Abstract
The insular cortex is the primary cortical site devoted to taste processing. A large body of evidence is available for how insular neurons respond to gustatory stimulation in both anesthetized and behaving animals. Most of the reports describe broadly tuned neurons that are involved in processing the chemosensory, physiological and psychological aspects of gustatory experience. However little is known about how these neural responses map onto insular circuits. Particularly mysterious is the functional role of the three subdivisions of the insular cortex: the granular, the dysgranular and the agranular insular cortices. In this article we review data on the organization of the local and long-distance circuits in the three subdivisions. The functional significance of these results is discussed in light of the latest electrophysiological data. A view of the insular cortex as a functionally integrated system devoted to processing gustatory, multimodal, cognitive and affective information is proposed.
Introduction
The gustatory cortex (GC) is the least understood among primary sensory cortices. GC's localization within the insular cortex (IC) was established only relatively recently in the history of neuroscience [1,2]. The rarity of gustatory symptoms in neurological conditions, the location of the insular cortex, nested deeply within the Sylvian sulcus, and the sparse responsiveness to gustatory stimuli in anesthetized preparations are only some of the reasons for this late discovery. The identification of the circuits responsible for taste processing has been further complicated by the inherently multimodal and affective nature of this sense. In more recent years, however, the interest in IC has been steadily increasing. Here we will discuss emerging evidence about the local and long distance circuits mediating the processing of gustatory experience in the IC. We will focus on results obtained from rodents and pertaining to the gustatory part of IC.
Insular Cortex
In primates the taste-processing part of the insular cortex occupies the portion of cerebral cortex above the claustrum and located deep within the Sylvian sulcus [3]. In rodents the same structure is located on the lateral portion of the hemisphere, mostly dorsal to the rhinal sulcus, with the exception of a small portion ventral to it and adjacent to the olfactory cortex [4–6].
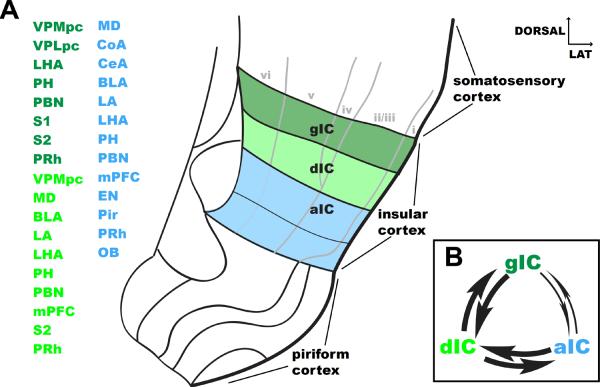
The insular cortex is divided into three cytoarchitectural subdivisions that can be identified in the dorso-ventral plane: the granular (dorsal), the dysgranular (intermediate) and the agranular (ventral) subdivisions [3,7,8] (Figure 1A). This nomenclature reflects the progressive disappearance of the granular layer (i.e. layer IV) and a change in laminar organization. Layer IV is present in the granular IC (gIC), dysmorphic in the dysgranular (dIC) and absent in the agranular (aIC). gIC has a classical six layered structure, dIC has a thinner and fainter layer IV and aIC appears as a tri-laminar structure, with no layer IV and a fusion between layers V and VI. Hence IC appears as a transitional area and its architecture progressively morphs from a standard neocortical template to a trilaminar-like structure as it approaches the paleocortex (Figure 1A).
Figure 1. Summary of long-distance and local connections of the insular cortex in the rat.
A. Coronal view of the region of the rat's brain containing the somatosensory cortex, the insular cortex and the piriform cortex. The three subdivisions of the insular cortex and the input areas are color coded: dark green corresponds to the granular insular cortex, light green to the dysgranular insular cortex and light blue to the agranular insular cortex. Overlaid in grey on the coronal view is a schematic outline of the cortical layers. The semi-transparent line between L5 and L6 in agranular insular cortex indicates the fading boundary between these two layers. Abbreviations: aIC: agranular insular cortex, BLA: basolateral amygdala, CeA: central amygdala, CoA: anterior cortical nucleus, dIC: dysgranular insular cortex, EN: endopiriform nucleus, gIC: granular insular cortex, LA: lateral amygdala, LHA: lateral hypothalamic area, MD: mediodorsal thalamic nucleus, mPFC: medial prefrontal cortex, OB: olfactory bulb, PBN: parabrachial nucleus, PH: posterior hypothalamic nucleus, Pir: piriform cortex, PRh: perirhinal cortex, S1: somatosensory cortex, area 1, S2: somatosensory cortex area 2, VPLpc: ventroposterolateral thalamic nucleus, parvicellular part, VPMpc: ventroposteromedial thalamic nucleus, parvicellular part. The numbers next to the afferent area indicate the references. B. Schematic of the feed-forward, feed-back interconnectivity between gIC, dIC and aIC. The smaller arrows connecting gIC with aIC reflect the sparser connection between these two areas.
