each study group. IL-1β protein expression in cerebral cortex of the non-ischemic control ovine fetuses (open bar, n=6), ischemic ovine fetuses exposed to 48 hours (gray bar, n=5), or 72 hours of reperfusion (closed bar, n=6); [ANOVA, main effect for treatment, F (2, 14) = 29.4, P < 0.001]. Values are m±SD, * P<0.05 versus ischemic control ovine fetuses.
Panel B shows: Representative Western immunoblots of IL-6 protein expression are shown for each study group. IL-6 protein expression in cerebral cortex of the non-ischemic control ovine fetuses (n=6), ischemic ovine fetuses exposed to 48 hours (n=6) or 72 hours of reperfusion [ANOVA, main effect for treatment, F (2,14) = 0.60, P = 0.56. Symbol legends as for A.
3. Results
3.1 Study subjects
The fetal sheep from which the regional brain samples were obtained for this study had baseline pH, blood gas, heart rate, and mean arterial blood pressure values, which were within the physiological range for our laboratory and those of others, and have been previously reported (Elitt et al., 2003; Petersson et al., 2002; Stonestreet et al., 2000; Unno et al., 1998).
3.2 IL-1β and IL-6 Protein Expression
Constitutive IL-1β protein expression was significantly higher in the cerebral cortex of the adult non-pregnant sheep and in the fetuses at 135–137 days than in the fetuses at 87–90 and 122–127 days of gestation (Fig. 1 A). In contrast, IL-1β expression did not differ in white matter between the fetal sheep at 87–90 and 135–137 days of gestation (P<0.06; Fig. 1 A). Constitutive IL-6 protein expression was higher in the cerebral cortex of the adult non-pregnant sheep than in the fetal sheep at 87–90, 122–127, and 135–137 days of gestation and in the fetal sheep at 122–127 than at 87–90 days of gestation (Fig. 1 B). IL-6 expression was significantly higher in the white matter of the fetal sheep at 135–137 days than 87–90 days of gestation.
Constitutive IL-1β protein expression was significantly higher in the cerebral cortex of the pregnant ewes at 135–137 days than at 87–90 days of gestation (Fig. 2 A). IL-1β protein was also higher in the white matter of the pregnant sheep at 135–137 days than at 87–90 days of gestation. IL-6 expression was significantly higher in the cerebral cortex of the non-pregnant adult and pregnant ewes at 135–137 days of gestation than in the pregnant ewes at 87–90 days of gestation (Fig. 2 B). IL-6 protein expression in white matter did not differ between the pregnant sheep 87–90 and 135–137 days of gestation (P=0.6).
IL-1β protein expression was significantly higher in the cerebral cortex of the fetal sheep exposed to 30 minutes of ischemia and reperfusion for 48 and 72 hours than in the non-ischemic fetal sheep at 122–127 of gestation (Fig. 3 A). In contrast, IL-6 expression was similar in the fetal sheep exposed to 30 minutes of ischemia and reperfusion for 48 and 72 hours and in the non-ischemic fetal sheep at 122–127 of gestation (Fig. 3 B).
