Abstract
Chondrocytes are mechanosensitive cells that require mechanical stimulation for proper growth and function in in vitro culture systems. Ultrasound (US) has emerged as a technique to deliver mechanical stress; however, the intracellular signaling components of the mechanotransduction pathways that transmit the extracellular mechanical stimulus to gene regulatory mechanisms are not fully defined. We evaluated a possible integrin/mitogen-activated protein kinase (MAPK) mechanotransduction pathway using Western blotting with antibodies targeting specific phosphorylation sites on intracellular signaling proteins. US stimulation of chondrocytes induced phosphorylation of focal adhesion kinase (FAK), Src, p130 Crk-associated substrate (p130Cas), CrkII, and extracellular-regulated kinase (Erk). Furthermore, pre-incubation with inhibitors of integrin receptors, Src, and MAPK/Erk kinase (MEK) reduced US-induced Erk phosphorylation levels, indicating integrins and Src are upstream of Erk in an US-mediated mechanotransduction pathway. These findings suggest US signals through integrin receptors to the MAPK/Erk pathway via a mechanotransduction pathway involving FAK, Src, p130Cas, and CrkII.
Keywords: Chondrocyte, Ultrasound, Mechanotransduction, Integrin, focal adhesion kinase (FAK), Src, p130 Crk-associated substrate (p130Cas), CrkII, extracellular-regulated kinase (Erk)
Introduction
Optimal in vitro culture of chondrocytes involves a combination of unique culture conditions, including growth factors and supplements in culture media, three-dimensional culture in scaffolds (Grundmann et al. 1980; Benya and Shaffer 1982; Noriega et al. 2012), and mechanical stimulation (Palmoski et al. 1980; Carver and Heath 1999a). Naturally, articular cartilage is subjected to frequent mechanical impacts as the joint performs its normal functions (Wang and Thampatty 2006). Chondrocytes sense these mechanical stresses and convert the mechanical signals into the activation of intracellular signaling molecules that ultimately affect the metabolic activity of chondrocytes (Fitzgerald et al. 2004; Fitzgerald et al. 2006). If cartilage in vivo does not receive adequate mechanical stresses, the cartilage tends to atrophy due to decreased metabolic activity of chondrocytes and decreased production of components of the extracellular matrix (ECM). For example, immobilization of a joint causes degenerative changes to the cartilage, such as reduced proteoglycan production by chondrocytes (Palmoski et al. 1979; Jortikka et al. 1997). Conversely, the application of mechanical force to cartilage by daily exercise can increase the thickness of cartilage and enhance the glycosaminoglycan content of joint cartilage (Kiviranta et al. 1988) and can enhance cartilage repair of injured joints (Todhunter et al. 1993). This mechanical stress-induced biological response of chondrocytes has been utilized to enhance in vitro chondrocyte culture systems, stimulating chondrocyte proliferation and production of components of the ECM. Multiple techniques can be used to apply mechanical stimulation to in vitro culture systems, including hydrostatic pressure (Carver and Heath 1999a, 1999b), hydrodynamic shear (Freed et al. 1993), static and dynamic compression (Buschmann et al. 1995; Bonassar et al. 2001; Fitzgerald et al. 2004; Fitzgerald et al. 2006), dynamic shear (Fitzgerald et al. 2006), and application of low-intensity ultrasound (US) (Parvizi et al. 1999; Nishikori et al. 2002; Zhang et al. 2002, 2003; Noriega et al. 2007; Hasanova et al. 2011). Low-intensity US stimulation has emerged as technique to enhance in vitro chondrocyte culture systems (Noriega et al. 2007; Hasanova et al. 2011), accelerate fracture healing, and shorten the duration of treatment needed to repair damaged cartilage in patients and in animal models (Heckman et al. 1994; Yang et al. 1996; Rubin et al. 2001; Pounder and Harrison 2008). Our previous work has shown that the expression levels of integrins α5 and β1, as well as chondrocytic markers, Sox5, Sox9, collagen II and aggrecan, were increased in chondrocytes exposed to a continuous US signal at 5.0 MHz (0.14 mW/cm2) (Hasanova et al. 2011).
Mechanical stimulation of cells can be detected by multiple mechanoreceptors, including stretch activated channels (SAC) (Wright et al. 1996), annexin V (von der Mark and Mollenhauer 1997; Haut Donahue et al. 2004), CD44 (Morris et al. 2010), and integrins (Zhou et al. 2004; Wang and Thampatty 2006). Integrin receptors physically adhere chondrocytes to the ECM (Wang and Thampatty 2006), and when stimulated integrins activate intracellular signaling pathways that promote survival (Coppolino and Dedhar 2000) and mediate ECM component production by chondrocytes (Takeuchi et al. 2008). When integrin receptors are activated they cluster with other integrins, adaptor proteins, and kinases such as focal adhesion kinase (FAK) to form a focal adhesion complex, which then activates intracellular signaling cascades that mediate cellular responses (Vuori 1998; Giancotti and Ruoslahti 1999). The positive effects of US on chondrocytes in culture, including increased proliferation and production of ECM components, are already well documented (Carver and Heath 1999a; Noriega et al. 2007; Hasanova et al. 2011). However, the intracellular signaling components of the mechanotransduction pathways that are responsible for transmitting the extracellular mechanical stimulus to gene regulatory mechanisms are not fully defined and require further research. This study began with multiple hypotheses of potential mechanotransduction pathways responsible for US-mediated effects on chondrocytes based on an extensive literature search of possible signaling molecules and phosphorylation sites. Then each potential component was systematically ruled out until the signaling molecules were narrowed down to the integrin/mitogen-activated protein kinase (MAPK) pathway presented in this manuscript.
Materials and Methods
Human chondrocyte culture
Adult human chondrocytes isolated from normal articular cartilage were purchased from Cell Applications Inc. (San Diego, CA, USA). These chondrocytes can be cultured for at least ten doublings according to the manufacturer, and they can produce collagen II protein and deposit extracellular collagen II fibers through passage five (data not shown). Cryopreserved chondrocytes were thawed and cultured in T75 flasks with RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 1 mM sodium pyruvate (Invitrogen), 23.8 mM sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA), and 1% antibiotic-antimycotic (Invitrogen) at 37°C, 5% carbon dioxide, and 99% humidity. To passage chondrocytes, we removed the medium, rinsed cells with 1x trypsin (Invitrogen) in Hank’s balanced salt solution (HBSS; Invitrogen), added 2.5 mL 1x trypsin in HBSS to the T75 flask, and incubated at 37°C for 3 minutes, rinsed the flask with RPMI, centrifuged cell suspension for 6 minutes at 200x gravity. After centrifugation, we removed supernatant, resuspended cells in RPMI, counted cells using a hemocytometer, and replated chondrocytes at 7.5×105 cells/T75 flask. Chondrocytes were passaged at about one week intervals and used between passages two and five.
