Abstract
To overcome the limitations of monomeric pH probes for acidic tumor environments, this study designed a mixed micelle pH probe composed of polyethylene glycol (PEG)-b- poly(L-histidine) (PHis) and PEG-b-poly(L-lactic acid) (PLLA), which is well-known as an effective antitumor drug carrier. Unlike monomeric histidine and PHis derivatives, the mixed micelles can be structurally destabilized by changes in pH, leading to a better pH sensing system in nuclear magnetic resonance (NMR) techniques. The acidic pH-induced transformation of the mixed micelles allowed pH detection and pH mapping of 0.2–0.3 pH unit differences by pH-induced “on/off”-like sensing of NMR and magnetic resonance spectroscopy (MRS). The micellar pH probes sensed pH differences in non-biological phosphate buffer and biological buffers such as cell culture medium and rat whole blood. In addition, the pH-sensing ability of the mixed micelles was not compromised by loaded doxorubicin. In conclusion, PHis-based micelles could have potential as a tool to simultaneously treat and map the pH of solid tumors in vivo.
Keywords: pH imaging, poly(L-histidine), micelle pH probe, NMR, MRS
INTRODUCTION
Various tools for biomedical imaging have been developed to diagnose diseases, monitor drug delivery, and predict treatment responses.1 pH imaging for tumor diagnosis has received particular interest due to the unique acidic environment of solid tumors resulting from its physiological and metabolical abnormality.2–4 Amongst the various means (such as electrodes,5, 6 fluorescence,7 luminescence,8 magnetic resonance (MR),2–4, 9, 10 and positron emission tomography (PET)4, 11) which have been investigated for monitoring tumor pH in vivo, MR Imaging/Spectroscopy (MRI/MRS) is one of the most promising imaging techniques due to its noninvasiveness and clinical applicability.2–4, 9, 10 pH imaging is attainable using techniques such as pH-dependent chemical shift12–15 for MRS and pH-dependent relaxivity16–18 and pH-sensitive chemical exchange saturation transfer (CEST)19–21 for MRI with proper low molecular weight (MW) probes or imaging agents. However, low MW chemicals are limited in clinical applications due to their nonspecific nature, fast elimination kinetics,12, 22–24 and the difficulty in estimating their in vivo concentrations.2, 25
To detect the extracellular pH (pHe) of a tumor using MRS, pH probes should have a pKa in the weak acidic-to-neutral pH range (pH 6.0–7.4).4 For this reason, low MW probes having a pH-sensitive imidazole group (e.g., 2-imidazol-1-yl-3-ethoxycarbonyl-propionate14,15 and 2-(imidazol-1-yl) succinic acid12) have been applied for 1H-MRS/MRSI-based pH measurements. In addition, Lee et al. recently imaged an acidic tumor or ischemic site in vivo using MR imaging agents (SPIO) loaded into a pH-sensitive polymeric micelle.26, 27
As reported previously by our group,28–30 poly(ethylene glycol)-b-poly(L-histidine) (PEG-PHis), with or without a second polymeric component of PEG-b-poly(L-lactic acid) (PEG-PLLA), forms pH-sensitive micelles in basic solutions but are physically dissociated under acidic conditions. It is hypothesized that the pH-sensitive structural transition between micelles and water-soluble polymers amplify the detection of environmental pH. In addition, nanoparticles generally have greater accumulation and longer retention in solid tumors than small molecules due to the enhanced permeation and retention (EPR) effect.23 PHis based pH-sensitive micelles can be systemically administered and are highly cytocompatible,29 but when loaded with an anti-tumor chemical drug such as doxorubicin (DOX) they can effectively kill tumor cells in vitro and in vivo.29
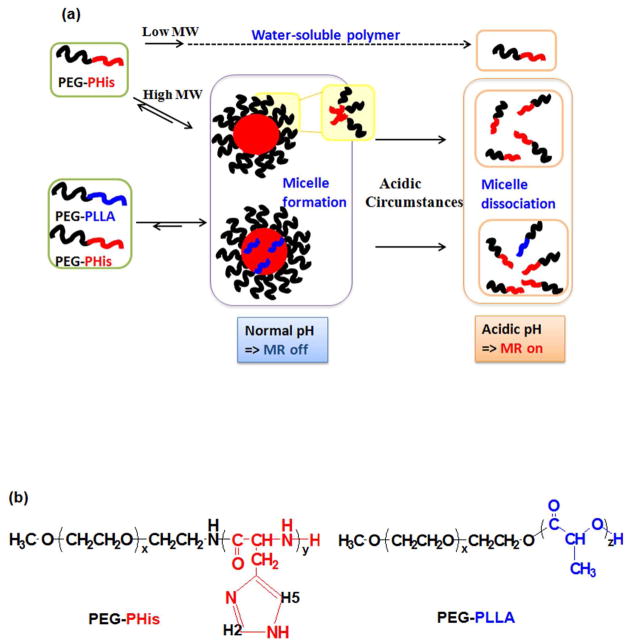
With the aformentioned attractive characteristics of PEG-PHis-based micellar constructs, this study explores their utility in pH detection and imaging. Our hypothesis was that pH-sensitive polymeric micelles at normal physiological pH (~pH 7.4) turns MR signaling from the pH-sensitive moiety (i.e., imidazole groups in PHis) off, whereas acidic environments (~ pH 6) will destabalize the pH-sensitive micelles, causing them to break apart into water-soluble polymers, and turn pH-sensitive MR signals on (Figure 1). Thus, in this study, we invesitgated whether PEG-PHis-based micelles detect and image pH in various environments such as phosphate buffer, cell culture medium and blood. In addition, we examined the micellar pH probe loaded with DOX, which can be applied for theragnosis (i.e., therapy and diagnosis) in future applications.
