Abstract
Impaired control of mediolateral body motion during walking is an important health concern. Developing treatments to improve mediolateral control is challenging, partly because the mechanisms by which muscles modulate mediolateral ground reaction force (and thereby modulate mediolateral acceleration of the body mass center) during unimpaired walking are poorly understood. To investigate this, we examined mediolateral ground reaction forces in eight unimpaired subjects walking at four speeds and determined the contributions of muscles, gravity, and velocity-related forces to the mediolateral ground reaction force by analyzing muscle-driven simulations of these subjects. During early stance (0-6% gait cycle), peak ground reaction force on the leading foot was directed laterally and increased significantly (p < 0.05) with walking speed. During early single support (14-30% gait cycle), peak ground reaction force on the stance foot was directed medially and increased significantly (p < 0.01) with speed. Muscles accounted for more than 92% of the mediolateral ground reaction force over all walking speeds, whereas gravity and velocity-related forces made relatively small contributions. Muscles coordinate mediolateral acceleration via an interplay between the medial ground reaction force contributed by the abductors and the lateral ground reaction forces contributed by the knee extensors, plantarflexors, and adductors. Our findings show how muscles that contribute to forward progression and body-weight support also modulate mediolateral acceleration of the body mass center while weight is transferred from one leg to another during double support.
Introduction
Impaired control of mediolateral motion during walking is an important problem for a variety of individuals. Abnormal mediolateral motion has been reported in children with cerebral palsy (Hsue et al., 2009), individuals with Down syndrome (Kubo and Ulrich, 2006; Agiovlasitis et al., 2009), and adolescents with scoliosis (Chockalingam et al., 2008). For elderly individuals, failure to control mediolateral motion can cause a sideways fall, a major risk factor for hip fracture (Greenspan et al., 1998). The direct medical cost of fall-related fractures was estimated to exceed $19 billion in the year 2000 in the United States (Stevens et al., 2006). One-year mortality rates for hip fractures range from 12-20% for women and 21-42% for men (Elliott et al., 2004). Approximately half of all falls among elderly community dwellers occur during locomotion (Ashley et al., 1977).
Treating impaired mediolateral balance during walking is challenging, in part, because the mechanisms used to modulate mediolateral motion during unimpaired gait are poorly understood. Several studies have measured kinematics, ground reaction forces, and joint moments related to mediolateral balance during walking (Nilsson and Thorstensson, 1989; MacKinnon and Winter, 1993; Lyon and Day, 1997; Orendurff et al., 2004; Andriacchi et al., 2005; Hof, 2007; Agiovlasitis et al., 2009; Kavanagh, 2009). Maintaining mediolateral balance requires active control by muscles (Kuo, 1999; Donelan et al., 2004), yet studies of gait kinematics and kinetics have not examined how muscles produce mediolateral accelerations.
How muscles control mediolateral balance can be examined by analyzing how muscles produce mediolateral ground reaction forces and the resulting acceleration of the body mass center (Chou et al., 2003; Yang et al., 2011). Muscles generate forces that propagate along the body segments via intersegmental forces to cause each foot to apply a force to the ground. The ground responds by applying an equal and opposite ground reaction force to each foot. The mediolateral acceleration of the mass center is equal to the mediolateral ground reation force divided by body mass (Davis and Kaufman, 2006). Pandy et al. (2010) identified the muscle groups contributing to mediolateral acceleration of the body mass center during free-speed walking. Walking speed affects muscle contributions to mass center acceleration (Liu et al., 2008; Neptune et al., 2008) and slower walking speeds have been reported in elderly individuals (Murray et al., 1969; Hageman and Blanke, 1986; Elble et al., 1991) and individuals with gait disorders (Turnbull et al., 1995; Abel and Damiano, 1996; Goldie et al., 1996; Dingwell et al., 2000). Thus, it is important to understand how muscle contributions to the mediolateral ground reaction force vary with speed, yet no study has investigated this.
Our study examined muscle contributions to the mediolateral ground reaction force during unimpaired walking to establish a baseline line for comparing muscle contributions to pathological populations. Our objectives were to (1) determine how mediolateral ground reaction force changes with walking speed, (2) identify the muscle groups that make the largest contributions to mediolateral ground reaction force across a range of speeds, and (3) determine how muscle contributions to mediolateral ground reaction force change with speed.
