Abstract
Background
Thirty years ago we reported our experience with abdominal vascular trauma, highlighting the critical role of hypothermia, acidosis, and coagulopathy. Damage control surgery was subsequently introduced to address this “lethal triad.” The purpose of this study is to evaluate outcomes from our most recent 6-year experience compared to 30 years ago.
Methods
Patients with major abdominal vascular injuries were examined; the most recent 6-year period was compared with archived data from a similar 6-year period three decades ago.
Results
The number of patients with major abdominal vascular injuries decreased from 123 patients (1975–1980) to 64 patients (2004–2009). The mean initial pH decreased from 7.21 to 6.96 (1975–1980 vs. 2004–2009]) for patients with overt coagulopathy. In spite of increasingly protracted acidosis, mortality attributable to refractory coagulopathy has decreased from 46% to 19% (1975–1980 vs. 2004–2009, χ2 = 4.36, p = 0.04). There was no significant difference in mortality due to exsanguinating injuries (43% vs. 62%, 1975–1980 vs. 2004–2009, χ2 = 1.96, p = 0.16). Prehospital transport times were unchanged (22 min vs. 20 min, 1975–1980 vs. 2004–2009). Despite the administration of additional clotting factors and the advent of DCS, the overall mortality remains largely unchanged (37% vs. 33%, 1975–1980 vs. 2004–2009, χ2 = 0.385, p = 0.53).
Conclusions
Adoption of damage control surgery, including the implementation of a massive transfusion protocol, is associated with a reduction in mortality for abdominal vascular injuries due to coagulopathy, however, patients continue to die from exsanguination.
Keywords: damage control surgery, abdominal vascular trauma, massive transfusion, coagulopathy, blunt trauma, penetrating trauma, exsanguination
INTRODUCTION
Thirty years ago we reported our experience with intra-abdominal vascular injuries (IAVI) due to trauma, highlighting the critical role of hypothermia, acidosis, and coagulopathy. Our group reported that 89% of deaths following abdominal vascular injury were due to uncontrollable hemorrhage, and of those, we identified 51% with overt coagulopathy, made worse by hypothermia and acidosis.1 When grouped together, hypothermia, acidosis, and coagulopathy comprise “the bloody vicious cycle,” ultimately coined “the lethal triad.” Since this snapshot in time, there have been many innovations in the field of trauma surgery, including markedly improved resuscitation strategies, pre-operative computed tomography (CT) imaging, angioembolization, and the promotion of damage control surgery (DCS).2–6
In 1982, our group was one of the first to administer fresh frozen plasma (FFP) empirically for patients anticipated to require massive red blood cell (RBC) transfusion. Aggressive and early administration of clotting factors, especially in military studies, has been shown to improve survival among patients requiring massive transfusion.7–10 However, the proposed 1:1 ratio of FFP:RBC has been recently scrutinized for its true efficacy and requires further investigation.11–12 Thus, the exsanguinating patient remains a challenge.
Advances in emergency medical services (EMS), early injury identification, and surgical approaches have continued to improve mortality in patients with blunt and penetrating trauma.13–15 Damage control surgery, defined as abbreviating the initial exploratory laparotomy, leaving the trauma patient’s abdomen open, and delaying definitive closure until hemodynamic stability and correction of coagulopathy can be established, is associated with improved survival in the coagulopathic patient.16–17 Early identification of injuries, by focused abdominal sonography for trauma (FAST) examination and CT, is a significant priority in the treatment of trauma. Furthermore, early physiologic parameters, including base deficit (or lactate) in the emergency department (ED) have been useful in identifying high risk patients that would benefit from expedited surgical intervention.18
Given these advances in trauma management, we examined our present patient population in order to determine their impact on mortality and cause of death. We hypothesized that advances in trauma care over the past 30 years would have reduced overall mortality and, in particular, early deaths due to acute blood loss.
