Abstract
Burns may represent one of the main indications for face allotransplantation. Severely disfigured faces featuring a devastating appearance and great functional impairments are not only seen as burn sequelae, but also occur as a result of other traumatic injuries, oncological surgical resections, benign tumors (e.g., neurofibromatosis), and major congenital malformations. To date, sixteen human face composite tissue allotransplantations have been performed with success. Despite the initial scepticism about its applicability, due mainly to ethical and technical reasons, the previous worldwide cases and their associated positive outcomes –including acceptable immunosuppressive regimens, excellent aesthetic and functional results, and good psychological acceptance by the recipient- , enable the conclusion that face composite tissue allotransplantation has become another therapeutic strategy in the reconstructive surgical armamentarium, which bears special consideration when dealing with severely disfigured burned patients. The aim of this review is to describe the basics of face composite tissue allotransplantation and give an overview of some of the cases performed until now, with special attention paid to debating the pros and cons of its applicability in burn patients.
Keywords: Face, allotransplantation, burn, immunosuppression, stem cells
1. Introduction to Composite Tissue Allotransplantation
A Composite Tissue Allotransplant or allograft (CTA), as opposed to a solid organ allotransplant, contains several cadaveric tissues such as skin -which is considered most antigenic- [1,2], mucosa, muscle, tendon, cartilage, bone, nerves, vessels, and/or immune cells. The main aim of a CTA is to restore structure and function in patients with devastating injuries and, therefore, improve their quality of life [3]. These multiple tissues are of ectodermal and mesodermal origin and represent a different antigenic load than a typical solid organ allograft [4]. Successful CTAs performed to date include hands, face, abdominal wall, larynx, tongue, uterus, penis, knees, flexor tendon apparatus, and nerves [5].
The first human CTA was a hand transplantation performed in 1964 in Ecuador by Gilbert [6]. Although it was a technical success, it ultimately failed because of ineffective immunosuppression. The first human hand transplantation in the modern era of immunosuppression was performed in 1998 in France by Dubernard, the same surgeon who performed the first human face transplantation in 2005 [7,8].
2. Face Transplantation: Fantasy becomes Reality
The face is one of the most important parts of the human anatomy: it plays a major role in basic physical functions, interactions with the environment and emotional expressions such as swallowing, breathing, seeing, hearing, smelling, smiling, kissing, etc. [9]. It also represents a key piece of one's identity and sense of self. Severely injured patients with devastating facial disfigurement in appearance and motor and sensory function suffer, not only huge chronic physical impairment, but also psychological and social sequelae [10]. Although traditional techniques in plastic and reconstructive surgery (including grafts and local and free flaps) may partially remedy those devastating injuries [11], the reconstruction of more than one aesthetic and functional facial unit still represents a complex challenge. The aforementioned techniques often imply multiple surgeries and increasing morbidity due to the harvesting of donor areas and usually offer poor aesthetic and functional outcomes, e.g., color and thickness mismatch and potential residual scar contractures [12]. Until recently, the autologous bipedicled scapular-parascapular flap has been considered the best option for coverage of a total facial defect [13]. However, the perfect match of facial skin texture, pliability, and color, as well as mimetic of function, can only be achieved by human face composite tissue allotransplantation [14]. Especially in severely burned patients, particularly with injuries in the peri-oral and peri-ocular regions, when adjacent skin is not suitable for donation and where free flaps tend to lead to an unsatisfactory cosmetic appearance and lack of facial expression, the FCTA becomes a less theoretical and more real therapeutic strategy to keep in mind, able to help many severely disfigured patients where other approaches have failed [15].
Regarding a survey of previous cases, human FCTAs have been performed in France (the pioneering country in 2005 in Amiens, with the third world-wide facial transplant in 2007 in Créteil), China (2006, the second one), United States (2008 in Cleveland, the fourth one, and in 2008 and 2011 in Boston), and Spain (2009 and 2010, three cases in three different hospitals and cities, Valencia, Sevilla, and Barcelona; this latter was the first full-face transplant in March 2010). Altogether, the first four facial composite tissue allotransplantations were performed in four different centers in three different countries [16-19]. All the face CTAs performed until now have been technically successful, although the second patient (China) died due to immunosuppressive treatment non-compliance during the second year posttransplantation [18]. A face and bilateral-hand CTA French recipient died later on in the hospital, after good graft take, because of sepsis [20].
When attempting FCTA, the reconstructive plastic surgeon would follow a detailed and lengthy preoperative plan, including the following: 1) due to the still experimental character of the procedure, a complete protocol including the lifelong immunosuppression planned regimen and the infection prophylaxis/immunization regimen is needed, and this has to be approved by institutional review boards; 2) finding appropriate recipients and donors (the matching between donor and recipient should, ideally, take into account all of the following: gender, age, race, blood type, and HLA – Human Leukocyte Antigen- type); 3) training, technical feasibility, and applicability (flap dissection, microsurgery, logistics); 4) respond to ethical, social, psychological, and psychiatric issues [21-25]. Of all of these, selecting the appropriate recipient seems to be the key step, due to the fact that one must be motivated enough to understand the high, long-lasting risk of such a non-vital procedure and the life-long requirement of immunosuppressive treatment, with its substantial potential side effects [19]. Despite all those handicaps, the currently surviving recipients are doing well and are satisfied with their new appearance, resuming the normal activities of their previous lives and, sometimes, even acquiring new ones [19, 26]. These positive outcomes are likely, at least in part, the result of psychiatric and psychological evaluation and treatment of the recipient patients and their families, prior to the operation and life-long, thereafter [10, 27-28].
3. Immunosuppression in CTA
Because CTAs are derived from genetically disparate cadaveric donors, recipients require immunosuppression for life to prevent rejection of the transplant [7, 26]. Although skin is thought to be more highly antigenic, the current immunosuppressive protocols applied to hand and facial CTAs are extrapolated from regimens used in solid organ transplantation (mainly kidney transplantation) with comparable or slightly higher doses [7, 29-31]. The most common maintenance immunosuppressant treatment consists of a triple therapy combining tacrolimus (previously known as FK506), mycophenolate mofetil (MMF), and corticosteroids (eg, prednisolone or prednisone) [26]. These lower dose maintenance regimens may be interspersed by short courses of intensive high-dose therapy to overcome any episodes of acute rejection. When treated adequately and effectively, acute rejection does not seem to impair graft function or long-term survival [32].
Moreover, this maintenance therapy is preceded by a potent induction protocol, including diverse combinations of drugs, eg. cyclosporine, tacrolimus, MMF, prednisolone, polyclonal anti-thymocyte globulins (ATG), anti-interleukin-2 receptor antibodies (such as daclizumab and basiliximab), and anti-CD3 monoclonal antibodies (such as OKT3) [3, 31].
Improvements in immunosuppression have allowed the transition of CTA from research models to clinical reality, but immunosuppression therapy has many disadvantages and risks, mainly infection, organ toxicity, and malignancy [7, 33]. Specifically, encountered side effects include opportunistic cytomegalovirus infections, Clostridium Difficile enteritis, herpes simplex infections, mucosis, diabetes, arterial hypertension, hyperlipidemia, nephrotoxicity, neurotoxicity, bone marrow aplasia, and avascular necrosis, among others (Table 1) [34,37]. Consequently, various recent modifications have been applied to the immunosuppressive protocols, including steroid sparing/avoidance, conversion from tacrolimus to rapamycin (also known as sirolimus, a mammalian target of rapamycin –mTOR- inhibitor) for long-term therapy, or the use of the topical route of administration to reduce the overall amount of systemic immunosuppression. [7, 38-41]. Furthermore, modern maintenance therapy aims to elicit synergistic effects of drugs in order to reduce dosages and minimise individual side effects [3].