Very little information is available on the functional organization of insular circuits involved in taste processing. While it is reasonable to expect that circuits in the gIC may share some similarities with its dorsal neighbor, the somatosensory cortex, such a priori assumption cannot be made for dIC and aIC. Recent optical imaging experiments relying on voltage sensitive dyes (VSD) directly compared the patterns of activation to intracortical stimulation of barrel cortex and insular cortex at its transition between the gIC and dIC [*9]. Electrical stimulation of layer IV of the barrel cortex and its equivalent depth in IC revealed differences and similarities between these structures. While stimulation elicited some degree of columnar activity in both cases, the vertical spread of activity in IC was more pronounced than in somatosensory cortex. Data showing correlated activity among neighboring neurons [10,11] as well as results from studies relying on optical imaging [*12,13] may confirm the columnar organization of IC.
Functional integration within insular circuits
Despite the evidence of a vertical spread of signals in gIC and dIC, the three subdivisions of IC should not be considered as independent compartments. Tracing studies have revealed a pattern of feedforward and feedback interconnectivity among these regions [7]. gIC projects diffusely to all the layers of dIC, dIC projects back to layers I and VI of gIC and diffusely to aIC. In addition aIC projects to layers I and VI of dIC and gIC (Figure 1B). No study has systematically investigated the functional strength of the feedforward/feedback loops between the different layers of the three divisions. VSD imaging data are however available to confirm the spread of activity across the different regions of IC [*9,14–17]. Electrical stimulation of the superficial layers (300 μm below the pial surface) of gIC, dIC or aIC results in spatiotemporal patterns of activation that spread to the entire IC, although activation of gIC appears to be less effective in exciting the superficial layers of aIC [14]. Stimulation of the intermediate layers of dIC induces a wave of activity that propagates vertically to the deep layers of dIC, travels obliquely to the deep layers of aIC and, to a lesser extent, horizontally to the intermediate layers of aIC [17]. In vivo VSD analysis of the spatiotemporal dynamics of excitation in IC further reveals that the extensive cross-talk between gIC, dIC and aIC occurs along both the dorso-ventral and rostro-caudal axes [14–16]. These spatiotemporal dynamics are shaped not only by horizontal excitatory projections, but also by local inhibitory circuits [14,15]. This result suggests the possibility that modulations of inhibition, likely mediated by noradrenergic or cholinergic transmission [18,19], could change the functional connectivity between gIC, dIC or aIC. The communication between the different subdivisions of IC can also be modulated by plastic changes in the circuit [16].
Bottom-up and top-down inputs of the insular cortex
The connections between IC and other regions are well described and add complexity to the anatomical and functional organization of this cortical area (Figure 1A). Gustatory information reaches IC via the parvicellular portion of the ventroposteromedial nucleus of the thalamus (VPMpc) and, to a lesser extent and only in rodents, via the parabrachial nucleus (PBN) located in the pons. gIC and dIC are the main recipients of gustatory information as they receive inputs from the VPMpc and PBN [4,20,21]; only a very small contingent of VPMpc fibers and a moderate projection from PBN reaches aIC [21,22]. Thalamic fibers terminate diffusely in all the cortical layers, with a slight bias for the intermediate layers [4,20].
Inputs to the gustatory portion of IC are not limited to taste. The thalamus (ventroposterolateral parvicellular nucleus -VPLpc) and PBN can also relay visceral information [4,6]. Other sensory areas are also densely connected with IC. The somatosensory cortex, for instance, sends projections carrying tactile information about the mouth and the tongue to gIC and dIC [7,23]. Electrophysiological and optical recordings of brain slices containing IC and somatosensory cortex revealed an oscillatory interplay between these two areas [24]. Sensory information pertaining to other sensory modalities, i.e. olfactory [22,25,26] and auditory [27,28], appears to preferentially target aIC. Particularly relevant from a functional standpoint are the projections from the olfactory system. The olfactory bulb and the piriform cortex project directly to aIC [22,25,26]. These inputs could play a large role in the integration and association between taste and olfaction that is known to involve also IC [*29].