Ultrasound stimulation
Human chondrocytes were passaged and cells were seeded in 6-well plates at 2.0×105 cells/well and allowed to attach and grow for 1–3 days in RPMI-1640 medium supplemented with 10% FBS, 1 mM sodium pyruvate, 23.8 mM sodium bicarbonate, and 1% antibiotic-antimycotic at 37°C, 5% carbon dioxide, and 99% humidity. The day prior to US stimulation the cells were deprived of serum by removing the RPMI medium with 10% FBS and replacing with 6 mL/well RPMI with 0.1% FBS, to reduce FBS-induced phosphorylation of intracellular signaling molecules that may otherwise mask the US-induced phosphorylation events (Takeuchi et al. 2008).
Serum-deprived chondrocytes were stimulated for 3 minutes with a continuous sinusoidal 5.0 MHz US signal using non-focused immersion transducers (Panametrics, V300, 12.7 mm diameter, 5 MHz center frequency; Waltham, MA, USA) immersed in the media in each well of the 6-well plates 6 mm away from the bottom of the well. The cells were in the near-field of the transducer, and the highly directive acoustic beam is just slightly wider than the transducer diameter at a distance of 6 mm from the transducer face. Wave reflection occurred exclusively at the well bottom, as the well diameter of 35 mm was much greater than that of the beam diameter of 12.7 mm. An air gap below the 6-well plate caused near complete reflection of the incident acoustic wave, resulting in a standing wave system with the cells located roughly 2/3 between a pressure antinode and a node, as predicted using numerical simulation (data not shown). The spatially averaged pressure, quantified using a needle hydrophone (Onda Corporation, CA, USA), was kept constant at 14 kPa.
Following US stimulations, the cells were incubated at 37°C for 5, 15, 30, or 60 minutes prior to protein extraction. One full 6-well plate was used for each condition with protein from all 6 wells combined for each sample. Cells that did not receive US stimulation were used as controls. For assays using inhibitor pre-incubation, 500 μg/mL Glycine-Arginine-Glycine-Aspartic Acid-Serine-Proline (GRGDSP) (AnaSpec Fremont, CA, USA), 50 μM 4-Amino-5-(methylphenyl)-7-(t-butyl)pyrazolo-(3,4-d)pyrimidine (PP1; Sigma-Aldrich), 50 μM PD98059 (Sigma-Aldrich), or vehicles alone for inhibitor-free controls were added to each well of the 6-well plate for four hours prior to US stimulation. All samples, including the non-US-stimulated control, received equal concentrations of water and DMSO vehicles that were used to reconstitute the inhibitors. Following four hours of inhibitor pre-incubation, the chondrocytes were treated with US as described above.
Protein extraction
Protein was extracted from the chondrocytes using Pierce IP lysis buffer (Thermo Scientific Pierce, Rockford, IL, USA) supplemented with 1x Halt protease and phosphatase inhibitor cocktail (Thermo Scientific Pierce) following a 5, 15, 30, or 60 minute incubation after US stimulation or from control chondrocytes that did not receive US stimulation. Chondrocytes were rinsed twice with cold HBSS, and then 200 μL of IP lysis buffer with Halt inhibitor was added to each well and incubated for at least 5 minutes prior to combining the lysis buffer from all six wells. The 1.2 mL of protein and lysis buffer was then lyophilized for 3 hours to a volume of around 300 μL to increase the concentration of the protein samples. The protein concentration was then analyzed using the bicinchoninic acid assay (Thermo Scientific Pierce) method.
Western blotting analysis
Protein (20–25 μg/well) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis using the NuPAGE system following the manufacturer’s instructions (Invitrogen). Proteins were combined with NuPAGE 4x lithium dodecyl sulfate sample buffer and NuPAGE 10x sample reducing agent (Invitrogen), and then heated at 100°C for 10 minutes in a heating block. Denatured and reduced proteins were separated on 10% or 4–12% NuPAGE gels (Invitrogen) and electrophoretically transferred to Immobilon-P polyvinyldifluoridene (PVDF) membranes (Millipore, Billerica, MA, USA) using a Pierce fast semi-dry blotter according to the manufacturer’s protocol (Thermo Scientific Pierce). Membranes were blocked with 5% dry milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for one hour, and then incubated with primary antibodies in TBST with 5% bovine serum albumin (BSA; Sigma-Aldrich) overnight at 4°C. Membranes were rinsed with TBST then incubated with secondary horseradish peroxidase (HRP)-linked antibodies for one hour at room temperature and rinsed with TBST. Protein bands were visualized by enhanced chemiluminescence (ECL) Western blotting substrate (Millipore or Thermo Scientific Pierce) and captured with GE Healthcare Amersham Hyperfilm ECL (GE Healthcare, Piscataway, NJ, USA). Primary antibodies used were: phospho-Y397-FAK (1:250, Cell Signaling Technology, Beverly, MA, USA), FAK (1:250, Cell Signaling Technology), phospho-Y416-Src (1:500, Cell Signaling Technology), Src (1:500, Cell Signaling Technology), phospho-Y249-p130 Crk-associated substrate (p130Cas) (1:500, Cell Signaling Technology), p130Cas (1:500, Millipore), phospho-Y221-CrkII (1:250, Cell Signaling Technology), CrkII (1:1000, Cell Signaling Technology), phospho-T202/Y204-extracellular-regulated kinase (Erk) (1:1000, Cell Signaling Technology), and Erk (1:1000, Cell Signaling Technology). β-actin (1:1000, Sigma-Aldrich) was used as a loading control. Secondary antibodies used were: anti-rabbit (1:5000; KPL, Gaithersburg, MD, USA) and anti-mouse (1:10000, KPL). Immunoblots were scanned and images were quantified using the open access software NIH ImageJ (Abramoff 2004).
Statistical analysis
Data were expressed as means ± standard error of the mean (SEM). The data were evaluated statistically by the analysis of variance (ANOVA), followed by the Tukey-test for paired observations. Significance was considered to be p < 0.05.
