Abstract
An extinguished conditioned response can sometimes be restored. Previous research has shown that this renewal effect depends on the context in which conditioning versus extinction takes place. Here we provide evidence that the dorsal hippocampus is critically involved in the representation of context that underscores the renewal effect. We performed electrolytic lesions in dorsal hippocampus, before or after extinction, in a conditioned taste aversion paradigm with rats. Rats that underwent all conditioning, extinction and testing procedures in the same experimental context showed no renewal during testing in the original context. In contrast, rats that underwent extinction procedures in a different experimental context than the one in which they had acquired the conditioned response, showed a reliable renewal effect during testing in the original context. When electrolytic lesion was performed prior to extinction, the context-dependent renewal effect was disrupted. When electrolytic lesion was undertaken after extinction, we observed a complex pattern of data including the blockage of the conventional renewal effect, and the appearance of an unconventional renewal effect. The implications of these results are discussed with respect to current views on the role of the dorsal hippocampus in processing context information.
Keywords: Conditioned taste aversion, Renewal effect, Lesion of dorsal hippocampus, Context
Introduction
A previously extinguished conditioned response (CR) can be renewed under some conditions. Such renewal suggests that temporal context information may somehow facilitate the restoration of a CR that was temporarily inactivated, but not obliterated (Bouton 1993). Based on the phenomenon of spontaneous recovery, it seems reasonable to suggest that the extinction of a particular response does not necessarily imply the loss of the CS–US association itself. Instead, behavioral extinction might be due to active inhibitory mechanisms that oppose the CS–US association (Holland 1984; Ross and Holland 1981), or to new associations with the CS, which take dominance over the presently “extinguished” association.
Presumably, a CR is acquired through the formation of excitatory associations between CS and US. With extinction procedures, new inhibitory associations may be formed between CS and US. Thus, different types of association could be mutually negated, CR exhibition repressed, and extinction would take place (Holland 1984; Ross and Holland 1981). One interpretation along these lines would be that the experimental context determines whether inhibition of the CS–US association takes place: If the extinction context is the same as the conditioning context, then the CS–US association would be inhibited, but if the extinction context is different, then the situation is more ambiguous, leading to the possibility that more complex adaptive mechanisms will occur.
Research on the neural mechanisms of extinction and renewal has suggested that the hippocampus is one of the most relevant brain areas. It has been reported that the hippocampus is related to the formation of associations among information concerning “context–specificity” and important behavioral events. Additionally, several researchers (Selden et al. 1991; Kim and Fanselow 1992; Phillips and LeDoux 1992; Rudy and O’Reilly 2001; Jeffery et al. 2004; Bouton et al. 2006; Bruchey and Gonzalez-Lima 2006; Ji and Maren 2007; Bruchey et al. 2007; Neumann and Longbottom 2008) have suggested that the hippocampus plays an important role in the recognition of differences between conditioning and extinction contexts. When the hippocampus is damaged, reinstatement is hindered (Frohardt et al. 2000). When the activity of the dorsal hippocampus is hindered through injection of muscimol during extinction, the renewal effect is not established (Corcoran and Maren 2001). Thus, the dorsal hippocampus appears to be involved in information processing concerning experimental context and renewal. Yet, the precise mechanisms remain unknown.
Here we aim to further investigate the role of the dorsal hippocampus in relation to the renewal effect, using conditioned taste aversion (CTA). In typical CTA procedures, a novel gustatory stimulus (CS) is associated with lithium chloride injection (US), which produces discomfort in the internal organs. Taste and visceral sensations are presumably processed in parallel via a neural network including the pons, the cortical taste area, and amygdala (Gallo et al. 1992; Spector 1995; Lamprecht et al. 1997; Yasoshima and Yamamoto 1997; Schafe and Bernstein 1998). At the same time, the hippocampus has been thought to play a relatively small role (Yamamoto et al. 1995; Bures et al. 1998). In the present study, we used CTA to examine the effects of electrolytic lesion of the dorsal hippocampus (before or after extinction) on context processing. The degree of renewal was used as the dependent measure.
Table 1 shows the experimental schedule, consisting of three experiments, with two groups of subjects in each experiment (one group undergoing all procedures in the same context, AAA, and one group undergoing extinction in a different context, ABA). Experiment 1 provided baseline data. In Experiment 2, electrolytic lesions were performed before extinction. In Experiment 3, the lesions followed after extinction.
Table 1.