Figure 1.
(a) Proposed concept of on/off switching pH probe using PEG-PHis-based micelles via MR (1H-NMR and 1H-MRS) and (b) Chemical structures of polymer components in PEG-PHis-based micelles
MATERIALS AND METHODS
Materials, Cells and Rat Blood
Boc-His (Dnp)-OH·isopropanol (99%) and methoxy PEG amine hydrochloride salt (mPEG-NH2·HCl, Mn 2 kDa) were purchased from Bachem Americas, Inc (Torrance, CA) and Jenkem Technology USA Inc. (Allen, TX), respectively. Toluene, diethyle ether, 1,4-dioxane, acetone, dimethyl sulfoxide (DMSO), N,N′-dimethylformamide (DMF), 2-mercaptoethanol, sodium carbonate, thionyl chloride, L-lactide, methoxy PEG-OH (mPEG-OH, Mn 2 kDa), stannous octoate, deutrium oxide (D2O), sodium tetraborate, boric acid, 3-(trimethylsilyl) tetradeutero sodium propionate (TSP), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), sodium bicarbonate, fetal bovine serum (FBS), D-glucose, penicillin-streptomycin antibiotics, RPMI1640 medium, Dulbecco’s phosphate buffer solution (DPBS), ethylenediaminetetraacetic acid (EDTA), calcium hydride (CaH2), doxorubicin (DOX), insulin, and trypsin-EDTA were bought from Sigma-Aldrich Companies (St. Louis, MO). PEG derivaties such as mPEG-OH and mPEG-NH2 were purified by azeotropic distillation in toluene prior to use. DMF stirred in the presence of CaH2 was distilled under reduced pressure prior to use. Other solvents and chemicals were used without further purification. Dialysis tubing with molecular weight cut-off (MWCO) of 3.5 kDa was purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Rat whole blood was purchased from Innovative Research (Novi, MI).
MCF7 cells (a human breast adenocarcinoma cell line) were cultured in RPMI1640 medium supplemented with D-glucose (2 g/L), insulin (4 mg/L), and 10% FBS under humidified air containing 5% CO2 at 37°C.
Polymer Synthesis
PEG-PHis was synthesized by a modified method with shorter steps (Figure S1) compared to that reported previously.26 First, for synthesis of Nim-Dnp-histidine carboxyanhydride hydrochloride (Dnp-NCA·HCl), thionyl chloride (4 mL) was added into Boc-His(Dnp)-OH·isopropanol (4 g) in 1,4-dioxane (50 mL) under N2 atmosphere and the reaction mixture was reacted for 1 h. Then, the precipitate was filtered and washed with dioxane and diethyl ether. Dnp-NCA·HCl was dissolved in acetone and re-purified by precipitation in diethyl ether.
Poly(Nim-Dnp-histidine) was synthesized by amine-initiated ring-opening polymerization (ROP) of Dnp-NCA·HCl. To synthesize mPEG-b-poly(Nim-Dnp-histidine) diblock copolymer, Dnp-NCA·HCl (4.3 g) and sodium carbonate (1.9 g) in DMF (60 mL) were stirred at room temperature (RT) for about 1 h under N2 atmosphere. Then, mPEG-NH2·HCl (0.75 g) in DMF (16 mL) was added into the reaction mixture. The reaction was lasted for 3 d at RT under reduced pressure and stirring. The reaction solution was poured into ethanol and re-purified by precipitation in diethyl ether. After filtration, mPEG-b-poly(Nim-Dnp-histidine) was dried in vacuo.
For PEG-PHis, Dnp deprotection of PEG-b-poly(Nim-Dnp-histidine) was carried out. In brief, 2-mercaptoethanol (20 mL) was added into PEG-b-poly(Nim-Dnp-histidine) (2.9 g) in DMSO (60 mL). The reaction solution was stirred for 12 h at RT. The solution was dialyzed against DMSO for 1 d and then against deionized water (DIW) for 2 d to remove the deprotected groups using a dialysis tube (MWCO 3.5 kDa). After lyophilization, a light yellow powder (PEG-PHis, 1.4 g) was yielded. Using 1H-NMR spectra, MW of PHis block was estimated based on CH2 peak of PEG for PEG-PHis.