Results
US-induced FAK phosphorylation
Following US-induced activation of integrin receptors and formation of the focal adhesion complex in chondrocytes, FAK autophosphorylates at tyrosine (Y) 397, which then acts as a binding site for Src family kinases (Schaller et al. 1994; Takeuchi et al. 2008). Human chondrocytes that were serum-deprived overnight were treated with 5.0 MHz US for three minutes, and then the cells were lysed to collect protein 5, 15, 30 and 60 minutes after the US mechanical stress. Western blotting was used to analyze the phosphorylation of FAK at Y397 compared to total FAK. US stimulation induced phosphorylation of FAK Y397 (Fig. 1).
Figure 1. US-induced phosphorylation of FAK at tyrosine 397.
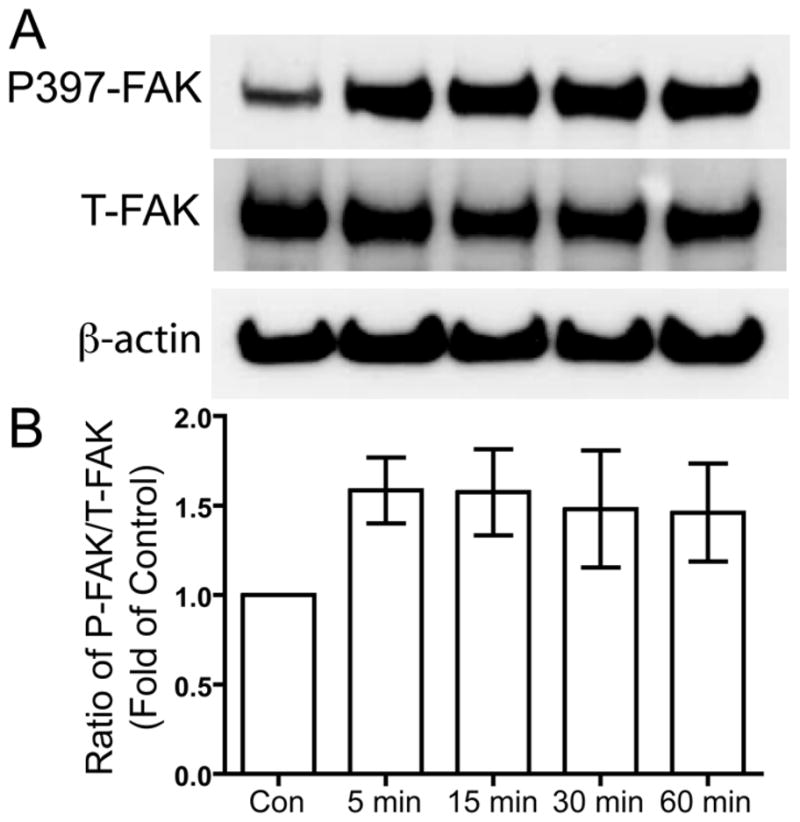
Serum-deprived primary human chondrocytes were treated with US for three minutes, then total cell lysates were collected 5, 15, 30, and 60 minutes after US treatment. Total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of FAK at tyrosine 397, total FAK and β-actin loading control were demonstrated by Western blotting (A). Western blotting data from six separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total FAK (B).
US-induced Src phosphorylation
Phosphorylated Y397 of FAK acts as a binding site for Src, which is then activated by autophosphorylation of Y416 in the activation loop of the kinase domain of Src (Cooper and MacAuley 1988; Schaller et al. 1994). Serum-deprived human chondrocytes were treated with 5.0 MHz US for three minutes. After the US mechanical stress, the cells were incubated for 5, 15, 30, and 60 minutes and then the cells were lysed to collect protein. Phosphorylation of Src at Y416 was compared to total Src was determined using Western blotting. US stimulation induced phosphorylation of Src Y416 (Fig. 2).
Figure 2. US-induced phosphorylation of Src at tyrosine 416.
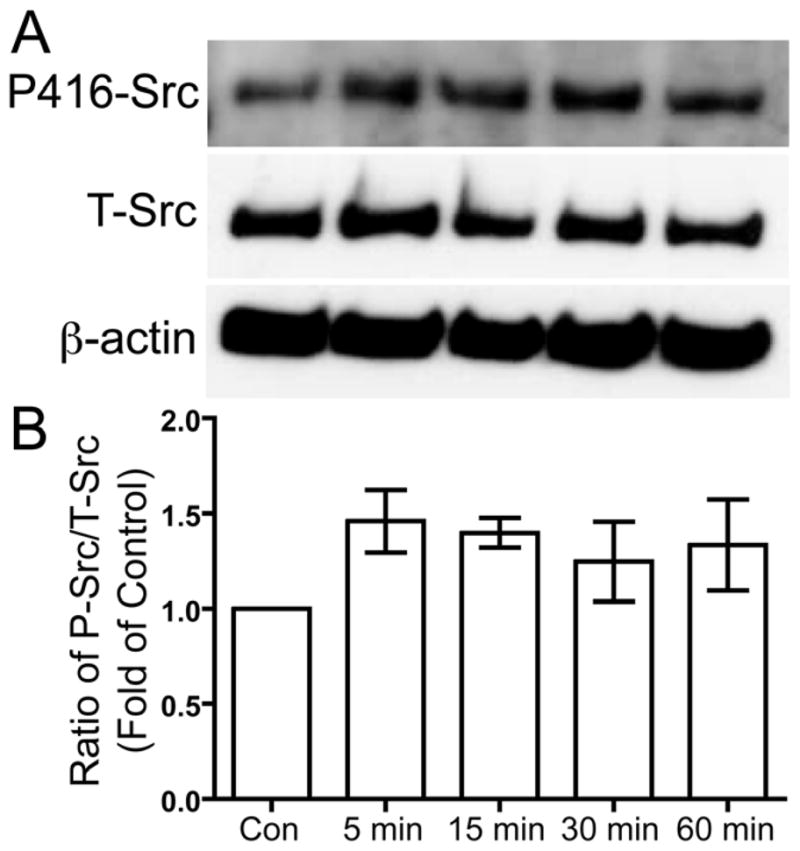
Serum-deprived primary human chondrocytes were treated with US for three minutes, then total cell lysates were collected 5, 15, 30, and 60 minutes after US treatment. Total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of Src at tyrosine 416 and total Src were demonstrated by Western blotting (A). Western blotting data from four separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total Src (B).