MATERIALS AND METHODS
After IRB approval, we queried our level I trauma center’s registry for all patients admitted to Denver Health Medical Center (DHMC) from 2004 to 2009 with abdominal injury. In order to make comparative analysis between groups, we maintained the same inclusion criteria as outlined in our 1982 study.1 Patients were selected based on having a primary injury to a major abdominal vessel, and the following patients were not included: (1) patients referred to DHMC from an outside facility, (2) patients with severe head injury, and (3) pre-operative deaths that occurred in the ED. At the time of the original study (1975–1980), DHMC did not accept out-of-town referrals, and for this reason we did not include referrals in our more recent cohort. Additionally, patients with devastating traumatic brain injury (TBI) were not included in the original study, so we did not include them here. We conducted a systematic review of the medical records of these patients and compared our findings with our experience between 1975 and 1980.
In our comparative analysis, we examined mechanism of injury, time to incision, vessels injured, blood product transfusion ratios, mortality, and cause of death attributable to coagulopathy. Cause of death, including coagulopathy, was assigned by the hospital trauma QI committee. In the most recent patient population we further analyzed the impact of DCS. DCS is used when the following circumstances exist: unachievable hemostasis due to refractory coagulopathy, injuries amenable to packing, limited access to major venous injury, anticipated need for a time-consuming procedure, and bowel edema.16 All statistical calculations, including Chi-square analysis and unpaired t-tests (one and two-tailed, type 3) were performed in Microsoft Excel (2011), and reported as mean ± standard deviation. Specifically we looked at mortality, FFP:RBC ratio, incidence of coagulopathy, international normalized ratio (INR), partial thromboplastin time (PTT) and base deficit at emergency department (ED) arrival and at 6 hours post injury.
Decisions concerning the restoration of intravascular volume after injury are made using a standard algorithm based on the patient’s initial hemodynamic status, response to crystalloid, and reassessment of hemodynamic stability by hemoglobin assays.19 Patients that present with hemodynamic instability (heart rate > 120/min, systolic blood pressure < 90, or ongoing hemorrhage) are infused with crystalloid. Transfusion of packed red blood cells is administered to patients who respond only transiently to crystalloid. For refractory cases, the massive transfusion protocol (MTP) is activated, i.e. patients are given FFP:RBC in a 1:2 ratio. This protocol has been in place at our institution since the early 1990s.
RESULTS
2004–2009 Patient Demographics
We identified 64 patients from 2004–2009 who were eligible for the study based on having sustained a primary abdominal vascular injury. Fifty-eight (91%) were men with a mean age of 32.3 ± 14.5 (range, 15 to 80). Mean injury severity score (ISS) was 27.3 ± 13.0 (range, 9 to 60). ISS was available for 62/64 patients. The majority (75%) sustained penetrating trauma and underwent DCS (53%). The mean ISS for penetrating trauma was 23.4 ± 9.4 and 41.7 ± 15.0 for blunt trauma patients (p < 0.001). ISS for survivors was 24.4 ± 12.5 versus 33.2 ± 12.4 for nonsurvivors (p < 0.05). Average ISS for DCS patients was 28.8 ± 13.1 versus 25.5 ± 13.0 in non-DCS patients (p = 0.16). Because ISS was not used as a patient evaluation tool at the time of our initial publication on IAVI, we were unable to compare values between cohorts. Similarly, abdominal injury scores (AIS) were not available for the 1975–1980 cohort. Instead, the only recorded indicator of associated injuries was the Abdominal Trauma Index (ATI), which reflects injuries within the abdominal cavity, but do not permit comparison to ISS in the more recent cohort.