Table 1. Most frequently used immunosuppressive drugs in FCTA and their main side effects.
| Drug | Family | Main Side effects | |
|---|---|---|---|
| Tacrolimus | Calcineurin inhibitor |
|
|
| Mycophenolate Mofetil | IMPD inhibitor |
|
|
| Sirolimus =Rapamycin | mTOR inhibitor |
|
|
| Prednisone | Corticosteroid |
|
|
IMPD = Inosine MonoPhosphate Dehydrogenase.
mTOR = mammalian Target Of Rapamycin.
Legend: Although overall functional and aesthetic outcome is acceptable, serious side effects and complications related to immunosuppresion are still challenges hindering progress in face CTA. The most frequent ones include diabetes, leucopenia, renal failure and hypertension. Face and hand CTA share equivalent immunosuppressive regimens.
Another disadvantage of chronic multidrug immunosuppression is the high cost due to the considerable amount of daily oral medication required, which may also lead to non-compliance [7, 42]. However, due to the fact that CTA recipients tend to be healthier than kidney transplantation recipients, the incidence of side effects and immunosuppression-associated mortality is lower within the CTA population [43].
Despite a high incidence of acute rejection in CTA, the occurrence of chronic rejection (a vasculopathy known as transplant arteriosclerosis, intimal hyperplasia, or endoarteritis) is much lower than that in solid organ transplantation. However, it should be noted that this incidence report may be skewed by the relatively few CTA cases reported to date (less than 200). Even so, periodic angiographies after the first year of CTA transplantation to screen for intimal thickening, and hence chronic rejection, have been strongly recommended [4].
Immune tolerance is a particularly appealing concept in CTA [44]. Chimerism is known to be a prerequisite for tolerance induction, and bone marrow is critical to establishing chimerism [7, 45]. CTA's contain immunocompetent elements such as bone marrow and lymph nodes that may hasten the rejection processes or result in graft-versus-host disease (GvHD) [46]. However, it is believed that high doses of bone marrow cells, infused in the absence of recipient conditioning with irradiation, do not induce GvHD [26, 47]. Immunomodulatory strategies are therefore being profusely studied, with potential phototherapy- [48] and mesenchymal stem cell-based protocols under consideration [49, 50], including donor bone marrow stem cells [51]. The use of mesenchymal stem cells in CTA seems to be promising for many reasons: 1) due to their immunoregulatory function, they might allow dose reduction of conventional immunosuppressive drugs, 2) they may prevent and treat GvHD, 3) they increase bone marrow engraftment and, 4) they may improve neural regeneration and, therefore, CTA functional outcomes [7]. Cell-based therapy including T regulatory cells appears to be another promising immunomodulative strategy [26, 52].
3.1. Infection Prophylaxis in CTA
FCTA's contain skin and mucosa, with accompanying donor flora exposed to the external environment. This fact, combined with the concomitant immunosuppressive treatment and the high risk of acquiring opportunistic infections, leads to a strong need for infection prophylaxis in these patients [53]. Accordingly, a triple prophylaxis against virus, bacteria, and fungi is the recommended approach [20].
Regarding viral infections, cytomegalovirus (CMV) plays the major role, involved in 56% of hand CTA infections [54]. This statistic is alarming, as CMV viremia is considered a risk factor for graft dysfunction [55]. Consequently, the ideal situation would be to avoid CMV donor-positive/ recipient-negative FCTA [56].
CMV prophylaxis is commonly achieved with valganciclovir for 6 months [57]. Neutropenia is a major side-effect of valganciclovir, hence foscarnet is usually then instituted. Caution should be exercised, however, because of the potential for nephrotoxic effects, as well as electrolyte disturbances and urogenital ulcers [20]. Face transplant recipients are also at risk of other herpes virus infections, such as herpes simplex type 1, Epstein-Barr, and hepatitis B and C [26]. Pretransplantation vaccination against hepatitis B is therefore mandatory [58].
Immediate post-transplantation prophylaxis may also include coverage for streptococci, anaerobes, capnocytophaga and candida species, e.g., with ampicillin-sulbactam [20]. Besides candida, other potential fungal infections include endemic mycoses such as histoplasmosis and coccidiomycosis, cryptococcosis and filamentous fungi such as Aspergillus (especially likely if paranasal sinuses are transplanted) and Mucor, among others [59]. One should take into account that azole antifungal agents increase calcineurin inhibitor (eg, tacrolimus) levels. Finally, oral candidiasis is very common, and most patients receive prophylaxis with nystatin or clotrimazole [20].
In summary, although CTA immunotherapy is quite analogous to solid organ transplantation therapy, the rate of infections reported to date is lower. However, it should be noted that severely burned populations would constitute a higher-risk group of potential recipients in terms of developing sepsis [19].
4. Criteria for Success after a FCTA
The simple survival of a FCTA might be considered a microsurgical, technical, medical, and immunological success, per se, but it only becomes a real success if that piece of transplanted tissue is able to restore function and morphology to certain degrees of improvement and perfection, restoring at least some facial mobility and expression [60]. Therefore, adequate facial nerve function is essential for optimal outcomes in FCTA [19].
As in hand and forearm CTA, the functional outcome after a FCTA depends on intensive, continuous, and patient-tailored rehabilitation programmes, which require a high degree of patient motivation and compliance. Unlike solid organ transplants, which provide metabolic function soon after revascularization, a CTA is viable after reperfusion, but activity of intrinsic muscles and sensation are absent. Hence, neuro-regeneration represents a unique challenge, as muscle degeneration occurs if not reactivated in a timely manner, and plasticity and rerouting in the central nervous system is required when reintegrating the graft in the sensory and motor cortex [26]. In general, motor function restoration has been achieved later than sensory function after a FCTA [19]. In patients compliant with immunosuppressive medication and rehabilitation, early and intermediate functional return after CTA have been considered as highly encouraging [26].
Regarding timing of outcomes, feeding, speech, sensory restoration, and facial expression all correlate with rate of successful facial reanimation, and have been therefore considered as “good” as early as 6 months post-operatively [8, 17, 19, 61, 62]. Although intelligible speech can be achieved by hard palate restoration [61], re-innervation of perioral musculature is required to accomplish proper phonation. Similarly, to achieve normal food bolus mobilization and also to restore smiling as a facial expression, buccal nerve re-innervation is required. This takes a minimum of 6 months, can take more than 2 years, and has even been described as an absent function or failure [19]. Recovery of passive and active lip movements was reportedly obtained between month 6 and 12 but differed between full or partial face transplantation [8, 17, 18, 26, 61, 62]. On the other hand, sensory restoration has been reported as normal by 3 to 6 months [8, 17, 19, 61, 62], even in cases of no or poor trigeminal neurorraphies. In these latter cases, extension of non-transplanted recipient nerves into graft territory or native nerve root re-innervation of graft tissues may have occurred. Thermic sensation recovery has been reported after 3-8 months by some groups [8, 17, 18, 19, 62]. Furthermore, facial CTA may alleviate hyperesthesia or chronic pain syndrome due to traumatic sequelae such as neuromas or nerve compression [19, 61].
Regarding aesthetic outcomes, to date, all patients self-report improvement in post transplantation appearance [8, 17-19, 61, 62]. A few patients have undergone additional surgery to improve contour with improved cosmetic outcomes reported [19]. Closely related to a patient's overall aesthetic satisfaction is the preservation of identity.
Although facial appearance is improved after CTA, ultimate success is determined, first, by the patient's subjective acceptance of the new face, and, second, by his or her commitment to societal reintegration. To date, all facial CTA patients have accepted their new faces and noted improvement in self-esteem and self-appearance within the first few weeks post-operation. Furthermore, all have been able to progressively integrate back into their previous lives following operation about 3-14 months after the procedure [8, 17-19, 61, 62]. However, before reaching premature conclusions, more detailed studies are still required to assess the psychological consequences of face transplantations [26], as well as further analyses for the long-term and continuous evaluation of general results elicited after a FCTA.