US-induced p130Cas phosphorylation
Activated Src kinase phosphorylates a multitude of targets such as p130Cas. We analyzed the phosphorylation of p130Cas at three potential phosphorylation sites Y165, Y249, and Y410 in response to US stimulation. Human chondrocytes were serum-deprived overnight and then treated with 5.0 MHz US for three minutes. Chondrocytes were lysed to collect protein 5, 15, 30, and 60 minutes after the US mechanical stress. Western blotting was used to analyze the phosphorylation of p130Cas at Y165, Y249, and Y410 compared to total p130Cas. US stimulation induced transient phosphorylation of p130Cas Y249, with a statically significant increase at 5 minutes compared to control (p < 0.05), and decrease back to basal levels by 60 minutes (p < 0.05) (Fig. 3). However, we did not observe US-induced phosphorylation at Y165 and Y410 (data not shown).
Figure 3. US-induced phosphorylation of p130Cas at tyrosine 249.
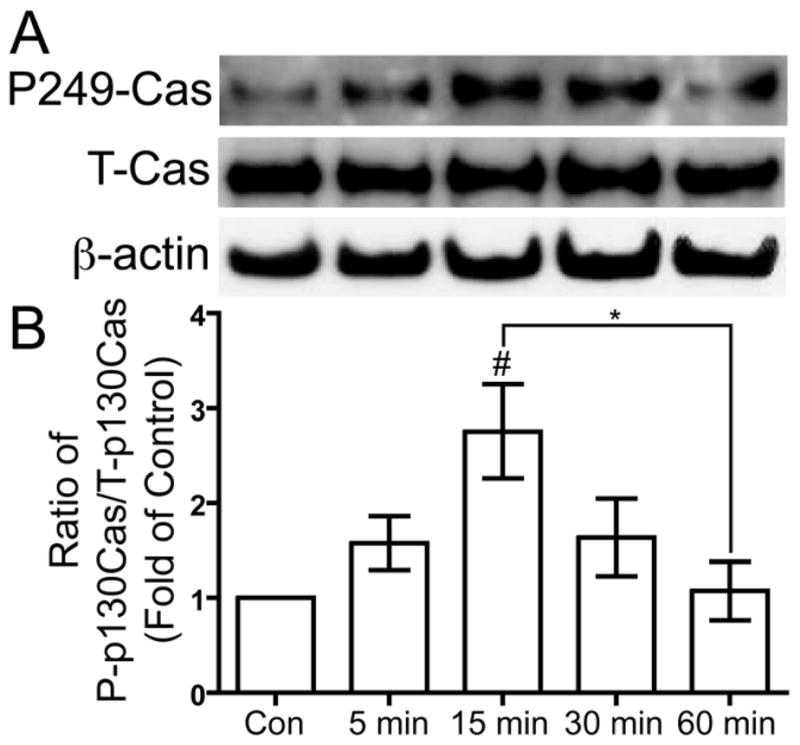
Serum-deprived primary human chondrocytes treated with US for three minutes, then total cell lysates were collected 5, 15, 30, and 60 minutes after US treatment. Total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of p130Cas at tyrosine 249, total p130Cas and β-actin loading control were demonstrated by Western blotting (A). Western blotting data from four separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total p130Cas (B). * p < 0.05. # p < 0.05 vs. control.
US-induced CrkII phosphorylation
Phosphorylated Y249 of p130Cas creates a binding site for the CrkII adapter protein (Shin et al. 2004; Modzelewska et al. 2006). To examine if CrkII plays a role in integrin-mediated US mechanotransduction, we measured the phosphorylation of CrkII at Y221. Serum-deprived human chondrocytes were treated with 5.0 MHz US for three minutes, then 5, 15, 30, and 60 minutes after the US mechanical stress the cells were lysed to collect protein. Phosphorylation of CrkII at Y221 was compared to total CrkII using Western blotting. US stimulation induced transient phosphorylation of CrkII at Y221, with a statically significant increase at 5 minutes compared to control (p < 0.05) (Fig. 4).
Figure 4. US-induced phosphorylation of CrkII at tyrosine 221.
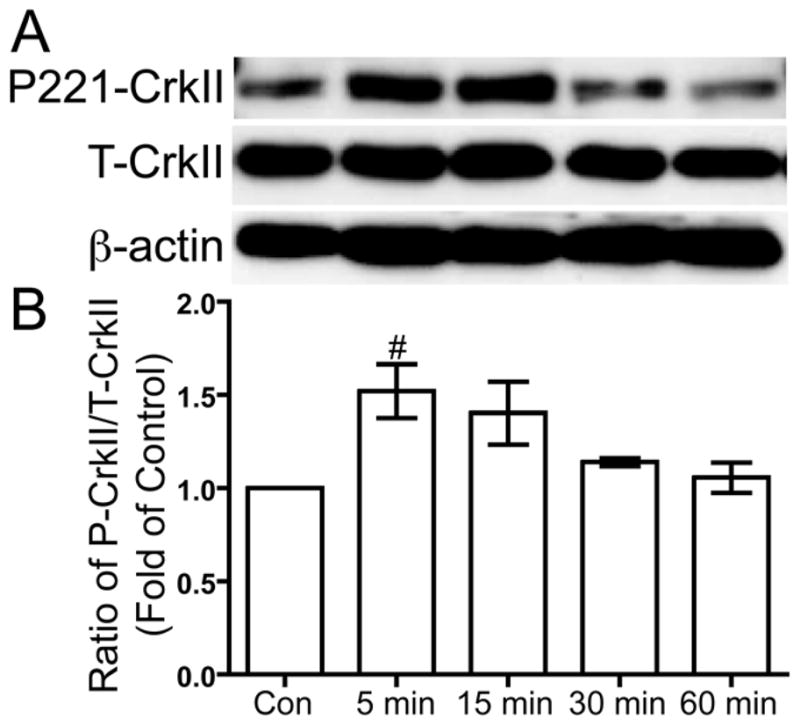
Serum-deprived primary human chondrocytes were treated with US for three minutes, then total cell lysates were collected 5, 15, 30 and 60 minutes after US treatment. Total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of CrkII at tyrosine 221, total CrkII and β-actin loading control were demonstrated by Western blotting (A). Western blotting data from three separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total CrkII (B). # p < 0.05 vs. control.