Demographic Comparison
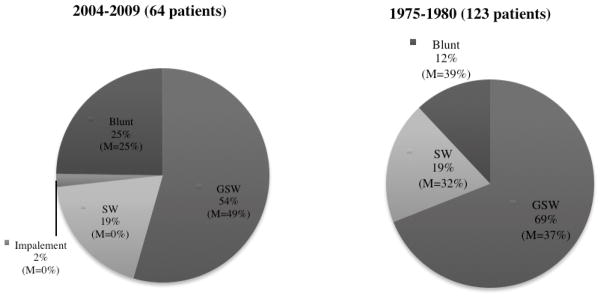
During the past 30 years, the incidence of major vascular injury has decreased (123 vs. 64 patients, 1975–1980 vs. 2004–2009). These data are summarized in Table 1, including a comparison of the number of vascular injuries subdivided by vessel. Although gunshot wounds (GSW) remain the predominant mechanism of injury (69% 1975–1980 vs. 55% 2004–2009), there is a relative increase in the number of patients presenting with injuries due to blunt trauma (12% vs. 25%, 1975–1980 vs. 2004–2009) (Figure 1). Overall survivability did not improve for either blunt or penetrating trauma (61% vs. 75%, blunt; and 64% vs. 65%, penetrating; 1975–1980 vs. 2004–2009). Prehospital transport times were unchanged (22 min vs. 20 min, 1975–1980 vs. 2004–2009).
Table 1.
Demographic Comparison and Vessel Injuries
| 2004–2009 | 1975–1980 | ISS (2004–2009) | |
|---|---|---|---|
| Demographics | |||
| Total | 64 | 123 | 27.3 |
| Age | 33 (15–80) | 29 (3–73) | |
| Male | 91% | 80% | 26.9 |
| Mechanism | |||
| Blunt | 15 (23%) | 15 (12%) | 41.7 |
| Penetrating | 49 (77%) | 108 (88%) | 23.5 |
| GSW | 35 (55%) | 85 (69%) | 25.7 |
| SW | 12 (19%) | 23 (19%) | 17.8 |
| Other P | 1 (2%) | N/A | 16.0 |
| Blunt | 15 (23%) | 15 (12%) | 41.7 |
| ED SBP, mm Hg | |||
| <60 | 14 (22%) | 62 (50%) | 30.6 |
| >60 | 50 (78%) | 46 (37%) | 26.4 |
| Major Vessel Injuries | |||
| Aorta | 5 | 18 | |
| Celiac Axis | 7 | 6 | |
| SMA | 10 | 6 | |
| IMA | 0 | 0 | |
| Renal A | 4 | 8 | |
| Iliac Artery | 16 | 19 | |
| IVC | 17 | 51 | |
| Renal V | 9 | 9 | |
| Iliac V | 22 | 21 | |
| Portal Vein | 3 | 9 | |
| Splenic Vein | 5 | 1 | |
| SMV | 5 | 10 | |
| IMV | 1 | 3 | |
| Mortality of Multiple Vascular Injuries | |||
| 1 | 19% | 24% | |
| >1 | 50% | 61% | |
| Overall Mortality | 33% | 37% | |
| <60 SBP | 86% | 61% | 29.4 |
| >60 SBP | 9% | 13% | 37.7 |
| Aorta + IVC | 100% | ||
| IVC | 75% | 39% | |
Figure 1. Mechanism of Injury.
The predominant mechanism of injury has changed over time, with a relative increase in blunt trauma. Gunshot wounds (GSW) remain the predominant mechanism of injury.
Physiologic Indices and Transfusion Comparison
For the recent cohort, mean systolic blood pressure (SBP) in the ED was 86.4 ± 49.0. Fourteen patients (22%) presented to the ED with a SBP less than 60 mm Hg; 12 patients had no palpable blood pressure on arrival. Thirty-six patients (56%) had a SBP greater than 90 mm Hg (120.5 ± 20.8). The overall mortality for patients with SBP less than 90 mm Hg was 57% (16 patients) versus 8% mortality in patients with a SBP of at least 90 mm Hg. A SBP less than 60 mm Hg was associated with a high mortality of 86%.
For patients with overt coagulopathy the mean pH declined from 7.21 to 6.96 (1975–1980 vs. 2004–2009). In the 2004–2009 cohort, the mean pH for all patients was 7.11 (N = 56). Overall the transfusion ratio of FFP:RBC in these patients was 1:2.6 (2004–2009) vs. 1:9 (1975–1980) (Table 2). The mortality of exsanguinating injuries has trended upward (43% vs. 62%, 1975–1980 vs. 2004–2009, χ2 = 1.96, p = 0.16).