4.1. Recipient Patient Selection in FCTA: The Key to Success
FCTA outcomes are highly dependent on careful patient selection, especially concerning pre-operative psychosocial screening of appropriate candidates, as well as continued post-operative psychiatric counselling [19, 26]. During the screening process, the transplant team should have a clear understanding of the issues that are motivating the candidate to seek a face transplant. Prospective patients that focus on physical and/or functional (rather than psychological) change are likely to be the most satisfied [25, 63]. Raised pre-operative levels of anxiety and neuroticism have been associated with poorer psychosocial outcomes up to three years postoperatively in other groups of transplant patients [64].
As people filter information about risks and benefits in very individual ways [65], and, because unrealistic optimism about risk and outcome in surgical patients is common [25], understanding of the information given should be carefully ascertained.
As adherence to a complex post-operative regimen (mainly, life-long immunosuppression and an estimated 2 years rehabilitation) is crucial to the successful outcome of the transplant, it would be advisable to assess the prospective patient's previous track record of compliance to medication or other forms of treatment [63]. In transplant recipients, levels of adherence have been shown to relate to personal characteristics, such as the age and educational level of the recipient, satisfaction with the outcome of the transplantation, beliefs about the consequences of non-adherence, side effects of the regimen, psychosocial status, and levels of practical and emotional support from family and friends [25]. Furthermore, good-quality social support will buffer patients against stressors, such as the surgery itself, their fear about unknown outcomes, and annoyance at social media attention [19]. Rating scales and questionaires need to be specifically adapted in order to be applicable for psychiatric assessment of face transplant recipients [26], and further research in this field is required.
Apart from being of general good physical and mental health and having adequate social support, patients with severe facial disfigurements suitable to receiving a FCTA should comply with a series of anthropomorphic and physical compatibilities to the donor, such as blood type, HLA, skin colour, texture, age, sex, race, and the size of the maxillofacial skeletal transplanted tissue or craneal perimeter, the latter in the case of full-face transplants [19, 25, 26].
Due to the experimental nature of the procedure and the high risks, patient selection and follow-up must be rigorous and managed by multi-disciplinary teams, including plastic and transplant surgeons, psychiatrists, psychologists, ethicists, radiologists, social workers, and renal medicine and infectious diseases physicians [19, 26].
5. What to do if a FCTA Fails
Unlike hand transplantations (where the transplant can be amputated) the salvage strategy in cases of face transplant failure (after emergent surgical revascularization attempt) necessitates an autologous coverage that may be very difficult or even impossible to procure [14, 60, 66, 67]. Another facial allograft donor will most probably not be available within the near future. A suitable functional and aesthetic prosthesis does not exist [19]. However, prosthetics may be useful after a failed CTA (or even when treating disfigured patients who would rather not undergo a facial CTA) to complement other flap reconstructions, minimize the amount of free tissue to be transferred, or just to improve the overall cosmetic result [68]. In addition, some defects that are impossible to reconstruct, such as the loss of an eye, can be replaced with an aesthetic prosthesis [69].
The facial defect that was subject to the allograft will have to be covered and obliterated by one or several autologous flap(s). This may in turn lead to a disfigurement worse than what the patient had previously [19]. In the near future, advances in stem cell therapies and tissue engineering may provide composite vascularized tissues to replace a full-facial defect. [69-71]. Failure of full-face transplantation would leave various other treatment options, which would include: a) the application of autologous skin grafts; b) the use of an artificial skin such as Integra (a dermal substitute) and later application of skin grafts; or c) a repeat transplant, which is more likely to be rejected due to prior sensitization [25]. The risk of free tissue transfer failure for technical reasons in experienced units is considered to be less than 5% [19]. The risk of failure of a free tissue flap from acute rejection is as yet not possible to quantify accurately, but it has been postulated to be on the order of 10%, based on the experience of solid organ transplantation [25].
6. Follow-up of Facial Composite Tissue Allotransplantations
6.1. The First Human Partial Face Transplantation
The first human partial face allograft contained only soft tissues and was performed on November 27, 2005 in Amiens, France, by a team directed by Dr Dubernard [17]. The recipient was a 38-year-old woman who had been previously severely bitten by her dog, resulting in amputation of her distal nose, both lips, her entire chin, and the adjacent parts of her right and left cheeks [8, 17]. The immunosuppressive regimen included thymoglobulins, tacrolimus, MMF, and prednisone. Medications were also given for prophylaxis of cytomegalovirus (CMV) infection and Pneumocystis jiroveci pneumonia. Two infectious complications occurred, a type 1 human herpes simplex virus infection of the lips and molluscum contagiosum. Donor bone marrow hematopoietic cells were infused into the recipient by the second week following surgery. Sequential biopsies were taken from the oral mucosa, the facial skin, and, particularly in this case, from a sentinel skin graft (a free radial forearm flap from the donor, anastomosed to the thoracodorsal vessels of the recipient and located in the left submammary fold, as a means of taking skin biopsies and therefore limiting damage to the grafted face) [8]. Sensory recovery was achieved prior to motor, at 6 and 10 months, respectively. Psychological acceptance of the graft progressed as function improved, and psychological support was continuously provided, as well as physical therapy, which was started on the second day after surgery. Rejection episodes occurred on days 18 and 214 after transplantation and were reversed with corticosteroid intravenous boluses and increases in the doses of oral prednisone, tacrolimus and MMF, as well as corticoid mouthwashes and topical clobetasol with or without tacrolimus ointment. Extracorporeal photochemotherapy was performed at 10 months to prevent recurrence of rejection. Nephrotoxicity led to a change in immunosuppressive regimen from tacrolimus to sirolimus at 14 months, although the improvement in renal function was rather slow. At 18 months, the most recent scientific published results, the immunosuppressive regimen included sirolimus (targeted trough level, 8 to 12 ng per millilitre), MMF (2 g per day), and prednisone (10 mg per day). One and a half years after the face allotransplantation, the patient was able to smile normally and she was satisfied with the aesthetic and functional results [8].
6.2. The First Human Full Face Transplantation
The first human full face allograft transplantation (type V of the Lengele's classification) was performed on March 27, 2010 in Barcelona, Spain, by a multidisciplinary team of the Vall d'Hebron University hospital directed by Dr. Barret, and contained, not only soft tissues, but also bony structures [72]. The recipient was a 30-year-old man that suffered a gunshot trauma with consequent severe facial deformity involving subtotal destruction of bilateral orbits and zigomatic bones, maxilla, and mandible, with a total lack of nose and partial lips. He also suffered devastating facial scars and severe functional impairment, with inability to speak, breath, or eat normally, with the permanent requirements of a tracheostomy and a percutaneous gastrostomy (PEG) tube (Table 2).
Table 2. Blood Group and Immunological Status of the first full face transplant performed in Barcelona, Spain.
| Donor | Recipient | |
|---|---|---|
| Blood Group | O− | AB+ |
| CMV | + | − |
| Epstein Barr | + | + |
| Varicella-Zoster | + | + |
| Herpes Virus 1 | + | − |
| Rubella | + | + |
| Toxoplasma Gondii | − | − |
| Treponema pallidum | − | − |
| Parotiditis | − | + |
| HIV, Hepatitis B,C | − | − |
| HLA | A11, A31, B35, B51 DRB 04, DRB 16 | A2, B15, B57, DRB 07, DRB 12 |
From [72]: Barret JP, Gavalda J, Bueno J, et al. Full face transplant: the first case report. Ann Surg 2011; 254(2): 252-6.