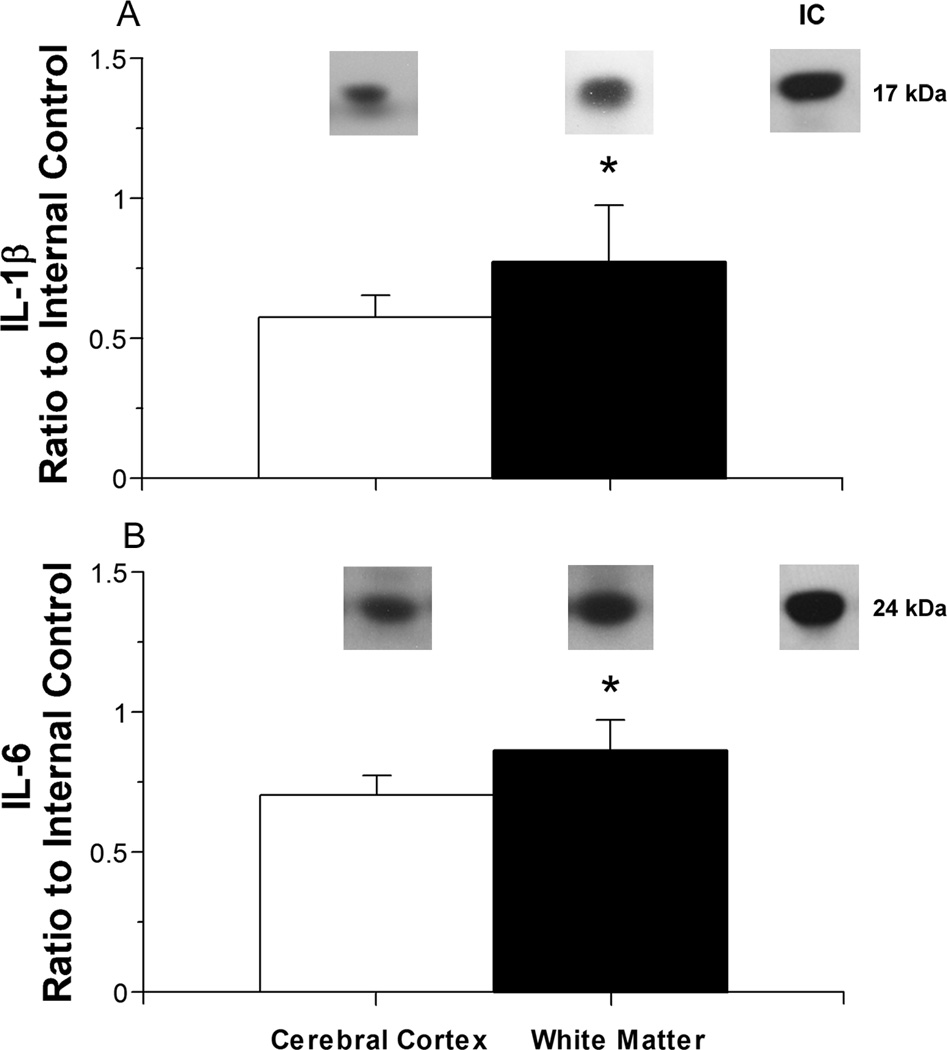
The IL-1β expression was higher in the white mater than in the cerebral cortex of the fetal sheep exposed to 30 minutes of ischemia and 72 hours of reperfusion in fetal sheep at 122–127 days of gestation (Fig. 4 A). Similarly, IL-6 expression was significantly higher in the white mater than in the cerebral cortex of the fetal sheep exposed to 30 minutes of ischemia and 72 hours of reperfusion (Fig. 4 B). White matter was not available from our former studies in the fetal sheep that were exposed to 30 minutes of ischemia and 48 hours of reperfusion, in the non-ischemic fetal sheep at 122–127 days of gestation or in the adult non-pregnant sheep.
Fig. 4.
Interleukin protein expression plotted as a ratio to the internal control protein (IC) in the cerebral cortex versus white matter of ovine fetuses at 122–127 days of gestation after 30 minutes of ischemia and 72 hours of reperfusion.
Panel A shows: Representative Western immunoblots of IL-1β protein expression are shown for each study group. IL-1β protein expression in the cerebral cortex (open bar, n=6) versus white matter (closed bar, n=6); Values are m±SD. *P<0.05 versus cerebral cortex.
Panel B shows: Representative Western immunoblots of IL-6 protein expression are shown for each study group. IL-6 protein expression in cerebral cortex (n=6) versus white matter (n=6). Symbol legends as for A. *P<0.05 versus cerebral cortex.
4. Discussion
There are four main findings of our study. First, IL-1β and IL-6 protein expression exhibited developmental regulation in cerebral cortex, and IL-6 in white matter of ovine fetuses. Second, IL-1 β and IL-6 expression increased in the cerebral cortex and IL-1β in the white matter of ewes with advancing gestation. Third, there are regional differences in IL-1β and IL-6 expression in normal fetal and maternal brain. Fourth, the response of IL-1β and IL-6 after ischemia was relatively greater in fetal white matter than cerebral cortex.
In this study, we have demonstrated that the constitutive expression of IL-1β and IL-6 proteins exhibits ontogenic increases from early in fetal life up to maturity in normal adult ovine brain. We showed that cerebral cortical, but not white matter, expression of IL-1β was up regulated in fetal sheep at 135–137 days of gestation and in non-pregnant adult sheep. Our findings are similar to results showing immunohistochemical staining of IL-1β-immunopositive cells of neuronal morphology in the cerebral cortex in fetal sheep between 100 and 140 days of gestation (Dziegielewska et al., 2000). However, our findings of elevated IL-1β expression in the adult cerebral cortex differ from their findings suggesting IL-1β-immunostaining was not present in adult ovine neocortex (Dziegielewska et al., 2000).