PEG-PLLA diblock copolymer was synthesized by ROP of L-lactide initiated by the hydroxy group of mPEG-OH in the presence of the catalyst stannous octoate. 31
Preparation of Polymeric Micelles
Polymeric micelles were constructed by using a typical diafiltration. PEG-PHis (18 mg) with or without PEG-PLLA (2 mg) for mixed micelles or homogenous micelles, respectively, was dissolved in DMSO (5 mL). Borate buffer solution (pH 9.2, 10 mM, 1 mL) was subsequently added dropwise into the polymer solution. The resulting solution was vigorously stirred for 1 h and then transferred into a dialysis membrane (MWCO 3.5 kDa). The dialysis was performed against borate buffer (pH 9.2, 10 mM) and the buffer was replaced with fresh buffer solution at 1, 4, and 12 h. After 24 h the formed micelles were lyophilized and then reconstituted prior to use. DOX-loaded micelles were prepared similarly with dissolution of DOX at the first stage. In addition, transmission electron microscopy (TEM) observations were performed by using a Philips TECNAI T12 electron microscope. To prepare the sample, one drop of the aqueous solution was deposited onto a Formvar-carbon coated copper grid. After 5 min, the excess solution was wicked away by touching a filter paper to the edge of the grid. The sample was then observed directly by TEM without staining.
To evaluate the structural transition of the micelles in phosphate buffer, rat whole blood and cultured cells, the micelle solution (10 mg/mL) was reconstituted at pH 7.4. All pH adjustments were performed using a pH meter to monitor pH changes by adding 1N DCl into samples. For NMR measurements and MRS phantoms in phosphate buffer, the micelle solution was prepared by DPBS in D2O and DPBS including 10% D2O, respectively. For evaluation of micelles in rat whole blood, micelle solution prepared by DPBS in D2O at each pH was added into the whole blood at the same pH and in the same proportions just prior to 1H-NMR evaluation.
To monitor micelles in a culture enviornment of MCF7 cells, the cells were seeded on 6-well plates at 2×105 cells/well and cultured for 1 d. Prior to adding the micelle solution, the culture medium with serum (1 mL) was replaced with fresh culture medium (pH 6.0 and pH 7.4) without serum. The micelle solution was added into the culture medium for 1H-NMR measurement.
1H-NMR Experiments
1H-NMR spectra were recorded on a Varian Unity 400 at 9 T with NaLoRAC Z-spec broadband probe for sample of DPBS in D2O and Varian 600 with a cryoprobe to allow for bio-samples with less D2O. TSP was used as an internal reference for 1H chemical shifts 1H-NMR spectra were acquired with the following parameters at RT: acquisition time (at) of 3.2 sec, delay time (d1) of 2 sec, spectral width (sw) of 8000 Hz, and 128 averages. A pulse sequence using CPMGT2 and Presat in the VNMRJ for 600 MHz NMR (Agilent Tech.) was exploited for the sample of blood and MCF7 cells.
The MWs of synthesized PHis were determined on the basis of NMR integration values with PEG with a known MW(2 kDa) used as a reference. Also, the relative integration of PHis depending on the pH was obtained from normalization of the integration value of imidazole peaks acquired after the PEG was adjusted to have the same integration value at each pH. The integration value of the imidazole peak at pH 2.0 was used as a reference for normalization because diblock polymers can be entirely dissolved at pH 2.0. For accurate determination of the relative integration value, we delicately performed quantitive NMR experiments with enough delay time, baseline correction and internalized double references with the same quantity of TSP and DMSO. The PEG integration value did not change in any pH aqueous solution as judged by comparison with integration value of DMSO and TMS, meaning that all of the PEG forming the outer shell of the micelle is exposed to maintain a stable micelle structure. Otherwise, the integration and peak shape of PEG in micelle can be changed if aggregation occurs during micelle preparation; similarly, PEG-PHis micelles prepared in the presence of Boc-histidine (hydrophillic small molecule), CH2 peak in the micelle at pH 9.0 was broader and smaller compared to a soluble copolymer at pH 5.0 (Figure S3).
MRS/MRI Experiments
MRS phantom studies were conducted using a 7 T small-bore animal MRI system (BioSpec 70/30 USR, Ettlingen, Germany) with a two-turn 1H surface coil. Point Resolved Spectroscopy (PRESS) sequence with a VAPOR sequence for water suppression was used with optimized relaxation delays. The 1H-MRS parameters were as follows: voxel size 2.8×2.8×8 mm3, TR=1500 ms, TE=13.92 ms, and 512 averages. The phantom consisted of 5 mm NMR tubes filled with 3.8 mM mixed micelle solution at different pH values. T1-weighted MR images were obtained using FLASH (Fast Low Angle Shot, gradient Echo pulse sequence): TR=60.2 ms, TE=15 ms, Flip Angle=30 deg, FOV= 3cm. The pixel matrix was 128 × 128. The pH values from each sample tube of the phantom were color-coded based on the H2 chemical shift using MATLAB.
RESULTS AND DISCUSSION
Although PHis-based polymers and nanoparticles have pH-sensitive characteristics such as proton buffering and structural transition,29, 30 their pH-sensing ability is not reported in literature. In addition, it is not known whether the imidazole groups in polymers retain the pH probing activity of monomeric imidazole groups. Thus, prior to the use of PHis-based micelles, the pH-sensitivity of PHis was first examined and compared with that of monomeric L-histidine. As shown in Figure S4, monomeric L-histidine and PHis showed similar pH sensitivity to the chemical shifts of H2 and H5 in imidazole rings, although the peaks in PHis were broader.