Legend: Recipient infections after all the FCTAs till now performed have involved cytomegalovirus (CMV), herpes simplex virus-1 (HSV-1) and Epstein-Barr virus (EBV). Antiviral treatment to prevent CMV and HSV-1 for at least 3 months post-transplantation is strongly recommended [26].
6.2.1. Surgical Technique
Harvest took 4.5 hours and the complete surgery (harvesting and implanting) lasted 24 hours. The entire face was harvested en-block, with skin, soft tissues and bony structures together, from the hairline to the neck midline and from the right to the left preauricular areas, pedicled on both external carotid arteries and jugular veins, as well as the right anterior jugular and the left retromandibular veins. The nerves included were sensory branches of the trigeminal (supraorbital, infraorbital, and mandibular nerves) and the buccal, zigomatic, orbicularis oculii, and frontal branches of the facial nerve. Osteotomies were last performed after the heart and lungs were harvested [73]. Total cold ischemia time was 2.5 hours. See reference [72] for more anatomical intraoperative details.
Concomitant to the harvest, other colleagues were removing all the scarred soft tissues and bony fragments from the recipient's face. Implantation of the facial allograft started by restoring the blood supply, performing bilateral end-to-end anastomosis of the right external carotid arteries, and the external jugular veins. An extra couple of end-to-side bilateral venous anastomoses were performed to the internal jugular veins. After eliciting complete perfusion of the allograft, bony rigid fixation was performed. Then, the intraoral mucosa and both palates were sutured. The next step consisted of end-to-end anastomosis between all sensory and motor nerves. Finally, the muscles, remaining soft tissues and skin were sutured. Excess skin in the neck and left preauricular regions was preserved to allow for postoperative tissue biopsies to monitor rejection.
6.2.2. Immunosuppression and Infection Control
The immunosuppression induction protocol included intravenous thymoglobulin (2 mg/kg 2 hours prior to surgery) and prednisone (1 g intra-operatively, just before the allograft reperfusion). Maintenance regimen consisted of prednisone (started intravenously, 1 mg/kg/24h and later orally, tapered to 10 mg/24h), oral tacrolimus (to target levels of 10-15 ng/ml) and mycophenolate mofetil (2 g/daily, also po = per os).
Infection prophylaxis strategies consisted of valganciclovir (for CMV) and cotrimoxazole (for pneumocystis), as well as antibiotics for gram-positive and gram-negative bacteria and intravenous antifungal drugs [72].
6.2.3. Challenges
There were no intra-operative complications. Post-operative complications included: venous anastomoses thrombosis, acute oro-cutaneous fistula, right parotid sialocele and two acute rejection episodes, which were resolved by surgical revision of the anastomosis, profuse irrigation, and immunotherapy adjustment (bolus administration of prednisone). The patient was discharged from the hospital at 4 months post-transplant with near-total sensation and partial-motor recovery, no psychological complications, and excellent acceptance of his new facial appearance. Psychological, psychiatric, and physical therapy has continuously been provided. He developed mild neutropenia and nephrotoxicity, which led to a change in the immunosuppressive regimen, withholding MMF, introducing sirolimus and weaning tacrolimus as well as adjusting prednisone to the minimal therapeutical dose.
At 12 months follow-up, the patient was able to eat, speak, and breathe normally. He is satisfied with the aesthetic and functional results and he has returned to usual, previous life activities as well as embarking on new projects, without social or psychological concerns.
6.3. Other Clinical Face Allotransplantations
The second case of human facial allotransplantation also contained bone, besides skin and other soft tissues, and was performed on April 13, 2006 in Xi'an, China, by Dr. Guo [18]. The recipient patient was a 30-year-old man from a remote Chinese village with a disfiguring facial injury resulting from a bear attack. The surgery included only one-side anastomosis of the right mandibular artery and anterior facial vein, with facial nerve anastomosis and complete repair of the entire nose, upper lip, parotid gland, front wall of the maxillary sinus, part of the infraorbital wall, and zygomatic bone. A quadruple immunosuppressive protocol was used, containing tacrolimus, MMF, corticosteroids, and humanized IL-2 receptor monoclonal antibody. Infections encountered included E Faecalis, S Epidermidis and Enterobacter Cloacae from sputum and oropharyngeal swabs. There were three acute rejection episodes (reversed with medication adjustments at 3, 5 and 17 months) and hyperglycaemia, which was controlled with insulin and, later, metformin. One may be tempted to explain the high incidence of acute rejection episodes (Table 3) by suggesting increased PRA (Panel Reactive Antibody) levels in the recipient, although this seems improbable because a protein A immuno-adsorption therapy was specifically used to decrease the PRA prior to the surgery. The more likely explanation would be non-compliance with the immunosuppressant treatment by 16 months post surgery, which eventually lead to graft loss and the recipient's death. X-ray irradiation, without infusions of donor bone-marrow cells, was used. Despite the unfortunate demise of the patient, at a 2-year follow-up study, the patient was able to eat, drink and talk normally, but the facial nerve was not fully functional; prior to that date, several minor surgeries were performed to improve the graft's cosmetic appearance [18, 74].
Table 3. BANFF criteria for acute rejection in CTA.
| Grade of acute rejection | Severity | Pathological findings |
|---|---|---|
| 0 | None | No or rare inflammatory infiltrates |
| I | Mild |
|
| II | Moderate |
|
| III | Severe | Dense inflammation and epidermal involvement with epithelial apoptosis, dyskeratosis and/or keratinolysis. |
| IV | Necrotizing | Frank necrosis of epidermis or other skin structures. |
From [105]: Am J Transplant 2008; 8(7): 1396-1400.
Legend: The Banff CTA-07 criteria are standardized and internationally recognized criteria to report severity and types of immune-mediated rejection in CTA. They were settled in a symposium held at the “Ninth Banff Conference on Allograft Pathology” in La Coruña, Spain, on June 2007.
Taking the encouraging short-term positive results of the first FCTA into account, other centers in multiple countries performed other facial transplantations, including a near-total human FCTA in the USA on December 9, 2008 (by Dr. Siemionow at the Cleveland clinic in Ohio [61]) and other cases in France (by Dr. Lantieri [62]) and Spain (Dr. Cavadas in Valencia and Dr. Gómez Cía in Sevilla).
Describing all of the 16 cases performed until now scopes outside the main aim of this review; instead, the reader may be directed to the references of the available published case reports [4, 8, 14, 16, 17, 61, 62, 75].
6.3.1. Face Allotransplantations Performed in Burn Patients: Indications, Types of Defects, and Results
To our knowledge, of the 16 face allotransplantations performed, only three have been performed in burn patients, and one of them died [19, 26, 66, 75].
The most recent FCTA case at the time of this publication was the first full face transplant in the USA, and it was performed on March 21, 2011 in Boston, the second FCTA performed at the Boston's Brigham and Women's Hospital (the former one was in April 2009, both on burn patients) [75]. More than 30 medical personnel participated in the 15-hour operation directed by Dr. Pomahac; the recipient was a 25-year-old man, a Texan construction worker that suffered high-voltage electrical burns many years ago, with severe facial deformities and dysfunctions.
In April 2009, Dr. Pomahac, et al., performed the second facial transplant in the United States and the first one on a burn patient in America. The recipient was a 59-year-old male with a complex bony and soft tissue mid-facial defect caused by a high-voltage electrical burn injury [19, 75]. He was previously reconstructed by means of a free anterolateral thigh flap (Figure 4). However, the patient was still unable to breathe, chew solid foods, or communicate. The patient was placed on the transplant wait-list in January 2009. The transplant was realized 3 months later. The allograft included the maxilla and zygomatic bones and the soft tissues of the midface, with facial, buccal, and infraorbital nerves [75, 76]. The donor procedure lasted 6 hours. Revascularization took 75 minutes and was done between external carotids on the left side and facial arteries on the right side. Total operative time was 17 hours. A radial sentinel forearm flap was used both to monitor allograft rejection and to release a first web-space contracture of the right hand [75].
