Abstract
Introduction
Salicylidene acylhydrazide compounds have been shown to inhibit bacterial pathogens, including Chlamydia and Neisseria gonorrhoeae. If such compounds could also target HIV-1, their potential use as topical microbicides to prevent sexually transmitted infections would be considerable. We determined the in vitro anti-HIV-1 activity, cytotoxicity and mechanism of action of several salicylidene acylhydrazides.
Methods
Inhibitory activity was assessed using TZMbl cells and primary peripheral blood mononuclear cells (PBMCs) as targets for HIV-1 infection. Anti-viral activity was measured against cell-free and cell-associated virus and in vaginal fluid and semen simulants. Since the anti-bacterial activity of salicylidene acylhydrazides is reversible by Fe2+, we determined whether Fe2+ and other cations could reverse the anti-HIV-1 activity of the compounds. We also employed real-time PCR to determine the stage affected in the HIV-1 replication cycle.
Results
We identified four compounds with 50% HIV-1 inhibitory concentrations of 1 to 7 μM. In vitro toxicity varied but was generally limited. Activity was similar against three R5 clade B primary isolates and whether targets for virus replication were TZMbl cells or PBMCs. Compounds inhibited cell-free and cell-associated virus and were active in vaginal fluid and semen simulants. Fe2+, but not other cations, reversed the anti-HIV-1 effect. Finally, inhibitory effect of the compounds occurred at a post-integration step.
Conclusions
We identified salicylidene acylhydrazides with in vitro anti-HIV-1 activity in the μM range. The activity of these compounds against other sexually transmitted pathogens makes them potential candidates to formulate for use as a broad-spectrum topical genital microbicide.
Keywords: Salicylidene acylhydrazides, HIV, microbicide, iron chelation
Introduction
A clinical trial of topical tenofovir, applied as a gel, has demonstrated a reduction in sexually acquired HIV infections among women [1]. The modest reduction associated with tenofovir gel establishes the utility of topical microbicides and underscores the need to develop more potent compounds. Compounds capable of simultaneously inhibiting both HIV and other sexually transmitted pathogens would constitute an ideal prevention strategy.
Previous studies have suggested that salicylidene acylhydrazides can inhibit the type III secretion apparatus of Yersinia pseudotuberculosis and of Chlamydia [2–4]. However, at least in the case of Chlamydia spp., the salicylidene acylhydrazides exert an antibacterial effect either directly or indirectly by limiting iron availability to host cells [4]. We have recently shown that sexually transmitted pathogens, including C. trachomatis and Neisseria gonorrhoeae, can be inhibited in vitro by μM concentrations of selected salicylidene acylhydrazides [5]. In the case of Chlamydia, these compounds were further shown to protect mice from a vaginal challenge [6].
Iron chelating compounds, including a salicylidene acylhydrazide have been previously shown to inhibit HIV infection [7–11]. It is thought that limiting iron uptake by cells susceptible to HIV slows cell-cycle progression and reduces integrated viral gene expression, although other mechanisms have also been proposed [7, 8, 12]. Given their anti-chlamydial, anti-gonococcal and iron-chelating activity, we sought to determine if the salicylidene acylhydrazides with activity against Chlamydia might also be effective in inhibiting HIV-1 infection. We conducted virus inhibition and toxicity assays using both primary CD4+ lymphocytes and a cell line that allows rapid detection of successful infection. Four compounds were identified with limited cytotoxicity and with antiviral activity in the low μM range. These compounds inhibited diverse HIV-1 strains, and inhibition was reversible by iron repletion.
Materials and Methods
Research on this project involved the use of blood samples taken from human subjects enrolled in the University of California, Irvine Normal Blood Donors Program. This program was approved by the University of California, Irvine Institutional Review Board. Written informed consent was obtained from each participant.