Methods
To determine the contributions of muscles to the mediolateral ground reaction force, we analyzed muscle-driven simulations of eight unimpaired subjects walking at four speeds (Liu et al., 2008). The subjects were aged 12.9 ± 3.3 years. The subject masses were 51.8 ± 19.2 kg. The subjects’ leg lengths were 0.81 ± 0.09 m. Schwartz et al. (2008) reported procedures for collecting and processing kinematics, ground reaction forces, and electromyographic (EMG) data. For each subject, the speed of each walking trial was categorized as very slow, slow, free, or fast using a dimensionless walking velocity , where v is absolute walking velocity, Lleg is leg length, and g is gravitational acceleration (Hof, 1996). Average dimensional walking speeds over all subjects were 0.54 m/s (very slow), 0.75 m/s (slow), 1.15 m/s (free), and 1.56 m/s (fast).
The procedures for generating and testing the simulations are described by Liu et al. (2008). We adapted these procedures to version 1.9.1 of OpenSim (Delp et al., 2007). A generic musculoskeletal model (Delp et al., 1990; Thelen and Anderson, 2006) with 19 degrees of freedom and 92 muscle-tendon actuators was scaled to match the size and mass of each subject. The degrees of freedom included in the model are pelvis position (3 degrees of freedom), pelvis orientation (3 degrees of freedom), lumbar joint (3 degrees of freedom), and for each leg, hip flexion-extension, abduction-adduction, and internal-external rotation, knee flexion-extension, and ankle plantarflexion-dorsiflexion. Computed muscle control (Thelen et al., 2003) was used for each walking trial to determine muscle excitations that drove the model to track the experimental kinematics while subject to the measured ground reaction forces. The excitation patterns were shown to be consistent with EMG patterns from the experiments (see Fig. 4 in Liu et al. (2008)). Computed muscle control generated a simulation for each of the eight subjects, walking at each of the four speeds. The data, setup files, and simulations are available at https://simtk.org/home/mspeedwalksims so that others can analyze the simulations and reproduce the results reported here.
In each simulation, the action forces (e.g., muscle forces) propagate along the body segments via intersegmental forces to cause each foot to apply a force to the ground. The ground responds by applying an equal and opposite reaction force to each pressing foot. We determined the ground reaction force (i.e., the force applied by the ground to each foot) as follows. In each trial, the subject achieved a double support phase with each foot striking a different force plate. Assuming symmetry, we time-shifted the ground reaction forces from the left and right feet using an averaging function to blend the data when they overlapped. This yielded a continuous ground reaction force from initial contact to terminal swing (Liu et al., 2008).
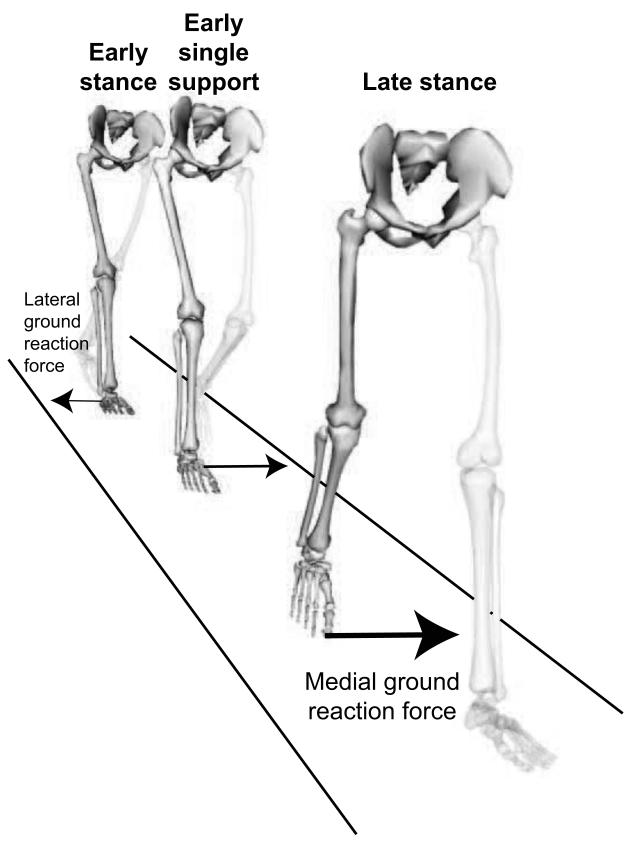
We identified the peak mediolateral ground reaction force for each subject and speed (very slow, slow, free, fast) during three periods: early stance (0-6% gait cycle) when the peak force on the leading foot is directed laterally, early single support (14-30% gait cycle) when the peak force on the stance foot is directed medially, and late stance (40-56% gait cycle) when the peak force on the trailing foot is directed medially (Fig. 1). In each period, we determined how peak mediolateral ground reaction force changed with walking speed as follows. We performed a one-way repeated measures analysis of variance (SPSS Inc., Chicago, IL, USA) using speed level as the within-subjects variable to test whether walking speed had a significant effect on the peak mediolateral ground reaction force during the period. If the effect of walking speed was significant, within-subjects repeated contrasts were analyzed to determine whether significant differences in peak mediolateral ground reaction force existed between any two speed levels. The significance level for all tests was α ≤ 0.05.