Beyond sensory inputs, IC also integrates affective, anticipatory and reward-related information coming from limbic areas. Regions like the mediodorsal thalamus (MD), the prefrontal cortex, the rostral insular cortex (an area adjacent to the orbitofrontal cortex), the basolateral nucleus of amygdala (BLA), the lateral hypothalamus (LHA), the ventral tegmental area (VTA) and the parahippocampal region are all known to project to the region of IC involved in processing taste [4,7,30–34]. With few exceptions, namely LHA which projects to all the subdivisions, inputs from these areas target mainly aIC and dIC [4].
To simplify the picture depicted above, one could conceptualize the inputs to IC as segregated into “bottom-up” gustatory afferents and “top-down” limbic or multisensory inputs. The former appear to target mostly gIC and dIC, while the latter project to dIC and aIC. On the grounds of this anatomical organization one could imagine the three subdivisions being equivalent to primary, secondary and tertiary gustatory cortex, each of which may process different aspects of the gustatory experience [7,35]. However, the robust reciprocal interactions between these areas, as well as anatomical data showing a more complex and overlapping pattern of connectivity with limbic and other sensory areas, argue in favor of a more integrative view. Since all three of the subdivisions are themselves sources of corticofugal fibers [4,7], it is reasonable to think of gIC, dIC and aIC as an integrated system working in parallel rather than serially. Therefore, no subdivision is just an input or an output stage, but instead each receives partially overlapping information, integrates it and feeds it back to their inputs as well as to other targets. The complex reciprocal connections with sensory, visceral and limbic areas are likely to mediate an extremely dynamic and integrated flow of information.
Coding of taste quality in IC
Most of the research on taste processing over the past years has been directed at understanding how neurons in IC process the basic taste qualities, i.e. sweet, salty, sour, bitter and umami. Extracellular recordings in anesthetized rodents [36–38,**39,40] have shown that while the majority of taste processing neurons are located in gIC and dIC, neurons in aIC can also display gustatory responses [36,41]. This result is consistent with evidence of direct VPMpc inputs to gIC and dIC and with indications that these two subdivisions might relay gustatory signals to aIC. While responses in all three subdivisions are generally broadly tuned [42,43], neurons in gIC appear more taste-selective than those in dIC [36]. Within each subdivision taste responsive neurons are distributed across all the cortical layers with a preference for the intermediate layers [**39] and L5 [37,41,44], where thalamic inputs appear to be denser [4,20].
Studies aimed at understanding how IC neurons process taste quality have traditionally relied on averaging firing responses over multiple seconds after stimulus delivery. This approach has neglected the fact that neural responses in IC are exquisitely time-varying [45,46]. Single neurons respond to gustatory stimuli with complex modulations in the time-course of their firing rates. Taste-evoked changes in firing rates can display different latencies, durations and rhythmic patterns [**39,47,48,**49,50,51]. The behavioral significance of these temporal profiles has been the focus of intense investigation (see below), however very little is known about how these dynamics map onto insular circuits. Recent results have shown that interneurons produce responses lasting longer than pyramidal cells [**39]. Given the organization of inputs to IC it is extremely likely that the position of neurons within insular circuits will also be a key factor associated with different response patterns. More work is however necessary to understand how firing dynamics relate to cell types, location within insular circuits (i.e. layers and subdivisions) and connectivity patterns.
While not much is known about how different elements in gIC, dIC ad aIC process taste, more attention has been devoted to the search for a spatial map of taste qualities on the surface of IC [*12,13,52]. Recent analyses of neural activity, using intrinsic or calcium imaging, show that responses to different taste qualities appear to be spatially organized in the superficial layers of gIC and dIC [*12,13,*53] (Figure 2A and B). The extent to which responses are spatially segregated is however unclear. Intrinsic imaging data show some functional segregation and a large degree of overlap between the areas activated by different taste qualities [13] (Figure 2B). This result was recently challenged by calcium imaging experiments in which sweet, salty, umami and bitter tastes appear to be coded by a small contingent of topographically isolated narrowly tuned neurons [*12] (Figure 2A). Since all of the mapping studies were performed in anesthetized preparations, the extent to which this spatial organization is reshaped by behavior in alert animals is at the moment unknown. The strong influence of learning on spatial mapping [*53] (Figure 2B) however suggests a high degree of behavioral- and state-dependent plasticity [*54].