1H-NMR-based pH sensing of PEG-PHis-based micelles in phosphate buffer
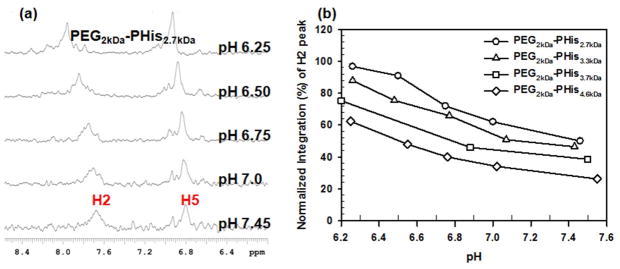
The aqueous solubility of PHis is decreased with increasing MW and is also pH-dependent due to different degrees of protonation of imidazole groups with varying pH. PHis homopolymers can be transformed from water-soluble polymers to hydrophobic aggregates by increasing pH. However, the formed PHis aggreggates would not be appropriate for tumor applications due to their uncontrolled size distribution which can extend into the micron range. Thus, hydrophilic PEG2kDa was chemically introduced into pH-sensitive PHis having different MWs (2.7 kDa ~ 4.6 kDa) to form water-soluble polymers at acidic tumor pHe and nanosized self-assembled micelles at pH 7.4 (Figure 1(a)). Using these PEG2kDa-PHis block copolymers, the MW effect of PHis on the chemical shifts of H2 and H5 in imidazole rings were evaluated by using 1H-NMR spectra. As expected, H2 and H5 peaks in PEG2kDa-PHis were shifted downfield with decreasing pH and the H2 peaks showed a more distinct pH-sensitive chemical shift change than the H5 peaks regardless of PHis’s MW because the protonation at 3-N of imidazole induces an electron deshieding effect with decreasing pH (Figure 2(a) and Figure S5).
Figure 2.
(a) 1H-NMR spectra of H2 and H5 peaks in PEG2kDa-PHis2.7kDa exposed at different pHs of phosphate buffer and (b) pH-dependent H2 peak’s integration of PEG2kDa-PHis with different MWs of PHis.
The normalized integration values of the H2 peaks in PEG2kDa-PHis polymers or micelles were evaluated, accounting for the pH-induced chemical shift and molecular weight dependency of PHis. As shown in Figure 2(b), the integration values near pH 7.4 decreased with increasing MW of PHis blocks (e.g., from 50% for PEG2kDa-PHis2.7kDa to 26% for PEG2kDa-PHis4.6kDa). This may occur because the longer PHis chain’s increased hydrophobicity causes the PHis blocks to strongly associate in the micelle’s solid core. Also, the normalized integrations of H2 peaks increased with decreasing pH (e.g., for PEG2kDa-PHis3.3kDa, from 46% at pH 7.4 to 88% at pH 6.25) when the MW of PHis in the PEG2kDa-PHis copolymer was held constant (Figure 2(b)). The result may be from the acid-induced dissociation of the micellar structures, which expose the protonated (i.e., hydrophilic) PHis blocks to the aqueous phase. These two complimentary factors improved pH sensitivity based on the normalized integration of the H2 peak. The difference between integration values at ~ pH 7.4 and ~ pH 6.3 was 47% for PEG2kDa-PHis2.7kDa, 42% for PEG2kDa-PHis3.3kDa, 37% for PEG2kDa-PHis3.7kDa, and 36% for PEG2kDa-PHis4.6kDa. However, at ~ pH 7.4 the high H2 peak’s normalized integration values (25%~50%) may have been a result of the colloidal instability of PEG2kDa-PHis micelles as reported previously.29 This might cause an unwanted signal in normal blood pH, leading to poor resolution of tumor pHe signal from the normal pH background.