Experimental schedule
| Group | Conditioning | Extinction | Test |
|---|---|---|---|
| Exp. 1 | |||
| AAA | A → US | A | A |
| ABA | A → US | B | A |
| Exp. 2 | |||
| AAA | A → US* | A | A |
| ABA | A → US* | B | A |
| Exp. 3 | |||
| AAA | A → US | A* | A |
| ABA | A → US | B* | A |
A context A; B context B
* Hippocampal lesion
Experiment 1
Experiment 1 was conducted to provide baseline data and to confirm with the present CTA that the renewal effect following extinction depends on the alteration of the experimental context.
Methods
Subjects
Eighteen Long Evans male rats 10 weeks old (280–320 g) were used. The breeding environment was maintained under conditions of a temperature of 23 ± 1 °C, humidity of 50 ± 10 %, and a light–dark cycle of 14/10 h (illumination at 7:00 a.m.). Subjects were raised with free intake of food and water until commencement of training. From then on, the food and water intake was controlled except for the period of recovery mentioned below. Prior to training, all subjects experienced 7 days handling. The treatment of laboratory animals was conducted in accordance with the “Guidelines for the Care and Use of Laboratory Animals” of Tamagawa University.
Devices and apparatus
For the conditioned taste aversion 3.5 % sucrose was used as a CS and 0.15 M lithium chloride (LiCl) was used as a US. For the experimental context, 2 different environments were used within sound-proof shield boxes. The primary experimental context consisted of a test cage (250 × 300 × 400 mm) created from acrylic boards. Two holes (20 mm in diameter) were opened so that stimuli could be administered through glass tubes in front, at 80 mm from the bottom and 105 mm from each end. The inside walls of these sound-proof boxes were darkened, and the house lights were switched off (context A). For the experimental context, white acrylic boards were attached to the test cage, the house lights were switched on, and paper towels and wire mesh were used to cover the floor of the test cage (context B).
Behavioral procedures
Procedures for this experiment comprised the following 4 periods: shaping, conditioning, extinction, and testing. 9 subjects experienced all procedures following intake training in the same context (AAA group) and 9 subjects experienced a different extinction context (ABA group).
For 5 days, shaping was undertaken such that all subjects received stimuli via glass tubes in context A and context B, with two session a day in each context (days 1–5). Water was offered to all subjects through 2 glass tubes over a period of 20 min in each context. The intake quantities were measured.
On the day following the end of intake training, conditioning was undertaken (day 6). 3.5 % sucrose (CS) was administered to all subjects through the glass tubes over 20 min in context A. Subsequently, 0.15 M lithium chloride (LiCl; 20 ml/kg) was intraperitoneally administered to all subjects as the US, and the subjects were returned to their home cages.
After the conditioning, there followed a 7-day recovery period (days 7–13). Then a 2-day Re-shaping was conducted again (days 14–15). Procedures were the same as those used during intake training. After confirmation that the subjects’ water drinking had recovered to normal quantities, 4-day extinction procedures were conducted presenting CS alone (days 16–19). During these extinction procedures, the experimental contexts were different for the two groups: context A for the AAA group, and context B for the ABA group. CS was administered for 20 min, and intake quantities were measured.
Additionally, after the extinction procedures had been carried out for 4 days, there followed again a 7-day recovery period (days 20–26). After the recovery period, a test was conducted (day 27). Testing was conducted in context A for all groups. After administration of CS alone for 20 min, intake quantities were measured.
Except during shaping, conditioning, extinction, and testing, food was applied at any time, and water was offered to all subjects over a period of 20 min from 9:00 to 11:00 in the home cage.
Results and discussion
The average water-drinking quantities during the period of intake training were 8.4 ml (SEM = 0.45) for the AAA group and 8.8 ml (SEM = 0.44) for the ABA group; the difference was not significant, t = 0.62, p > 0.05. Additionally, the average sucrose-drinking quantities for each group on the day of conditioning (day 6) were 12.8 ml (SEM = 0.62) for the AAA group and 12.2 ml (SEM = 0.53) for the ABA group; the difference was not significant, t = 0.75, p > 0.05.