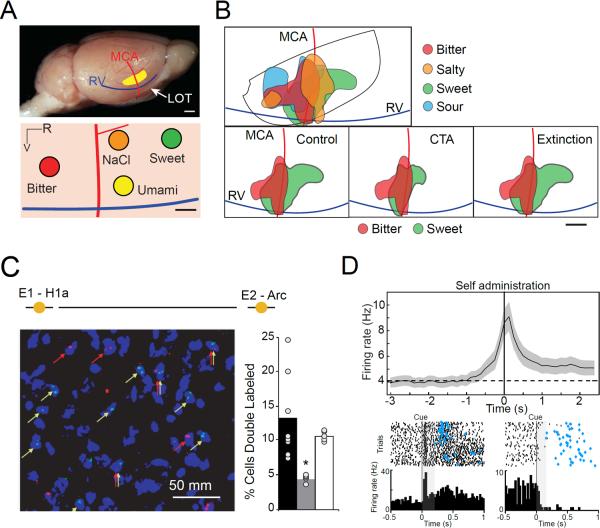
Figure 2. Neural responses in the insular cortex.
A. Hot spots of neurons narrowly tuned to specific taste qualities in the gustatory region of the insular cortex. Top. Diagram of the location of GC (yellow region) in the rodent brain. MCA: middle cerebral artery; RV: rhinal veins; LOT: lateral olfactory tract. Scale bar: 1 mm. Bottom. Diagram of GC indicating four hot spots each containing about 30% of neurons that are narrowly tuned for one specific taste quality (red: bitter; green: sweet; orange: salty; yellow: umami). The representations for each quality appear spatially segregated. Scale bar: 0.5 mm. B. Overlap and plasticity of taste maps. Top. Map of the region of insular cortex activated by four taste qualities: bitter (red), sweet (green), salty (orange) and sour (light blue). The maps show a large spatial overlap between qualities. Bottom. Plasticity of taste maps following aversive conditioning of sucrose. Left: baseline maps of the regions activated by bitter (red) and sweet (green) stimuli. Middle: regions activated by bitter and sweet stimuli following aversive conditioning to sucrose. Note the increase in overlap between sucrose and quinine representations. Right: restoration of the original map following extinction of the aversive memory. Scale bar: 0.5 mm. C. Cues activated taste responsive neurons in the gustatory portion of rodent insular cortex. Left. Double labeling of neurons in the gustatory cortex using two immediate early genes (H1a: red; c-fos: green). Neurons were double labeled with H1a and c-fos allowing for the identification of populations of neurons that were activated by two stimuli delivered at successive time points (epoch 1 E1 or epoch 2 E2). Right. Bar plot representing the proportion of neurons double labeled in response to: exposure to the same tastant (black), exposure to a predictive cue and the predicted tastant (white); exposure to a non-predictive cue and a tastant (grey). Note that the predictive cue activates the same population of neurons that were activated by the stimulus associated with the cue. D. Anticipatory firing in gustatory cortex. Top. Population peri-stimulus time histogram (PSTH) of gustatory cortical neurons in response to tastants self-administered following an auditory cue. Time = 0 corresponds to the time of self-delivery. Note the ramp of anticipatory activity preceding self-administration. Shading around the trace indicates the standard error of the mean. Bottom. Raster plots (above) and PSTHs (below) of two neurons in response to anticipatory cues. Time = 0 corresponds to the onset of the auditory cue. The left panel depicts a neuron with an excitatory response to the cue, the right panel shows a neuron with an inhibitory response. Blue diamonds indicate the timing of self-delivery. Panels were taken and modified from A: [*12]; B: [42]; C: [**57] and D: [**60].
Multiplexing of gustatory information in IC
The variety of inputs to IC, ranging from gustatory to multisensory and limbic, and the functional integration between gIC, dIC and aIC suggest that the gustatory portion of IC might play a role that goes beyond the “simple” coding of taste quality. The results obtained from experiments on alert animals are entirely consistent with this view and argue in favor of the functional integration of multiple sensory, cognitive and affective signals within IC.