Figure 1.
This study examines the mediolateral component of the ground reaction force during three periods: early stance, early single support, and late stance. The images show the direction of the peak mediolateral ground reaction force during each period.
To identify the muscle groups making the largest peak contributions to the mediolateral ground reaction force, we performed an induced accelerations analysis on each simulation. We extended the induced accelerations analysis in OpenSim version 2.4 to compute the reaction forces representing the interaction of each foot with the ground. The induced accelerations analysis computed the contributions of action forces (i.e., muscle forces, gravity, and forces due to Coriolis and centripetal accelerations) to the mediolateral ground reaction force of each foot as follows. The interaction of each foot with the ground was modeled by a rolling-without-slipping constraint (Hamner et al., 2010). At each time instant during the simulation, each action force (e.g., a muscle force) was applied individually and the constraint reaction force, which is the action force’s contribution to the ground reaction force, was calculated. We verified that the sum of the contributions of all action forces to the ground reaction force was within one standard deviation of the measured ground reaction force (Supplementary Figs. 1-3). Contributions from the residual force and moment acting on the pelvis (Delp et al., 2007) to the ground reaction force were negligible (Supplementary Fig. 4). Individual muscle forces were summed into muscle groups to facilitate presentation (Table 1).
Table 1.
Groups of contributors to mediolateral ground reaction force.
| Description | |
|---|---|
| ABD (Abductors) | Gluteus medius (anterior, intermediate, and posterior compartments), gluteus minimus (anterior, intermediate, and posterior compartments), and tensor fasciae latae |
| DF (Dorsiflexors) | Tibialis anterior, extensor hallucis longus |
| HAMS (Hamstrings) | Semimembranosus, semitendinosus, biceps femoris long head, and biceps femoris short head |
| VAS (Vasti) | Vastus medialis, intermedius, lateralis |
| ADD (Adductors) | Adductor longus, adductor brevis, adductor magnus (three compartments), pectineus, and gracilis |
| GMAX (Gluteus maximus) | Anterior, intermediate, and posterior compartments of gluteus maximus |
| GAS (Gastrocnemius) | Medial and lateral heads of gastrocnemius |
| SOL (Soleus) | Soleus |
| Back + abs | Erector spinae, internal oblique, and external oblique |
| GRAV (Gravity) | Gravitational force |
| VEL (Velocity) | Coriolis and centripetal forces |
To determine how muscle contributions to the mediolateral ground reaction force changed with walking speed, we performed a one-way repeated measures analysis of variance in each period, once for each action force of interest, with the peak contribution of the action force to the ground reaction force during this period as the dependent variable, and with speed level as the within-subjects variable. This analysis revealed whether walking speed had a significant effect on each action force’s peak contribution to the mediolateral ground reaction force during each period.
Results
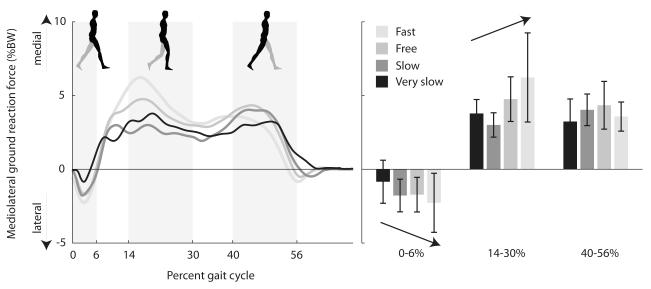
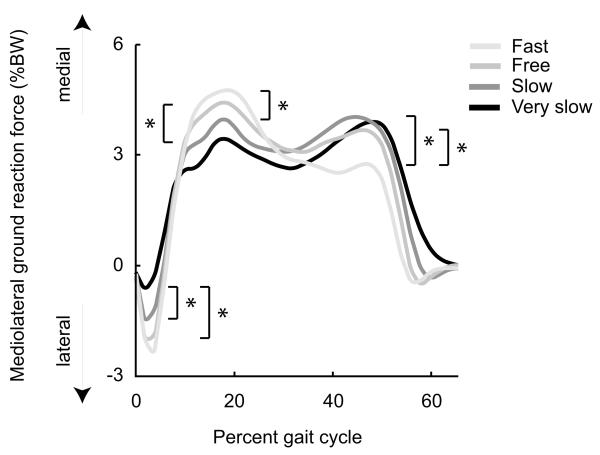
Mediolateral ground reaction force changed systematically with walking speed. During early stance (0-6% gait cycle), the peak ground reaction force on the leading foot was directed laterally and increased significantly with speed (Fig. 2). During early single support (14-30% gait cycle), the peak ground reaction force on the stance foot was directed medially and increased significantly with speed. During late stance (40-56% gait cycle), the peak ground reaction force on the trailing foot was directed medially; we detected no significant relationship between speed and peak medial ground reaction force during late stance.