Constitutive IL-6 levels exhibited developmental up regulation in the cerebral cortex of the normal fetus with higher expression at 122–127 days of gestation and in adult brain. In contrast to IL-1β, IL-6 expression was higher in the white matter of normal fetuses at 135–137 than 87–90 days of gestation. Our results are similar to recent work in rodents showing that IL-6, but not IL-1β, concentrations are higher in the brain on postnatal day 12 than on day one (Brochu et al., 2011). The differential expression patterns that we found in the ovine brain and observed by others in rodents (Brochu et al., 2011) may be related to different regulatory functions of the proteins and/or differences in developmental programming. Interleukins are expressed in normal embryonic cells at early stages of development (Moro et al., 2008). Microglia, which secret IL-1β and IL-6, have been identified in the developing rodent brain at the onset of neurogenesis and are thought to regulate cell survival and pruning during normal development (Deverman and Patterson, 2009; Munoz-Fernandez and Fresno, 1998). Our findings are relevant because they provide comparative results for a species widely used in physiological studies (Back et al., 2006). They suggest that there could be ontogenetic regulatory roles for these cytokines in normal fetal brain, and that there are regional differences in the IL-1β and IL-6 expression under normal conditions that need to be considered when examining pro-inflammatory cytokine expression under pathological conditions across different animal models.
Even though cytokines are critical in establishing and maintaining normal pregnancy (Hanna et al., 2006; Hanna et al., 2000; Makhseed et al., 2000; Thaxton and Sharma, 2010) and change in the placenta during gestation (Hanna et al., 2006; Hanna et al., 2000), changes in cytokine expression have not been reported in maternal brain during gestation in any species. We found that there were both gestational age- and brain region-dependent changes in the IL-1β and IL-6 expression patterns with advancing gestation in the brain of pregnant ewes. To our knowledge, our results are the first to identify changes in cytokine expression in the maternal brain during pregnancy. These findings support the contention that brain cytokines also participate in interactions between maternal immune and reproductive systems, and suggest that these interactions could extend to functions within the normal maternal brain during gestation (Hill, 1992; Wegmann et al., 1993). We speculate that the relevance of our novel findings of these gestation-related changes in maternal cerebral cortex and white matter result from changes in the maternal hormonal milieu in preparation for labor and delivery, because estrogens have been shown to regulate neuro-inflammatory genes via estrogen receptors in the frontal cortex of middle-aged female rats (Sarvari et al., 2011).
Although we cannot be certain of the physiological significance of the higher IL-6 in levels in the fetuses and ewes during gestation, work in rodents suggests that IL-6 is constitutively expressed in the embryonic cortex, but that it is highest in the adult suggesting that this cytokine could be a factor regulating brain development (Pousset, 1994). Moreover, fetal electrocortical activity exhibits maturational changes over a similar time-frame in gestation, as the observed increases in IL-1β and IL-6, suggesting that increases in these cytokines represent a normal component of increasing cerebral cortical complexity during development in the fetus (Keen et al., 2011). However, we cannot determine from our temporal measurements of IL-1β and IL-6 expression if the changes in cytokines regulate fetal brain development and, hence, subsequent brain maturation, or if they represent effects secondary to the changes in brain development.
We found elevations in IL-1β, but not IL-6 in the cerebral cortex of fetal sheep, 48 and 72 hours after ischemic-injury. Our findings are consistent with recent work in rodents, showing that IL-1β protein was elevated 48 hours after hypoxia-ischemia on postnatal days 1 and 12 (Brochu et al., 2011). However, our findings that IL-6 did not increase in the fetal cerebral cortex after ischemia differ from findings in rodents suggesting that IL-6 was elevated 24 and 48 hours after hypoxia-ischemia. We also found relatively higher levels of both IL-1β and IL-6 in the white matter than in the cerebral cortex 72 hours after ischemic injury supporting the contention that there are differential regional cytokine responses to the same insult in the developing brain. Taken together with the findings in rodents (Brochu et al., 2011), our results suggest that there could be cytokine, age, species, insult and brain region specific differences in cytokine responses in the immature brain, and strengthens the contention that the stage of brain and immune system development are important in the modulation of CNS neuro-inflammatory responses (Brochu et al., 2011).