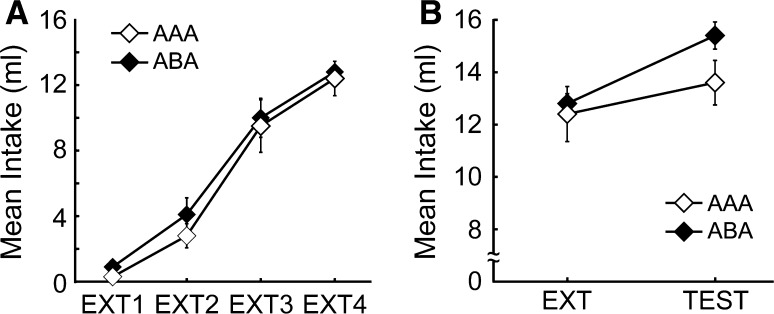
The average sucrose-drinking quantities for the AAA group and for the ABA group from the 1st day until the 4th day of extinction (EXT1-EXT4) are shown in Fig. 1A. Analysis of variance was performed on these data with groups (AAA vs. ABA) and sessions (1–4) as factors. A main effect for sessions was obtained, F(3,48) = 225.17, p < 0.000. However, there was neither a main effect for groups, F(1,16) = 0.66, nor an interaction effect, F(3,48) = 1.57. The results suggested that the difference concerning experimental context did not have an influence on extinction.
Fig. 1.
The basic renewal effect. A AAA group experienced all procedures following intake training in the same context and ABA group experienced a different extinction context. Mean (±SEM) intake sucrose-drinking for the AAA group and ABA group form the 4 days of extinction (EXT1–EXT4). B Mean (±SEM) intake sucrose-drinking last day of extinction (EXT) and testing (TEST)
In order to review the renewal effect, sucrose-drinking quantities on the last day of extinction were compared with those at the time of testing as shown in Fig. 1B. Analysis of variance with groups and sessions as factors showed neither a main effect for group, F(1,16) = 0.17 nor a main effect for sessions, F(1,16) = 2.07. However, the interaction between groups and sessions was highly significant, F(1,16) = 25.89, p < 0.0001. Post hoc analyses showed that the ABA group drank more sucrose on the last day of extinction than at the time of testing (p < 0.05), whereas the AAA group showed the opposite pattern, drinking more sucrose at the time of testing than on the last day of extinction (p < 0.05). These results confirmed that the intake quantities of sucrose during testing depended on the extinction context. Thus, a renewal effect was observed with the ABA group.
Zelikowsky et al. (2011) showed a context-dependent extinction using a procedure with the two contexts reversed as compared to our experiment. Their study provides further support to suggest that our results are due to context specificity.
Experiment 2
Having established a renewal effect with CTA in Experiment 1, Experiment 2 was conducted to examine the role of the dorsal hippocampus in the context-dependent renewal effect. Electrolytic lesion of the dorsal hippocampus was undertaken after conditioning, but before extinction. In all other respects, the experimental procedures were the same as in Experiment 1.
Methods
Subjects
Fourteen Long Evans male rats aged 10 weeks (280–320 g) were used. The breeding environment for the subjects was the same as that of experiment 1.
Devices and stimulation
The devices and stimulation for the behavioral procedures were the same as those in Experiment 1. For the electrolytic lesion of the dorsal hippocampus, we employed electrodes with stainless and headless pins (0.3 mm in diameter and 40 mm in length; Shiga Konchu Fukyu Company, Tokyo, Japan). The pins were insulated via electrochemical polishing up to 0.5 mm from the sharpened tip.
Surgery
Prior to the behavioral experiment, we embedded a stainless cannula for insertion of the electrodes to perform the electrolytic lesion. Ketamine (100 mg/kg, i.p.), xylazine (7 mg/kg, i.p.), and atropine sulfate (0.1 mg/kg, i.p.) were administered intraperitoneally for anesthesia. Subsequently, the heads of the subjects were fixed in a stereotaxic frame (Kopf Instruments). Four holes of 2.0 mm in diameter were opened: two at 2.5 mm from the bregma in the posterior direction, and 2.3 mm laterally (one in each hemisphere); and two at 4.5 mm from the bregma in the posterior direction, and 3.0 mm laterally (one in each hemisphere). Stainless cannulae (2.0 mm in diameter and 5 mm in length) were inserted in the holes. Subsequently, the holes were plugged and fixed with dental cement. After the operation and after a 7-day recovery period, water-drinking training commenced. During the recovery period, food and water were available without restriction.
Behavioral procedures
Dorsal hippocampal lesion was undertaken between conditioning and extinction. The behavioral paradigm was the same as that of Experiment 1. Six subjects were assigned to the AAA group and 8 subjects were assigned to the ABA group. For 5 days, intake training was undertaking, as in Experiment 1 (days 1–5). Immediately following the end of intake training, conditioning was undertaken in context A (day 6).
On day 7, the electrolytic lesion of dorsal hippocampus was performed by applying an electric current of 1.5 mA for 20 s. After lesion the subjects were given a 6-day recovery period (days 8–13). After recovery, a 2-day intake training period was conducted again (days 14–15).