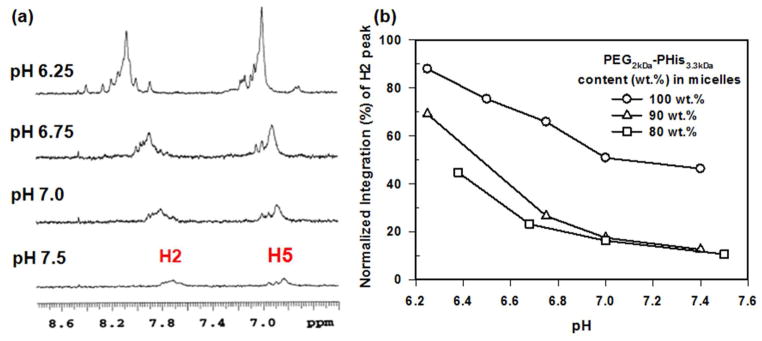
According to our previous reports, the mixed micelles composed of PEG2kDa-PHis5kDa and PEG2kDa-b-poly(L-lactic acid) (PEG2kDa-PLLA3kDa) are stable at pH 7.4 but unstable in the weak acidic pH range of 6.6~7.2 (which is found in the tumor extracellular space) depending on the mixing content of two block copolymers.30 To increase the colloidal stability, and thus H2 peak pH sensitivity of the micelles, candidate formulations of PEG2kDa-PHis3.3kDa and PEG2kDa-PLLA3kDa were used to prepare mixed micelles for use as pH probes (Figure 1). When prepared, these micelles were spherical and monodisperse (Figure S2). The chemical shift of H2 peaks was not influenced by increasing PEG2kDa-PLLA3kDa content in mixed micelles (Figure S6). Interestingly, however, the H2 peak’s normalized integration values of the two mixed micelles of PEG2kDa-PHis3.3kDa having PEG2kDa-PLLA3kDa (90 wt.%/10 wt.% and 80 wt.%/20 wt.%) were dramatically reduced compared with that of homomicelles of PEG2kDa-PHis3.3kDa (Figure 3(b)). Their reduced integration values may have resulted from the addition of PEG2kDa-PLLA3kDa into mixed micelles, which improved colloidal stability, and decreased the pH at which micelle destabilization occurs.30 In mixed micelles made of PEG2kDa-PHis3.3kDa (90 wt.%) and PEG2kDa-PLLA3kDa (10 wt.%), the improved colloidal stability at pH 7.4 generated a large drop in the H2 peak’s normalized integration value. This value could could fall to as little as 12% of the value from homogenous micelles PEG2kDa-PHis3.3kDa (100 wt.%). Similarly, the value at pH 6.3 was 69% of the mixed micelles and 88% of the homogenous micelles. The H2 peak’s integration difference ratio between pH 7.4 and pH 6.3 showed 136% better pH sensitivity for the mixed micelles than homogenous micelles. This finding indicates that the mixed micelles have great pH sensitivity. Furthermore, the mixed micelles represented the same H2 peak integration and intensity at a given pH over a 7 day colloidal stability test indicating that the pH sensitivity seems not to be changed over time (Figure S7).
Figure 3.
(a) 1H-NMR spectra of H2 and H5 peaks in mixed micelles of PEG2kDa-PHis3.3kDa (90 wt.%) having PEG2kDa-PLLA3kDa (10 wt.%) exposed at different pHs of phosphate buffer and (b) Comparison of pH-dependent H2 peak’s integration of homogenous micelles of PEG2kDa-PHis3.3kDa (100 wt.%) and mixed micelles of PEG2kDa-PHis3.3kDa (90 wt.%) having PEG2kDa-PLLA3kDa (10 wt.%).
1H-NMR-based pH sensing of PEG-PHis-based micelles in cell culture medium and whole blood
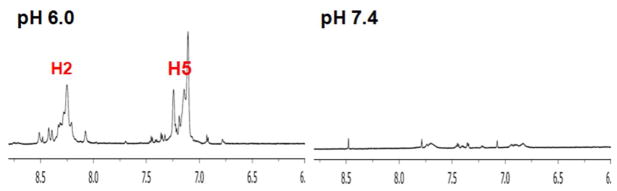
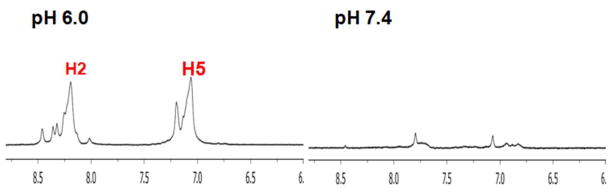
PEG-PHis-based mixed micelles in phosphate buffer were chosen for the next phase of the study on the basis of its superior pH-sensing ability. A mixed micelle system composed of PEG2kDa-PHis3.3kDa (90 wt.%) and PEG2kDa-PLLA3kDa (10 wt.%) was applied to a culture medium of MCF7 cells and to rat whole blood to evaluate whether the micelle still senses pH values in biological conditions. First, the micelles were tested with cultured MCF7 cells in two different pH media (pH 6.0 and pH 7.4). 1H-NMR experiments were performed using spinecho (CPMGT2) with water suppression (Presat) within 30 min. At pH 7.4 there was almost no signal in the aromatic range, whereas the H2 peak’s signal of PEG2kDa-PHis3.3kDa in the micelles was clearly observed at 8.25 ppm at pH 6.0 (Figure 4 and Figure S8). It is unclear whether the micelle pH probe exists in extracellular or intracellular environments because the given chemical shift of the micelle represented their average value. However, PEGylated nanoparticles are generally known to have delayed cellular uptake and improved tumor extracellular retention due to EPR and the ability of PEG-modified surfaces to resist cell and protein binding.23, 32 Thus, the prompt 1H-NMR results in our experiment most likely represent the extracellular pH.
Figure 4.
1H-NMR spectra of H2 and H5 peaks in mixed micelles of PEG2kDa-PHis3.3kDa (90 wt.%) having PEG2kDa-PLLA3kDa (10 wt.%) exposed at two different pH media (pH 6.0 and pH 7.4) of cultured MCF7 cells.