Neurons in IC are inherently multimodal [43]. Nociceptive [11], thermal [55], visceral [22,56], somatosensory [28,48,55], olfactory [**57], visual [58] and auditory [27,28,59,**60] signals are all known to be processed in IC. Particularly well-studied is the modulation from somatosensory inputs [48,**49]. Recordings from the area of IC that processes taste unveiled two patterns of somatosensory activity: phasic tactile responses to intraoral delivery of solutions [48] and licking-related rhythmic activity [**49]. Phasic and short-latency tactile responses to stimulus delivery are believed to communicate the arrival of a fluid in the mouth. Licking-related rhythmic activity, on the other hand, favors the processing of gustatory information, enhances the ability to discriminate taste quality and is modulated by learning. It is important to note that somatosensory and chemosensory information are not necessarily segregated in IC; indeed the same neurons can be modulated by both modalities.
IC neurons can also be activated by auditory and olfactory stimulation [28,**57,59,**60]. These signals are known to reach aIC and, to some extent, dIC. Auditory and olfactory cues become particularly effective in activating IC when they anticipate the availability of a gustatory reward [**57,59,**60]. Double labeling analyses of immediate early gene expression reveal an overlap in the neural population activated by sucrose and by the cue anticipating it (Figure 2C). Electrophysiological recordings from the insular cortex of behaving animals confirm this result [**60], generalize it to the visual domain [58], and uncover its functional implications [**60]. Auditory cues instructing the subject to press a bar for gustatory stimulation trigger expectation-related responses in insular neurons (Figure 2D). Cues induce patterns of insular cortical activity similar to those evoked by gustatory stimuli. This anticipatory priming of cortical activity results in faster encoding of gustatory information [**60]. These results are consistent with prior suggestions of a tight relationship between pre-stimulus changes in background firing activity, anticipation and gustatory processing [**49,**61,62–64]. These results also confirm that degree to which neurons respond exclusively to one modality is limited; indeed anticipatory/attentional cues of multiple modalities can effectively activate neurons that are also involved in the processing of chemosensory information [**57,59,**60]. Little is known of the neural basis underlying anticipatory cue-responses. However recent results convincingly point to the BLA as a necessary source of anticipatory signals [**60]. Other areas involved in the processing of rewards, i.e. MD, frontal cortices and LHA, as well as areas responsible for controlling the state of cortical networks, are also likely to play a fundamental role.
Another example of how information from multiple sources is integrated in IC comes from studies showing that neurons that encode taste quality can also respond to the hedonic value of taste [50,51]. Learning the aversiveness [51] or the desirability [*29] of a novel stimulus alters its perceived palatability without modifying its chemosensory identity. While perceptually independent, both these variables can be processed by the same neurons along the time course of its firing response [47,51,65]. This integration is likely mediated by functionally overlapping inputs from VPMpc and amygdala (and possibly other reward-related areas). The convergence on the same neurons of these two inputs has not been studied yet, however multiple lines of evidence support the involvement of amygdala in shaping taste-evoked IC activity [51,65,*66].
Finally, another effect of the convergence of multiple inputs on IC is the state dependency of taste responses. Work from different groups has shown how changes in the physiological [67] and psychological state [50] of the animal results in modifications of IC activity. Both satiety and arousal affect taste processing, with the latter resulting in dramatic modifications of how palatability is perceived and encoded by neural ensembles.
Conclusion
The results described in the previous paragraph, together with evidence of how learning and behavioral states shape the processing of gustatory experience, are the likely consequence of the anatomical organization of IC. A variety of inputs carrying gustatory, multisensory, reward-related and affective information enter IC through different routes (gIC, dIC and aIC). As it gets processed, this information propagates within all the subdivisions and generates time-varying responses. According to the electrophysiological evidence from alert animals, the extent to which inputs in IC are functionally segregated in multiple sensory or behavioral labeled-lines appears to be minimal. Instead, information is multiplexed in the temporal structure of single neuron firing and is adjusted to the behavioral state of the animal. The final outcome is a cortical structure whose perceptual and behavioral function is multifaceted and dependent upon the context [*54,68]. How this integration is achieved by insular circuits is currently unknown. While a lot of work and multiple approaches will be needed to address how insular circuits process the various aspects of gustatory experience, the recent surge in interest for this area promises exciting times ahead.