After confirmation that the rats’ drinking was back to normal, extinction procedures were undertaken over 4 days (days 16–19), with the same experimental context (A) for the AAA group, or with a different experimental context (B) for the ABA group. On these 4 extinction days sucrose was administered without the US for 20 min in each session. After the extinction, a 7-day recovery period was established (days 20–26). Finally, on day 27, a test was undertaken in context A to establish the presence or absence of a renewal effect. After administration of sucrose without the US for 20 min, intake quantities were measured.
Histology
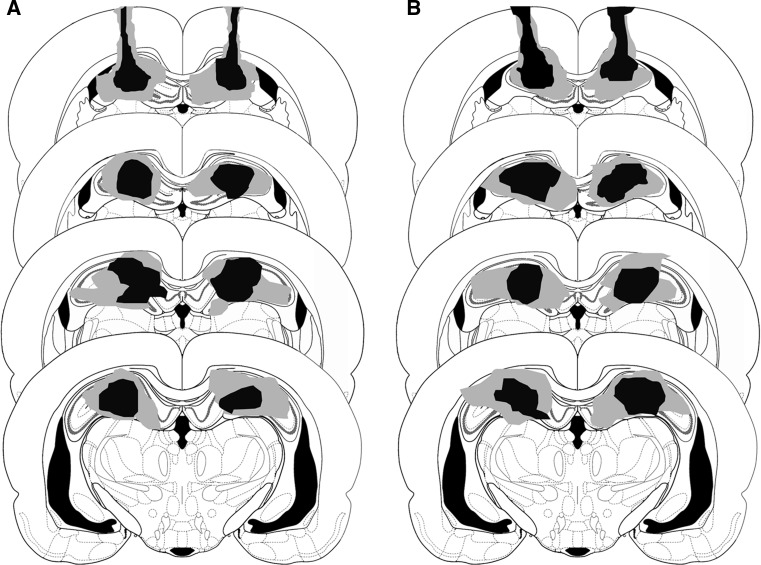
Upon completion of the behavioral experiment, pentobarbital (120 mg/kg, i.p.) was administered for deep pentobarbital anesthesia. Perfusion fixation was conducted by means of 10 % formalin solution, and then the brain was removed. Over the next few days, 10 % sucrose, 20 % sucrose, and 30 % sucrose were added incrementally. The removed brains were frozen and sliced to a thickness of 60 μm (CM3050S, Leica). The frozen sections were dyed with cresyl violet, and lesion locations were confirmed through visual inspection. An example of the electrolytic lesion of dorsal hippocampus is shown in Fig. 2.
Fig. 2.
An example lesioned area in dorsal hippocampus verified by histology (the arrowhead). A lesion area before extinction in experiment 2. B lesion area just after extinction in experiment 3. Minimum (black) and maximum (gray) extent of dosal hippocampus lesions in animals Experiments. The lesions were reconstructed on successive coronal sections (−2.4, −3.0, −3.6, and −4.2 mm to bregma) from Paxinos and Watson (2005)
Results and discussion
The average water-drinking quantities for each group during initial training prior to conditioning were 9.1 ml for the AAA group (SEM = 0.53) and 8.7 ml (SEM = 0.40) for the ABA group; the difference was not significant, t = 0.56, p > 0.05. Additionally, the average sucrose-drinking quantities for each group on the day of conditioning (day 6) were 12.9 ml for the AAA group (SEM = 0.95) and 12.4 ml (SEM = 0.61) for the ABA group; the difference was not significant, t = 0.44, p > 0.05.
The average sucrose-drinking quantities for the AAA group and for the ABA group during the period of extinction are shown in Fig. 3A. An analysis of variance with groups and sessions as factors produced neither a main effect for groups, F(1,12) = 0.57, nor an interaction effect, F(3,36) = 0.13. However, a main effect was observed for sessions, F(3,36) = 109.07, p < 0.000.
Fig. 3.