In addition, the mixed micelles were added into rat whole blood. Like cell culture medium, almost no signal in the aromatic range was detected at pH 7.4 except the original peaks from the blood, whereas H2 and H5 signals of the mixed micelles at pH 6.0 were apparent even in whole blood including biological substances such as proteins and lipids (Figure 5 and Figure S9). Although there was a slight peak broadening in blood, the chemical shifts at pH 6.0 were almost identical to those obtained in phosphate buffer and cell culture medium. Also, most of the peaks in blood, including the mixed micelle probe, coincided with the spectrum of blood itself. This may indicate that blood components did not interact with the mixed micelles. However, some aggregates were seen in the NMR tube after several days in the refrigerator.
Figure 5.
1H-NMR spectra of H2 and H5 peaks in mixed micelles of PEG2kDa-PHis3.3kDa (90 wt.%) having PEG2kDa-PLLA3kDa (10 wt.%) exposed at two different pHs (pH 7.4 and pH 6.0) of rat whole blood.
1H-MRS-based pH sensing of PEG-PHis-based micelles in phosphate buffer
The findings in non-biological (i.e., phosphate buffer) and biological (i.e., cell culture medium and whole blood) environments showed that PEG2kDa-PHis3.3kDa (90 wt.%) and PEG2kDa-PLLA3kDa (10 wt.%) mixed micelles can be effective pH probes in 1H-NMR, providing superior image resolution and pH sensitivity; however, 1H-NMR is rarely used in clinical applications. 1H-MRS, on the other hand, is much more useful in a clinical setting, meaning that a clincally useful pH probe should be effective in 1H-MRS measurements.
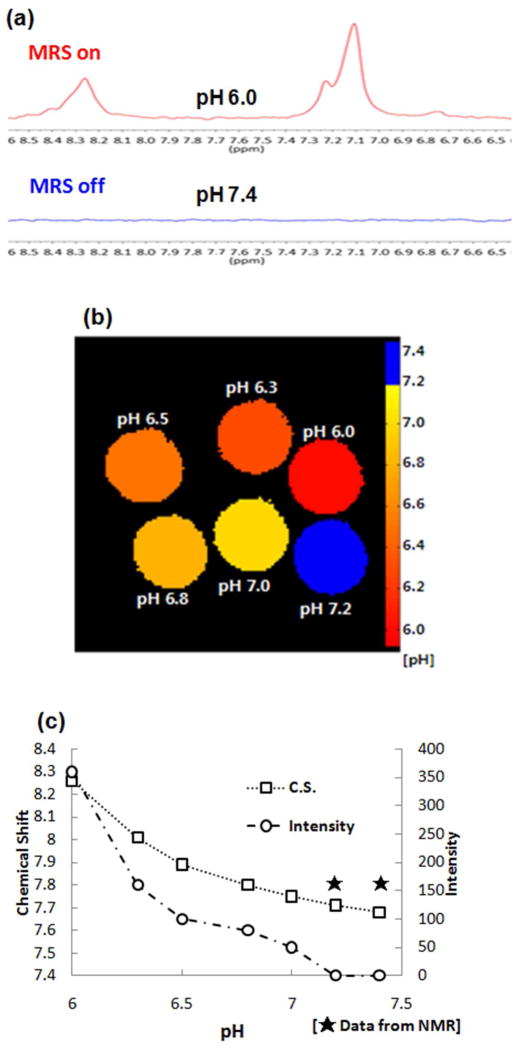
The ability of the micelle probes was further evaluated by using 1H-MRS to map pH and monitor the pH-dependent chemical shift and the intensity of the H2 peaks. MRS phantom data of the mixed micelles showed clear discrimination between pH 7.4 and pH 6.0 (Figure 6(a)) as seen in the 1H-NMR results. In addition, the pH of phosphate buffer containing the mixed micelles was sequentially decreased by 0.2–0.3 pH units from pH 7.4 to map MRS phantoms of the mixed micelles exposed to different pH enviornments. As expected, 1H-MRS of the H2 proton in the mixed micelles produced a pH map of the phantom (Figure 6(b)) and showed pH-dependent distinguishable spectra (Figure S10). The results clearly demonstrated the mixed micelles give pH-monitoring ability within acidic tumor pH ranges.
Figure 6.
(a) Phantom MRS of the mixed micelle composed of PEG2kDa-PHis3.3kDa (90 wt.%) and PEG2kDa-PLLA (10 wt.%): Single voxel MR spectra (PRESS, TR/TE (1500/13.9 ms), nt=512, VOI 2.8×2.8×8 mm3), (b) Phantom pH map based on the chemical shift values, which was encoded as a color scale on MatLab (blue indicates that no signal was detected), and (c) pH-dependent chemical shift and intensity of H2 peak monitored by 7.1T MRS.
From the 1H-MRS spectra of the micelles prepared at different pH values, the pH-dependent chemical shift and intensity changes of the H2 peaks were examined. As shown in Figure 6(c), when the mixed micelles were exposed to neutral pH conditions (pH 7.2–7.4) the H2 peak in the hydrophobic core of the micelles was not detected due to hidden PHis blocks in the micelle core; however, the H2 peak’s intensity was increased with decreasing pH and a one pH unit difference between pH 7.0 and pH 6.0 caused an approximately 7-fold intensity difference. These results are consistent with the 1H-NMR spectroscopic data.