Compounds
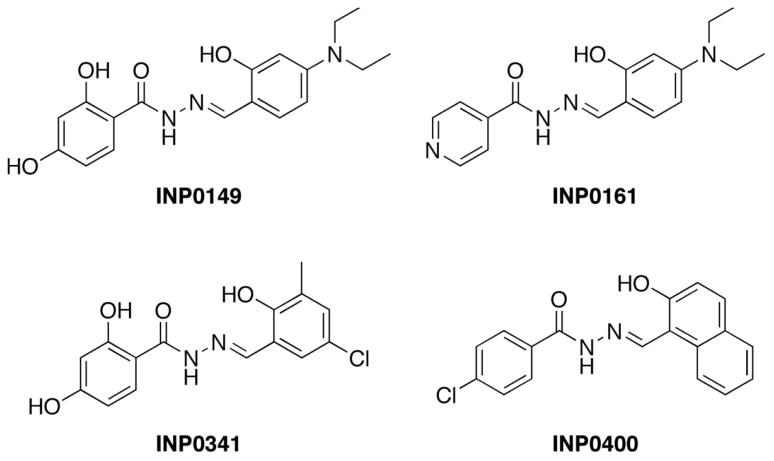
The salicylidene acylhdrazides N′-(4-diethylamino-2-hydroxybenzylidene)- 2,4- dihydroxybenzhydrazide (INP0149), N′-(4-diethylamino-2-hydroxybenzylidene)- isonicotinicacid hydrazide (INP0161), N′-(5-chloro-2-hydroxy-3-methylbenzylidene)-2,4- dihydroxybenzhydrazide (INP0341), and N′-(2-hydroxy-1-naphtalenylmethylene)-4- chlorobenzhydrazide (INP0400) were the kind gift of Creative Antibiotics, Umeå, Sweden (Figure 1). For use in the assays described, compounds were dissolved in dimethyl sulfoxide (DMSO; Fisher Scientific, Fair Lawn, NJ). The compounds were synthesized from the corresponding salicylic aldehydes and hydrazides according to published procedures [13]. The compounds were analyzed by nuclear magnetic resonance spectroscopy and mass spectrometry.
Figure 1.
Structures of salicylidene acylhydrazides
Virus inhibition assays
Virus inhibition was measured using either TZMbl luciferase reporter cells or phytohemagglutinin (PHA)-activated primary peripheral blood mononuclear cells (PBMCs) as target cells. TZMbl cells, at 5 × 104/well, were plated into 96-well microtiter plates and incubated overnight until confluent. Compounds, diluted in DMSO, were next added to the monolayers, followed immediately by polybrene (10 μg/ml) and HIV-1 (MOI = 0.02). Note that the use of polybrene increased the yield of virus as expected but had little or no impact on the magnitude of inhibition due to test compounds (data not shown). 24-hours post-infection, monolayers were washed three times with medium to remove the compounds. Wells were then repleted with fresh medium (without compound), and the plates were incubated for an additional 24 hours. To determine virus yield, cell culture supernatant was removed from the wells, the monolayers were lysed with 40 μl of a 1X solution of cell lysis buffer (Promega) for 10 minutes, and 25 μl of the cell lysate was transferred to a white opaque microtiter plate for luminescence reading. A Biotek Synergy 2 multi-purpose plate reader with automatic injectors was used to inject 100 μl of Luciferase 1000 assay substrate (Promega) in each well, and relative light units (RLUs) were read with a 1 second integration time one well at a time. Inhibition was calculated as follows: 100 × (RLUDMSO control− RLUtest compound)/RLUDMSO control. 50% inhibitory concentrations (IC50) were determined from the linear portion of the % inhibition-logarithmic concentration curve.
In some experiments, compounds were first mixed for 15 minutes with a vaginal fluid simulant at pH 4.5, 5.5, or 6.5, a semen simulant at pH 7.7, or a 1:1 (vol/vol) combination of vaginal fluid simulant (pH 4.5) and semen simulant (pH 7.7) prior to incubating with cells. In these experiments, compound in vaginal fluid or semen simulant was left on the target cells for 24 hours, washed, and replaced with fresh medium. Vaginal fluid and semen simulants were prepared by following the formulas proposed by Owen & Katz [14, 15].
To evaluate the impact of salicylidene acylhydrazides on limiting infection initiated by infected cells, as opposed to cell-free virus, CEM.NKr.CCR5 cells were exposed to HIV-1US657 for 3 days. After washing to remove cell-free virus, the infected CEM.NKR.CCR5 cells were added to TZMbl cells treated with compounds as above.
PBMCs were separated from whole blood of healthy volunteers via Ficoll-Paque (GE Healthcare, Piscataway, NJ). PBMCs were PHA stimulated for 2–3 days, washed three times with medium and plated into round bottom 96-well microtiter plates. The compounds and HIV-1US657 were added as above. After washing 24 hours post-infection to remove salicylidene acylhydrazides and repleting with fresh medium, plates were incubated for an additional 6 days. On day 7, HIV-1 was quantified in a p24 ELISA (ZeptoMetrix). Inhibition was calculated by dividing the concentration of p24 in wells with compound by the concentration of p24 in control (DMSO) wells.
Iron and other cations
Experiments with added iron, magnesium, or calcium were set up similarly to the virus inhibition assays with the addition of 250 μM final concentrations of FeSO4, MgCl2, or CaCl2 during the first 24-hour incubation period.