Table 2.
Mortality and Coagulopathy Comparison
| 2004–2009 | 1975–1980 | |
|---|---|---|
| Number of Patients | 64 | 123 |
| Damage Control Surgery | 34 (53%) | 0 (0%) |
| Received >20 Units of RBC | 20 (31%) | 35 (28%) |
| Overall Mortality | 21 (33%) | 46 (37%) |
| Deaths due to Exsanguination | 13 (62%) | 20 (43%) |
|
| ||
| Overt Coagulopathy | ||
| Deaths due to Coagulopathy | 4 (19%) | 21 (46%), p < 0.05 |
| Mean pH | 6.96 | 7.21 |
| Mean Temperature (°C) | 35.7 | 31.3 |
| RBC:FFP | 2–3:1 | 9:1 |
Eighteen patients, from the 2004–2009 cohort, initially presented to the ED with an INR >1.5 (2.97 ± 1.14; mortality, 66.7%), but within 24 hours of admission there were 41 patients that had at least one INR greater than 1.5 (2.64 ± 1.26; mortality, 41.5%). Patients that required an increasing number of units of RBC showed reduced survivability (Figure 2).
Figure 2.
There is a trend toward increased mortality with increased blood transfusion. In the most recent cohort, patients who received more than 20 units of packed red blood cells had a mortality of 60%
For patients that underwent DCS, there was no significant difference in the FFP:RBC ratio between survivors and nonsurvivors (1:2.4 vs. 1:2.1, p = 0.60). However, among patients that didn’t require or failed to undergo DCS, survivors received a higher proportion of clotting factors relative to packed RBCs (1:2.6 vs. 1:6.8, p < 0.01).
Outcomes Comparison
Overall mortality remains largely unchanged (37% vs. 33%, 1975–1980 vs. 2004–2009, p = 0.53). Mortality attributable to refractory coagulopathy has decreased from 46% to 19% in spite of increasingly protracted acidosis (21/46 1975–1980 vs. 4/21 2004–2009, p < 0.05). In the present cohort, 81% of deaths were attributable to acute blood loss, and of those deaths, coagulopathy was identified in 24%. Despite increased administration of blood components, the average time to death in patients with coagulopathy was 1 hour and 50 minutes. In this group of patients, the mean ISS for coagulopathic patients was 26.83 ± 8.01 (N = 6), 21 ± 7.07 in survivors (N = 2), and 29.75 ± 7.5 in nonsurvivors (N = 4) (2004–2009). Mortality was 67% in patients with overt coagulopathy. ISS values were comparable between patients with overtly identifiable coagulopathy and all other patients (p = 0.90). There was no significant difference in ISS between survivors and nonsurvivors among coagulopathic patients (p = 0.28).
Patients who received more than 20 units of packed red blood cells (N = 20) had an overall mortality of 60% (Figure 2). In the present cohort, 48% of deaths occurred during the first 3 hours of admission, while only 30% of deaths occurred between 3 and 6 hours of admission. Only one death occurred between 6 and 9 hours of admission, and the three remaining deaths (15%) occurred after 12 hours (range, 14 hours to 12 days). Time to death was unavailable for one patient. The high percentage of early deaths (48% within three hours of admission) similarly reflects the upward trend in mortality of exsanguinating patients.