Figure 2.
Mediolateral component of ground reaction force as a percentage of body weight at four walking speeds, averaged over eight unimpaired subjects. The left panel shows a brief laterally directed ground reaction force in early stance followed by a medially directed ground reaction force. The right panel shows the peak ground reaction forces during three time periods (0-6%, 14-30%, and 40-56% gait cycle). Error bars span ± one standard deviation. Arrow indicates significant effect of speed on mediolateral ground reaction force.
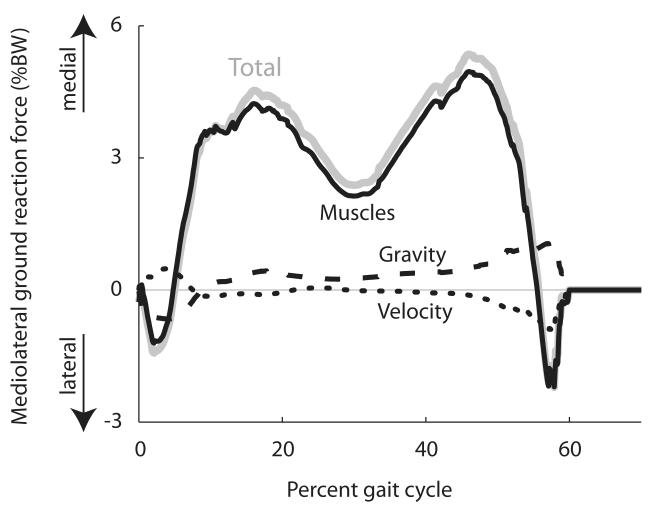
Muscles contributed 92% of the total mediolateral ground reaction force, on average, over all walking speeds (Fig. 3). At all walking speeds, gravity contributed a lateral ground reaction force immediately after foot contact followed by a medial ground reaction force. Velocity-related forces (i.e., forces due to centrifugal and Coriolis accelerations) opposed this pattern, providing a medial ground reaction force immediately after foot contact followed by a lateral ground reaction force. Overall, the contributions of passive dynamics (gravity and velocity-related forces) to the ground reaction force were much smaller than the contributions made by muscles.
Figure 3.
Total mediolateral ground reaction force as a percentage of body weight and contributions to mediolateral ground reaction force from muscles, gravity, and velocity-related forces, averaged over the free-speed trials of all eight unimpaired subjects.
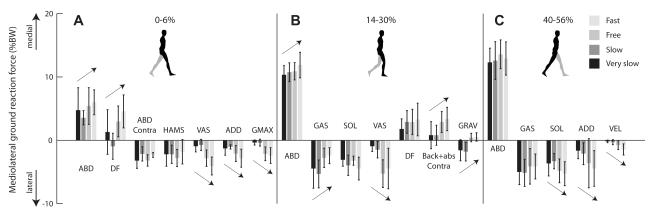
At all speeds, in all periods, abductors contributed the largest peak medial ground reaction force (Fig. 4). During early stance (0-6% gait cycle), dorsiflexors were the second largest contributors to medial ground reaction force at free and fast speeds (Fig. 4A). During the same period, hamstrings and contralateral abductors were among the largest contributors to lateral ground reaction force at all speeds. Vasti, adductors, and gluteus maximus were other large contributors to lateral ground reaction force at free and fast speeds.
Figure 4.
