Abstract
Insulin-like growth factor 1 (IGF-1), the most abundant growth factor in the bone matrix, regulates bone mass in adulthood. We report that IGF-1 released from bone matrix stimulates osteoblastic differentiation of mesenchymal stem cell (MSCs) by activation of mTOR during bone remodeling. Mice knockout of IGF-1 receptor (Igf1r) in the preosteoblastic cells exhibited low bone mass and reduced mineral deposition rates. The MSCs recruited to the bone surface were unable to differentiate into osteoblasts. In age-related osteoporosis in humans, we found that marrow IGF-1 levels were 40% lower than controls. Similarly, the levels of IGF-1 in the bone matrix and marrow of aged rats were also decreased and directly correlated with the age-related decrease in bone mass. Notably, injection of IGF-1 with IGF binding protein 3 (IGFBP3), not IGF-1 alone, increased the level of IGF-1 in the bone matrix and stimulated new bone formation in old rats. Thus, IGF-1 released during bone resorption from bone matrix activates mTOR to induce osteoblast differentiation of MSCs in maintaining bone micro-architecture and mass.
INTRODUCTION
Bone mass peaks in mid and late adolescence, plateaus for several years and then inexorably declines to a point, usually in older individuals, during which skeletal fragility is enhanced and osteoporosis is established1–4. Acquisition of peak bone mass (PBM) is thought to reduce the subsequent risk of fracture whereas impaired peak acquisition or loss of bone during adolescence is associated with greater fracture risk. IGF-1 is a key factor in the endocrine regulation of body composition and is integral not only to the acquisition of PBM but also to the maintenance of bone mineral density5–9. In particular, IGF-1 and several of its binding proteins positively correlate with bone mass and can act as independent predictors for the risk of osteoporosis and incident fractures10–13.
The maintenance of adult bone mass is accomplished by skeletal remodeling3,14. This remodeling is precisely coordinated by the activities of osteoblasts and osteoclasts15, 16. During bone remodeling, osteoclasts resorb bone, followed by recruitment of bone marrow MSCs for subsequent differentiation and bone formation. We have previously shown that TGF-β1 recruits MSCs to the bone resorptive sites in response to osteoclastic bone resorption coupling bone resorption and formation17. The recruited MSCs at bone resorption sites then undergo differentiation for bone formation. However, the osteogenic nature of the microenvironment at bone resorptive sites is not well known. IGF-1 is the most abundant growth factor deposited in the bone matrix12, 18–20 and has been implicated in the coupling process through its actions on MSC differentiation21. In this study, we found that the level of bone marrow IGF-1 was decreased during aging in rats and closely associated with the bone volume whereas serum levels of IGF-1 were relatively steady. IGF-1 released from bone matrix during bone resorption generates an osteogenic microenvironment and induces differentiation of recruited MSCs for new bone formation. Notably, IGF-1 activates mTOR through the PI3K-Akt pathway to induce differentiation of MSCs into osteoblasts. Thus we postulate that a primary function of IGF-1 in the bone matrix is to maintain bone mass and skeletal homeostasis during bone remodeling.
RESULTS
Knockout of Igf1r reduces bone formation during bone remodeling
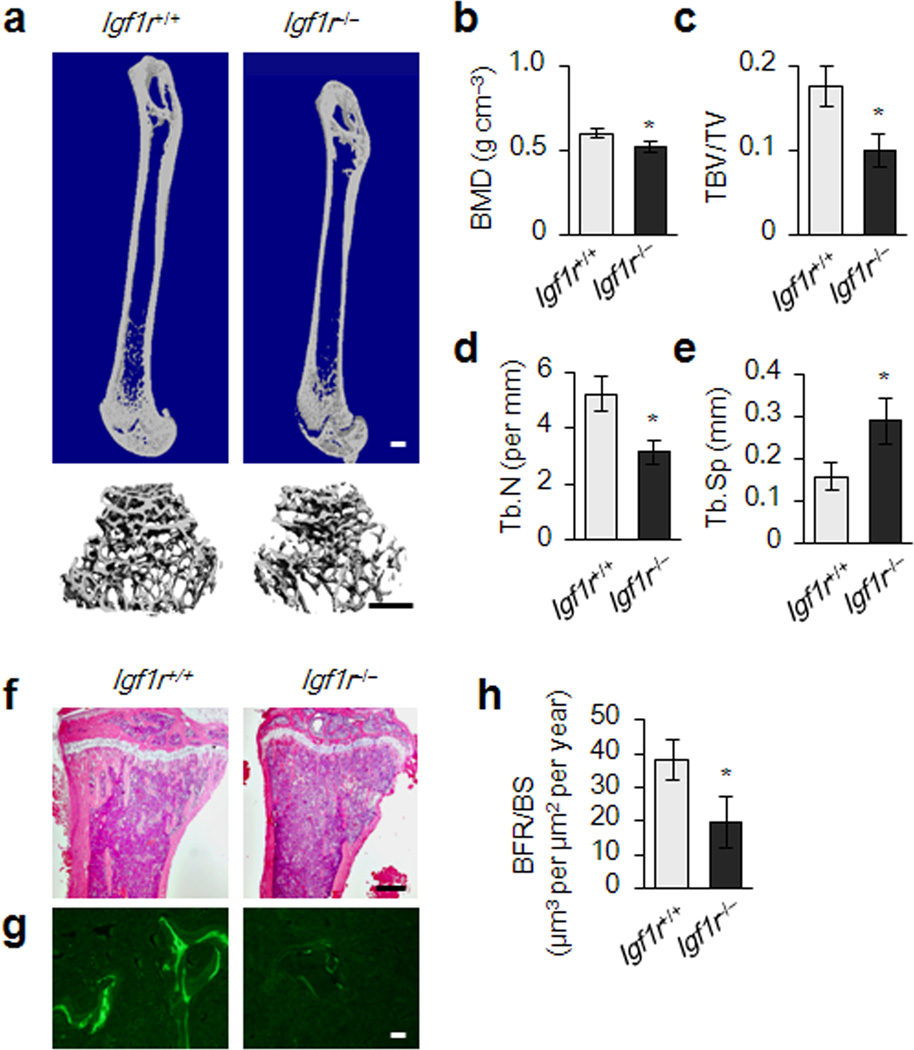
Floxed Igf1r mice were crossed with the Osx-GFP:Cre mice under the transcriptional regulation of the Osx1 promoter22 to generate conditional Igf1r knockout mice Osx-Cre; Igf1rfl/fl (Igf1r−/−)(Supplementary Fig. 1a,b). The sizes of newborn Igf1r−/− mice are similar for both genders to those of their wild-type littermates Osx-Cre (Igf1r+/+) (Supplementary Fig. 1c–f). Bone mineral density was decreased in 2 month old Igf1r−/− mice relative to their wild-type littermates (Fig. 1a,b). Particularly, Igf1r−/− mice exhibited a significant loss in trabecular bone volume and thickness, and greater trabecular bone space relative to their wild-type littermates (Fig. 1c–e). Similar results were observed in histomorphometric analysis (Supplementary Fig. 2). The trabecular bone deficiency in female Igf1r−/− mice was more impressive than their male Igf1r−/− littermates (Supplementary Fig. 2), likely due to estrogen dependency of IGF-1 action in bone23. Analysis of calcein double-labeling demonstrated a decreased dynamic bone formation rate (BFR) in Igf1r−/− mice (Fig. 1g,h). Interestingly, periosteal bone formation was not affected in Igf1r−/− mice (Supplementary Fig. 2g). The results suggest a critical role of IGF-1 in maintaining bone homeostasis in adult mice.