Dorsal hippocampal lesion, before extinction, blocks the renewal effect. A Mean (±SEM) intake sucrose-drinking for the AAA group and ABA group form the 4 days of extinction (EXT1-EXT4). B Mean (±SEM) intake sucrose-drinking last day of extinction (EXT) and testing (TEST)
To examine the renewal effect, sucrose-intake quantities on the last day of extinction were compared with those at the time of testing (see Fig. 3B). Analysis of variance with groups and sessions as factors showed neither a main effect for groups, F(1,12) = 1.48, nor an interaction effect, F(1,12) = 3.19. However, there was a highly reliable main effect of sessions, F(1,12) = 23.03, p < 0.0004. These results show that the dorsal hippocampal lesion did not influence extinction. Instead, the lesion had a detrimental impact on the renewal effect, with rats performing in the same way regardless of whether the extinction had taken place in the same context (A) or in a different context (B) than the one used for conditioning and testing (A). The data supported the hypothesis that the dorsal hippocampus is critically involved in the representation of context that underscores the renewal effect.
Experiment 3
Given that lesion of the dorsal hippocampus prior to extinction inhibits the renewal effect, it is safe to conclude that the dorsal hippocampus plays a role in the representation of context at the time of extinction. There are two possible hypotheses regarding such a role. First, the dorsal hippocampus may be related specifically to the encoding or acquisition of hierarchical, context-dependent control of CS–US inhibition. By this “acquisition hypothesis,” the dorsal hippocampus would serve to discriminate the differential context and to establish differential context-dependent mechanisms, such that CS–US inhibition would occur in a different (extinction) context, but not in the original (conditioning) context, unless extinction and conditioning took place in the same context. Consequently, if the dorsal hippocampus is damaged after the acquisition has taken place, the renewal effect should not be disrupted.
Alternatively, the role of the dorsal hippocampus might be particularly crucial at the time of retrieval, or the actual CR restoration at the time of testing. According to this “retrieval hypothesis,” it should not matter whether the lesion of the dorsal hippocampus occurred before or after extinction—in both cases, the damage to the dorsal hippocampus should disrupt the reactivation (or disinhibition) of CR. Of course, it is theoretically possible that the dorsal hippocampus is involved in both acquisition and retrieval, in which case, again, the CR should be disrupted even if the lesion takes place after extinction. To examine these different possibilities, we undertook Experiment 3, performing the dorsal hippocampal lesion after the extinction of conditioned taste aversion, but prior to testing, using behavioral procedures that were the same as in the previous experiments.
Methods
Subjects
Fourteen Long Evans male rats aged 10 weeks (280–320 g) were used. The breeding environment for subjects, the light–dark cycle, and the handling procedures were the same as those of Experiments 1 and 2.
Devices and stimulation
The devices and stimulation were the same as those used in Experiments 1 and 2.
Surgery and behavioral procedures
The surgery undertaken was the same as that of Experiment 2. Except for the timing of the lesion of the dorsal hippocampus, the experimental conditions and procedures were the same as those of Experiment 2. Seven subjects were assigned to the AAA group and 7 subjects were assigned to the ABA group. After the end of extinction procedures (day 20), the lesion of the dorsal hippocampus was performed. Following the lesion, the rats were given a 6-day recovery period (days 21–26).
Histology
The procedures for histology were the same as those of Experiment 2.
Results and discussion
The average water-drinking quantities for each group during the period of intake training were 8.7 ml for the AAA group (SEM = 0.59) and 9.1 ml (SEM = 0.44) for the ABA group; the difference was not significant, t = 0.49, p > 0.05. Additionally, the average sucrose-drinking quantities on the day of conditioning (day 6) were 12.9 ml for the AAA group (SEM = 0.95) and 12.4 ml (SEM = 0.61) for the ABA group; again, the difference was not statistically significant, t = 0.67, p > 0.05.
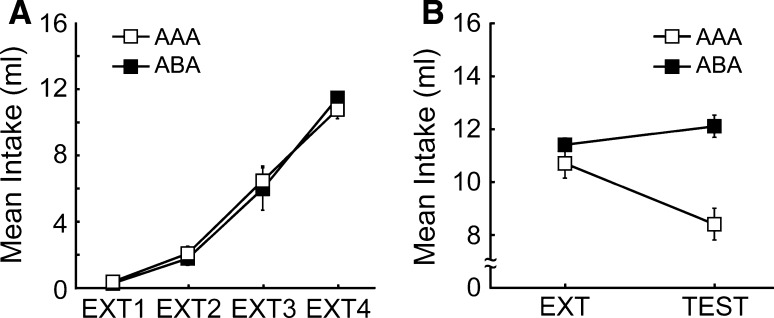
The average sucrose-drinking quantities for each group during the period of extinction are shown in Fig. 4A. An analysis of variance with groups and sessions as factors produced a main effect of sessions, F(3,36) = 95.05, p < 0.000. There was neither a main effect for groups, F(1,12) = 0.59, nor an interaction effect, F(3,36) = 0.27.
Fig. 4.