Cytotoxicity assays
Compound cytotoxicity was measured by lactate dehydrogenase (LDH) release and by uptake of propidium iodide (PI). For LDH release, the Cytotox 96® non-radioactive cytotoxicity assay (Promega Corporation, Madison, WI) was used. TZMbl cells were seeded into 96-well microtiter plates at a concentration of 5 × 104 cells per well. Cells were incubated at 37°C in 5% CO2 using RPMI media with 10% FBS, 4 mM of glutamate and 50 μg/ml of gentamicin. Subsequently, concentrations of salicylidene acylhydrazides ranging from 0.78 μM to 100 μM diluted in DMSO were added along with 10 μg/ml of polybrene to confluent monolayers. The final DMSO concentrations of controls were the same as those of the test compounds in all experiments. After 24-hours of incubation, media was collected to assay LDH release, and cells were lysed for total LDH estimation. Media and cell lysates were diluted with PBS-1% FBS to obtain the linear range of LDH activity. Aliquots of 50 μl of each sample were combined with the substrate and transferred to a flat bottom 96-well plate. Reactions were incubated for 30 minutes at room temperature in the dark following the addition of 50 μl of 1 M acetic acid. Each sample was assayed in triplicate. All assays were performed on three separate occasions. Reactions were read at 492 nm using a Spectra MAX 340 plate reader (Molecular Devices, Sunnyvale, CA). DMSO-treated samples were used as a background control and salicylidene acylhydrazide-treated cells were used to calculate the percentage of LDH leakage (LDH activity in medium/total LDH activity × 100).
To determine compound cytotoxicity by PI uptake, TZMbl cells were plated into 24-well tissue culture plates and incubated overnight until confluent. Concentrations of salicylidene acylhydrazides ranging from 3.125 to 100 μM were added to the wells, incubated for 24 hours, washed, and replaced with fresh medium. Plates were then incubated for an additional 24 hours. Subsequently, cells were trypsinized and resuspended in 1x phosphate buffered saline (PBS). Cytotoxicity was determined by adding PI and determining the percentage of cells containing PI from the FL3 channel of an Accuri C6 flow cytometer using CFlow Plus software. Toxicity due to the compounds was expressed relative to PI positive cells treated with DMSO.
Quantification of early reverse-transcription products
DNA was extracted (Dneasy Blood and Tissue kit, QIAgen, Valencia, CA) from trypsinized TZMbl cells that had been treated with compound and infected with HIV-1US657 as above but in 24-well plates. The following primers and probe were used to quantify R-U5 DNA by real-time PCR: R-U5 sense M667 (5′-GGCTAACTAGGGAACCCACTG-3′); R-U5 antisense AA55 (5′-CTGCTAGAGATTTTCCACACTGAC-3′); and RU5 probe (5′-6-FAM-TGTGTGCCCGTCTGTTGTGTGACT-DDQI-3′). The human apolipoprotein B (HAPB) gene was quantified in the same reaction to adjust for experimental variability using the following primers and probe: HAPB forward primer (5′-TGAAGGTGGAGGACATTCCTCTA-3′); HAPB reverse primer (5′-CTGGAATTGCGATTTCTGGTAA-3′); and HAPB probe (5′-JOE-CGAGAATCACCCTGCCAGACTTCCGT-TAMRA-3′). Real-time PCR was done with a RotoGene 3000 (Corbett Research, Sydney, Australia) using cycling parameters of 10 minutes at 95°C followed by 45 cycles of 30 seconds at 95°C, 30 seconds at 58°C, and 30 seconds at 72°C.
Quantification of integrated HIV-1 DNA
DNA was extracted from trypsinized TZMbl cells that had been treated with compound and infected with HIV-1US657 as above. Alu-Gag (chromosomally integrated) DNA was amplified using the following primers: Alu forward primer (5′-GCCTCCCAAAGTGCTGGGATTACAG-3′) and HIV Gag reverse primer (5′-GTTCCTGCTATGTCACTTCC-3′). PCR cycling parameters were 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C, 15 seconds at 50°C, and 3.5 minutes at 72°C. Once completed, 2 μL from this first PCR was used to amplify RU5 and HAPB in real time PCR as above. PCR standards consisted of DNA extracted from a known number of 8E5/LAV cells (AIDS Research and Reference Reagent Program, Germantown, MD from Dr. Thomas Folks), which contain one integrated copy of HIV-1 DNA per cell. Standards were nested and treated the same way as unknowns.
Results
Anti-HIV-1 activity of salicylidene acylhydrazides
We initially screened a total of 25 salicylidene acylhydrazide compounds for anti-HIV-1 activity at concentrations from 50 to 6.25 μM using HIV-1US657, a primary, clade B R5 isolate. In these assays, target cells consisted of TZMbl cells, which allow for rapid quantification of infection. In addition, compound cytotoxicity was measured by LDH release and by microscopic examination of cells. Four compounds, INP0149, INP0161, INP0341 and INP0400 (structures illustrated in Figure 1), inhibited virus, had limited cytotoxicity, and were evaluated further (see below).