Figure 1. Reduced bone formation during bone remodeling in Igf1r−/− (Osx-Cre; Igf1rfl/fl) mice.
(a) Representative μCT images of femora from a 3-month-old female Igf1r−/− (Osx-Cre; Igf1rfl/fl) mouse and wild type littermate Igf1r+/+ mouse (Osx-Cre). Scale bars: 1 mm. (b–e) Quantitative μCT analysis of the secondary spongiosa of proximal tibiae. Volumetric bone mineral density (BMD) (b), trabecular bone volume fraction (TBV/TV) (c), trabecular number (Tb. N) (d), and trabecular separation (Tb. Sp) (e). (f) H&E histological sections of tibiae from 3-month-old Igf1r+/+ and Igf1r−/− mice. Scale bar: 1 mm. (g) Calcein double labeling of the metaphyseal trabecular bone at distal femora (Scale bar: 1 mm). (h) Bone formation rate per bone surface (BFR/BS). Data represent the mean ± SEM. n = 10. *p < 0.05.
Mature osteoblasts are reduced at the bone remodeling surface of Igf1r−/− mice
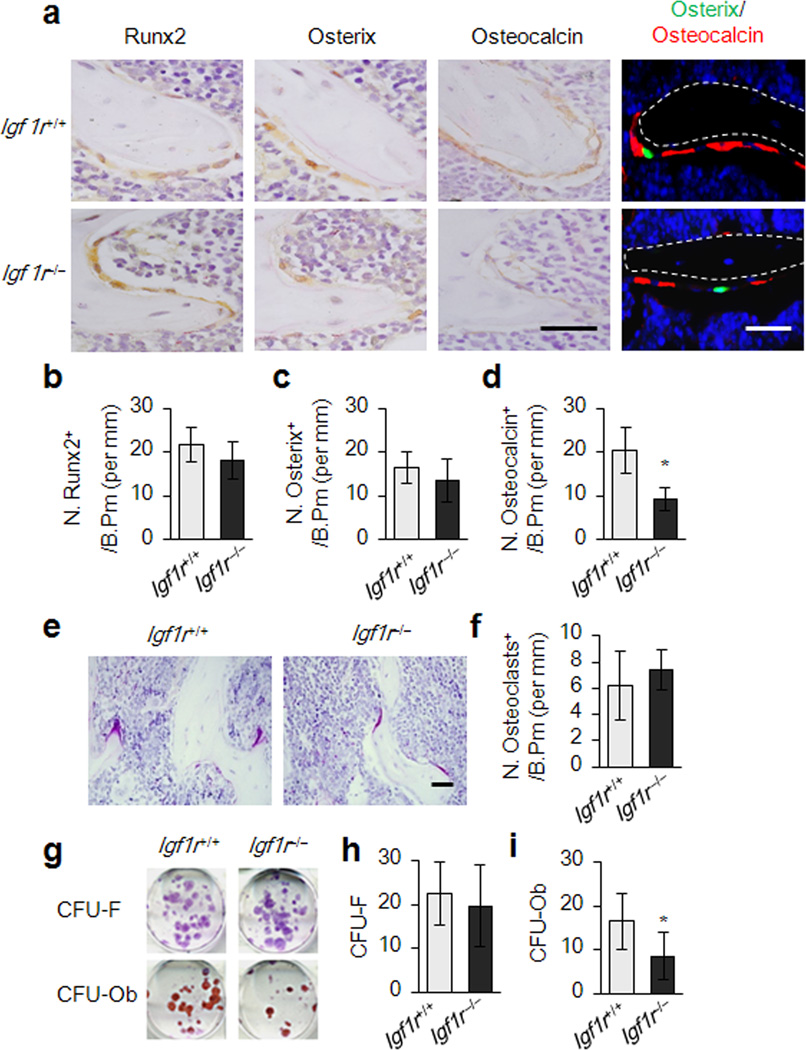
The number of osteoblastic cells at different stages of osteoblast differentiation was measured by immunostaining of femur sections of Igf1r−/− mice and their littermates (Igf1r−/−). Runx2-positive and Osterix-positive osteoprogenitors on bone surfaces of Igf1r−/− mice were not significantly different from those of wild-type littermates (Fig. 2a–c), Athough, the number of osteocalcin-positive mature osteoblasts on the bone surfaces were decreased significantly in Igf1r−/− mice (Fig. 2a,d). Additionally, the Igf1r−/− mice with Osterix-Cre-mediated expression of GFP allowed us to visualize endogenous cells with specific deletion of Igf1r in the osteoblastic lineage in vivo. The Igf1r deficient osteoprogenitors were therefore GFP-positive and were primarily found at the bone surface. However the number of mature osteoblasts which were osteocalcin-positive was much less than that of wild type mice (Fig. 2a). TRAP-positive mature osteoclasts in the Igf1r−/− mice were not significantly different from their wild-type littermates (Fig. 2e,f). Colony forming unit-fibroblast (CFU-F) and colony forming unit-osteoblast (CFU-Ob) assays showed that CFU-F of the Igf1r−/− mice was not significantly different from their wild-type littermates (Fig. 2g,h), but CFU-Ob was reduced in the Igf1r−/− mice (Fig. 2g–i), further indicating that mature osteoblasts on bone surfaces were reduced due to the inhibited differentiation of MSCs recruited to the bone remodeling surface.