DISCUSSION
Of greatest significance, the results of this study demonstrate that adoption of damage control is associated with reduced mortality due to coagulopathy in patients with abdominal vascular trauma. Our data show that of the deaths due to uncontrollable hemorrhage, 24% were attributable to coagulopathy, whereas in 1983 we found that half of hemorrhagic deaths occurred after surgical repair of major bleeding sites. Despite the administration of additional clotting factors, better approaching the debated 1:1–2 goal administration ratio of FFP:RBC, and the advent of DCS, the overall mortality remains largely unchanged.20 While adoption of damage control has reduced mortalities for abdominal vascular injuries due to coagulopathy, patients continue to die from exsanguination. These patients represent an ongoing challenge for the future. Our experience is not unique. High mortality associated with exsanguination was similarly reported by Asensio et al in 2003.21
Although a recent case series study showed improved survival with DCS in a mixed cohort of patients with either solid organ injuries or abdominal vascular injuries undergoing DCS, identifying specific patients with isolated intra-abdominal vascular injuries (IAVI) who will benefit from this practice remains unclear.22 Recently, Paul et al. compared their experience with IAVI to a historical cohort, and found no difference in mortality, in spite of the improvements in prehospital care, aggressive administration of clotting factors and the advent of DCS.23 In this cohort, most deaths were due to exsanguination rather than refractory coagulopathy, which can be inferred from number of early deaths (48%) occurring within 3 hours of admission. This phenomenon has been observed in the trauma population at-large (Morton A, Moore EE, Wohlauer MV, et al. Revisiting early post-injury mortality: are they bleeding because they are dying or dying because they are bleeding? Journal of Surgical Research. Under Review.) Additionally, one may wonder if there is a threshold of RBC transfusion that could predict futile care. There was not a number of RBCs transfused above which no one survived. For example, one person received 90 units of RBCs in 6 hours and still died. The patient with the next highest number of RBCs transfused (75 units) survived.
In addition to future FFP studies, the efficacy of blood substitutes in trauma resuscitation warrants further investigation. Our institution previously examined the benefit-to-risk ratio associated with the use of the oxygen carrier substitute, PolyHeme, and determined that its use is potentially beneficial when blood is needed but unavailable.24 Currently, we are studying prehospital plasma transfusion as a method to improve survival in patients with life-threatening hemorrhage.
This study from a single institution has several limitations. First, not all data were available for analysis and comparison from the 1975–1980 study. For example, although data suggest mortality for IAVI has not changed in 30 years, this may be due to a shift in deaths from the ED to the OR. We were unable to investigate the significance of this shift because ED deaths were not included, and ISS was not calculated in the original study. However, ISS alone is limited because it can underestimate the severity of isolated, devastating injuries. We also suspect that the explanation might be attributable to the increased proportion of patients sustaining blunt trauma. In contrast to the precise focus of injury that occurs with penetrating abdominal trauma, the force is dispersed in blunt trauma, leading to widespread endothelial injury that can be more difficult for the surgeon to identify and manage. Also, due to the retrospective nature of this case series, causality cannot be shown; rather we can only show an association between adoption of DCS and decreased coagulopathic deaths. Additionally, we recognize that clinical coagulopathy in the context of hypothermia is difficult to asses by laboratory testing because tests are performed at 37° C rather than body temperature.25
In summary, although adoption of damage control is associated with reduced mortality from abdominal vascular injuries due to coagulopathy, patients continuing to die from exsanguination represent a persistent challenge. Additional studies are warranted to differentiate between patients that have “acute coagulopathy due to trauma” and patients that are dying from consumptive coagulopathy due to surgical bleeding. Death from exsanguination eclipses coagulopathy as a primary cause of death in these patients, and remains a major problem.
Footnotes
Presented as a Quick Shot Presentation at the 7th Annual Academic Surgical Congress, February 14–16, 2012 in Las Vegas, NV
Disclaimer: The content is solely responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Disclosure of Financial Interests and Potential Conflicts of Interest. There are no biomedical financial interests or potential conflicts of interest.