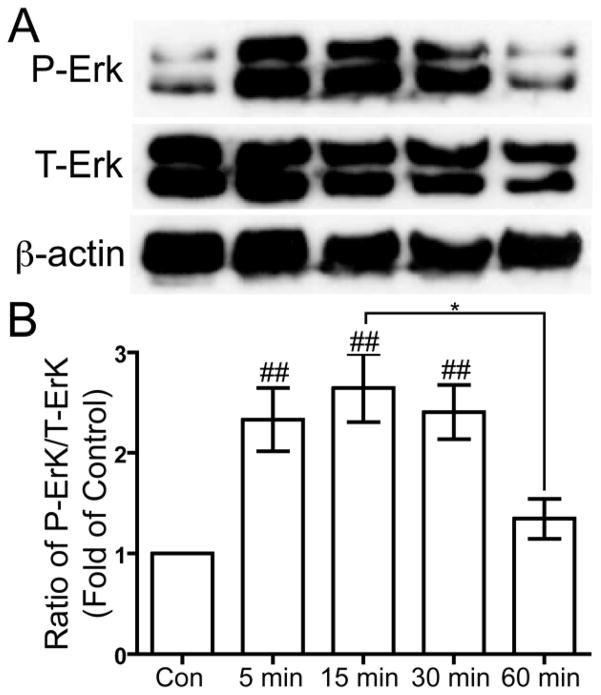
US-induced Erk1/2 phosphorylation
Guanine nucleotide exchange factors, including C3G (Knudsen et al. 1994; Matsuda et al. 1994), Son of Sevenless (SOS) (Matsuda et al. 1994), and dedicator of cytokinesis (DOCK180) (Hasegawa et al. 1996), may bind to CrkII and lead to the activation of the MAPK pathway and ultimately the phosphorylation and activation of Erk. Human chondrocytes that were serum-deprived overnight were treated with 5.0 MHz US for three minutes, and then the cells were lysed to collect protein 5, 15, 30, and 60 minutes after the US mechanical stress. Western blotting was used to analyze the phosphorylation of Erk1/2 at threonine (T) 202/Y204 of Erk1 and T185/Y187 of Erk2 compared to total Erk1/2. US stimulation induced transient phosphorylation of Erk1/2, with a statically significant increase at 5, 15, and 30 minutes compared to control (p < 0.01), and decrease to near basal levels by 60 minutes (p < 0.05) (Fig. 5).
Figure 5. US-induced phosphorylation of Erk at threonine 202 and tyrosine 204.
Serum-deprived primary human chondrocytes were treated with US for three minutes, then total cell lysates were collected 5, 15, 30 and 60 minutes after US treatment. Total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of Erk1/2 at threonine 202 and tyrosine 204, total Erk1/2 and β-actin loading control were demonstrated by Western blotting (A). Western blotting data from six separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total Erk (B). * p < 0.05. ## p < 0.01 vs. control.
Inhibitors of integrins, Src, and MEK reduce US-induced Erk1/2 phosphorylation
We demonstrated that FAK, Src, p130Cas, CrkII, and Erk are all phosphorylated in response to US stimulation; thus, it is plausible that they are involved in an US-mediated mechanotransduction pathway. However, without blocking the downstream phosphorylation of Erk by inhibiting the factors upstream we cannot conclude that these phenomena are a part of an interconnected mechanotransduction pathway. Thus, we pre-treated serum-deprived human chondrocytes with 500 μg/mL GRGDSP, a peptide inhibitor of integrins, 50 μM PP1, an inhibitor of Src, 50 μM PD98059, an inhibitor of MAPK/Erk kinase (MEK), the kinase immediately upstream of Erk, or vehicles alone for inhibitor-free controls for four hours prior to US stimulation. Following inhibitor pre-incubation, the chondrocytes were treated with 5.0 MHz US for three minutes, and then the cells were lysed to collect protein 15 minutes after the US mechanical stress. Western blotting was used to analyze the phosphorylation of Erk1/2 at T202/Y204 of Erk1 and T185/Y187 of Erk2 compared to total Erk1/2. US stimulation induced phosphorylation of Erk1/2 in cells that received US treatment without inhibitor pre-treatment, and both GRGDSP and PP1 reduced Erk1/2 phosphorylation to varying degrees with PD98059 as a positive control for preventing Erk1/2 phosphorylation (Fig. 6). Similar levels of inhibition of Erk1/2 phosphorylation were also observed using lower concentrations of inhibitors: 100 μg/mL GRGDSP, 10 μM PP1, and 20 μM PD98059 (data not shown). These findings suggest that integrin receptors and Src are both upstream of US-mediated Erk1/2 phosphorylation.
Figure 6. Inhibitors of integrins, Src and MEK reduced levels of US-induced phosphorylation of Erk at threonine 202 and tyrosine 204.
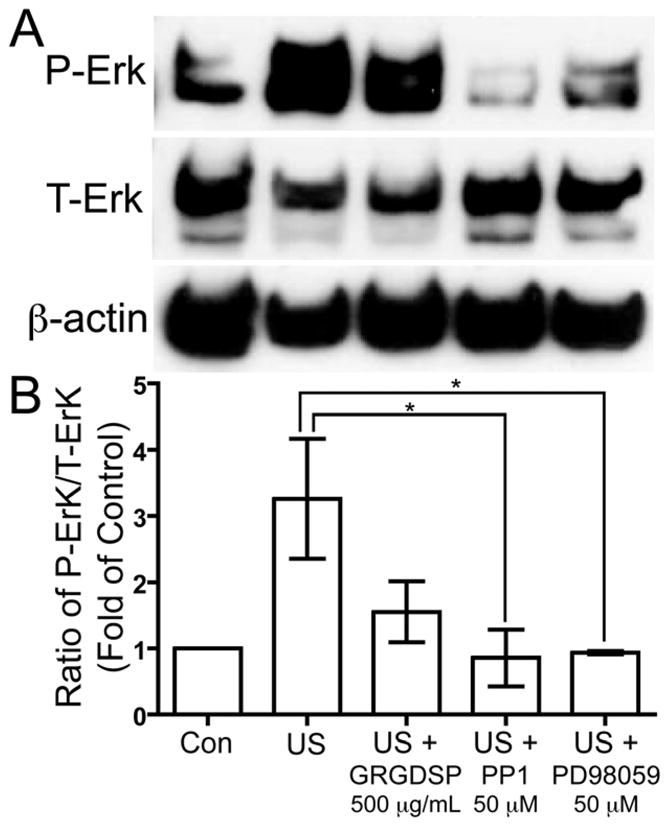
Serum-deprived primary human chondrocytes were pre-treated for 4 hours with 500 μg/mL GRGDSP, a peptide inhibitor of integrins, 50 μM PP1, an inhibitor of Src, and 50 μM PD98059, a MEK inhibitor, then cells were treated with US for three minutes. Total cell lysates were collected 15 minutes after US treatment, and total cell lysate for control was collected from chondrocytes that did not receive US treatment. Phosphorylation of Erk1/2 at threonine 202 and tyrosine 204, total Erk1/2 and β-actin loading control were demonstrated by Western blotting (A). Western blotting data from three separate experiments were quantified using Image J and averages are shown as the ratio of phosphorylated to total Erk (B). * p < 0.05.