IL-1β and IL-6 are elevated in many brain disorders including ischemia and exhibit both pro- and anti-inflammatory functions (Hagberg et al., 1996; Matousek et al., 2011; Samland et al., 2003; Szaflarski et al., 1995; Thorns et al., 2002). IL-6 up regulates neuroprotective acidic fibroblast growth factor and reduces glutamate-induced cytotoxicity, whereas, IL-1β augments excitotoxicity, but prolonged IL-1β exposure also increases IL-6 release, which increases acidic fibroblast growth factor and decreases excitotoxicity (Thorns et al., 2002). On the other hand, IL-6 and inflammation in the CNS not only causes spontaneous neurodegeneration but also enhances neurotoxic insults resulting in neurological impairment (Samland et al., 2003), whereas IL-1β could also have a neuroprotective role in Alzheimer’s disease (Matousek et al., 2011). Hence, IL-1β and IL-6 in normal brain and under pathophysiological conditions have both pro- and anti-inflammatory roles that influence cellular survival in the brain (Thorns et al., 2002). We cannot determine from our studies what proportion of the constitutive or ischemia related changes in IL-1β and IL-6 expression represent pro- or anti-inflammatory effects in the ovine brain.
There are several limitations to our study. We did not have white matter available from the adult non-pregnant sheep, non-ischemic fetal sheep at 122–127 days of gestation or fetal sheep exposed to 48 hours of reperfusion. Hence, we could only compare the relative responses of IL-1β and IL-6 in cerebral cortex and white matter after ischemia and reperfusion for 72 hours. Nonetheless, although hypoxia-ischemia commonly leads to global brain injury, evidence suggests that dividing oligodendrocytes in developing white matter are sensitive to ischemia (Riddle et al., 2006; Segovia et al., 2008) and potentially cytokine mediated inflammation. Although we did not measure white matter damage in this study, we have shown in the same model that ischemia and reperfusion for 48 and 72 h results in white matter damage (Elitt et al., 2003; Petersson et al., 2002). The white matter lesion that we observed included a dramatic reduction in myelin basic protein and reactive gliosis (Petersson et al., 2002). Although it would have been interesting to correlate IL-1β and IL-6 expression with our reported brain injury scores and quantitative white matter damage after ischemic injury, we did not have residual tissue from enough animals to permit correlations between the IL-1β and IL-6 expressions in this report with our reported injury scores (Petersson et al., 2002). In addition, it would have been interesting to measure other mediators of ischemic white matter injury such as reactive oxygen species and nitric oxide that are also important components of ischemic damage (Bagenholm et al., 1998; Marks et al., 1999; Oka et al., 1993). However, we did not have sufficient residual tissue from our previous studies.
Periventricular white matter injury is one of the major causes of neurological impairment in premature newborns (Kaur and Ling, 2009). Although the etiology of this disorder is multifaceted, hypoxia-ischemia and overproduction of pro-inflammatory cytokine represent underlying factors (Kaur and Ling, 2009). Moreover, inflammatory processes that begin in utero most likely are antecedents of brain damage in premature infants because elevations in inflammation-related proteins including cytokines in the first days after preterm birth predict the risk of sonographic white matter damage in premature infants (Leviton et al., 2011). Therefore, our observations of relatively higher levels in IL-1β and IL-6 expression in white matter than the cerebral cortex could be indicative of factors mediating the white matter vulnerability previously observed in the ovine fetus (Back et al., 2006; Back et al., 2007; Elitt et al., 2003; Petersson et al., 2002). Consequently, ischemia-related elevations pro-inflammatory expression in the fetal white matter could predispose premature infants to white matter damage as previously suggested (Yoon et al., 1997). We speculate that neuroprotective agents targeted at limiting ischemia-related inflammation could be beneficial in preventing white matter related brain damage in neonates (Carty et al., 2011; Tuzun et al., 2011). In this regard, it would be of great interest to modulate the IL-1β and IL-6 expression levels with pharmacological interventions and determine the effect of such modulations on post-ischemic changes in the fetal brain.
5. Conclusion
We conclude that IL-1β and IL-6 exhibit developmental regulation in fetal brain, exhibit increased expression in the brain of ewes with advancing gestation, regional differences in normal fetal and maternal brain, and differential responses after ischemia in fetal cerebral cortex and white matter.
Highlights.