Highlights
-
1)
Gustatory information is processed by the insular cortex
-
2)
The insular cortex is composed by three subdivisions (granular, dysgranular and agranular)
-
3)
Gustatory inputs to gIC/dIC are integrated with limbic inputs to aIC via intracortical connections
-
4)
This integration generates responses that are multimodal, multidimensional and state dependent
Acknowledgements
This work was supported by National institute of Deafness and Other Communication Disorders Grants R01-DC010389 and by a Klingenstein Foundation Fellowship (AF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penfield W, Jasper HH. Epilepsy and the functional anatomy of the human brain. edn [1st Little; Boston: 1954. [Google Scholar]
- 2.Motta G. I fattori centrali delle disgeusie. Tipografia Luigi Parma; Bologna: 1958. [Google Scholar]
- 3.Gallay DS, Gallay MN, Jeanmonod D, Rouiller EM, Morel A. The insula of reil revisited: multiarchitectonic organization in macaque monkeys. Cereb Cortex. 2012;22:175–190. doi: 10.1093/cercor/bhr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 5.Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- 6.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- 7.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M. Macroscopic connection of rat insular cortex: anatomical bases underlying its physiological functions. Int Rev Neurobiol. 2011;97:285–303. doi: 10.1016/B978-0-12-385198-7.00011-4. [DOI] [PubMed] [Google Scholar]
- *9.Sato H, Shimanuki Y, Saito M, Toyoda H, Nokubi T, Maeda Y, Yamamoto T, Kang Y. Differential columnar processing in local circuits of barrel and insular cortices. J Neurosci. 2008;28:3076–3089. doi: 10.1523/JNEUROSCI.0172-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, which relies on VSD imaging techniques, the authors show that stimulation of the intermediate layers of IC results in a vertical spread of activity. They further show that horizontal activation is regulated by inhibition.
- 10.Yokota T, Satoh T. Three-dimensional estimation of the distribution and size of putative functional units in rat gustatory cortex as assessed from the inter-neuronal distance between two neurons with correlative activity. Brain Res Bull. 2001;54:575–584. doi: 10.1016/s0361-9230(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Ogawa H. Columnar organization of mechanoreceptive neurons in the cortical taste area in the rat. Exp Brain Res. 2002;147:114–123. doi: 10.1007/s00221-002-1228-0. [DOI] [PubMed] [Google Scholar]
- *12.Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using two-photon calcium imaging of single neurons in the insular cortex of anesthetized mice this study shows a topographic map for sweet, salty, bitter and umami stimuli. The authors find that each taste quality selectively activates spatially segregated small groups of narrowly tuned neurons.
- 13.Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita S, Adachi K, Koshikawa N, Kobayashi M. Spatiotemporal dynamics of excitation in rat insular cortex: intrinsic corticocortical circuit regulates caudal-rostro excitatory propagation from the insular to frontal cortex. Neuroscience. 2010;165:278–292. doi: 10.1016/j.neuroscience.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Fujita S, Koshikawa N, Kobayashi M. GABA(B) receptors accentuate neural excitation contrast in rat insular cortex. Neuroscience. 2011;199:259–271. doi: 10.1016/j.neuroscience.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi N, Fujita S, Koshikawa N, Kobayashi M. Spatiotemporal dynamics of long-term potentiation in rat insular cortex revealed by optical imaging. Neurobiol Learn Mem. 2011;96:468–478. doi: 10.1016/j.nlm.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Fu W, Sugai T, Yoshimura H, Onoda N. Convergence of olfactory and gustatory connections onto the endopiriform nucleus in the rat. Neuroscience. 2004;126:1033–1041. doi: 10.1016/j.neuroscience.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Koyanagi Y, Yamamoto K, Oi Y, Koshikawa N, Kobayashi M. Presynaptic interneuron subtype- and age-dependent modulation of GABAergic synaptic transmission by beta-adrenoceptors in rat insular cortex. J Neurophysiol. 2010;103:2876–2888. doi: 10.1152/jn.00972.2009. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Koyanagi Y, Koshikawa N, Kobayashi M. Postsynaptic cell type-dependent cholinergic regulation of GABAergic synaptic transmission in rat insular cortex. J Neurophysiol. 2010;104:1933–1945. doi: 10.1152/jn.00438.2010. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res. 2000;36:297–309. doi: 10.1016/s0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 21.Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Res. 1982;242:33–40. doi: 10.1016/0006-8993(82)90493-0. [DOI] [PubMed] [Google Scholar]
- 22.Krushel LA, van der Kooy D. Visceral cortex: integration of the mucosal senses with limbic information in the rat agranular insular cortex. J Comp Neurol. 1988;270:39–54. 62–33. doi: 10.1002/cne.902700105. [DOI] [PubMed] [Google Scholar]