Figure 2. Suppressed osteoblast maturation in Igf1r−/− (Osx-Cre; Igf1rfl/fl) mice.
(a) Immunohistochemical analysis of Runx2, Osterix, and Osteocalcin performed on trabecular bone sections from distal femora of 3-month-old female Igf1r−/− (Osx-Cre; Igf1rfl/fl) mouse and wild type littermate mouse Igf1r+/+ (Osx-Cre). Osx-GFP expressing cells observed by direct fluorescence microscopy appear green, immunofluorencent staining for osteocalcin visualize red (far right panel). Scale bar: 100 µm. (b–d) Numbers of Runx2, Osterix, and Osteocalcin positive cells on bone surface, measured as cells per millimeter of perimeter in sections. n = 5. *p < 0.05. (e,f) Light micrographs of tartrate-resistant acid phosphatase (TRAP)-staining performed on trabecular bone sections from distal femora of mice. Number of osteoclasts per tissue area (N.Oc/T,Ar) was measured. Data represent the mean ± SEM. n = 10. *p < 0.05. (Scale bar: 100 µm) (g) CFU-F and CFU-Ob assays from harvested bone marrow of the mice as indicated. Representative images of CFU-Fs stained with crystal violet (top panels). Representative images of CFU-Obs stained with Alizarin Red (bottom panels). (h,i) Quantifications of the CFU-F and CFU-Ob assays. Data represent the mean ± SEM. of triplicate cultures of bone marrow nucleated cells pooled from five individual mice. *p < 0.05.
IGF-1 induces osteoblast differentiation of Sca-1+ MSCs through activation of mTOR
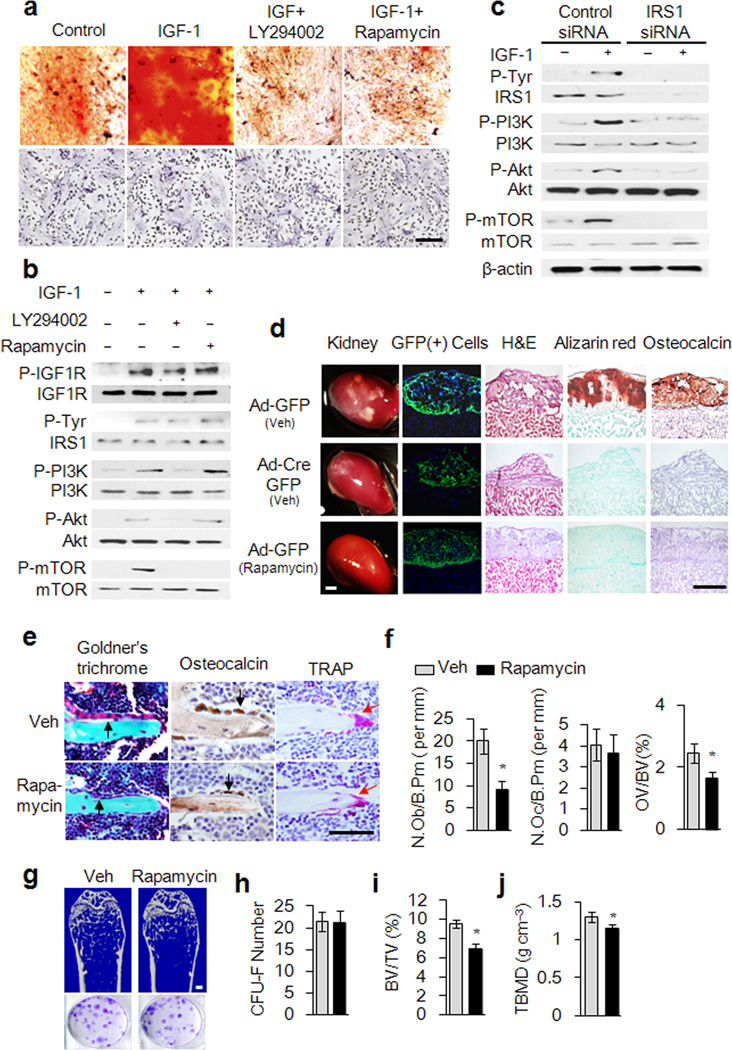
We wished to elucidate the signaling mechanism of IGF-1 induced Sca-1+ MSCs to osteogenic differentiation. IGF-1 stimulates MSCs-mediated mineralization in Alizarin red staining (Fig. 3a). Inhibitor LY294002 (10 µM) for PI3K or rapamycin (20 nM), an inhibitor of mTOR, impaired the mineralization but did not affect cell growth and survival (Fig. 3a). Moreover, IGF-1 stimulates phosphorylation of IGF1R, IRS1, PI3K, Akt and mTOR of Sca-1+ MSCs. While the phosphorylation of PI3K, Akt and mTOR were reduced by a PI3K inhibitor, rapamycin only inhibited phosphorylation of mTOR (Fig. 3b), indicating that IGF-1 activates mTOR through the PI3K-Akt pathway in Sca-1+ MSCs. When Irs1 was knocked down in Sca-1+ MSCs with Irs1 targeting siRNA, phosphorylation of PI3K, Akt and mTOR induced by IGF-1 (20 ng ml−1, 15 minutes) was inhibited (Fig. 3c), confirming that IRS1 mediates IGF-1 induced activation of mTOR. Importantly, rapamycin inhibited IGF-1 induced expression of markers of osteoblast differentiation including Osterix (Sp7), Runx2, Alkaline phosphatise (Alp), Osteocalcin (Bglap), Osteoglycin (Ogn) and Osteoactivin (Gpnmb) (Supplementary Fig. 3).