IL-1β and IL-6 exhibit developmental regulation in fetal brain
Expression of IL-1β and IL-6 increases in the brain of ewes with advancing gestation
IL-1β and IL-6 exhibit regional differences in normal fetal and maternal brain
IL-1β and IL-6 exhibit differential responses after ischemia in fetal cerebral cortex and white matter
Acknowledgments
This work was supported by NIH R01-HD34618 and 1R01-HD-057100 from the NIH National Institutes for Child Health and Human Development (NICHD) and the National Institutes for Neurological Diseases and Stroke (NINDS) and RI-INBRE P20RR016457-11 from the National Center for Research Resources (NCRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J. Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Bagenholm R, Nilsson UA, Gotborg CW, Kjellmer I. Free radicals are formed in the brain of fetal sheep during reperfusion after cerebral ischemia. Pediatr. Res. 1998;43:271–275. doi: 10.1203/00006450-199802000-00019. [DOI] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J. Comp. Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Prog. Brain Res. 1967;26:60–77. doi: 10.1016/S0079-6123(08)61419-3. [DOI] [PubMed] [Google Scholar]
- Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J Neuroinflammation. 2011;8:55–69. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Reinebrant HE, Gobe G, Colditz PB, Buller KM. Ibuprofen inhibits neuroinflammation and attenuates white matter damage following hypoxia-ischemia in the immature rodent brain. Brain Res. 2011;1402:9–19. doi: 10.1016/j.brainres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Cook CJ, Gluckman PD, Johnston BM, Williams C. The development of the somatosensory evoked potential in the unanaesthetized fetal sheep. J Dev Physiol. 1987;9:441–455. [PubMed] [Google Scholar]
- Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Duncan AR, Sadowska GB, Stonestreet BS. Ontogeny and the effects of exogenous and endogenous glucocorticoids on tight junction protein expression in ovine cerebral cortices. Brain Res. 2009;1303:15–25. doi: 10.1016/j.brainres.2009.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- Elitt CM, Sadowska GB, Stopa EG, Pinar H, Petersson KH, Stonestreet BS. Effects of antenatal steroids on ischemic brain injury in near-term ovine fetuses. Early Hum. Dev. 2003;73:1–15. doi: 10.1016/s0378-3782(03)00030-6. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Gilland E, Bona E, Hanson LA, Hahin-Zoric M, Blennow M, Holst M, McRae A, Soder O. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr. Res. 1996;40:603–609. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, Sharma S. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am. J. Reprod. Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J. Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- Hill JA. Cytokines considered critical in pregnancy. Am. J. Reprod. Immunol. 1992;28:123–126. doi: 10.1111/j.1600-0897.1992.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Hu XL, Yang Y, Hunt JS. Differential distribution of interleukin-1 alpha and interleukin-1 beta proteins in human placentas. J. Reprod. Immunol. 1992;22:257–268. doi: 10.1016/0165-0378(92)90047-8. [DOI] [PubMed] [Google Scholar]
- Kameda T, Matsuzaki N, Sawai K, Okada T, Saji F, Matsuda T, Hirano T, Kishimoto T, Tanizawa O. Production of interleukin-6 by normal human trophoblast. Placenta. 1990;11:205–213. doi: 10.1016/s0143-4004(05)80266-8. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog. Neurobiol. 2009;87:264–280. doi: 10.1016/j.pneurobio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Keen AE, Frasch MG, Sheehan MA, Matushewski B, Richardson BS. Maturational changes and effects of chronic hypoxemia on electrocortical activity in the ovine fetus. Brain Res. 2011;1402:38–45. doi: 10.1016/j.brainres.2011.05.043. [DOI] [PubMed] [Google Scholar]
- Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and alpha1- and beta1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod. Fertil. Dev. 2006;18:413–423. doi: 10.1071/rd05114. [DOI] [PubMed] [Google Scholar]
- Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr. 2011;158:897–903. doi: 10.1016/j.jpeds.2010.11.059. e891-895. [DOI] [PubMed] [Google Scholar]