Figure 4.
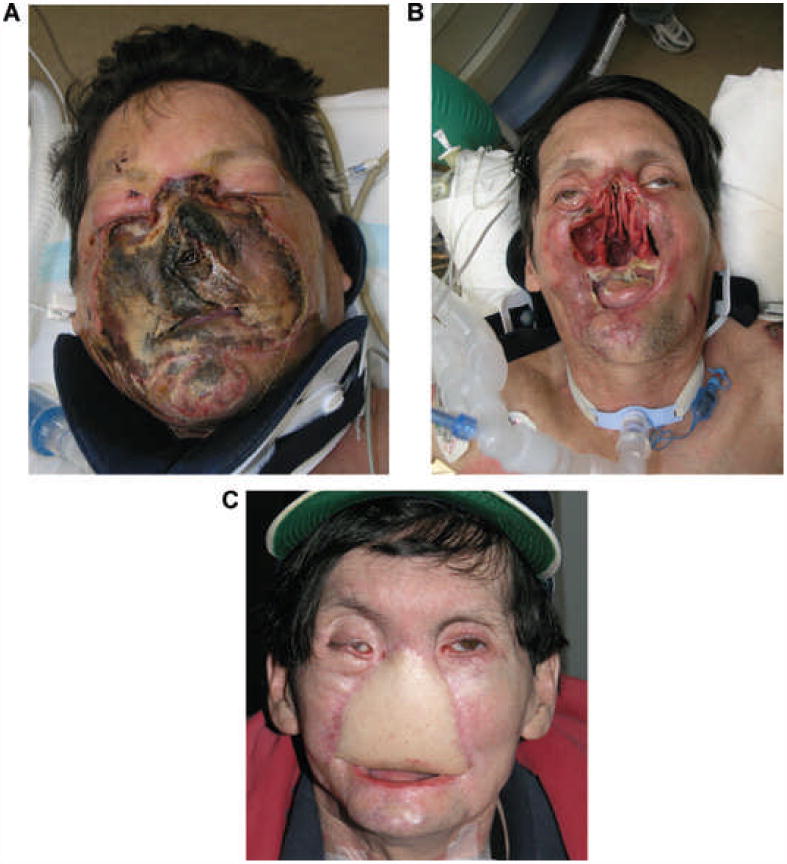
FCTA after severe disfigurement from burn injury:
The recipient, before the transplant.
From [75[: Am J Transplant 2011, 11: 386-93.
Legend: Full-thickness electrical burn affecting mainly the midface, including peri-oral and peri-orbital regions, before (A) and after (B) debridement. Outcome after reconstruction previous to FCTA, including ALT (anterolateral thigh) free flap.
(Photographs used with permission of Dr. Pomahac B).
Early postoperative recovery proceeded without complications and the patient was discharged from the hospital on post-operative day 15. The patient suffered rosacea transferred from the donor, which complicated the differential diagnosis with acute rejection episodes during the first 3 months following the transplant. The facial skin biopsy at 6 months showed no signs of rejection. At that time, a secondary revision surgical procedure was performed, to resect redundant cheek skin. The immunosuppression protocol included tacrolimus, mycophenolate mofetil, and prednisone, with diabetes as a side-effect. No renal or infectious complications were described. The patient was able to breathe spontaneously , eat, and talk. Sensory and motor functions were gained gradually, with the former being restored first [75].
At the 1 year follow-up, the surgeons concluded that restoration of facial form and function was achieved with minimal and well-tolerated immunosuppression [19]. Patient's satisfaction was also high, and he became reintegrated into society [75]. He was able to smile symmetrically. The control CT performed at 15 months showed skeletal facial bony union between donor and recipient (Figure 5).
Figure 5.
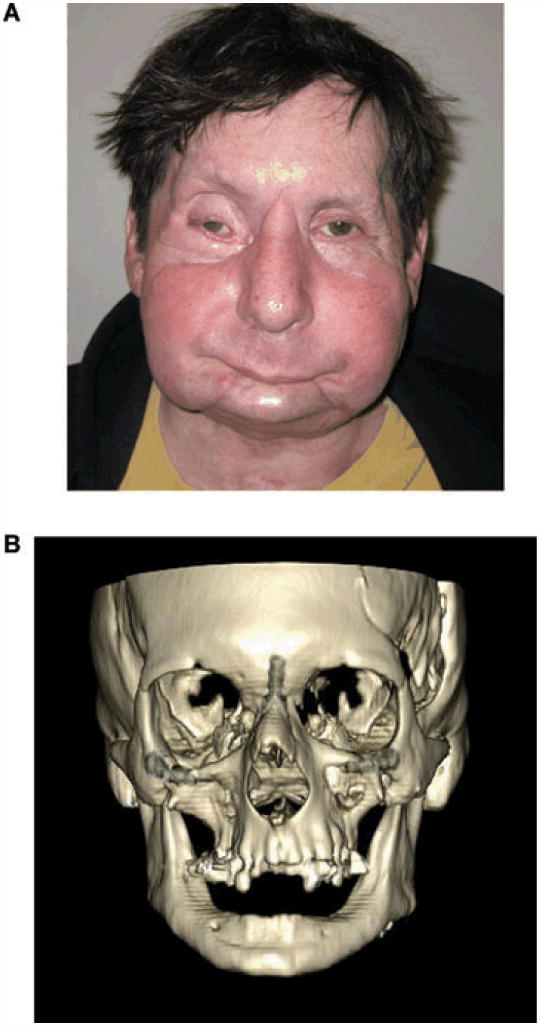
FCTA after severe disfigurement from burn injury:
Results at 1 year after face allotransplantation, clinically (A) and radiologically (B).
From [75[: Am J Transplant 2011, 11: 386-93.
Legend : Clinical appearance and 3D CT obtained 12 months after FCTA, showing skeletal integration of transplanted donor maxillary bone.
(Photographs used with permission of Dr. Pomahac B).
In April 2009, Lantieri, et al., transplanted the upper two-thirds of the face and bilateral hands to another burn recipient [66]. Of note, upper or total face transplantations indicated for burn patients are considered more difficult than lower ones. This patient was a 37 year-old man with full-thickness burns on his scalp, upper, and middle face, with ocular affection. Although there were no acute rejection episodes described, the patient suffered a multi-resistant Pseudomonas Aeruginosa infection on post-operative day 15, affecting tissue of the face and left hand allograft, with partial septic necrosis of the facial graft and septic rupture on a radial bypass. Immunosuppression was decreased, tapering down tacrolimus and withholding mycophenolate mofetil, and extensive antibiotic therapy was administered. The left hand was amputated and the necrotic upper part of the facial allograft was removed. Unfortunately, the patient died at 65 days after the transplant, due to anoxic cardiac arrest from tracheostomy obstruction in this context of infectious complications [66].
7. The Important Role of Face Allotransplantation in Burns
7.1. Burn Injury Dimensions
Although burns represent a small number of all traumatic injuries, they are responsible for significant morbidity and mortality worldwide, leading to chronic devastating sequelae, not only of a physical nature, but also psychological and social [77]. A burn injury represents one of the most severe forms of trauma, occurs in more than two to three million people in North America each year, and is the fourth leading cause of death from unintentional injury [78]. A severe burn constitutes an extensive, devastating traumatic event, affecting nearly every organ system and leading to significant morbidity and mortality, especially in the developing world [79]. Sepsis and multi-organ failure are the main mortality causes [80]. Although mortality has declined over the past few years due to improved medical care and promotion of burn prevention, management of major burns still remains a challenge, even in modern, developed countries. The increasing numbers of burn survivors are at risk for developing long-term psychological and physical sequelae, with potentially devastating consequences to them, their families, and society in general. Furthermore, burns have grave human and material costs [80].