Four active compounds inhibit HIV-1 on both the TZMbl cell line and on primary CD4+ lymphocytes
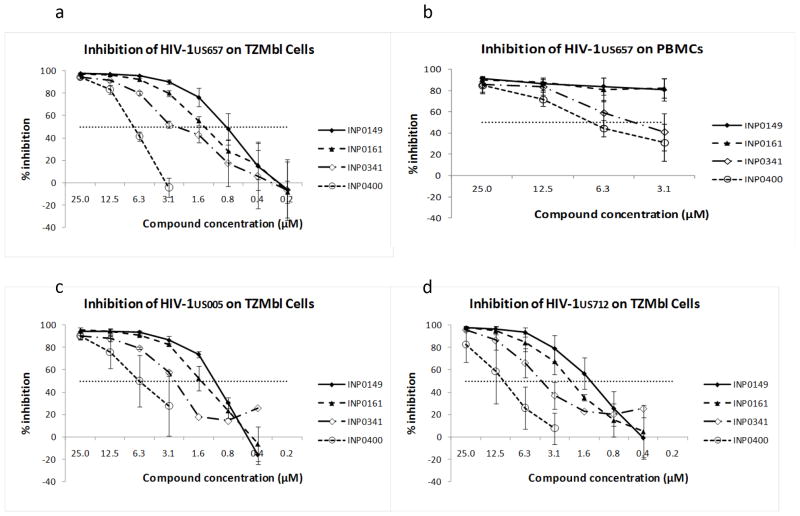
Serial dilutions of INP0149, INP0161, INP0341 and INP0400 were next tested for HIV-1 inhibitory activity on TZMbl cells. Compounds were added to the cells immediately prior to infection, and the compounds were washed off 24 hours later. Virus yield was quantified by luminescence (see Methods) 48 hours after infection. On TZMbl cells, all four compounds inhibited virus with 50% inhibitory concentrations between about 0.9 and 7 μM (figure 2a; table 1). In these experiments, the TZMbl cells were confluent and were infected in the presence of polybrene. Similar results were obtained with infection of 50%-confluent cells or of confluent cells in the absence of polybrene (data not shown).
Figure 2.
HIV-1 is inhibited by salicylidene acylhydrazides using TZMbl indicator cells (a) or PHA-stimulated primary PBMCs (b) as targets for virus growth. A clinical clade B R5 strain (HIV-1US657) was used as cell-free virus with both target cells. Similar virus inhibition was observed with two additional clade B R5 strains, HIV-1US005 (c) and HIV-1US712 (d). The compounds also inhibit HIV-1 after exposure of TZMbl target cells to cell-associated virus (e), where CEM.NKr-CCR5 cells, infected for three days with HIV- 1US657 were incubated with TZMbl cells in the presence of compounds INP0149 or INP0341. Shown are means ± standard errors of assays repeated two or three times. Horizontal dotted line indicates 50% virus inhibition.
Table 1.
Anti-HIV-1 activity of salicylidene acylhydrazides1.
| Compound | IC50 (μM) | Cyt25 (μM) |
|---|---|---|
| INP0149 | 0.9 | 12.5 |
| INP0161 | 1.3 | >25 |
| INP0341 | 2.3 | >25 |
| INP0400 | 7.3 | >25 |
Compounds were assayed against HIV-1US657, a primary R5 isolate, using TZMbl target cells. Percentage inhibition was calculated based on a DMSO control (no compound). IC50 refers to concentration of compound resulting in ≥50% virus inhibition; Cyt25 refers to concentration resulting in ≥25% cytotoxicity of TZMbl cells by propidium iodine staining.
We also measured inhibition on primary, PHA-stimulated PBMCs, with virus yield determined seven days after infection by measuring p24 in supernatant fluid. In the absence of compound, virus yield ranged from 751 to 1015 pg/ml. Maximum inhibition on PBMCs was somewhat less than on the TZMbl cells, but the potency of the individual compounds relative to each other was similar on both cell types (with INP0149>0161>0341>0400 (figure 2b).
Compounds inhibit diverse clade B strains of HIV-1
An effective topical microbicide must be active against a broad range of clinical HIV-1 strains. Using TZMbl cells as targets, we tested the four active compounds against two additional R5 clade B strains of HIV-1, HIV-1US005 and HIV-1US712 (figure 2c and 2d). These two additional strains were inhibited at concentrations similar to those that inhibited HIV-1US657.