- Makhseed M, Raghupathy R, Azizieh F, Farhat R, Hassan N, Bandar A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Hum. Reprod. 2000;15:2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- Malaeb SN, Sadowska GB, Stonestreet BS. Effects of maternal treatment with corticosteroids on tight junction protein expression in the cerebral cortex of the ovine fetus with and without exposure to in utero brain ischemia. Brain Res. 2007;1160:11–19. doi: 10.1016/j.brainres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KA, Mallard CE, Roberts I, Williams CE, Gluckman PD, Edwards AD. Nitric oxide synthase inhibition and delayed cerebral injury after severe cerebral ischemia in fetal sheep. Pediatr. Res. 1999;46:8–13. doi: 10.1203/00006450-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Matousek SB, Ghosh S, Shaftel SS, Kyrkanides S, Olschowka JA, O'Banion MK. Chronic IL-1beta-Mediated Neuroinflammation Mitigates Amyloid Pathology in a Mouse Model of Alzheimer's Disease without Inducing Overt Neurodegeneration. J Neuroimmune Pharmacol. 2011;7:156–164. doi: 10.1007/s11481-011-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro JA, Carretero J, Alonso MI, Martin C, Gato A, Mano Ade L. Prenatal expression of interleukin 1beta and interleukin 6 in the rat pituitary gland. Cytokine. 2008;44:315–322. doi: 10.1016/j.cyto.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J. Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler. Thromb. Vasc. Biol. 1998;18:503–513. doi: 10.1161/01.atv.18.4.503. [DOI] [PubMed] [Google Scholar]
- Petersson KH, Pinar H, Stopa EG, Faris RA, Sadowska GB, Hanumara RC, Stonestreet BS. White matter injury after cerebral ischemia in ovine fetuses. Pediatr. Res. 2002;51:768–776. doi: 10.1203/00006450-200206000-00019. [DOI] [PubMed] [Google Scholar]
- Pousset F. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Brain Res. Dev. Brain Res. 1994;81:143–146. doi: 10.1016/0165-3806(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Riddle A, Luo NL, Manese M, Beardsley DJ, Green L, Rorvik DA, Kelly KA, Barlow CH, Kelly JJ, Hohimer AR, Back SA. Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 2006;26:3045–3055. doi: 10.1523/JNEUROSCI.5200-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron NP, Kazianis JA, Padbury JF, Brown CM, McGonnigal BG, Sysyn GD, Sadowska GB, Stonestreet BS. Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex. Reprod. Fertil. Dev. 2005;17:535–542. doi: 10.1071/rd03044. [DOI] [PubMed] [Google Scholar]
- Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. American journal of physiology. Heart and circulatory physiology. 2010;298:H179–H188. doi: 10.1152/ajpheart.00828.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Stopa EG, Stonestreet BS. Ontogeny of connexin 32 and 43 expression in the cerebral cortices of ovine fetuses, newborns, and adults. Brain Res. 2009;1255:51–56. doi: 10.1016/j.brainres.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J. Neurosci. Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Hrabovszky E, Kallo I, Solymosi N, Toth K, Liko I, Szeles J, Maho S, Molnar B, Liposits Z. Estrogens regulate neuroinflammatory genes via estrogen receptors alpha and beta in the frontal cortex of middle-aged female rats. J Neuroinflammation. 2011;8:82–92. doi: 10.1186/1742-2094-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am. J. Physiol. 1999;276:R283–R289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–R477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26:1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorns V, Walter GF, Licastro F. Effects of IL6 and IL1beta on aFGF expression and excitotoxicity in NT2N cells. J. Neuroimmunol. 2002;127:22–29. doi: 10.1016/s0165-5728(02)00072-3. [DOI] [PubMed] [Google Scholar]
- Tseng YT, Yano N, Rojan A, Stabila JP, McGonnigal BG, Ianus V, Wadhawan R, Padbury JF. Ontogeny of phosphoinositide 3-kinase signaling in developing heart: effect of acute beta-adrenergic stimulation. American journal of physiology. Heart and circulatory physiology. 2005;289:H1834–H1842. doi: 10.1152/ajpheart.00435.2005. [DOI] [PubMed] [Google Scholar]
- Tuzun F, Kumral A, Dilek M, Ozbal S, Ergur B, Yesilirmak DC, Duman N, Yilmaz O, Ozkan H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J Matern Fetal Neonatal Med. 2011;25:849–854. doi: 10.3109/14767058.2011.587917. [DOI] [PubMed] [Google Scholar]
- Unno N, Wu WX, Wong CH, Bennett PR, Shinozuka N, Nathanielsz PW. Prostaglandin regulation of fetal plasma adrenocorticotropin and cortisol concentrations in late-gestation sheep. Biol. Reprod. 1998;58:514–519. doi: 10.1095/biolreprod58.2.514. [DOI] [PubMed] [Google Scholar]
- Volpe J. Hypoxic-Ischemic Encephalopathy, Neurology of the Newborn, 4th ed. Philadelphia, PA: W. B. Saunders Company; 2001. pp. 260–313. [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am. J. Obstet. Gynecol. 1997;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]