Peak contributions of major muscle groups to mediolateral ground reaction force, as a percentage of body weight, at four walking speeds during (A) 0-6%, (B) 14-30%, and (C) 40-56% gait cycle. Peak contributions for each speed were determined after averaging contributions across all eight unimpaired subjects. Error bars span ± one standard deviation. Arrow indicates significant effect of speed on contribution. Table 1 lists the muscles indicated by each abbreviation. “Contra” indicates muscles on the contralateral limb; all other muscles are on the ipsilateral limb.
During early single support (14-30% gait cycle), gastrocnemius and soleus were the largest lateral contributors at slow and very slow speeds (Fig. 4B). Soleus and vasti were the largest lateral contributors at free and fast speeds. The dorsiflexors and contralateral back and abdominal muscles were the next largest medial contributors at free and fast speeds. During late stance (40-56% gait cycle), gastrocnemius, soleus, and adductors were the largest lateral contributors across all speeds (Fig. 4C).
Our analysis revealed how contributions from muscles and passive dynamics changed with walking speed. During early stance (0-6% gait cycle), the vasti, adductors, and gluteus maximus contributed laterally directed ground reaction forces that increased significantly with speed. In contrast, the abductors and dorsiflexors contributed medially directed ground reaction forces that increased significantly with speed (Fig. 4A). During early single support (14-30% gait cycle), the medial contribution of the abductors increased significantly as walking speed increased, while the lateral contribution of vasti increased and of gastrocnemius decreased significantly as speed increased (Fig. 4B). The medial contributions of the contralateral back and abdominal muscles increased significantly as speed increased. The contribution due to gravity changed significantly with walking speed, from lateral at very slow and slow speeds to medial at free and fast speeds. During late stance (40-56% gait cycle), the lateral contributions of soleus, adductors, and velocity-related forces increased significantly with speed (Fig. 4C).
Discussion
Our study has revealed that, over a range of walking speeds, the medial ground reaction force generated by the abductors opposes the lateral ground reaction forces contributed by the vasti, gastrocnemius, and soleus muscles, which play major roles in modulating forward progression and providing body-weight support. The abductors support body weight (Liu et al., 2008) and contribute a large medial ground reaction force at all speeds. The vasti, gastrocnemius, and soleus influence forward progression and body-weight support (Liu et al., 2008) and contribute lateral ground reaction forces, along with the adductors, at all speeds.
It may seem suboptimal for muscles to provide opposing contributions to the mediolateral ground reaction force, but humans indeed expend modest metabolic energy to control mediolateral motion. When the body is externally stabilized in the mediolateral direction, humans choose a narrower step and expend less metabolic energy (Donelan et al., 2004). To maintain mediolateral balance during walking, humans may employ active control that increases metabolic energy expenditure (Wezenberg et al., 2011). Further investigation is needed to determine whether external stabilization reduces the opposition between muscle contributions to mediolateral ground reaction force and whether this reduction lowers metabolic energy expenditure.
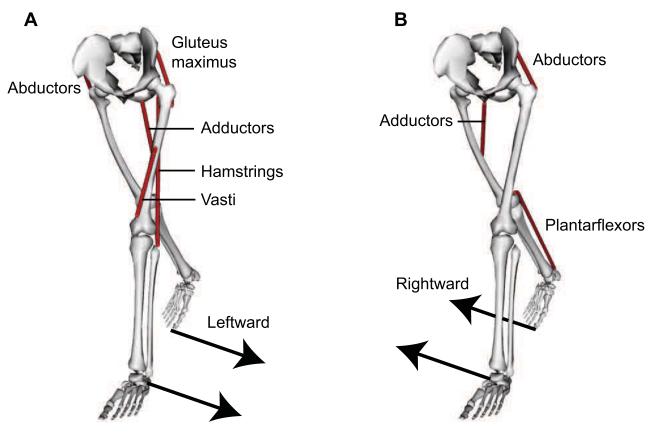
Our study reveals which muscles modulate mediolateral ground reaction forces to transfer weight from one leg to another during double support. Consider a double support phase when weight is transferred from the right (trailing) leg to the left (leading) leg (Fig. 5). As double support progresses, the direction of the left leg’s ground reaction force changes from lateral (leftward) to medial (rightward), while the direction of the right leg’s ground reaction force changes from medial (leftward) to lateral (rightward) before toe-off. These changes in ground reaction forces are largely due to i) an increase in the left abductors’ medial (rightward) contribution to the ground reaction force and ii) a decrease in the right abductors’ medial (leftward) contribution to the ground reaction force. The increase in the left abductors’ medial (rightward) contribution overcomes the lateral (leftward) contributions from the left hamstrings, gluteus maximus, and vasti, which support body weight during early stance (Liu et al., 2008). The decrease in the right abductors’ medial (leftward) contribution allows lateral contributors on the right leg to generate a brief lateral (rightward) ground reaction force before toe-off. This rightward force on the right foot assists in shifting the direction of the net mediolateral acceleration from rightward at the beginning of double support to leftward at the end of double support.