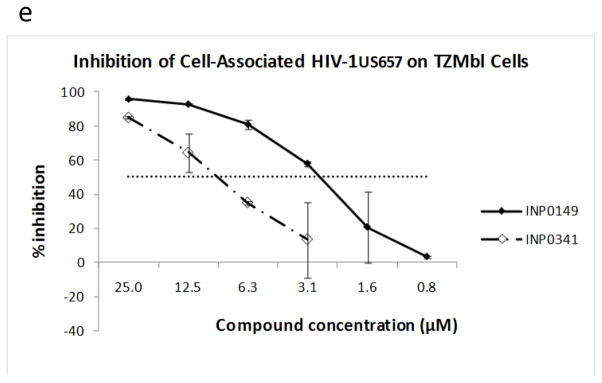
Salicylidene acylhydrazides inhibit virus replication initiated by HIV-1-infected cells
It is possible that both infected cells and cell-free virus initiate sexual transmission of HIV infection. To explore the impact of the compounds on infection by cell-associated virus, we incubated infected CEM.NKr-CCR5 cells (which had been washed to remove cell-free virus) with TZMbl target cells in the presence or absence of salicylidene acylhydrazides. The two compounds tested (INP0149 and INP0341) both resulted in inhibition of HIV-1US657 (figure 2e). However, 50% inhibitory concentrations were somewhat higher than those seen with cell-free virus (compare with figure 2a), likely because virus yield on the TZMbl cells was about ten-times greater (based on RLUs) after exposure to infected cells (data not shown).
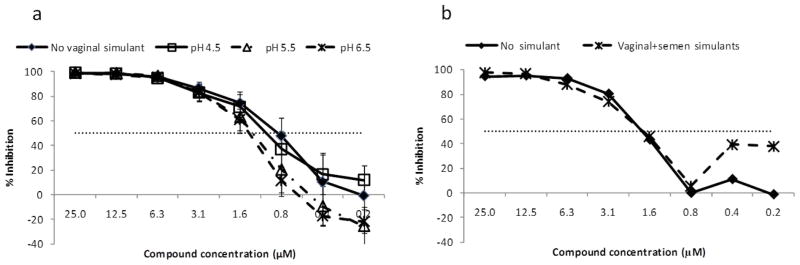
Compounds are effective inhibitors of HIV-1 in vaginal fluid simulant, in semen simulant and in combined vaginal fluid-semen stimulants
Since a topical microbicide must retain activity on the vaginal mucosa, we used a simulant of vaginal fluid to evaluate the anti-viral activity and cytotoxicity of the salicylidene acylhydrazides. The compounds were incubated with the simulant adjusted to a pH of 6.5, 5.5, or 4.5. Under these conditions, all four compounds continued to have similar activity against HIV-1US657 (figure 3a) and demonstrated no increased toxicity (data not shown). Similarly, using either semen simulant (data not shown) or semen simulant in combination with vaginal fluid simulant (figure 3b) had little effect on the anti-HIV-1 activity of the compounds.
Figure 3.
Vaginal fluid simulant at pH 4.5, 5.5 or 6.5 has little effect on inhibition of HIV-1 by the compounds (a). Inhibition of HIV-1US657 by compound INP0149 is shown, and similar results were obtained with compounds INP0161, INP0341, and INP0400. Similarly, combined vaginal (pH 4.5) and semen (pH 7.7) simulants at a 1:1 vol/vol ratio (b) have little effect on compound activity. Horizontal dotted line indicates 50% virus inhibition.
Compounds have limited cytotoxicity
Cytotoxicity was measured by LDH release and by PI staining of TZMbl cells under the same conditions used to measure virus inhibition. By LDH release, no compound resulted in more than 20% cytotoxicity at concentrations up to 100 μM. Furthermore, in the active HIV-1 inhibitory range (1 – 7 μM), cytotoxicity was <3% for all four compounds (data not shown). Based on PI staining, three of the four compounds (INP0161, INP0341 and INP0400) gave <25% cytotoxicity (Cyt25) at concentrations up to 25 μM, whereas compound INP0149 resulted in about 25% cytotoxicity at a concentration of 12.5 μM (table 1). At concentrations up to 100μM, cytotoxicity did not reach 50% for any of the four compounds when determined by PI staining. However, some of the compounds began to precipitate at concentrations above 50 μM. We therefore calculated a therapeutic index (TI) based on 25% cytotoxicity concentrations, rather than the more standard 50% cytotoxicity concentration. Compounds INP0149, INP0161 and INP0341 had TIs (Cyt25/IC50) of ≥10 and compound INP0400 had a TI of >3.4. It should be noted that PI staining was conducted at 48 hours following a wash at 24 hours. Unattached cells would have been washed away at that time, and PI staining might have therefore underestimated the number of cells staining with PI. To explore this possibility, we quantified the number of unattached TZMbl cells at 24 hours after the addition of compounds. Controls treated with DMSO alone demonstrated about 8.8% of unattached cells at 24 hours (compared to all cells). At concentrations of 25 μM and 12.5μM, respectively, unattached cells with INP0149 were 14.7% and 9.4%, with INP0161 12.3% and 9%, with INP0341 7% and 6%, and with INP0400 15.7 and 15.7%. Thus, in some cases, there may have been a slight underestimation of cytotoxicity by the PI staining due to the unattached cells that would have not been accounted for. These results engender some concern about toxicity; however, the LDH results, as well as histological findings in mice [6], suggest that the toxicity of the compounds is limited.