Dorsal hippocampal lesion, after extinction, affects reinstatement as well as renewal. A Mean (±SEM) intake sucrose-drinking for the AAA group and ABA group form the 4 days of extinction (EXT1-EXT4). B Mean (±SEM) intake sucrose-drinking last day of extinction (EXT) and testing (TEST)
To examine the renewal effect, the average intake quantities on the last day of extinction were compared to those at the time of testing; the data are shown in Fig. 4B. An analysis of variance with groups and sessions as factors produced neither a main effect of group, F(1,12) = 1.77, nor a main effect of sessions, F(1,12) = 1.51. However, there was a highly reliable interaction between groups and sessions, F(1,12) = 19.23, p < 0.0009. Based on multiple comparisons, significant differences in intake quantities between the groups were recognized at the time of testing (p < 0.05). Post hoc analyses showed that the AAA group drank more sucrose on the last day of extinction than at the time of testing (p < 0.05), whereas the ABA group showed the opposite pattern, drinking more sucrose at the time of testing than on the last day of extinction (p < 0.05).
The data suggested that the renewal effect was abolished for the ABA group following lesion of the dorsal hippocampus after extinction, providing evidence against the selective “acquisition hypothesis.” On the other hand, CR restoration was observed with the AAA group. Obviously, for this group there had been no context alteration at the time of extinction, and so this CR restoration deviates from the conventional renewal effect.
General discussion
The effects of context on memory acquisition, retrieval, and the renewal effect with a conditioned taste aversion procedure have been reported previously with respect to different mechanisms (e.g., latent inhibition; Quinteroa et al. 2011) and in other brain areas (e.g., Bouton and Bolles 1985; Orsini et al. 2011). The present study addressed the influence of hippocampal lesion on the renewal effect in a conditioned taste aversion paradigm with rats. Experiment 1 provided baseline data, establishing that the context-dependent renewal effect can be obtained with CTA. Lesion of the dorsal hippocampus prior to extinction disrupted the renewal effect in Experiment 2. This observation supported the hypothesis that dorsal hippocampus is critically involved in the representation of context that underscores the renewal effect. In Experiment 3, the role of dorsal hippocampus was further examined by evaluating the “acquisition” versus “retrieval” hypotheses. Performing the lesion after extinction, we again observed blockage of the conventional renewal effect in Experiment 3. However, we also observed spontaneous recovery of CR in rats that had always experienced the same experimental context throughout conditioning, extinction and testing. This unconventional renewal effect could not easily be captured by extant theories on the role of the dorsal hippocampus in the representation of context.
The results indicating that the renewal effect is inhibited due to lesion of the dorsal hippocampus are in accord with reports that the dorsal hippocampus is related to recall of fear memories related to contexts (Corcoran and Maren 2001; Holt and Maren 1999; Corcoran and Maren 2004; Ji and Maren 2005). However, our results depart from a previous report (Wilson et al. 1995), which suggested that even when electrolytic or pharmacological lesions were applied to the hippocampal fimbria and fornix prior to conditioning, there was no influence on renewal effect. In all likelihood, the discrepancy with the current data is due to the difference in the areas of the lesions.
As to the reason why lesion of the dorsal hippocampus prior to extinction procedures hindered the renewal effect, we propose that the hierarchical, context-dependent control of US-CS inhibition was blocked. Given that the blockage was observed in Experiment 3 as well, with lesion following extinction, it seems clear that the dorsal hippocampus must have a retrieval function relating to the context-dependent inhibition, either independent of, or in addition to, an acquisition function.