Financial disclosures: Supported in part by NIH P50GM49222 and T32GM08315
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kashuk JL, Moore EE, Millikan JS, et al. Major abdominal vascular trauma—a unified approach. J Trauma. 1982;22:672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Feliciano DV, Mattox KL, Jordan GL., Jr Intra-abdominal packing for control of hepatic hemorrhage: a reappraisal. J Trauma. 1981;21:285–90. doi: 10.1097/00005373-198104000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–5. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz JJ, Jr, Cullinane DC, Dutton WD, et al. The management of the open abdomen in trauma and emergency general surgery: part 1—damage control. J Trauma. 2010;68:1425–38. doi: 10.1097/TA.0b013e3181da0da5. [DOI] [PubMed] [Google Scholar]
- 5.Nicholas JM, Rix EM, Easley KA, et al. Changing patterns in the management of penetrating abdominal trauma: the more things change, the more they stay the same. J Trauma. 2003;55:1095–108. doi: 10.1097/01.TA.0000101067.52018.42. [DOI] [PubMed] [Google Scholar]
- 6.Higa G, Friese R, O’Keeffe T, et al. Damage control laparotomy: a vital tool once overused. J Trauma. 2010;69:53–9. doi: 10.1097/TA.0b013e3181e293b4. [DOI] [PubMed] [Google Scholar]
- 7.Borgman M, Spinella P, Perkins M, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–6. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Tien H, Nascimento B, Jr, Callum J, et al. An approach to transfusion and hemorrhage in trauma: current perspectives on restrictive transfusion strategies. Can J Surg. 2007;50:202–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyt DB. Blood and war—lest we forget. J Am Coll Surg. 2009;209:681–6. doi: 10.1016/j.jamcollsurg.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Chambers LA, Chow SJ, Shaffer LE. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011;136:364–70. doi: 10.1309/AJCPH16YXJEFSHEO. [DOI] [PubMed] [Google Scholar]
- 12.Nunez TC, Young PP, Holcomb JB, et al. Creating, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. J Trauma. 2010;68:1498–505. doi: 10.1097/TA.0b013e3181d3cc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozycki GS, Ochsner MG, Schmidt JA, et al. A prospective study of surgeon-performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma. 1995;39:492–8. doi: 10.1097/00005373-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Henneman PL, Marx JA, Moore EE, et al. Diagnostic peritoneal lavage: accuracy in predicting necessary laparotomy following blunt and penetrating trauma. J Trauma. 1990;30:1345–55. [PubMed] [Google Scholar]
- 15.Aprahamian C, Thompson BM, Towne JB, et al. The effect of a paramedic system on mortality of major open intra-abdominal vascular trauma. J Trauma. 1983;23:687–90. doi: 10.1097/00005373-198308000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Moore EE, Thomas G. Orr Memorial Lecture. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg. 1996;172:405–10. doi: 10.1016/s0002-9610(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 17.Balogh Z, McKinley BA, Cox CS, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock. 2003;20:483–92. doi: 10.1097/01.shk.0000093346.68755.43. [DOI] [PubMed] [Google Scholar]
- 18.Eachempati SR, Robb T, Ivatury RR, et al. Factors associated with mortality in patients with penetrating abdominal vascular trauma. J Surg Res. 2002;108:222–6. doi: 10.1006/jsre.2002.6543. [DOI] [PubMed] [Google Scholar]
- 19.Cothren CC, Biffl WL, Moore EE. Trauma. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter John G, Matthews JB, Pollock RE, editors. Schwartz’s Principles of Surgery. 9e. McGraw-Hill Companies, Inc; 2010. pp. 135–95. [Google Scholar]
- 20.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma: packed red blood cells the answer? J Trauma. 2008;65:261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 21.Asensio JA, Petrone P, Roldan G, et al. Analysis of 185 Iliac Vessel Injuries. Arch Surg. 2003;138:1187–1194. doi: 10.1001/archsurg.138.11.1187. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261–9. doi: 10.1097/00005373-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Paul JS, Webb TP, Aprahamian C, et al. Intraabdominal vascular injury: are we getting any better? J Trauma. 2010;69:1393–7. doi: 10.1097/TA.0b013e3181e49045. [DOI] [PubMed] [Google Scholar]
- 24.Moore EE, Johnson JL, Moore FA, et al. The USA Multicenter Prehospital Hemoglobin-based Oxygen Carrier Resuscitation Trial: scientific rationale, study design, and results. Crit Care Clin. 2009;25:325–56. doi: 10.1016/j.ccc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed RL, 2nd, Johnson TD, Hudson JD, et al. The disparity between hypothermic coagulopathy and clotting studies. J Trauma. 1992;33:465–70. doi: 10.1097/00005373-199209000-00022. [DOI] [PubMed] [Google Scholar]