7.2. The Severely Facially-Burned Patient
Deep burns, especially after flame or electrical injuries, may profoundly harden the face, not only disrupting the physical appearance and functionality, but also altering the psychosocial sphere of the patient [15]. Furthermore, surgical treatment may only elicit similar disappointing results, with limited reconstructive options, especially when dealing with pan-facial burns. Deep facial burns that alter more than one aesthetic facial unit still remain a challenge for the reconstructive surgeon, who has to do his/her best to achieve the most favourable result, particularly in this setting, due to the key role of the human face [75].
In fact, the face is one of the most important parts of the human anatomy; it plays a major role in basic physical functions and emotions such as swallowing, breathing, seeing, hearing, smelling, smiling and kissing, to name only a few [9]. It represents the central organ of communication, usually the first focus of sexual attractiveness, and the means of immediate recognition by others [81]. Debridement and coverage of deep dermal or full-thickness facial burns with traditional plastic surgery techniques often leads to poor functional and aesthetic outcomes, with potentially severe, disfiguring burn scar contractures [12,19]. Indeed, burn scar contractures in general represent the main complication in burn survivors. When located on the face, they lead to severe functional and cosmetic sequelae (e.g., microstomia, ectropion, nose avulsion, etcetera), eventually leading not only to physical, but also to psychological and social disturbances, drastically reducing the quality of life of the burn patient, who has to cope with these chronic injuries long-life [82, 83]. The contractures may be treated surgically, but usually with less than ideal functional and aesthetic outcomes, many operative sessions over the course of many years, ongoing complication risks, and high costs. Accordingly, a unique cost analysis of conventional facial reconstruction procedures compared to face allotransplantation in one patient showed that they had similar costs, arguing in favour of the latter [84]. The potential alleviation of psychological and physiological suffering, exceptional functional recovery, and fulfilment of long-lasting hope for social reintegration achieved with the FCTA “may be priceless”, according to Siemionow [84].
Regarding facial burns in children, vital structures such as the orbits, teeth and nose may become deformed due to contractures, leading to severe complications such as microstomia and occlusion amblyopia [85, 86]. Furthermore, because young children are actively developing the concept of self, severe facial burns can alter a child's sense of identity and place him/her at high risk for future emotional and psychological problems [19, 86]. To our knowledge, no FCTA have been performed in children up to date, although the allotransplantation of solid organs has had great success in the pediatric population, with over 80% survival rate at young adulthood [87].
7.3. Face, Body Image, Self-Esteem and Burns
To really understand the psychosocial suffering of the severely facially burned patient, which may be the trigger for seeking a FCTA, we would first like to describe some related terms in more detail:
The link between facial disfigurement, impaired body image and self-esteem, and sociopathy has been demonstrated in several research studies, although with some discrepancy [81]. Indeed, facial disfigurement has been described as “the last bastion of discrimination” and a potential cause of grievous distress [88]. Coping depends on a complex signaling network between the individual and the environment, and is greatly dependent on the individual. As a general rule, those who confront their anxieties directly seem to have better psychosocial outcomes than those who avoid them [89].
Body image has been described as “the picture of our body which we form in our mind”; that is to say, “the way in which our body appears to ourselves”, as well as “the loose mental representation of the body”, among other definitions [90, 91]. Body image is dynamic, depending on age, mood or clothing, etc. When one is younger, acceptance from others leads to acceptance of oneself; however, after adolescence, media, society and culture play a more important role [92]. Price proposed “the body image model”, comprising body reality, body presentation, and body ideal [93]. Disfigurement following facial burns alters body reality (how they objectively are); individuals may adapt to this by changing their body ideal (how they would like to be) or body presentation (how they dress or behave to society) [89]. It is important to note that most studies about body image focus on eating disorders and not facial disfigurement, though [92].
Regarding the latter, the key point is not altered identity per se, but repeated alteration in appearance. People who have undergone disfiguring injuries, such as burns, comment on the length of time for resolution (maybe years), to achieve concordance between identity and appearance, and to recognize the new face as their own [94]. In the case of transplant recipients (whether it be kidney or face), this tends to occur as the new organ or tissue begins to function (measured by improved diuresis or functional cortical MRI data, respectively) [26, 81].
Self-esteem refers to “our assessment of our social worth” [93]. Self-esteem differs from body image, although body image influences our self-esteem, as well as our perception of other's opinion of ourselves. The lower a person's self-esteem becomes, he or she will be more likely to perceive negative social interactions. Furthermore, burn patients often come from lower socioeconomic backgrounds and have lower pre-morbid levels of self-esteem and social support [95].
“Altered body image” is defined as “any significant alteration to body image occurring outside the realms of expected human development” [93]. For some individuals, this may constitute a crisis; whereas, others seem to adapt well. Coping strategies, the cause of altered body image, the impact on lifestyle, and the degree of social support (in the case of burn patients, mostly the family) influence this adaptation [81].
“Facial disfigurement” describes the visual effect of scars, skin grafts, asymmetry, or altered pigmentation in different grades. It may cause disruption to body image and, especially if there is loss of self-recognition or guilt because others have died or been injured, constitute a major life crisis [96]. The reaction to facial disfigurement is comparable to the stages of bereavement (denial, anger, bargaining, depression, and acceptance) described by Kubler-Ross [97].
The major problem reported by those with facial disfigurement relates to social interaction: they may feel anxious, threatened by others, and preoccupied with their appearance [80]. Using Newell's fear-avoidance model for disfigured people may avoid activities that induce anxiety or conceal disfigurement [99]. Although this potentially provides short-term psychological relief, a pattern of avoidance may ensue, thus reinforcing anxiety-avoiding behavior. This may prevent habituation to curiosity and hinder the development of adequate coping strategies and the discrediting of falsely held beliefs [100]. Camouflage creams or makeup, hats and prosthesis may facilitate social interaction in the short-term, but they usually do not address the underlying problem [101]. Moreover, burn patients have to cope with the social impairment of wearing pressure garments and particularly in the face, silicone masks for a long period of time. Furthermore, an immobile or distorted face may impede verbal and nonverbal communication, provoking unease in others. The development of social skills may be difficult. Negative coping strategies (e.g. aggression, social withdrawal, or alcohol misuse) can develop, reinforced by reciprocation and discrimination in personal and working relationships [10, 81].
The “buffering hypothesis” argues that social support (essentially, family and friends) is the most powerful factor in ameliorating stressful events, such as burns [102]. Social support results in high self-esteem, which may buffer emotional turbulence [92].
In summary, severe facial burns represent a significant facial disfigurement event that the individual may cope with in either a positive (the ideal way, with social support and interaction, and non-avoidant strategies, [81]) or negative (with anxiety and social disturbances) manner. Both the individual and the environment influence the outcome and counterbalance one another. On one hand, people who are psychologically vulnerable and distressed are prone to seek appearance-enhancing surgical treatments with unrealistic expectations, and changing their cognitive behavior may help; on the other hand, society should modify its stereotypes and reactions to those with facial disfigurement [25, 92]. Achieving this modification seems rather difficult, though.
Furthermore, the quality of life when living with severe facial disfigurement may be so poor as to be compared to living with end-stage renal disease or human immunodeficiency virus (HIV) [103].
Severely facially burned patients suffer from devastating physical, social, and physical sequelae, including functional and aesthetic ones. They may not be able to perform such basic human functions as eating orally, breathing nasally, or expressing any facial emotion. Sometimes, they have even been described as having “no faces” at all [60]. The alleviation of psychological and physiological suffering, reintegration into society, the potential of full functional recovery, and the renewed hope given to the patient [84] are all reasons why face allotransplantation, despite high risks and controversies, plays a major role in burns and should be carefully considered.