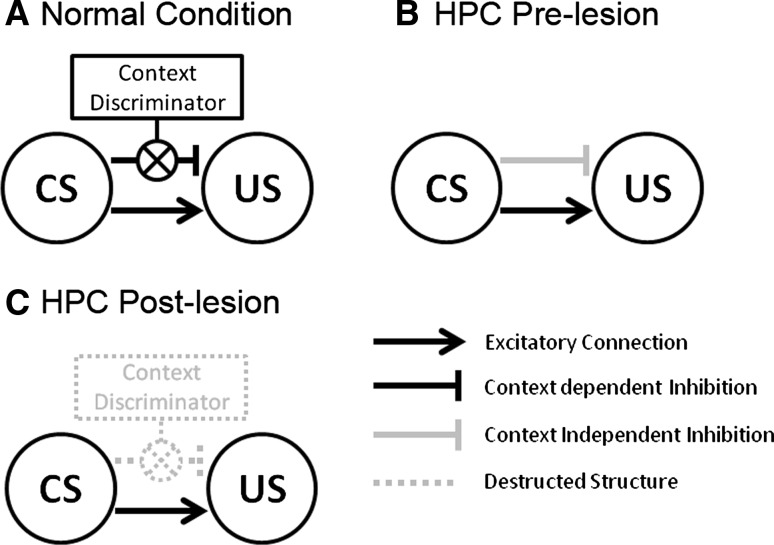
To explain “the hierarchical context-dependent control” of CS–US association, we present a schematic drawing in Fig. 5, on the basis of previous research (Bouton and Bolles 1985; Bouton 1994, Holland et al. 1999; Buenoa and Holland 2008). As shown in Fig. 5A, when the subjects receive CS at the time of testing after extinction procedures, this would activate, or lead to the retrieval of, both excitatory associations regarding US, acquired at the time of conditioning, and inhibitory associations acquired at the time of extinction. CR inhibition would be due to the mutual operation of such associations. As for the renewal effect, the CS–US inhibition would be context-dependent, occurring in the extinction and test context difference. Thus, context alteration would prevent the inhibitory association in the original (conditioning) context. As shown in Fig. 5B, lesion of the dorsal hippocampus, however, would disrupt this process. As a consequence, inhibitory associations are no longer bound to the extinction context, and may occur for the original (conditioning) context. It is quite possible that the hierarchical, context-dependent control is acquired without the dorsal hippocampus. However, with the loss of the ability to retrieve context-related information, inhibitory associations would prevail at the time of testing, and so the renewal effect would emerge.
Fig. 5.
Hierarchical context-dependent control of CS–US. A If the test context is identical to the extinction one, then the gate between CS and US connects to inhibit the US representation, otherwise the inhibitory connection does not work (e.g., ABA). This gating mechanism represents “hierarchical control” driven by Hippocampus. B A context-independent (hippocampal-independent) type of inhibitory connection is established during the extinction. C The context-dependent hierarchical controller has been removed by hippocampal lesion
As shown in Fig. 5C, the blocking of the renewal effect for the ABA group in Experiment 3 was consistent with the “retrieval hypothesis.” However, we also obtained an unpredicted result with the recovery of CR exhibited by the AAA group. This finding does not match with the conventional renewal effect, and cannot easily be accounted for with hierarchical, context-dependent control of inhibition of CS–US associations. Though the observation warrants further examination, some preliminary thoughts may be useful to guide future research efforts. One idea is that the unconventional renewal effect represents a form of spontaneous recovery, governed by the temporal dynamics of post-lesion neural reorganization. Indeed, Bouton (1993) pointed out that time course can be viewed as a dimension with a similar function as the physical experimental context. However, for present purposes it remains unclear how the time course could have a differential impact for the AAA group (which showed spontaneous recovery) as compared to the ABA group—unless spontaneous recovery took place in the ABA group as well, but was overshadowed by a more dominant blockage of the conventional renewal effect.
Alternatively, one might explain the entire pattern of data by suggesting that brain areas other than the hippocampus can be recruited to support general, but not context-dependent extinction mechanisms. To elaborate on this proposal, we first consider the data with the AAA groups, which experienced all experimental procedures in the same context. If hippocampal lesion occurs before extinction, then other brain areas (e.g., the amygdala) might be recruited to compensate for the lost hippocampal function in establishing mechanisms for the acquisition and/or retrieval of extinction (Herry et al. 2008). Consequently, in terms of behavioral expression, everything remains the same as compared to the normal case, without lesion. This explains why the data for the AAA group in Experiment 2 (lesion before extinction) were similar to those of the AAA group in Experiment 1 (no lesion). On the other hand, if hippocampal lesion occurs after extinction, then hippocampal would have performed its default role in establishing extinction mechanisms; however, following the lesion, these mechanisms can no longer be retrieved. As a consequence, the CR would reappear, as it did for the AAA group in Experiment 3 (lesion after extinction).
Now, turning to the ABA groups, which experienced a different context for extinction than the one in which conditioning and testing took place, we note that brain areas other than the hippocampus might not have shown the potential to replace the specific role of hippocampus in context representation. Thus, in Experiment 2 (lesion before extinction), other brain areas could not compensate for the loss of context-dependent control of extinction. Consequently, the dorsal hippocampal lesion would lead to indiscriminate extinction, that is, absence of CR during testing, or blockage of the renewal effect. This was indeed the case for the ABA group in Experiment 2. The same logic applies to Experiment 3 (lesion after extinction), with the further specification that the role of dorsal hippocampus must pertain, at least in part, to retrieval processes, given that the loss of context-dependent control of extinction occurred even when there had been ample opportunity for the acquisition of this type of control.