1H-MRS-based pH sensing of DOX-loaded PEG-PHis-based micelles in phosphate buffer
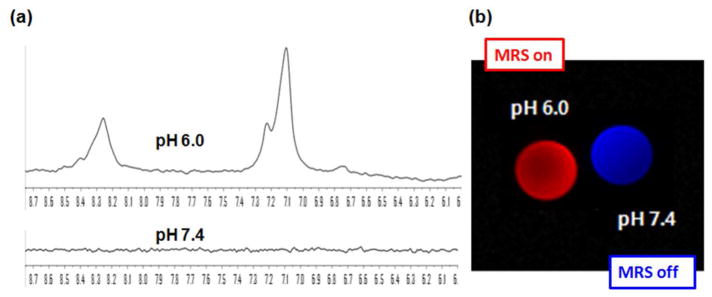
Recently, dual delivery systems using a single carrier have recieved attention as a tool for theragnosis.33, 34 The PEG-PHis-based mixed micelles proposed as an acidic pH probe in this study have been reported to be effective and biocompatible drug carriers to solid tumors29, 35–37. Thus, it was investigated whether the loaded DOX can interfere with the pH sensing of PEG-PHis in the mixed micelles (composed of PEG2kDa-PHis3.3kDa (90 wt.%) and PEG2kDa-PLLA3kDa (10 wt.%)). DOX-loaded mixed micelles added into two different pH phosphate buffers (pH 6.0 and pH 7.4) showed pH-distingushable characteristics in the aromatic range of 1H-MRS spectra (Figure 7(a)). Like the 1H-NMR and 1H-MRS spectra of the mixed micelles without DOX in biological or non-biological environments, DOX-loaded mixed micelles at pH 7.4 did not show any detectable signals in the aromatic range of chemical shift, whereas at pH 6.0, the pH sensing components (i.e., H2 and H5 peaks in imidazole groups of PHis) of the micelles were clearly detectable. In addition, based on MRS spectra of the DOX-loaded mixed micelles exposed to pH 7.4 and pH 6.0, MR images were mapped and expressed as different colors (Figure 7(b)). The findings are similar to the results of the mixed micelles without DOX and indicates that DOX does not interfere with the PEG-PHis-based mixed micelle’s ability to detect acidic pH.
Figure 7.
MRS of theragnosis-applicable Dox-loaded mixed micelle. (a) Single voxel MR spectra (PRESS, TR/TE(1500/13.9 ms), nt=512, VOI 2.8×2.8×8 mm3) and (b) MR Image colored on MatLab.
The pH-sensing ability of the imidazole group in monomeric histidine was extended into its homopolymer (i.e., PHis) and block copolymer (i.e., PEG-PHis). PHis-containing micelles also recognized small differences of 0.2–0.3 pH unit based on chemical shift and integration values of pH-sensitive protons (i.e., H2 and H5 of imidazole group in this study) in non-biological and biological environments. Unlike monomers, the chemical exchange processes in the polymer itself cause both peak broadening and rapid T2 relaxation.13 The fact that higher pH induces shorter T213 may be advantageous because it creates a distinct difference in the MR on/off peak occurrence when using micelle pH probes. The mixed micelles are stable at normal cellular and blood pH 7.4, resulting in no 1H-MRS detection, whereas acidic conditions destabilize the mixed micelles, leading to a strong 1H-MRS signal. The pH-activated on-off operation of the PHis-based mixed micelles provides good pH-sensing capability and could endow high resolution diagnosis of solid tumors against a non-tumor tissue background (i.e., high signal-to-noise). In addition, PEGylated micelles could improve tumor extracelluar retention because PEG interferes with the cellular uptake of the micelles making the micelles highly specific to the extracellular compartment.
CONCLUSION
pH-sensitive PHis in polymers and micelles showed pH-sensing capability and was able to distinguish small pH differences in phosphate buffer, cell culture medium and rat whole blood by using MR techniques such as NMR and MRS. Therapeutic drugs in the mixed micelle pH probes did not compromise its pH-sensitivity. PHis-containing nanoparticular drug delivery systems have great potential to be a pH sensing probe for acidic tumor environments as well as an effective therapeutic platform.
Supplementary Material
Acknowledgments
This study was partially supported by National Institutes of Health, USA (NIH CA101850 and CA140348).
Footnotes
Notes
The authors declare no competing financial interest.
Supporting Information Available.