Discussion
Mechanotransduction is a process in which mechanical stimuli are sensed by cells and are converted into an alteration of intracellular signaling molecules that induce a cellular response. Mechanical stimulation can be detected by multiple mechanoreceptors, including SAC (Wright et al. 1996), annexin V (von der Mark and Mollenhauer 1997; Haut Donahue et al. 2004), CD44 (Morris et al. 2010), and integrins (Zhou et al. 2004; Wang and Thampatty 2006), and redundant mechanisms for US mechanotransduction in chondrocytes involving multiple mechanoreceptors may exist. However, for this study, we focused only on the role of integrin receptors in the mechanotransduction of low-intensity continuous US in human chondrocytes in vitro. Inhibition of integrins using an Arginine-Glycine-Aspartic Acid (RGD)-containing peptide reduced downstream US-induced Erk1/2 phosphorylation (Fig. 6), and an integrin-blocking antibody reduced Erk1/2 phosphorylation in a human chondrocyte cell line (Choi et al. 2007). Furthermore, inhibition of integrins using an integrin-blocking antibody or RGD peptide was reported to reduce US-induced proliferation of fibroblasts (Zhou et al. 2004), demonstrating the role of integrins in US-mediated mechanotransduction. Integrins are transmembrane receptors that adhere cells to the ECM via short amino acid sequences, such as RGD motifs, and bind to various intracellular cytoskeletal-adaptor proteins and signaling molecules (Giancotti and Ruoslahti 1999). Integrins act as mechanotransducers that activate intracellular signaling pathways after binding to the ECM or sensing mechanical stimulation. However, integrins do not possess intrinsic catalytic activity; thus, signals from integrin-ECM interactions or mechanical stimuli are transduced into the cell via interactions with and activation of integrin-associated proteins (Mitra and Schlaepfer 2006).
When integrin receptors are activated, they cluster with other integrins, structural adaptor proteins, and kinases to form a focal adhesion complex which then activates intracellular signaling cascades that mediate cellular responses (Vuori 1998; Giancotti and Ruoslahti 1999). Focal adhesion complexes are formed by the interaction of the cytoplasmic tail of integrins with proteins such as paxillin, talin, vinculin, actin, and FAK (Chen et al. 1995; Ezzell et al. 1997; Giancotti and Ruoslahti 1999; Schaller 2001). Following activation of integrins, FAK autophosphorylates at Y397, generating a high-affinity binding site for the Src homology 2 (SH2) domain of Src family kinases (Schaller et al. 1994). Mutation of Y397 of FAK to phenylalanine abolished cyclic strain-induced phosphorylation of Erk2 in osteoblasts (Boutahar et al. 2004), demonstrating the role of FAK phosphorylation at Y397 in integrin-mediated mechanotransduction.
Multiple lines of evidence suggest that Src, a non-receptor tyrosine kinase that consists of a SH3 domain, SH2 domain, and tyrosine kinase domain, is involved in integrin-mediated mechanotransduction of US mechanical stimulation of chondrocytes. Src has previously been shown to bind to phosphorylated Y397 of FAK via the SH2 domain (Schaller et al. 1994; Schlaepfer et al. 1994; Boutahar et al. 2004). Furthermore, mutation of Y397 to phenylalanine prevented stable binding of Src to FAK (Schaller et al. 1994) and suppressed mechanical strain-induced Erk2 phosphorylation in osteoblasts (Boutahar et al. 2004), demonstrating that Src-Y397-FAK interaction is a prospective component of integrin-mediated mechanotransduction. US stimulation induced Src autophosphorylation of Y416 of Src as demonstrated in Figure 2; moreover, other studies reported US-induced Src Y416 phosphorylation in fibroblasts (Zhou et al. 2004) and cyclic strain-induced Src Y416 phosphorylation in osteoblasts (Boutahar et al. 2004). Inhibition of Src by PP1 prevented US-induced downstream phosphorylation of Erk1/2 in chondrocytes as demonstrated in Figure 6 as well as in fibroblasts (Zhou et al. 2004). Taken together this evidence suggests that Src plays a role in integrin-mediated mechanotransduction of chondrocytes in vitro after US mechanical stimulation.
The p130Cas docking protein has been shown to bind to FAK via a SH3 domain and this interaction has been implicated in integrin-mediated signaling (Polte and Hanks 1995, 1997). A Src binding domain, RPLPSPP, near the C terminus of p130Cas acts as a direct binding site for Src via the SH3 domain (Nakamoto et al. 1996), and p130Cas contains Tyr-X-X-Pro motifs, including Y165, Y249, and Y410, that when phosphorylated may act as binding sites for SH2 domains of numerous proteins, such as Crk (Ruest et al. 2001). FAK acts as a docking protein for both Src and p130Cas, with the SH2 domain of Src binding to phosphorylated Y397 of FAK and the SH3 domain of p130Cas binding to FAK (Ruest et al. 2001). Src may then bind p130Cas through the SH3 domain of Src, enhancing substrate recognition prior to phosphorylation of tyrosine residues on p130Cas (Nakamoto et al. 1996), and possibly strengthening the FAK-Src-p130Cas complex. Following the formation of the FAK-Src-p130Cas complex, Src phosphorylates p130Cas on Tyr-X-X-Pro motifs, such as Y165, Y249, and Y410; however, we were only able to detect phosphorylation of p130Cas at Y249 but not at Y165 or Y410 (Fig. 3; data not shown).