Figure 3. IGF-1 induces osteoblastic differentiation of MSCs through the IRS-PI3K-Akt-mTOR pathway.
(a) Alizarin red staining showing Osteoblastic differentiation of Sca-1+ MSCs induced by IGF-1 as indicated (top panels). Alive cells number was determined by hematoxylin staining (bottom panels). Scale bar: 100 µm. (b) Western blot analysis of IGF-1 induced phosphorylation of IGF1R, IRS1, PI3K, Akt, and mTOR in Sca-1+ MSCs treated with IGF-1 (20 ng ml−1) or vehicle in the presence or absence of LY294002 (10 µM) or rapamycin (20 nM) for 15 minutes as indicated. (c) Western blot analysis of IGF-1-induced phosphorylation of IRS1, PI3K, Akt, and mTOR in Sca-1+ MSCs treated by IGF-1 after transfected with Irs1 siRNA or control siRNA. (d) IGF-1 induced Sca-1+ MSCs differentiation underneath renal capsules. The renal sections were analyzed by direct GFP fluorescence visualization, H&E staining, Alizarin red staining or immunohistology for osteocalcin. Scale bar: 100 µm. (e–g) Rapamycin impairs trabecular bone formation. Representative images of mouse distal femora sections with staining of Golder’s Trichrome, osteocalcin or TRAP (e). Scale bar: 100 µm. Histomorphometric analysis of remodeling trabecular bone after treated with rapamycin: number of osteoblast per bone perimeter (f left), number of osteoclast per bone perimeter (f center), Osteoid volume/bone volume (f right), μCT Representative images of distal femora (g top), Scale bar: 1 mm. CFU-F assays (g,h), trabecular bone volume fraction (TBV/TV) (i) trabecular bone mineral density (TBMD) (j). Data represent the mean ± SEM. n = 5. *p < 0.05.
To investigate the role of mTOR in IGF-1 induced MSC differentiation in vivo, we isolated Sca-1+ MSCs from Igf1rfl/fl mice and deleted Igf1r by adenoviral-mediated expression of Cre with GFP (Ad-Cre-GFP) or GFP only (Ad-GFP) as a control. The Igf1r−/− or Igf1rfl/fl MSCs embedded in matrigel were transplanted underneath the renal capsule of immune-deficient Rag2−/− mice injected with rapamycin (3 mg kg−1 per day) or vehicle daily for 4 weeks (Fig. 3d). Igf1rfl/fl MSCs underwent osteoblast differentiation and mineralization underneath the renal capsule as shown by H&E, Alizarin red and immuno-histology of osteocalcin staining (Fig. 3d). Similar to the results of Igf1r−/− MSCs, osteoblast differentiation and mineralization of Igf1rfl/fl MSCs were inhibited by rapamycin (Fig. 3d). Furthermore, 6 week old wild type C57BL/6 mice were subcutaneously injected with rapamycin daily (3mg kg−1 per day) for 4 weeks. The number of osteocalcin-positive osteoblasts on the bone surface decreased significantly relative to their vehicle-injected littermates (Fig. 3e,f), whereas the number of osteoclasts remained unchanged (Fig. 3e,f). Significant reduction of new bone formation by Trichrome staining (Fig. 3e) and trabecular bone loss by μCT analysis (Fig. 3g,i,j and Supplementary Table 1) were also observed, but the number of CFU-Fs was not affected by introduction of rapamycin (Fig. 3g,h). Taken together, IGF-1 activates mTOR through the IRS1-PI3K-Akt pathway to regulate osteoblast differentiation of MSCs for bone formation.
IGF-1 released from bone matrix induces osteoblast differentiation of MSCs
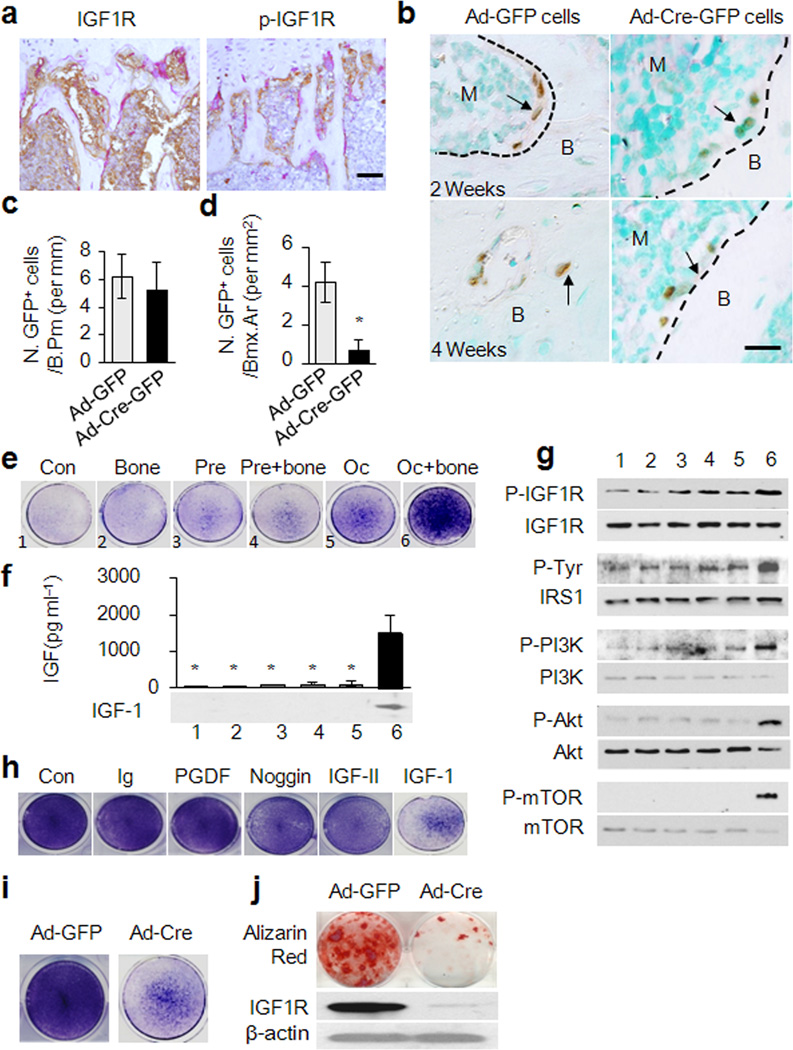
The recruited MSCs at bone resorptive sites undergo differentiation for new bone formation but the osteogenic nature of the microenvironment at bone resorptive sites is not well known. We therefore examined the levels of phosphorylated IGF1R in the bone resorption areas by co-staining with TRAP-positive osteoclasts. The phosphorylated IGF1R is primarily found at the bone surfaces along the bone resorptive sites as defined by the presence of mature TRAP-positive osteoclasts while the IGF1R positive cells are evenly distributed in the bone marrow (Fig. 4a), suggesting that active IGF-1 is released during osteoclastic bone resorption. Transplanted GFP-labeled mouse Sca-1+ MSCs on bone surfaces were identified by immunostaining with an anti-GFP antibody and quantified. There was no significant difference on the bone surface between Ad-Cre-GFP MSCs and Ad-GFP MSCs at 2 weeks after injection (Fig. 4b,c). The embedded GFP-positive Igf1rfl/fl MSCs into the bone matrix were significantly higher relative to the Igf1r−/− MSCs at 4 weeks after injection (Fig. 4b,d). The survival rate of the injected GFP-labeled MSCs in the bone marrow was analyzed by flow cytometry 2 weeks after transplantation and the results indicate that deletion of IGF1R did not affect the survival rate of the MSCs in the bone marrow (Supplementary Fig. 4).