HIV-1 inhibitory activity is reversed by Fe2+
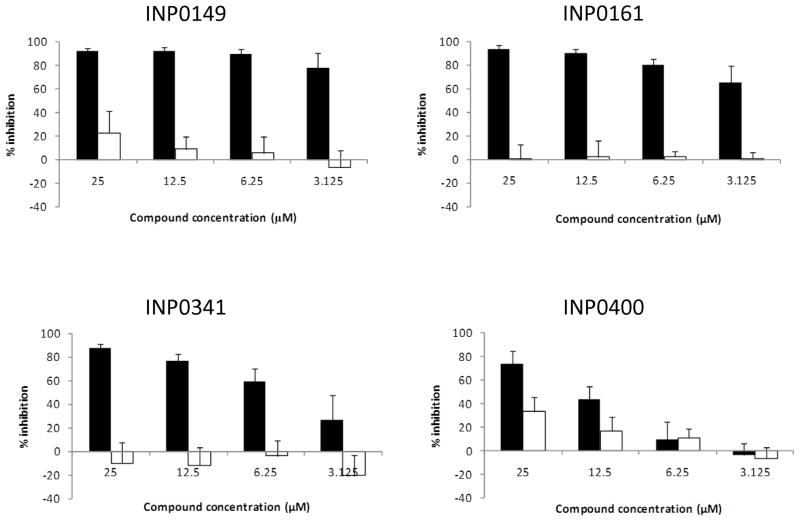
The salicylidene acylhydrazides have been previously shown to limit iron availability [4], and iron depletion can result in inhibition of HIV-1 replication [7–11]. We therefore added Fe2+ (FeSO4 250 μM) to the medium prior to adding compounds and infecting TZMbl cells. At this concentration, Fe2+ was able to almost substantially reverse the inhibition of all four compounds (figure 4). Interestingly, reversal by Fe2+ was most modest for the least active compound (INP0400). The use of the cations Mg2+ and Ca2+ did not reverse inhibition of HIV-1 (data not shown), suggesting that chelation of Fe2+ is involved in the mechanism underlying the HIV-1 inhibitory activity of the salicylidene acylhydrazides.
Figure 4.
Fe2+ reverses the HIV-1 inhibitory activity of salicylidene acylhydrazides. Compounds were added in the absence (solid bars) or presence (open bars) of FeSO4 (250 μM). Virus yield was determined using TZMbl cells. Data represent the mean and standard errors of 2–4 separate experiments.
HIV-1 inhibition occurs at a post-entry, post-reverse transcription, post-integration step
To determine the stage of the HIV-1 replication cycle inhibited by the compounds, we measured R-U5 DNA after infection of TZMbl cells in the presence or absence of compound. R-U5 DNA is reverse-transcribed from the HIV-1 RNA genome very early after virus entry. Using real-time PCR, we demonstrated that compounds did not inhibit R-U5 DNA levels relative to the DMSO control, indicating that neither virus entry nor early reverse transcription were affected by the compounds (table 2).
Table 2.
Effect of salicylidene acylhydrazides on an early HIV-1 reverse transcription product (R-U5 DNA) and on integrated viral DNA1.
| Compound | R-U5 DNA/HAPB | Integrated HIV DNA/HAPB |
|---|---|---|
| Control (no compound) | 17.3 | 9.8 |
| INP0149 | 17.5 | 9.0 |
| INP0161 | 30.6 | ND |
| INP0341 | 39.2 | 7.7 |
| INP0400 | 27.3 | ND |
All compounds were tested at 25μM. Data are reported as DNA copies of HIV sequence per HAPB copy. HAPB serves as a “housekeeping” gene to adjust for the number of TZMbl cells from which DNA was extracted. ND = not done.
We next measured integrated HIV-1 DNA using primers specific for both Alu (which occurs frequently in chromosomal DNA) and HIV-1. Again, we found similar amounts of integrated HIV-1 DNA in the presence or absence of the two salicylidene acylhydrazides tested (table 2). Thus, the compounds must be acting at steps in the virus replication cycle subsequent to integration.