Phosphorylated Y249 of p130Cas creates a binding site for the CrkII adapter protein (Shin et al. 2004; Modzelewska et al. 2006). CrkII is an adapter protein that binds multiple guanine nucleotide exchange factors such as C3G (Knudsen et al. 1994; Matsuda et al. 1994), SOS (Matsuda et al. 1994), and DOCK180 (Hasegawa et al. 1996; Kiyokawa et al. 1998), which can signal to the MAPK/Erk pathway via Rap (Gotoh et al. 1995; Peyssonnaux and Eychene 2001), Ras (Schlaepfer et al. 1998; Giancotti and Ruoslahti 1999), and Rac (Eblen et al. 2002) GTP-binding proteins, respectively. To examine the activation of CrkII, we measured the phosphorylation of CrkII at Y221, which when phosphorylated causes intramolecular binding of Y221 with the SH2 domain of CrkII (Feller et al. 1994; Rosen et al. 1995), disrupting CrkII binding to p130Cas (Kain and Klemke 2001) and downstream guanine nucleotide exchange factors such as C3G (Huang et al. 2008). Even though phosphorylation of Y221 is considered to be a negative regulator of CrkII, phosphorylation of Y221 demonstrates CrkII may be involved in an US-mediated mechanotransduction pathway.
The MAPK/Erk pathway is a well-documented signaling pathway that influences numerous cellular processes including proliferation, survival, migration, and a wide range of gene regulation that control events such as integrin expression in osteoblasts and cartilage ECM remodeling, via alterations in the activity of transcription factors (Cobb 1999; Lai et al. 2001; Pearson et al. 2001; Beier and Loeser 2010). Our results along with other reports indicate that Erk is phosphorylated in response to US mechanical stimulation via an integrin-mediated mechanotransduction pathway (Zhou et al. 2004; Choi et al. 2007; Pounder and Harrison 2008). Erk1/2 is phosphorylated in response to mechanical stimulation by US as demonstrated in Figure 5. US-induced Erk1/2 phosphorylation is downstream of integrin receptors, as demonstrated by the reduction of Erk1/2 phosphorylation in chondrocytes by an RGD-containing peptide (Fig. 6) and an integrin-blocking antibody (Choi et al. 2007). Furthermore, mechanical strain-induced Erk phosphorylation was reduced by the mutation of Y397 of FAK to phenylalanine in osteoblasts (Boutahar et al. 2004), demonstrating the role of FAK phosphorylation at Y397 in integrin-mediated mechanotransduction. Src has also been shown to be upstream of US-induced Erk1/2 phosphorylation in chondrocytes (Fig. 6) and fibroblasts (Zhou et al. 2004) by inhibition with PP1.
In this study, we present a possible mechanotransduction pathway for US mechanical stimulation of chondrocytes in vitro. US stimulation of chondrocytes induced phosphorylation of FAK, Src, p130Cas, CrkII, and Erk; thus, these intracellular signaling molecules may be involved in an US-mediated mechanotransduction pathway. Pre-incubation of chondrocytes with inhibitors of integrin receptors, Src and MEK reduced US-induced Erk phosphorylation levels, indicating integrins and Src are upstream of Erk in a mechanotransduction pathway of US-stimulated mechanical stress of chondrocytes. However, dominant-negative expression or site-specific mutation of the phosphorylation sites of FAK, Src, p130Cas, CrkII, and Erk are needed to further verify the signaling pathway proposed in this study. Furthermore, the results presented in this study remain to be confirmed in primary chondrocytes or cartilage tissue explants.
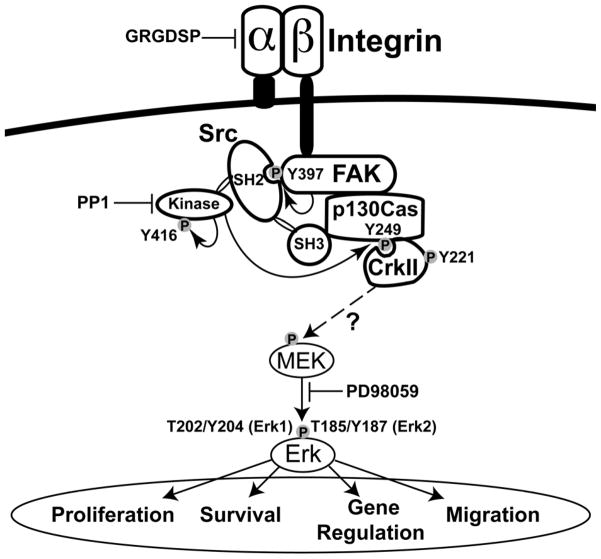
Figure 7. Schematic illustration of the proposed signaling pathway for US activation of chondrocytes via integrin receptors.
Following US-induced activation of integrin receptors, FAK autophosphorylates at Y397, which is a binding site for Src (Schaller et al. 1994; Takeuchi et al. 2008). Src binds to FAK and is activated by autophosphorylation of Y416 in the activation loop of the kinase domain of Src (Cooper and MacAuley 1988; Schaller et al. 1994). The p130Cas docking protein binds to FAK and Src, forming an FAK-Src-p130Cas complex, and then Src phosphorylates p130Cas on Y249 (Nakamoto et al. 1996; Ruest et al. 2001), which is a binding site for the CrkII adapter protein (Shin et al. 2004; Modzelewska et al. 2006). We propose that the binding and activation of CrkII leads to the phosphorylation and activation of Erk, which influences numerous cellular processes including proliferation, survival, migration, and a wide range of gene regulation; however, the pathway through which CrkII signals to Erk has not been elucidated in this study.