Figure 4. Osteoclastic bone resorption-conditioned medium (BRCM) induces osteogenic differentiation of MSCs.
(a) Immunohistochemical analysis of the trabecular bone sections of mouse distal femora with antibodies against IGF1R (Left) and p-IGF1R (Right). Scale bar: 200 µm. (b) Immunohistochemical analysis of femora sections of 3 months old mice transplanted with GFP-labeled mouse MSCs with GFP antibody. Scale bar: 100 µm. (c,d) Quantification of GFP+ cells on bone surface 2 weeks after transplantation or in bone matrix 4 weeks after transplantation. n=5. *p< 0.05. (e) ALP staining for the differentiation potential of MSCs cultured in various conditioned media as indicated. 1-medium only, 2-Bone slice only, 3-Osteoclast precursor culture, 4-Osteoclast precursors cultured with bone slice, 5-Osteoclast culture, 6-Osteoclasts cultured with bone. (f) ELISA analysis of IGF-1 levels in BRCM. n = 3. *p < 0.05 versus Oc+ bone group. (g) Western blot analysis of the effect of various condition media on phosphorylation of IGF1R, IRS1, PI3K, Akt, and mTOR in MSCs. (h) ALP staining for the differentiation potential of MSCs cultured in BRCM with addition of individual neutralizing antibodies (Ab) or noggin, as indicated. (i) ALP staining and (j) Alizarin red staining for the effect of BRCM on differentiation potential of Sca-1+ MSCs isolated from Igf1rfl/fl by infection with adenovirous-Cre (Ad-Cre-GFP) or Ad-GFP (j, top). Western blot analysis of IGF1R in MSCs (j, bottom).
We reasoned that the IGF-1 released from bone matrix comprises the osteogenic microenvironment for the induction of osteoblast differentiation. Osteoclast precursors or mature osteoclasts were cultured with or without bone slices in vitro and the culture media was collected to examine their effects on the osteoblastic differentiation of MSCs. Bone resorption-conditioned media (BRCM) from mature osteoclasts with bone have the highest capability to induce alkaline phosphatase activity, a marker for osteoblast differentiation (Fig. 4e). IGF-1 was detected only in the BRCM from osteoclastic bone resorption but not in other conditioned media (Fig. 4f). Osteoclastic bone resorption media stimulated phosphorylation of IGF1R, IRS1, PI3K, Akt and mTOR within 1 hour after treatment (Fig. 4g). Moreover, addition of an antibody specific for IGF-1 to the BRCM significantly inhibited the activity of alkaline phosphatase, whereas noggin (an antagonist for BMPs) and the antibodies against IGF-II and PGDF had no or minimal effects on alkaline phosphatase activity (Fig. 4h), suggesting that IGF-1 is the primary factor for the osteogenic microenvironment at bone resorptive sites. Moreover, deletion of IGF1R in MSCs isolated from Igf1r floxed mice blocked their osteoblast differentiation induced by BRCM, evident by both alkaline phosphatase staining and Alizarin red staining (Fig. 4i,j). Thus, IGF-1 released from the bone matrix induces osteoblast differentiation of MSCs recruited by TGF-β1 in the process of coupling bone resorption and formation.