Discussion
We have demonstrated the anti-HIV-1 activity of four salicylidene acylhydrazides. These compounds inhibit diverse clade B HIV-1 strains in the low μM range, are active with primary CD4+ lymphoblast target cells and maintain their activity in the presence of vaginal fluid and semen simulant. Moreover, we have shown that HIV-1 inhibitory activity is reversible when high concentrations of Fe2+ are added to the medium. Finally, the compounds appear to inhibit infection at a post-integration stage.
An ideal vaginal microbicide would have inhibitory activity against a number of sexually transmitted pathogens. Notably, the four compounds we have tested inhibit three different R5 clinical isolates of HIV-1 and have similar inhibitory potency against C. trachomatis [5]. Moreover, compound INP0341was also active against five of six N. gonorrhoeae strains with minimal bactericidal concentrations of 6.3 μM [5]. In the case of Chlamydia, compound INP0341 has been shown to be active in a mouse model of vaginal infection [5]. While inhibiting a number of pathogens, the compounds tested do not have inhibitory activity (at concentrations up to 100μM) against two of the most common hydrogen peroxide-producing members of the vaginal normal flora, Lactobacillus jensenii and Lactobacillus crispatus [5]. These two organisms are considered crucial to the maintenance of normal vaginal flora. Thus, the specificity of these compounds in targeting pathogens and sparing essential organisms make them well-suited for development as topical vaginal microbicides.
The four compounds had activity against HIV-1 in primary CD4+ lymphoblasts obtained from healthy donors. Since CD4+ lymphocytes are the major target cell for HIV-1 replication, compound activity could translate into in vivo efficacy. However, we have not measured the effect of the salicylidene acylhydrazides on other cells, such as macrophages and dendritic cells, which are likely very early targets following mucosal exposure. Moreover, although a vaginal fluid simulant at three different pH values, as well as semen simulant and a combination of semen and vaginal fluid simulants had little or no effect on activity, it remains to be determined if the compounds will inhibit HIV infection after exposure to intact or inflamed vaginal mucosa.
In the absence of details regarding the exact nature of the inoculum during natural sexual transmission of HIV, effective preventions should be active against incoming virus in both the cell-free and cell-associated forms. In this regard, the compounds tested were able to inhibit virus after exposure of target cells to either cell-free or cell- associated virus.
Our experiments clearly show that the addition of iron can reverse the inhibitory effect of the four compounds tested. Previous studies have also found that iron chelating agents, including the salicylidene acylhydrazide 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazine, inhibit HIV-1 [7–11]. Moreover, increased iron stores associated with a number of conditions, adversely affect HIV infection [16–18]. Our results are also consistent with those of others who have observed that iron depletion affects HIV replication at the level of transcription and that such an effect is related to perturbations in cell cycling [7, 10, 11]. We have previously observed that the inhibition of C. trachomatis and N. gonorrhoeae by salicylidene acylhydrazides is also reversible by iron [4, 5]. Finally, a group of acylhydrazones has been shown to inhibit SIV, bind to HIV-1 capsid, and inhibit capsid assembly [19]. Whether the compounds we tested also target capsid assembly or solely act as iron chelators is unknown.
By likely acting on a post-integration step in virus replication, the compounds tested will allow the entry of virus and the integration of its genome. This implies that, in order to be effective, the compounds will need to be maintained at inhibitory concentrations for the duration of the life of infected cells. In this regard, we are in the process of testing the compounds in a humanized mouse model to gauge there in vivo efficacy. Moreover, the mechanism of inhibition will likely require the use of these compounds in combination with substances that inhibit early steps in the virus life cycle.
The compounds used in our study have been shown to slow cell progression, but as demonstrated by the LDH and PI results, there is minimal toxicity to the cells at the active concentrations. We note that the TI values are rather small, particularly for INP0400. However, because of compound precipitation at high concentrations, TI’s were calculated using an unconventional 25% cytotoxicity value, rather than the usual 50% value. In addition, 1mM of INP0341 applied intravaginally to mice on three separate occasions over three days did not result in histological abnormalities [6]. In a previous study, INP0161, 0341 and 0400 also showed minimal toxicity to HeLa 299 cells when measured by mitochondrial dehydrogenase activity; however, in that study, INP0149 resulted in 9.4% and 81.1% cytotoxicity at 20μM and 50μM [4]. Additonal in vivo studies will be required to further establish the safety profile of these compounds.