In short, the entire pattern of data can be explained by assuming (1) that the dorsal hippocampus is the default structure for the acquisition and retrieval of extinction mechanisms, (2) that the dorsal hippocampus can exert context-dependent control over the acquisition and retrieval of extinction, and (3) that other brain areas (e.g., the amygdala) might compensate for general, but not context-dependent, acquisition and retrieval of extinction mechanisms when the dorsal hippocampus is damaged (i.e., when extinction procedures are undertaken after dorsal hippocampal lesion). Of course, further research is required to corroborate and spell out the ramifications of these propositions, for instance, by examining the effects of lesion in the amygdala, and by examining neuronal firing rates in hippocampus versus amygdala in the present CTA paradigm. In the meantime, however, the present data already show that the current line of investigation will enable researchers to chart, in detailed neural circuits, the excitatory versus inhibitory connections among various representations that underscore conditioning, extinction and the role of context.
Acknowledgments
We thank Dr. Satoshi Fujii in Yamagata University School of Medicine for valuable discussions and advice on physiological experiments. This work was supported by MEXT: Tamagawa University GCOE program, Supported Program for the Strategic Research Foundation at Private Universities (2009–2013), and Grant-in-Aid for Scientific Research on Innovative Areas (No. 4103–21120006).
References
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and classical conditioning. Psychol Sci. 1994;3(2):49–53. [Google Scholar]
- Bouton ME, Bolles RC (1985) Context, event-memories, and extinction. In Balsam PD, Tomie A (eds) Cotext and learning, Hillsdale, NJ, Erlbaum, pp 133–166
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Brain activity associated with fear renewal. Eur J Neurosci. 2006;24(12):3567–3577. doi: 10.1111/j.1460-9568.2006.05229.x. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145(2):423–437. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenoa JLO, Holland PC. Occasion setting in Pavlovian ambiguous target discriminations. Behav Process. 2008;79(3):132–147. doi: 10.1016/j.beproc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned taste aversion: memory of a special kind. New York: Oxford University Press; 1998. [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21(5):1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11(5):598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114(2):227–240. doi: 10.1037/0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gallo M, Roldan G, Bures J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res. 1992;52(1):91–97. doi: 10.1016/S0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Holland PC. Differential effects of reinforcement of an inhibitory feature after serial and simultaneous feature negative discrimination training. J Exp Psychol Anim Behav Process. 1984;10(4):461–475. doi: 10.1037/0097-7403.10.4.461. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9(2):143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19(20):9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neurosci Biobehav Rev. 2004;28(2):201–218. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12(3):270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17(9):749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 1997;17(21):8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DL, Longbottom PL. The renewal of extinguished conditioned fear with fear-relevant and fear-irrelevant stimuli by a context change after extinction. Behav Res Ther. 2008;46(2):188–206. doi: 10.1016/j.brat.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31(47):17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates 5th edition. Elsevier Academic Press, London, UK
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037/0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quinteroa E, Díaza E, Vargasa JP, Schmajukb N, Lópeza JC, Dela-Casaa LG. Effects of context novelty vs. familiarity on latent inhibition with a conditioned taste aversion procedure. Behav Process. 2011;86(2):242–249. doi: 10.1016/j.beproc.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Ross RT, Holland PC. Conditioning of simultaneous and serial feature-positive discriminations. Anim Learn Behav. 1981;9(3):293–303. doi: 10.3758/BF03197835. [DOI] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82. doi: 10.3758/CABN.1.1.66. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion. II. Insular (gustatory) cortex. Brain Res. 1998;800(1):40–47. doi: 10.1016/S0006-8993(98)00492-2. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Spector AC. Gustatory function in the parabrachial nuclei: implications from lesion studies in rats. Rev Neurosci. 1995;6(2):143–175. doi: 10.1515/REVNEURO.1995.6.2.143. [DOI] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109(5):828–836. doi: 10.1037/0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neurosci Res. 1995;22(1):31–49. doi: 10.1016/0168-0102(95)00875-T. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Yamamoto T. Rat gustatory memory requires protein kinase C activity in the amygdala and cortical gustatory area. Neuro Report. 1997;8(6):1363–1367. doi: 10.1097/00001756-199704140-00009. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Pham DL, Fanselow MS. (2011) Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. doi:10.1002/hipo.20954 (on line) [DOI] [PMC free article] [PubMed]