Figure 5.
Muscle groups modulating mediolateral ground reaction forces to transfer weight from the right leg to the left leg during double support. (A) The abductors on the right leg act with the hamstrings, vasti, adductors, and gluteus maximus on the left leg to generate leftward ground reaction forces. (B) The plantarflexors and adductors on the right leg, along with the abductors on the left leg, generate rightward ground reaction forces.
Our results support the findings of Pandy et al. (2010) who reported the medial contribution of the abductors and the lateral contributions of the vasti, plantarflexors, and adductors. Consistent with our finding that the vasti are major contributors to lateral acceleration in early to mid-stance, these muscles are active at this time (Perry, 1992). The plantarflexors are most active in the latter half of stance (Cappellini et al., 2006), consistent with our finding that the plantarflexors are major contributors to the mediolateral ground reaction force during that period. The abductors exhibit increased activity with increasing walking speed (Cappellini et al., 2006). This, combined with the abductors’ large force-generating ability (Ward et al., 2009), is consistent with our identification of the abductors as an important contributor to mediolateral ground reaction force. The role of abductors in modulating mediolateral motion was also reported by MacKinnon and Winter (1993). The contributions to mediolateral ground reaction force at free speed we found for gravity and velocity-related forces agree with Pandy et al. Our study identifies that the contribution from gravity shifts from medial to slightly lateral with increasing speed and the lateral contribution from velocity-related forces increases with walking speed. Our study also reveals significant changes in muscle contributions as walking speed increases (e.g., the increasing medial contributions of abductors and the increasing lateral contributions of vasti).
We identified a significant increase in the laterally directed ground reaction forces contributed by soleus, adductors, and velocity in late stance, but did not detect an effect of speed on mediolateral ground reaction force in late stance. To determine whether our failure to detect a change in mediolateral ground reaction force in late stance was due to the relatively small number of subjects for which we generated simulations (n = 8), we examined mediolateral ground reaction forces in 80 subjects drawn from Schwartz et al. (2008), which included our eight subjects. These eight subjects were chosen from the 80 subjects by Liu et al. (2008) for achieving at least one double support phase at each walking speed, enabling analysis of muscle contributions during double support. With this larger number of subjects, as speed increased, we detected a significant decrease in medially directed ground reaction force in late stance (Fig. 6), most likely arising from the larger lateral ground reaction forces contributed by soleus, adductors, and velocity in late stance and the constant medial ground reaction force contributed by the abductors (Fig. 4). The analysis of the larger number of subjects confirmed the changes in peak ground reaction force with speed detected in early stance and early single support (compare Figs. 2 and 6).
Figure 6.
Mediolateral ground reaction force, as a percentage of body weight, averaged over 80 subjects drawn from Schwartz et al. (2008) at four walking speeds. * indicates significant difference (p < 0.05) between speeds. In early stance, peak lateral ground reaction force increased significantly with speed (very slow to slow, very slow to free). In early single support, peak medial ground reaction force increased significantly with speed (very slow to free, slow to fast). In late stance, peak medial ground reaction force decreased significantly with speed (slow to fast, free to fast).
Our findings illustrate the roles of gravity and muscles in modulating mediolateral ground reaction forces in slow-walking individuals. Gravity plays a larger role in mediolateral motion control at slower walking speeds than at faster speeds, while abductors play a smaller role at slow speeds. Our results show that in slow walking, during single support, gravity contributes a larger, laterally directed ground reaction force than in faster walking, and abductors contribute a smaller, medial ground reaction force than in faster walking. Together, these patterns contribute to a smaller medial ground reaction force during single support at slower speeds compared to faster speeds. Individuals with weak abductors may have difficulty generating the larger medial ground reaction forces required for faster walking. Further investigation is needed to determine whether individuals with weak abductors prefer to walk at slower speeds, and whether strengthening abductors enables individuals to walk faster.