Age-related bone loss is associated with IGF-1 in the bone matrix of rats
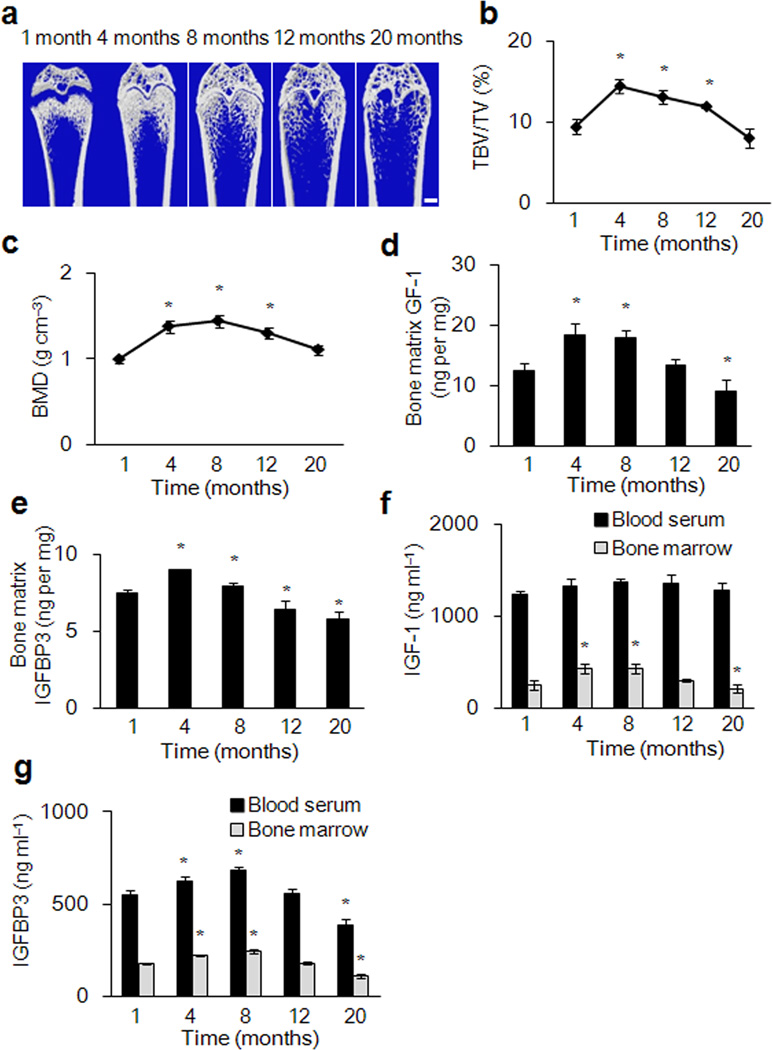
We assessed the level of IGF-1 and IGFBP3 in the bone matrix and its potential correlation with bone mass of rats at ages 1, 4, 8, 12, and 20 months. We found that the bone volume and bone mass steadily increased from 1 to 8 months after birth and then decreased continuously (Fig. 5 a–c). PBM was also observed at 8 months of age. Notably, changes of IGF-1 and IGFBP3 levels in the bone matrix are well correlated with the changes in bone mass cited above (Fig. 5d,e) and the number of osteoblasts also decreased with increased age (Supplementary Fig. 5a,b). In contrast, The level of IGF-1 in the serum was much higher than that in bone marrow and did not exhibit considerable decreases with aging (Fig. 5f). However, IGFBP3 showed an age-dependant decrease in sera (Fig. 5g). Interestingly, in humans, IGF-1 and IGFBP3 levels in the bone marrow were also correlated with the changes in bone mass during aging. Notably, in osteoporotic individuals with hip fractures, IGF-1 level of bone marrow was 40% lower relative to controls with normal bone mass (Supplementary Fig. 5d and Supplementary table 2).
Figure 5. Analysis of IGF-1 and IGFBP3 levels in blood, bone marrow and bone matrix in relation to bone mass during aging of rats.
(a) Representative μCT images of distal femora from rats of 1, 4, 8, 12, and 20 months. Scale bars: 1 mm. (b, c) Quantitative μCT analysis of the distal femur. trabecular bone volume fraction (TBV/TV) (b)Trabecular bone mineral density (BMD) (c). (d) IGF-1 concentrations in bone matrix extraction. (e) IGFBP3 concentration in bone matrix extraction. (f, g) Levels of IGF-1(f) and IGFBP3 (g) in bone marrow and peripheral blood serum at different ages. Data represent mean ± SEM of triplicate repeat for each sample and 10 individual rats for each time point. *p < 0.05 versus 1 month group.
To determine whether aging affects the ability of IGF1R activation on MSCs, bone marrow cells were isolated from rats at 1, 4 and 20 months for the CFU-F assay. A single colony was picked and expanded, cultured and treated with IGF-1 (20 ng ml−1) for 10 minutes. There was no significant difference in IGF1R activation in MSCs from young, adult and old rats by Western blot analysis (Supplementary Fig. 5e). The correlation of bone matrix IGF-1 levels with bone mass measurements suggests an important role of bone matrix IGF-1 in maintaining bone mass.
Increase of IGF-1 in the bone matrix attenuates bone loss in aged animals
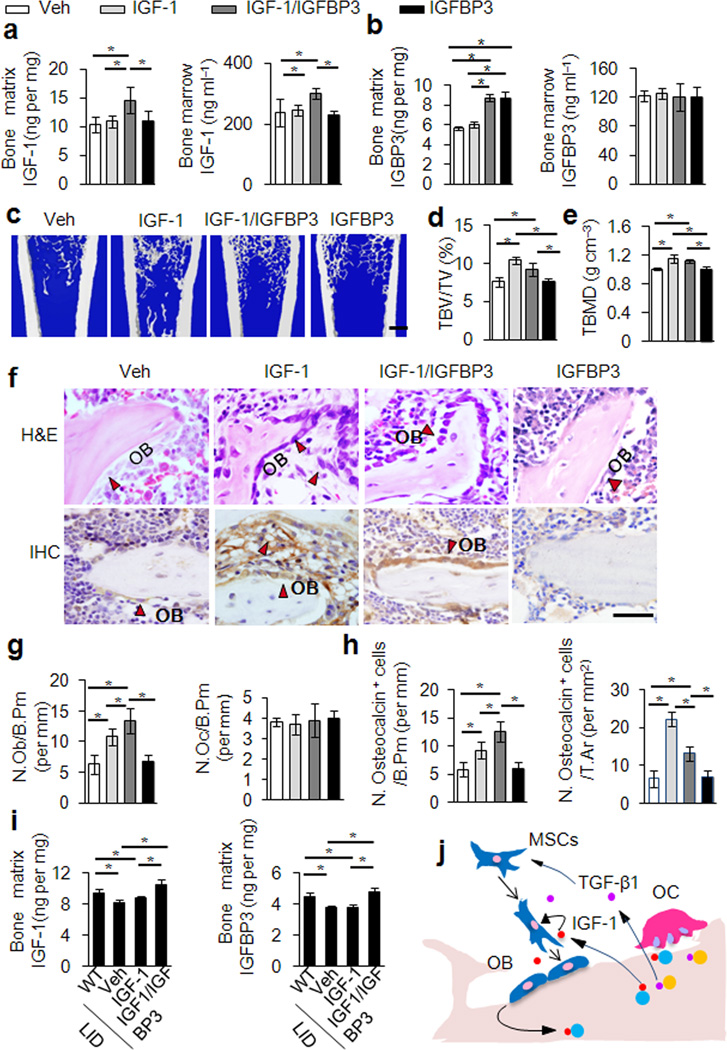
To assess whether IGFBP3 binds IGF-1 in the extracellular matrix and enhances its activity, we injected either vehicle, IGF-1, IGFBP3, or IGF-1 plus IGFBP3 into the distal femur cavity of 20 month old rats once a week for 4 weeks. The level of IGF-1 and IGFBP3 in the serum, marrow, and bone matrix were measured 10 days after the last injection. IGF-1 levels in the bone matrix and marrow were significantly higher in rats with injection of IGF-1 plus IGFBP3 than in rats injected with IGF-1, IGFBP3 only or vehicle (Fig. 6a). The IGFBP3 levels in bone matrix also increased with injection of IGF-1 plus IGFBP3 and IGFBP3 only relative to the vehicle group (Fig. 6b). Serum IGF-1 and IGFBP3 levels remain unchanged in all injection groups (Supplementary Fig. 5f). This suggests that IGFBP3 regulates IGF-1 deposition in the bone matrix and its lifespan in the bone marrow.