In summary, we have identified salicylidene acylhydrazides with in vitro anti-HIV-1 activity in the μM range. Although not as potent as some anti-retroviral agents, these compounds appear to have relatively low cytotoxicity in vitro and in vivo at well above inhibitory concentrations [6]. Finally, the activity of the compounds against other sexually transmitted pathogens makes them, likely in combination with other agents, potential candidates for formulation and use in a broad-spectrum topical microbicide.
Acknowledgments
The authors wish to thank Dr. Christopher Öberg for technical assistance.
Funding: This project was supported by award number R21AI079775 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Competing Interests: None
Ethical Approval: Approval given by the University of California, Irvine Institutional Review Boad (HS# 2002-2430)
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordfelth R, Kauppi AM, Norberg HA, Wolf-Watz H, Elofsson M. Small-molecule inhibitors specifically targeting type III secretion. Infect Immun. 2005;73:3104–3114. doi: 10.1128/IAI.73.5.3104-3114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey L, Gylfe A, Sundin C, Muschiol S, Elofsson M, Nordstrom P, et al. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 2007;581:587–595. doi: 10.1016/j.febslet.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Slepenkin A, Enquist PA, Hagglund U, de la Maza LM, Elofsson M, Peterson EM. Reversal of the antichlamydial activity of putative type III secretion inhibitors by iron. Infect Immun. 2007;75:3478–3489. doi: 10.1128/IAI.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu H, Slepenkin A, Elofsson M, Keyser P, de la Maza LM, Peterson EM. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseriagonorrhoeae. Int J Antimicrob Agents. 2010;36:145–150. doi: 10.1016/j.ijantimicag.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slepenkin A, Chu H, Elofsson M, Keyser P, Peterson EM. Protection of Mice From a Chlamydia trachomatis Vaginal Infection Using a Salicylidene Acylhydrazide, a Potential Microbicide. J Infect Dis. 2011;204:1313–1320. doi: 10.1093/infdis/jir552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debebe Z, Ammosova T, Jerebtsova M, Kurantsin-Mills J, Niu X, Charles S, et al. Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology. 2007;367:324–333. doi: 10.1016/j.virol.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debebe Z, Ammosova T, Breuer D, Lovejoy DB, Kalinowski DS, Karla PK, et al. Iron chelators of the di-2-pyridylketone thiosemicarbazone and 2-benzoylpyridine thiosemicarbazone series inhibit HIV-1 transcription: identification of novel cellular targets--iron, cyclin-dependent kinase (CDK) 2, and CDK9. Mol Pharmacol. 2011;79:185–196. doi: 10.1124/mol.110.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traore HN, Meyer D. The effect of iron overload on in vitro HIV-1 infection. J Clin Virol. 2004;31 (Suppl 1):S92–98. doi: 10.1016/j.jcv.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HS, Marx JJ, van Asbeck BS. Inhibition of human immunodeficiency virus type 1 replication in human mononuclear blood cells by the iron chelators deferoxamine, deferiprone, and bleomycin. J Infect Dis. 2000;181:484–490. doi: 10.1086/315223. [DOI] [PubMed] [Google Scholar]
- 11.Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D’Alliessi Gandolfi D, Park MH, et al. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Asbeck BS, Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HS, Marx JJ. Anti-HIV effect of iron chelators: different mechanisms involved. J Clin Virol. 2001;20:141–147. doi: 10.1016/s1386-6532(00)00122-0. [DOI] [PubMed] [Google Scholar]
- 13.Dahlgren MK, Zetterstrom CE, Gylfe S, Linusson A, Elofsson M. Statistical molecular design of a focused salicylidene acylhydrazide library and multivariate QSAR of inhibition of type III secretion in the Gram-negative bacterium Yersinia. Bioorg Med Chem. 2010;18:2686–2703. doi: 10.1016/j.bmc.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 15.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 16.Gordeuk VR, Delanghe JR, Langlois MR, Boelaert JR. Iron status and the outcome of HIV infection: an overview. J Clin Virol. 2001;20:111–115. doi: 10.1016/s1386-6532(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 17.Rawat R, Humphrey JH, Ntozini R, Mutasa K, Iliff PJ, Stoltzfus RJ. Elevated iron stores are associated with HIV disease severity and mortality among postpartum women in Zimbabwe. Public Health Nutr. 2009;12:1321–1329. doi: 10.1017/S136898000800390X. [DOI] [PubMed] [Google Scholar]
- 18.McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, et al. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr. 2007;46:498–507. doi: 10.1097/qai.0b013e31815b2d4b. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Tan Z, He M, Tian B, Tang S, Hewlett I, et al. SAR and molecular mechanism study of novel acylhydrazone compounds targeting HIV-1 CA. Bioorg Med Chem. 2010;18:2135–2140. doi: 10.1016/j.bmc.2010.02.003. [DOI] [PubMed] [Google Scholar]