8. When is FCTA Indicated in Burns?
Burn patients are ideal candidates for facial composite tissue allotransplantation, as they often present with severe facial deformities involving the peri-oral and peri-orbital regions [16, 62]. Although there is, as of yet, a paucity of scientific evidence, we suggest that FCTA is indicated in severe facially disfiguring burns, with peri-oral and peri-orbital disruption, in a psychologically stable, healthy, compliant, socially-supported and well-informed patient. As Pomahac assured, in the following decades, face transplantation may become the treatment of choice in general for patients with severe facial disfigurements that represent considerable functional and social impairment [19]. Each patient will present with a unique defect that carries a particular set of challenges and difficulties. Full face burn defects involving cutaneous and osseous tissue with severe disfigurement may be a clear indication for FCTA. The same panorama, but with only skin affection, may also be repaired by means of FCTA or traditional surgical techniques, depending on the grade of functional impairment, essentially.
When dealing with burned children, we suggest not to recommend this procedure as freely at the present moment, awaiting amelioration of immunosuppressive regimens with the potential arrival of stem cell therapy and tissue engineering techniques [19]. However, due to the fact that these techniques are still very novel, research is still ongoing, and the wait may be too long for a severely disfigured child. Therefore, every candidate should be carefully studied, with all ethical and medical concerns being strictly analyzed with the proper authorities, to make an individually-based decision.
9. Disadvantages of FCTA in Burns
Severely disfigured facial burn patients that plan to undergo a FCTA must agree to undertake daily and life-long mild- to severe risks, including minor side effects, opportunistic infections, malignancies, and death derived from current immunosuppression regimens [25]. Other risks include rejection, acute graft-versus-host disease, and any other potential risks of a technique still in the pioneering stage, with only short follow-up periods on record as of yet [66]. Furthermore, during the transplant procedure and peri-transplant days, there is also an additional risk of technical complications, such as venous thrombosis and partial or total flap necrosis, with the greatest threat being an urgent need for a second face transplant and cross-match [19]. Moreover, the complex surgical technique of the procedure, the pre-operative study and selection of the appropriate patient, the need for accurate compliance with multiple medications (immunosuppression therapies and infection prophylaxis), potential complications, and the need for periodic clinical visits, make this procedure extremely expensive. Another particular concern specific to the burn patient population is the high prevalence of psychiatric comorbidity and drug abuse, as well as high risk of septic complications [19, 75].
However, if we take into account the cost of numerous previous reconstructive surgeries that a burn patient may have undergone over a long period of time, with several admissions, and the professional, personal, and social breakthroughs achieved so far by the few patients that have undergone facial transplants, we would probably be inclined to perform more FCTAs.
10. Advantages of FCTA in Burns
On the other hand, the high quality achieved with this kind of reconstruction (FCTA), in only one surgical step and with long-lasting results, encourages us to conclude that FCTA is arising as a promising new surgical strategy to help severely disfigured facially burned patients, becoming the gold standard of major facial reconstruction [19, 26, 66, 75]. In fact, facial transplantation represents a rather ideal form of restoration by “replacement” of “like with like” tissue instead of “reconstruction” [19].
Due to the versatility of the graft, which may include only soft tissues or also bone structures, it is applicable to the treatment of many different defects. Moreover, the technique is becoming more accessible thanks to the increasing opportunity for surgeons to train their transplant-related dissection skills with cadavers and the general spread of microsurgical skills [14]. When compared to healing by secondary intention, grafts, or local and free flaps, FCTA represents a substantial improvement in terms of appearance, cosmesis, and sensory and motor function recovery. These physical and functional improvements lead to the resolution of psychosocial distress and a state of satisfaction, happiness, and gratefulness that only such patients of such unique circumstances may really understand.
11. Society and FCTA: The Debate is Served
FCTA represents an innovative treatment and, as such, stimulates social debate. Facial transplantation is possible, but is it an acceptable treatment for severe facial pathology? Most current answers will probably be affirmative, although it still remains controversial, with social concerns that may lead to misinformation and emotional-rather than intellectual-based reports [81]. That's why there is an urgent need for objective, scientific analyses of the pros and cons of this new technique, defining the population that might benefit and at what point in their treatment might this surgery be appropriate [104]. Main social concerns against facial tissue allotransplantation derive from moral and ethical stances. It has been described in the literature that some individuals still think that facial transplant represents an identity exchange, although this is not true at all, and this belief may represent the most emotive barrier to face transplantation [19, 81, 92]. Due to the fact that transplanted faces –as well as transplanted hands- are visible in a way that solid organs are not, the family of potential donors are likely to have the biggest concern about the extent to which his or her loved one can be recognized in the new face of a stranger [25, 81]. Regarding ethics, there is also major social concerns related to waiting lists of a non-vital organ or tissue transplant, or when dealing with the potential problem of instability of the donor during FCTA harvest and possible disruption of the other donor vital organs [19]. Conceptually, whether or not a procedure can be considered ethically justified depends on the relationship between risk and benefit, and the ability of the surgical team to convey that to the recipient [81].
It has been postulated that the largest impediment to facial transplantation might not be ethical or medical issues, though, but financial restrictions [84]. If we don't take economics into account, immunosuppression remains the major limitation of CTA [19]. The questions regarding face transplantation will turn to, “who is responsible for paying and how?”. These questions are quite relevant considering recent global concern about health care costs [84].
The cost of the first U.S. face transplant is similar to multiple conventional reconstructions. Since the cost of health care and quality of life differs worldwide, it is not possible to make objective cost comparisons, and any conclusions derived from this study are only speculative. If future research finds that the cost of face transplantation is comparable to conventional methods, the prolonged reconstructive course (and its associated morbidity) may make face transplantation the first-line choice in a select group of severely disfigured patients [84].
However, many questions still remain unanswered: Are multiple reconstructive surgeries and life-long disfigurement more or less affordable than early transplantation with life-long immunosuppression? To what extent should society finance “elective” facial CTA for defects caused by self-destructive etiologies? [19].
12. Concluding Remarks
Face composite tissue allotransplantation is currently a feasible therapeutic strategy able to reconstruct severe facial disfigurements, and it may become the new gold standard for full-face reconstruction. Although there are associated ethical, social, surgical, technical, and immunological concerns, the positive outcomes demonstrated by the FCTAs performed until now encourage many patients who suffer from chronic devastating facial sequelae (as a result of burns, trauma, tumors, or congenital malformations) to keep their hopes high.
Improvements in the immunosuppressive regimens and innovative and ongoing research on immune tolerance, along with the potential use of stem cells to decrease the risks associated with life-long immunosuppressive drugs and improve functional and cosmetic outcomes, may facilitate more FCTAs in the near future. However, it is still a complex and ever-lasting process, which begins even prior to the surgery –with the appropriate recipient candidate selection, who should take into account the high and long-lasting risks, as well as the mandatory requirement to keep a chronic immunosuppressive protocol and clinical control compliance, with a special personalized consent form- and continues during the patient's entire life. The first cases of solid-organ transplantation were also received with skepticism in the late 1950s, as well as the clinical application of perforator free flaps 20 years ago; however, as time went by and more and more procedures were performed with success, these new surgical milestones were implemented more and more worldwide, with even better refinement methods.
Overall, FCTA is in its beginning phase, and it is still regarded as an experimental procedure. Due to the complexity involved, its indications appear to be currently limited to only severely disfiguring facial defects, in the case of burns, particularly when involving the peri-orbital and/or peri-oral regions, with severe functional and cosmetic impairment. Immune tolerance and stem cell research may contribute to amelioration of the immunosuppressive risks and enhance the already demonstrated feasibility of this new reconstructive technique. Whereas tissue engineering may be the next promising step for the most ideal form of facial reconstruction, FCTA emerges currently as the new gold standard in this setting, with carefully personalized evaluation of applicability in each potential subject.