Figure 6. Increase of IGF-1 in the bone matrix attenuates bone loss.
(a,b) IGF-1 and IGFBP3 concentrations in bone matrix extraction (a and b left) and bone marrow (a and b right) of 20 months old rats locally injected with vehicle (Veh), IGF-1, IGF-1 plus IGFBP3 (IGF-1/IGFBP3) or IGFBP3 only. (c) Representative images of three dimensional μCT of distal femora injected with Veh, IGF-1, IGF-1/IGFBP3 or IGFBP3. Scale bar: 1 mm. (d,e) Quantitative μCT analysis of the distal femora. trabecular bone volume fraction (TBV/TV) (d), Trabecular volumetric bone mineral density (TBMD) (e). (f) H&E (top) and immunostaining for osteocalcin (bottom) of femur sections from the rats with indicated injection. Osteoblast cells are shown with red arrows. Scale bar: 100 µm. (g) Number of osteoblasts (left) and osteoclasts (right) of remodeling trabecular bone quantified by histomorphometric analysis. (h) Quantification of osteocalcin-positive cells on bone surface (left) and in total tissue area (right (i) Concentrations of IGF-1 (left) and IGFBP3 (right) in bone matrix extraction of LID mice or their littermates infused with Veh, IGF-1 only or IGF-1 plus IGFBP3 by osmotic pumps. All data represent the mean ± SEM. n = 10. *p < 0.05 (j) Schematic diagram of bone matrix IGF-1 induced osteoblast differentiation of MSCs during bone remodeling. TGF-β1 recruits MSCs to the bone resorptive site in response to osteoclastic bone resorption, and IGF-1 released from bone matrix comprises the osteogenic microenvironment for differentiation of recruited MSCs.
Of note, both bone volume and bone density were increased in rats with injection of IGF-1 only or IGF-1 plus IGFBP3 (Fig. 6d,e). However, bone formation in the IGF-1 only group occurred in clusters structurally deficient of natural trabecular bone whereas injection with IGF-1 plus IGFBP3 allowed for both improvements in bone mass and micro-architecture (Fig. 6c–f). Analysis of contralateral controls indicated that such changes in bone are limited to the injected femur (Supplementary Fig. 5g–i). H&E and immunohistology for osteocalcin of femur sections showed that a significant number of osteoblast-like cells or osteocalcin-positive osteoblasts were scattered throughout the bone marrow (Fig. 6c) in IGF-1 only injected groups. Importantly, in the IGF-1 plus IGFBP3 injected rats, osteocalcin-positive osteoblasts were primarily found on the bone surface (Fig. 6g,h). Osteoclast numbers did not change significantly. The results indicate that IGFBP3 mediated the association of IGF-1 to the bone extracellular matrix for de novo bone formation at the bone surface. To test whether circulating IGF-1 and IGFBP3 can target and become immobilized in the bone matrix. Either IGF-1, IGF-1 plus IGFBP3, or vehicle was infused into the circulation of 4 week old liver-specific IGF-1 gene deletion (LID) mice with osmotic pumps for 4 weeks. Infusion of IGF-1 plus IGFBP3 significantly increased both IGF-1 and IGFBP3 in the bone matrix (p < 0.05). (Fig. 6i) and enhanced trabecular bone formation relative to LID mice infused with IGF-1 alone or a vehicle (p < 0.05) (Supplementary Table 3). Our results suggest that IGFBPs such as IGFBP3 facilitate the deposition of IGF-1 in the bone matrix for its function during bone remodeling.
DISCUSSION
Bone formation is an energy consuming metabolic process in which there is significant bone matrix synthesis and mineralization by osteoblasts. IGF-1 regulates this new bone formation by acting more as a differentiation factor than a mitogen for osteoblasts. We found that IGF-1 induces osteoblast differentiation of Sca-1+ MSCs through activation of mTOR. Inhibition of mTOR activity by rapamycin blocked IGF-1 induced osteoblast differentiation of Sca-1+ MSCs and mineralization. mTOR is critical as a signaling molecule relative to both whole organ and cellular energy metabolism in response to nutrient availability and several environmental stimuli24. The mTOR pathway when genetically down-regulated increases life span and stem cell homeostasis in evolutionarily diverse organisms including mammals24. IGF-1 is also an important determinant of body size and lifespan in animals. Our data demonstrating that the regulation of osteoblast differentiation from MSCs in the bone remodeling unit by IGF-1 through mTOR may help explain the mechanism of IGF-1 regulation of body size and longevity25, 26. In addition, the mTOR complex has emerged as a key regulator of cell migration and chemotaxis27, suggesting that IGF-1 may also facilitate the recruitment of MSCs in the coupling process during bone remodeling. In the coupling process, active IGF-1, released from bone matrix, induces differentiation of MSCs recruited by TGF-β1 (Fig. 6j).