Our results should be interpreted in light of several limitations. First, the contributions to mediolateral ground reaction force we computed for each muscle are dependent on the forces exerted by those muscles in each simulation; since measuring muscle forces during walking is infeasible, we do not have a direct test for the accuracy of the simulated muscle forces. Second, our subjects were seven children and adolescents and one young adult, while a motivation for this study was to understand mediolateral balance in adults. The kinematics, joint moments, and muscle activations of our simulations agree with measurements for adults (Liu et al., 2008). Moreover, all of our subjects were age seven or older (mean age was 12.9 years), and walking kinematics and joint moments become adult-like by age seven (Sutherland, 1997). Thus, we believe the reported muscle contributions are representative of healthy adults. However, elderly adults may perform more work at the hip and less work at the knee and ankle than young adults (DeVita and Hortobagyi, 2000); future work should investigate whether muscle contributions to mediolateral motion differ from our results due to these differences. Finally, to approximate the limited subtalar motion that occurs during level walking on a smooth surface, our simulations were performed without subtalar motion. We did not include subtalar motion because precise measurement and tracking of subtalar motion was infeasible and we did not have accurate recordings of the EMG activity of tibialis posterior and the peroneal muscles; thus, it was infeasible to test the accuracy of simulated activation patterns for these muscles. Although inversion-eversion occurs at the subtalar joint, the movement is typically less than 5 degrees (Jenkyn et al., 2010). Ignoring the subtalar joint may have reduced our calculated contributions of the ankle inverters and everters to mediolateral acceleration of the mass center. Other studies (MacKinnon and Winter, 1993; Pandy et al., 2010) also suggest that the inverter and everter contributions are smaller than the contributions from abductors, gastrocnemius, and soleus when walking on a level surface.
Our study has identified the muscle groups contributing to the mediolateral ground reaction force during walking across a range of speeds. Our finding that the abductors, vasti, and plantarflexors make large contributions to mediolateral ground reaction force suggests that these muscles may be valuable targets for strength training in subjects with impaired mediolateral balance and muscle weakness. Our analysis of unimpaired walking provides baseline data for future studies analyzing muscle contributions to mediolateral motion in elderly subjects and individuals with gait pathologies and balance impairments.
Supplementary Material
Acknowledgements
We thank Ayman Habib, Melanie Fox, Samuel Hamner, Gary Beaupré, Michael Sherman, Paul Mitiguy, Katherine Steele, Richard Neptune, Edith Arnold, Tim Dorn, Melinda Cromie, Sonia Goel, and Ian Stavness for productive input and assistance with OpenSim. This work was supported by NIH grants U54 GM072970, R01 NS55380, R24 HD065690, R01 HD033929, and GM63495, and the Achievement Rewards for College Scientists Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement None of the authors had any financial or personal conflict of interest with regard to this study.
References
- Abel MF, Damiano DL. Strategies for increasing walking speed in diplegic cerebral palsy. J Pediatr Orthop. 1996;16:753–758. doi: 10.1097/00004694-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Agiovlasitis S, McCubbin JA, Yun J, Mpitsos G, Pavol MJ. Effects of Down syndrome on three-dimensional motion during walking at different speeds. Gait Posture. 2009;30:345–350. doi: 10.1016/j.gaitpost.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Johnson TS, Hurwitz DE, Natarajan R. Musculoskeletal Dynamics, Locomotion, and Clinical Applications. In: Mow, Huiskes, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. 3rd ed Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 91–122. [Google Scholar]
- Ashley MJ, Gryfe CI, Annies A. A longitudinal study of falls in an elderly population. II. Some circumstances of falling. Age Ageing. 1977;6:211–220. doi: 10.1093/ageing/6.4.211. [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol. 2006;95:3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Chockalingam N, Bandi S, Rahmatalla A, Dangerfield PH, Ahmed E-N. Assessment of the centre of pressure pattern and moments about S2 in scoliotic subjects during normal walking. Scoliosis. 2008;3:6. doi: 10.1186/1748-7161-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou L-S, Kaufman KR, Hahn ME, Brey RH. Medio-lateral motion of the center of mass during obstacle crossing distinguishes elderly individuals with imbalance. Gait Posture. 2003;18:125–133. doi: 10.1016/s0966-6362(02)00067-x. [DOI] [PubMed] [Google Scholar]