- 23.Guldin WO, Markowitsch HJ. Cortical and thalamic afferent connections of the insular and adjacent cortex of the rat. J Comp Neurol. 1983;215:135–153. doi: 10.1002/cne.902150203. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura H, Kato N, Sugai T, Honjo M, Sato J, Segami N, Onoda N. To-and-fro optical voltage signal propagation between the insular gustatory and parietal oral somatosensory areas in rat cortex slices. Brain Res. 2004;1015:114–121. doi: 10.1016/j.brainres.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 25.Lo L, Anderson DJ. A cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shipley MT, Geinisman Y. Anatomical evidence for convergence of olfactory, gustatory, and visceral afferent pathways in mouse cerebral cortex. Brain Res Bull. 1984;12:221–226. doi: 10.1016/0361-9230(84)90049-2. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A, Imbe H, Donishi T. Efferent connections of an auditory area in the caudal insular cortex of the rat: anatomical nodes for cortical streams of auditory processing and cross-modal sensory interactions. Neuroscience. 2010;166:1140–1157. doi: 10.1016/j.neuroscience.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers KM, Benison AM, Klein A, Barth DS. Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex. 2008;18:2941–2951. doi: 10.1093/cercor/bhn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, Katz DB. State dependence of olfactory perception as a function of taste cortical inactivation. Nat Neurosci. 2010;13:158–159. doi: 10.1038/nn.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]; This very elegant study shows the importance of the insular cortex in influencing olfactory perception. Using behavioral and pharmacological methods the authors show the involvement of insular cortex in flavor perception, a result which provides a functional perspective to the connectivity between gustatory and olfactory areas.
- 30.Ohara PT, Granato A, Moallem TM, Wang BR, Tillet Y, Jasmin L. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J Neurocytol. 2003;32:131–141. doi: 10.1023/b:neur.0000005598.09647.7f. [DOI] [PubMed] [Google Scholar]
- 31.Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- 32.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 33.Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald CJ, Meck WH, Simon SA, Nicolelis MA. Taste-guided decisions differentially engage neuronal ensembles across gustatory cortices. J Neurosci. 2009;29:11271–11282. doi: 10.1523/JNEUROSCI.1033-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sewards TV, Sewards MA. Cortical association areas in the gustatory system. Neurosci Biobehav Rev. 2001;25:395–407. doi: 10.1016/s0149-7634(01)00021-5. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa H, Hasegawa K, Murayama N. Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas, in rats. Exp Brain Res. 1992;91:415–424. doi: 10.1007/BF00227838. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol. 1984;51:616–635. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. II. Information processing of taste quality. J Neurophysiol. 1985;53:1356–1369. doi: 10.1152/jn.1985.53.6.1356. [DOI] [PubMed] [Google Scholar]
- **39.Yokota T, Eguchi K, Hiraba K. Functional properties of putative pyramidal neurons and inhibitory interneurons in the rat gustatory cortex. Cereb Cortex. 2011;21:597–606. doi: 10.1093/cercor/bhq126. [DOI] [PubMed] [Google Scholar]; This study analyzes the shape of extracellularly recorded spike waveforms to separate GC neurons in different populations. Putative pyramidal neurons and putative interneurons show different responses to gustatory stimulation in anesthetized rats.
- 40.Ogawa H, Ito S, Murayama N, Hasegawa K. Taste area in granular and dysgranular insular cortices in the rat identified by stimulation of the entire oral cavity. Neurosci Res. 1990;9:196–201. doi: 10.1016/0168-0102(90)90004-x. [DOI] [PubMed] [Google Scholar]
- 41.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 42.Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa H, Murayama N, Hasegawa K. Difference in receptive field features of taste neurons in rat granular and dysgranular insular cortices. Exp Brain Res. 1992;91:408–414. doi: 10.1007/BF00227837. [DOI] [PubMed] [Google Scholar]
- 45.Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proc Natl Acad Sci U S A. 2007;104:18772–18777. doi: 10.1073/pnas.0705546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller P, Katz DB. Stochastic transitions between neural states in taste processing and decision-making. J Neurosci. 2010;30:2559–2570. doi: 10.1523/JNEUROSCI.3047-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz DB, Nicolelis MA, Simon SA. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol. 2002;12:448–454. doi: 10.1016/s0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- 48.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.Gutierrez R, Simon SA, Nicolelis MA. Licking-induced synchrony in the taste-reward circuit improves cue discrimination during learning. J Neurosci. 2010;30:287–303. doi: 10.1523/JNEUROSCI.0855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; These article analyzes how licking modulates neuronal firing in IC of freely licking rats engaged in a go/no-go task. The authors find neurons in IC that are entrained by licking and whose entrainement increases with learning.