Although most fracture prevention efforts for osteoporosis have been directed at inhibition of age-related bone loss, evidence is growing that bone maintenance during early adult life is an important contributor to bone strength during aging12, 28–31. IGF-1 is the most abundant factor deposited in the bone matrix throughout life32. Levels of IGF-1 in the circulation and in bone matrix decline significantly with age in both men and women33–35. This is most likely due to a reduction in growth hormone secretion. Notably, skeletal IGF-1 content in human bones also decline almost 60% between the ages of 20 and 60 years36. However, mice circulating IGF-1 is much higher than that of humans and the level of human IGF-II in circulation is higher than that of IGF-1. Furthermore, IGF-1 levels serum blood do not change with age in mice whereas in humans they progressively decrease with age to less than 100 ng ml−1 around 60 years of age. Importantly, bone matrix IGF-1 in both humans and rodents decrease with age. In our study we found a significant reduction in bone marrow IGF-1 levels amongst osteoporotic individuals with very low bone density undergoing hip replacements. Although both osteoarthritis and osteoporosis subjects were age matched, one limitation was that we could not evaluate healthy aged controls. In spite of this limitation and consistent with our findings, in a previous clinical trial hip fracture individuals treated with an IGF-1 plus IGFBP3 complex had significant functional improvements and a blunting in femoral bone loss post fracture compared to vehicle-treated osteoporotic control subjects after hip fracture37, whereas, IGF-1 administration alone has not been shown to enhance bone mass or improve functional outcomes in older individuals. Taken together, the clinical observations and our rodent data suggest that the essential pool of IGF-1 in the bone matrix may not be sufficiently available for new bone formation during the aging process. Therefore, modulation of IGF-1 deposition in the bone matrix could potentially be a therapeutic approach to delay or prevent osteoporosis.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by National US Institutes of Health Grant AR 053973 (XC).
Footnotes
AUTHOR CONTRIBUTIONS
L.X., X.W., M.L. and L.P performed the majority of the experiments, analyzed data and prepared the manuscript. T.Q. maintained mice, collected tissue samples and helped with μCT analyses. L.P. helped with the in vitro transwell migration assay. J.P.R finished the human sample detection. X.J. and L.Z. assisted with rat in vivo experiments. J.C., F.F., C.J.R., S.Y., S. X., A.E and M.W. provided suggestions for the project and critically reviewed the manuscript. X.C. supervised the project and wrote most of the manuscript.
REFERENCE LIST
- 1.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Schettler AE, Gustafson EM. Osteoporosis prevention starts in adolescence. J. Am. Acad. Nurse Pract. 2004;16:274–282. doi: 10.1111/j.1745-7599.2004.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 4.Zaidi M. Skeletal remodeling in health and disease. Nat. Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 5.Agnusdei D, Gentilella R. GH and IGF-I as therapeutic agents for osteoporosis. J. Endocrinol. Invest. 2005;28:32–36. [PubMed] [Google Scholar]
- 6.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 7.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakar S, Rosen CJ. From mouse to man: redefining the role of insulin-like growth factor-I in the acquisition of bone mass. Exp. Biol. Med. (Maywood.) 2003;228:245–252. doi: 10.1177/153537020322800302. [DOI] [PubMed] [Google Scholar]
- 9.Yakar S, Pennisi P, Wu Y, Zhao H, LeRoith D. Clinical relevance of systemic and local IGF-I. Endocr. Dev. 2005;9:11–16. doi: 10.1159/000085718. [DOI] [PubMed] [Google Scholar]
- 10.Amin S, et al. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J. Bone Miner. Res. 2007;22:799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- 11.Ohlsson C, et al. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seck T, et al. Concentration of insulin-like growth factor (IGF)-I and -II in iliac crest bone matrix from pre- and postmenopausal women: relationship to age, menopause, bone turnover, bone volume, and circulating IGFs. J. Clin. Endocrinol. Metab. 1998;83:2331–2337. doi: 10.1210/jcem.83.7.4967. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, et al. Serum levels of insulin-like growth factor (IGF); IGF-binding proteins-3, -4, and -5; their relationships to bone mineral density and the risk of vertebral fractures in postmenopausal women. Calcif. Tissue Int. 2006;78:18–24. doi: 10.1007/s00223-005-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill PA. Bone remodelling. Br. J. Orthod. 1998;25:101–107. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- 15.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 16.Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–443. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautista CM, Mohan S, Baylink DJ. Insulin-like growth factors I and II are present in the skeletal tissues of ten vertebrates. Metabolism. 1990;39:96–100. doi: 10.1016/0026-0495(90)90154-5. [DOI] [PubMed] [Google Scholar]
- 19.Canalis E, Pash J, Gabbitas B, Rydziel S, Varghese S. Growth factors regulate the synthesis of insulin-like growth factor-I in bone cell cultures. Endocrinology. 1993;133:33–38. doi: 10.1210/endo.133.1.8319580. [DOI] [PubMed] [Google Scholar]
- 20.Pfeilschifter J, et al. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J. Clin. Invest. 1995;96:767–774. doi: 10.1172/JCI118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden JM, Mohan S, Baylink DJ. The insulin-like growth factor system and the coupling of formation to resorption. Bone. 1995;17:93S–98S. doi: 10.1016/8756-3282(95)00186-h. [DOI] [PubMed] [Google Scholar]
- 22.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 23.Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol. 2010;207:127–134. doi: 10.1677/JOE-10-0209. [DOI] [PubMed] [Google Scholar]
- 24.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Parent CA. Review series: TOR kinase complexes and cell migration. J.Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 29.Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica Mex. 2009;51(Suppl 1):S5–S17. doi: 10.1590/s0036-36342009000700004. [DOI] [PubMed] [Google Scholar]
- 30.Liu JM, et al. IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal women. J. Bone Miner. Metab. 2008;26:159–164. doi: 10.1007/s00774-007-0799-z. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson C, et al. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. J. Bone Miner. Res. 2011;26:865–872. doi: 10.1002/jbmr.281. [DOI] [PubMed] [Google Scholar]
- 32.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 1986;261:12665–12674. [PubMed] [Google Scholar]
- 33.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 34.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 35.Ziv E, Hu D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res. Rev. 2011;10:201–204. doi: 10.1016/j.arr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Mohan S, Baylink DJ. Serum insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 levels in aging and age-associated diseases. Endocrine. 1997;7:87–91. doi: 10.1007/BF02778070. [DOI] [PubMed] [Google Scholar]
- 37.Boonen S, et al. Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J. Clin. Endocrinol. Metab. 2002;87:1593–1599. doi: 10.1210/jcem.87.4.8426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.