- Davis RB, Kaufman KR. Kinetics of Normal Walking. In: Rose, Gamble, editors. Human Walking. 3rd ed Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 53–76. [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;55:1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Sternad D, Cavanagh PR. Slower speeds in patients with diabetic neuropathy lead to improved local dynamic stability of continuous overground walking. J Biomech. 2000;33:1269–1277. doi: 10.1016/s0021-9290(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Thomas SS, Higgins C, Colliver J. Stride-dependent changes in gait of older people. J Neurol. 1991;238:1–5. doi: 10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- Elliott ME, Drinka PJ, Krause P, Binkley NC, Mahoney JE. Osteoporosis assessment strategies for male nursing home residents. Maturitas. 2004;48:225–233. doi: 10.1016/j.maturitas.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Goldie PA, Matyas TA, Evans OM. Deficit and change in gait velocity during rehabilitation after stroke. Arch Phys Med Rehabil. 1996;77:1074–1082. doi: 10.1016/s0003-9993(96)90072-6. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Myers ER, Kiel DP, Parker RA, Hayes WC, Resnick NM. Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med. 1998;104:539–545. doi: 10.1016/s0002-9343(98)00115-6. [DOI] [PubMed] [Google Scholar]
- Hageman PA, Blanke DJ. Comparison of gait of young women and elderly women. Phys Ther. 1986;66:1382–1387. doi: 10.1093/ptj/66.9.1382. [DOI] [PubMed] [Google Scholar]
- Hamner SR, Seth A, Delp SL. Muscle contributions to propulsion and support during running. J Biomech. 2010;43:2709–2716. doi: 10.1016/j.jbiomech.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof AL. Scaling gait data to body size. Gait Posture. 1996;4:222–223. [Google Scholar]
- Hof AL. The equations of motion for a standing human reveal three mechanisms for balance. J Biomech. 2007;40:451–457. doi: 10.1016/j.jbiomech.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Hsue B-J, Miller F, Su F-C. The dynamic balance of the children with cerebral palsy and typical developing during gait. Part I: Spatial relationship between COM and COP trajectories. Gait Posture. 2009;29:465–470. doi: 10.1016/j.gaitpost.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Jenkyn TR, Shultz R, Giffin JR, Birmingham TB. A comparison of subtalar joint motion during anticipated medial cutting turns and level walking using a multi-segment foot model. Gait Posture. 2010;31:153–158. doi: 10.1016/j.gaitpost.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ. Lower trunk motion and speed-dependence during walking. J Neuroengineering Rehabil. 2009;6:10. doi: 10.1186/1743-0003-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Ulrich B. Coordination of pelvis-HAT (head, arms and trunk) in anterior–posterior and medio-lateral directions during treadmill gait in preadolescents with/without Down syndrome. Gait Posture. 2006;23:512–518. doi: 10.1016/j.gaitpost.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robot Res. 1999;18:917–930. [Google Scholar]
- Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. J Biomech. 2008;41:3243–3252. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115:345–356. doi: 10.1007/pl00005703. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26:633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. J Gerontol. 1969;24:169–178. doi: 10.1093/geronj/24.2.169. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture. 2008;28:135–143. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Thorstensson A. Ground reaction forces at different speeds of human walking and running. Acta Physiol Scand. 1989;136:217–227. doi: 10.1111/j.1748-1716.1989.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES, Kadel NJ. The effect of walking speed on center of mass displacement. J Rehabil Res Dev. 2004;41:829–834. doi: 10.1682/jrrd.2003.10.0150. [DOI] [PubMed] [Google Scholar]
- Pandy MG, Lin Y-C, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech. 2010;43:2055–2064. doi: 10.1016/j.jbiomech.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. SLACK Inc.; Thorofare, NJ: 1992. [Google Scholar]
- Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. J Biomech. 2008;41:1639–1650. doi: 10.1016/j.jbiomech.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and nonfatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. The development of mature gait. Gait Posture. 1997;6:163–170. [Google Scholar]
- Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. J Biomech. 2006;39:1107–1115. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. J Biomech. 2003;36:321–328. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- Turnbull GI, Charteris J, Wall JC. A comparison of the range of walking speeds between normal and hemiplegic subjects. Scand J Rehabil Med. 1995;27:175–182. [PubMed] [Google Scholar]
- Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture correct? Clin Orthop Relat Res. 2009;467:1074–1082. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezenberg D, de Haan A, van Bennekom CA, Houdijk H. Mind your step: metabolic energy cost while walking an enforced gait pattern. Gait Posture. 2011;33:544–549. doi: 10.1016/j.gaitpost.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Yang F, Bhatt T, Pai Y-C. Limits of recovery against slip-induced falls while walking. J Biomech. 2011;44:2607–2613. doi: 10.1016/j.jbiomech.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.