- 50.Fontanini A, Katz DB. State-dependent modulation of time-varying gustatory responses. J Neurophysiol. 2006;96:3183–3193. doi: 10.1152/jn.00804.2006. [DOI] [PubMed] [Google Scholar]
- 51.Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimura H, Sugai T, Fukuda M, Segami N, Onoda N. Cortical spatial aspects of optical intrinsic signals in response to sucrose and NaCl stimuli. Neuroreport. 2004;15:17–20. doi: 10.1097/00001756-200401190-00005. [DOI] [PubMed] [Google Scholar]
- *53.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci U S A. 2008;105:4010–4015. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using intrinsic imaging in anesthetized rats the authors show spatial mapping of taste responses in the insular cortex. The representations of different taste qualities are overlapping and plastic. Aversive learning induces a rearrangement of sucrose spatial representation that results in an increase in its overlap with quinine's map.
- *54.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review discusses the importance of behavior in shaping network states and sensory responses. It proposes a novel framework for studying trial-to-trial variability of neural responses. Variability of taste responses in IC is interpreted against this backgroud.
- 55.Yamamoto T, Yuyama N, Kawamura Y. Cortical neurons responding to tactile, thermal and taste stimulations of the rat's tongue. Brain Res. 1981;221:202–206. doi: 10.1016/0006-8993(81)91075-1. [DOI] [PubMed] [Google Scholar]
- 56.Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol. 1998;79:2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- **57.Saddoris MP, Holland PC, Gallagher M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. J Neurosci. 2009;29:15386–15396. doi: 10.1523/JNEUROSCI.3233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report relies on analysis of immediate early gene (H1a and c-fos) colocalization to identify neurons activated by stimuli separated by a temporal interval. The authors find that anticipatory cues activate a subset of IC neurons that are also activated by the predicted tastant.
- 58.Ifuku H, Nakamura T, Hirata S, Ogawa H. Neuronal activities in the reward phase in primary and higher-order gustatory cortices of monkeys. Neurosci Res. 2006;55:54–64. doi: 10.1016/j.neures.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.Samuelsen CL, Gardner MPH, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012 doi: 10.1016/j.neuron.2012.02.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article the authors describe responses to auditory cues in the insular cortex of alert rats. Multelectrode recordings are used to show that antipatory cues prime GC in a way similar to that achieved by unexpected tastants. This cue-dependent priming of cortical activity, which depends upon amygdalar inputs, correlates with more rapid processing of gustatory information.
- **61.Yoshida T, Katz DB. Control of prestimulus activity related to improved sensory coding within a discrimination task. J Neurosci. 2011;31:4101–4112. doi: 10.1523/JNEUROSCI.4380-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using multielectrode techniques in animals trained to perform a gustatory discrimination task, the authors show that encoding of gustatory stimuli depends on whether the animal is task-oriented or not. Analysis of pre-stimulus activity suggests a fundamental relationship between background activity and responses to stimuli in GC.
- 62.Stapleton JR, Lavine ML, Nicolelis MA, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Front Neurosci. 2007;1:161–174. doi: 10.3389/neuro.01.1.1.012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, Simon SA. Rapid taste responses in the gustatory cortex during licking. J Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Sensory inputs from the oral region to the cerebral cortex in behaving rats: an analysis of unit responses in cortical somatosensory and taste areas during ingestive behavior. J Neurophysiol. 1988;60:1303–1321. doi: 10.1152/jn.1988.60.4.1303. [DOI] [PubMed] [Google Scholar]
- 65.Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J Neurosci. 2009;29:2486–2495. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *66.Stone ME, Maffei A, Fontanini A. Amygdala stimulation evokes time-varying synaptic responses in the gustatory cortex of anesthetized rats. Front Integr Neurosci. 2011;5:3. doi: 10.3389/fnint.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the synaptic potentials evoked by BLA inputs onto insular cortical neurons. Using in vivo intracellular techniques the authors show that amygdala stimulation results in a fast excitatory response followed by a longer lasting inhibitory potential.
- 67.de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:195–199. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]