Supporting information includes: details about the synthesis of PEG-PHis; TEM image of mixed micelles; NMR spectra of PEG-PHis micelles with or without PEG-PLLA in various buffer conditions. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Weissleder R, Pittet MJ. Nature. 2008;452:580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. NMR Biomed. 2011;24:582–91. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Lin Y, Gillies RJ. J Nucl Med. 2010;51:1167–70. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher FA, Kettunen MI, Brindle KM. NMR Biomed. 2011;24:1006–15. doi: 10.1002/nbm.1742. [DOI] [PubMed] [Google Scholar]
- 5.Wike-Hooley JL, Haveman J, Reinhold HS. Radiother Oncol. 1984;2:343–66. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 6.Gerweck LE, Seetharaman K. Cancer Res. 1996;56:1194–8. [PubMed] [Google Scholar]
- 7.Hassan M, Riley J, Chernomordik V, Smith P, Pursley R, Lee SB, Capala J, Gandjbakhche AH. Mol Imaging. 2007;6:229–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Schreml S, Meier RJ, Wolfbeis OS, Landthaler M, Szeimies RM, Babilas P. Proc Natl Acad Sci U S A. 2011;108:2432–7. doi: 10.1073/pnas.1006945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khemtong C, Kessinger CW, Gao J. Chem Commun (Camb) 2009:3497–510. doi: 10.1039/b821865j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malet-Martino M, Holzgrabe U. J Pharm Biomed Anal. 2011;55:1–15. doi: 10.1016/j.jpba.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Vavere AL, Biddlecombe GB, Spees WM, Garbow JR, Wijesinghe D, Andreev OA, Engelman DM, Reshetnyak YK, Lewis JS. Cancer Res. 2009;69:4510–6. doi: 10.1158/0008-5472.CAN-08-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provent P, Benito M, Hiba B, Farion R, Lopez-Larrubia P, Ballesteros P, Remy C, Segebarth C, Cerdan S, Coles JA, Garcia-Martin ML. Cancer Res. 2007;67:7638–45. doi: 10.1158/0008-5472.CAN-06-3459. [DOI] [PubMed] [Google Scholar]
- 13.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. Magn Reson Med. 1999;41:743–50. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Gil S, Zaderenzo P, Cruz F, Cerdan S, Ballesteros P. Bioorg Med Chem. 1994;2:305–314. doi: 10.1016/s0968-0896(00)82186-0. [DOI] [PubMed] [Google Scholar]
- 15.Gil MS, Cruz F, Cerdan S, Ballesteros P. Bioorg Med Chem Lett. 1992;2:1717–1722. doi: 10.1016/s0968-0896(00)82186-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Wu K, Sherry AD. Angew Chem Int Ed Engl. 1999;38:3192–3194. [PubMed] [Google Scholar]
- 17.Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. J Am Chem Soc. 2001;123:7601–9. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]
- 18.Martinez GV, Zhang X, Garcia-Martin ML, Morse DL, Woods M, Sherry AD, Gillies RJ. NMR Biomed. 2011;24:1380–1391. doi: 10.1002/nbm.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Nat Med. 2003;9:1085–90. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 20.Ward KM, Balaban RS. Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Longo DL, Dastru W, Digilio G, Keupp J, Langereis S, Lanzardo S, Prestigio S, Steinbach O, Terreno E, Uggeri F, Aime S. Magn Reson Med. 2011;65:202–11. doi: 10.1002/mrm.22608. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Martin ML, Herigault G, Remy C, Farion R, Ballesteros P, Coles JA, Cerdan S, Ziegler A. Cancer Res. 2001;61:6524–31. [PubMed] [Google Scholar]
- 23.Maeda H, Bharate GY, Daruwalla J. Eur J Pharm Biopharm. 2009;71(3):409–19. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Bae YH, Park K. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayr M, Burkhalter F, Bongartz G. J Magn Reson Imaging. 2009;30:1289–97. doi: 10.1002/jmri.21975. [DOI] [PubMed] [Google Scholar]
- 26.Gao GH, Lee JW, Nguyen MK, Im GH, Yang J, Heo H, Jeon P, Park TG, Lee JH, Lee DS. J Control Release. 2011;155:11–17. doi: 10.1016/j.jconrel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Gao GH, Im GH, Kim MS, Lee JW, Yang J, Jeon H, Lee JH, Lee DS. Small. 2010;6:1201–1204. doi: 10.1002/smll.200902317. [DOI] [PubMed] [Google Scholar]
- 28.Lee ES, Na K, Bae YH. J Control Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee ES, Na K, Bae YH. J Control Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Yin H, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. J Control Release. 2008;126:130–8. doi: 10.1016/j.jconrel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SK, Na K, Bae YH. Colloid Surface A Physicochem Eng Aspects. 2003;214:49–59. [Google Scholar]
- 32.Oyewumi MO, Yokel RA, Jay M, Coakley T, Mumper RJ. J Control Release. 2004;95:613–626. doi: 10.1016/j.jconrel.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Kang HC, Bae YH. Biomaterials. 2011;32:4914–24. doi: 10.1016/j.biomaterials.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon HY, Koo H, Choi KY, Lee SJ, Kim K, Kwon IC, Leary JF, Park K, Yuk SH, Park JH, Choi K. Biomaterials. 2012;33:3980–9. doi: 10.1016/j.biomaterials.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. Angew Chem Int Ed Engl. 2008;47:2418–21. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Lee ES, Park K, Kwon IC, Bae YH. Pharm Res. 2008;25:2074–82. doi: 10.1007/s11095-008-9603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Small. 2008;4:2043–50. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.