Acknowledgments
We kindly acknowledge Dr. Sanjukta Guha Thakurta, Dr. Karl Kador, MinJeong Schneider and Leonard Akert who provided technical support for this work. Trish Fenster, Kandra Hahn, Peggy Pedersen and Karen Spath provided outstanding administrative support. This work was supported, in part, by the American Recovery and Reinvestment Act of 2009 research grant 1R21RR024437-01A1 from the Department of Health and Human Services, and a State of Nebraska grant Stem Cell 2011-04.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem. 2010;110:573–80. doi: 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–7. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–99. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108 ( Pt 4):1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999a;62:166–74. [PubMed] [Google Scholar]
- Carver SE, Heath CA. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999b;5:1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–9. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Choi BH, Choi MH, Kwak MG, Min BH, Woo ZH, Park SR. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc Inst Mech Eng H. 2007;221:527–35. doi: 10.1243/09544119JEIM201. [DOI] [PubMed] [Google Scholar]
- Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Cooper JA, MacAuley A. Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci U S A. 1988;85:4232–6. doi: 10.1073/pnas.85.12.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. Bi-directional signal transduction by integrin receptors. Int J Biochem Cell Biol. 2000;32:171–88. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–33. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzell RM, Goldmann WH, Wang N, Parashurama N, Ingber DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. Embo J. 1994;13:2341–51. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279:19502–11. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095–103. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257–64. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Hattori S, Nakamura S, Kitayama H, Noda M, Takai Y, Kaibuchi K, Matsui H, Hatase O, Takahashi H, et al. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol Cell Biol. 1995;15:6746–53. doi: 10.1128/mcb.15.12.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann K, Zimmermann B, Barrach HJ, Merker HJ. Behaviour of epiphyseal mouse chondrocyte populations in monolayer culture. Morphological and immunohistochemical studies Virchows. Arch A Pathol Anat Histol. 1980;389:167–87. doi: 10.1007/BF00439484. [DOI] [PubMed] [Google Scholar]
- Hasanova GI, Noriega SE, Mamedov TG, Thakurta SG, Turner JA, Subramanian A. The effect of ultrasound stimulation on the gene and protein expression of chondrocytes seeded in chitosan scaffolds. J Tissue Eng Regen Med. 2011;5:815–22. doi: 10.1002/term.384. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, Kurata T, Matsuda M. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996;16:1770–6. doi: 10.1128/mcb.16.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut Donahue TL, Genetos DC, Jacobs CR, Donahue HJ, Yellowley CE. Annexin V disruption impairs mechanically induced calcium signaling in osteoblastic cells. Bone. 2004;35:656–63. doi: 10.1016/j.bone.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183:711–23. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortikka MO, Inkinen RI, Tammi MI, Parkkinen JJ, Haapala J, Kiviranta I, Helminen HJ, Lammi MJ. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Ann Rheum Dis. 1997;56:255–61. doi: 10.1136/ard.56.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain KH, Klemke RL. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J Biol Chem. 2001;276:16185–92. doi: 10.1074/jbc.M100095200. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Tammi M, Jurvelin J, Saamanen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6:188–95. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–6. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen BS, Feller SM, Hanafusa H. Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J Biol Chem. 1994;269:32781–7. [PubMed] [Google Scholar]
- Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–50. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Hashimoto Y, Muroya K, Hasegawa H, Kurata T, Tanaka S, Nakamura S, Hattori S. CRK protein binds to two guanine nucleotide-releasing proteins for the Ras family and modulates nerve growth factor-induced activation of Ras in PC12 cells. Mol Cell Biol. 1994;14:5495–500. doi: 10.1128/mcb.14.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Modzelewska K, Newman LP, Desai R, Keely PJ. Ack1 mediates Cdc42-dependent cell migration and signaling to p130Cas. J Biol Chem. 2006;281:37527–35. doi: 10.1074/jbc.M604342200. [DOI] [PubMed] [Google Scholar]
- Morris HL, Reed CI, Haycock JW, Reilly GC. Mechanisms of fluid-flow-induced matrix production in bone tissue engineering. Proc Inst Mech Eng H. 2010;224:1509–21. doi: 10.1243/09544119JEIM751. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J Biol Chem. 1996;271:8959–65. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- Nishikori T, Ochi M, Uchio Y, Maniwa S, Kataoka H, Kawasaki K, Katsube K, Kuriwaka M. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. J Biomed Mater Res. 2002;59:201–6. doi: 10.1002/jbm.1226. [DOI] [PubMed] [Google Scholar]
- Noriega S, Mamedov T, Turner JA, Subramanian A. Intermittent applications of continuous ultrasound on the viability, proliferation, morphology, and matrix production of chondrocytes in 3D matrices. Tissue Eng. 2007;13:611–8. doi: 10.1089/ten.2006.0130. [DOI] [PubMed] [Google Scholar]
- Noriega SE, Hasanova GI, Schneider MJ, Larsen GF, Subramanian A. Effect of Fiber Diameter on the Spreading, Proliferation and Differentiation of Chondrocytes on Electrospun Chitosan Matrices. Cells Tissues Organs. 2012;195:207–21. doi: 10.1159/000325144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M, Perricone E, Brandt KD. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22:508–17. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- Palmoski MJ, Colyer RA, Brandt KD. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980;23:325–34. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Wu CC, Lewallen DG, Greenleaf JF, Bolander ME. Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res. 1999;17:488–94. doi: 10.1002/jor.1100170405. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci U S A. 1995;92:10678–82. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Hanks SK. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130(Cas)) are elevated in cytoskeleton-associated fractions following adhesion and Src transformation. Requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–9. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–8. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rosen MK, Yamazaki T, Gish GD, Kay CM, Pawson T, Kay LE. Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature. 1995;374:477–9. doi: 10.1038/374477a0. [DOI] [PubMed] [Google Scholar]
- Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83-A:259–70. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol Cell Biol. 2001;21:7641–52. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–72. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–8. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–91. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–85. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem. 2004;279:38331–7. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- Takeuchi R, Ryo A, Komitsu N, Mikuni-Takagaki Y, Fukui A, Takagi Y, Shiraishi T, Morishita S, Yamazaki Y, Kumagai K, Aoki I, Saito T. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res Ther. 2008;10:R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todhunter RJ, Minor RR, Wootton JA, Krook L, Burton-Wurster N, Lust G. Effects of exercise and polysulfated glycosaminoglycan on repair of articular cartilage defects in the equine carpus. J Orthop Res. 1993;11:782–95. doi: 10.1002/jor.1100110603. [DOI] [PubMed] [Google Scholar]
- von der Mark K, Mollenhauer J. Annexin V interactions with collagen. Cell Mol Life Sci. 1997;53:539–45. doi: 10.1007/s000180050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K. Integrin signaling: tyrosine phosphorylation events in focal adhesions. J Membr Biol. 1998;165:191–9. doi: 10.1007/s002329900433. [DOI] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wright M, Jobanputra P, Bavington C, Salter DM, Nuki G. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: evidence for the presence of stretch-activated membrane ion channels. Clin Sci (Lond) 1996;90:61–71. doi: 10.1042/cs0900061. [DOI] [PubMed] [Google Scholar]
- Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, Bolander ME. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996;14:802–9. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Huckle J, Francomano CA, Spencer RG. The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: an explant study. Ultrasound Med Biol. 2002;28:1547–53. doi: 10.1016/s0301-5629(02)00659-2. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Huckle J, Francomano CA, Spencer RG. The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med Biol. 2003;29:1645–51. doi: 10.1016/j.ultrasmedbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem. 2004;279:54463–9. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]