Acknowledgments
The author wishes to acknowledge the substantial work of colleagues at Vall d'Hebron University Hospital who made possible the first human full face transplant, the donors families for their selflessness, and the FCTA recipients for their continuous fight to improve and enhance their lives, struggling with us to engage in new and ongoing research and forming projects aimed at offering the best quality of life to our patients.
Footnotes
Conflicts of Interest and Source of Funding: None of the authors has any financial interest whatsoever in any of the drugs, treatments, techniques or instruments mentioned in this article, nor any source of funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WPA, Yaremchuk MJ, Pan YC, et al. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401–11. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Murray JE. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast Reconstr Surg. 1971;47:425–31. doi: 10.1097/00006534-197105000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Madani H, Hettiaratchy S, Clarke A, Butler PEM. Immunosuppression in an emerging field of plastic reconstructive surgery: composite tissue allotransplantation. J Plast Reconstr Aesthet Surg. 2008;61(3):245–9. doi: 10.1016/j.bjps.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Petruzzo P, Kanitakis J, Badet L, et al. Long-term follow-up in composite tissue allotransplantation: In-depth study of five (hand and face) recipients. Am J Transplant. 2001;11:808–16. doi: 10.1111/j.1600-6143.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 5.Hettiaratchy S, Randolph MA, Petite F, et al. Composite tissue allotransplantation: a new era in plastic surgery? Br J Plast Surg. 2004;57(5):381–91. doi: 10.1016/j.bjps.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert R. Transplant is successful with a cadaver forearm. Med Trib Med News. 1964;5:20–2. [Google Scholar]
- 7.Brandacher G, Gorantla VS, Lee WPA. Hand Allotransplantation. Semin Plast Surg. 2010;24(1):11–17. doi: 10.1055/s-0030-1253243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubernard JM, Lengele B, Morelon E, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. 2007;357:2451–60. doi: 10.1056/NEJMoa072828. [DOI] [PubMed] [Google Scholar]
- 9.Siemionow M, Bassiri Gharb B, Rampazzo A. The face as a sensory organ. Plast Reconstr Surg. 2011;127:652–62. doi: 10.1097/PRS.0b013e3181fed6fd. [DOI] [PubMed] [Google Scholar]
- 10.Soni CV, Barker JH, Pushpakumar SB, et al. Psychosocial considerations in facial transplantation. Burns. 2010;36:959–64. doi: 10.1016/j.burns.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.McIndoe AH. Total reconstruction of the burned face. The bradshaw lecture 1958. Br J Plast Surg. 1983;36(4):410–20. doi: 10.1016/0007-1226(83)90119-4. [DOI] [PubMed] [Google Scholar]
- 12.Latifoglu O, Ayhan S, Atabay K. Total face reconstruction: Skin graft versus free flap. Plast Reconstr Surg. 1999;103:1076. [PubMed] [Google Scholar]
- 13.Angrigiani C, Grilli D. Total face reconstruction with one free flap. Plast Reconstr Surg. 1997;99(6):1566–75. [PubMed] [Google Scholar]
- 14.Siemionow M, Unal S, Agaoglu G, Sari A. A cadaver study in preparation for facial allograft transplantation in humans: part I. What are alternative sources for total facial defect coverage? Plast Reconstr Surg. 2006;117:864–72. doi: 10.1097/01.prs.0000204875.10333.56. [DOI] [PubMed] [Google Scholar]
- 15.Pushpakumar SB, Barker JH, Soni CV, et al. Clinical considerations in face transplantation. Burns. 2010;36:951–58. doi: 10.1016/j.burns.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Hui-Chou HG, Nam AJ, Rodriguez ED. Clinical facial composite tissue allotransplantation: A review of the first four global experiences and future implications. Plast Reconstr Surg. 2010;125:538–46. doi: 10.1097/PRS.0b013e3181c722a8. [DOI] [PubMed] [Google Scholar]
- 17.Devauchelle N, Badet L, Lengele B, et al. First human face allograft: early report. Lancet. 2006;368:203–9. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 18.Guo S, Han Y, Zhang X, et al. Human facial allotransplantation: a 2 year follow-up study. Lancet. 2008;372:631–8. doi: 10.1016/S0140-6736(08)61276-3. [DOI] [PubMed] [Google Scholar]
- 19.Pomahac B, Nowinski D, Diaz-Siso JR, et al. Face transplantation. Curr Probl Surg. 2011;48:293–357. doi: 10.1067/j.cpsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Gordon CR, Aver RK, Abouhassan W, et al. Cytomegalovirus and other infectious issues related to face transplantation: specific considerations, lessons learned and future recommendations. Plast Reconstr Surg. 2011;127:1515–23. doi: 10.1097/PRS.0b013e318208d03c. [DOI] [PubMed] [Google Scholar]
- 21.Morris PJ, Bradley JA, Doyal L, et al. Facial transplantation: A working party report from the Royal College of Surgeons of England. Transplantation. 2004;77(3):330–8. doi: 10.1097/01.TP.0000113810.54865.BE. [DOI] [PubMed] [Google Scholar]
- 22.Hettiaratchy S, Butler PE. Face transplantation: Fantasy or the future? Lancet. 2002;360:5. doi: 10.1016/S0140-6736(02)09361-3. [DOI] [PubMed] [Google Scholar]
- 23.Petit F, Paraskevas A, Minns AB, et al. Face transplantation: Where do we stand? Plast Reconstr Surg. 2004;113(5):1429–33. doi: 10.1097/01.prs.0000112747.85388.39. [DOI] [PubMed] [Google Scholar]
- 24.Siemionow M, Ozwen S, Demir Y. Prospects for facial allograft transplantation in humans. Plast Reconstr Surg. 2004;113(5):1421–8. doi: 10.1097/01.prs.0000112792.44312.a5. [DOI] [PubMed] [Google Scholar]
- 25.Morris P, Bradley A, Doyal L, et al. Face transplantation: A review of the technical, immunological, psychological and clinical issues with recommendations for good practice. Transplantation. 2007;83:109–128. doi: 10.1097/01.tp.0000254201.89012.ae. [DOI] [PubMed] [Google Scholar]
- 26.Schneeberger S, Landin L, Jableki J, et al. Achievements and challenges in composite tissue allotransplantation. Transplant Int. 2011;24(8):760–9. doi: 10.1111/j.1432-2277.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarwer DB, Bartlett SP, Whitaker LA, et al. Adult psychological functioning of individuals born with craniofacial anomalies. Plast Reconstr Surg. 1999;103(2):412–8. doi: 10.1097/00006534-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Klapheke MM, Marcell C, Taliaferro G, et al. Psychiatric assessment of candidates for hand transplantation. Microsurgery. 2000;20:453–7. doi: 10.1002/1098-2752(2000)20:8<453::aid-micr18>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2010;90(12):1590–4. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 30.Whitaker IS, Duggan EM, Alloway RR, et al. Composite tissue allotransplantation: a review of relevant immunological issues for plastic surgeons. J Plast Reconstr Aesthet Surg. 2008;61:481–92. doi: 10.1016/j.bjps.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Gorantla VS, Barker JH, Jones JW, Jr, et al. Immunosuppressive agents in transplantation: mechanisms of action and current anti-rejection strategies. Microsurgery. 2000;20:420–9. doi: 10.1002/1098-2752(2000)20:8<420::aid-micr13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Lanzetta M, Petruzzo P, Dubernard JM, et al. Second report (1998-2006) of the international Registry of Hand and Composite Tissue Transplantation. Transpl Immunol. 2007;18:1–6. doi: 10.1016/j.trim.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Cendales L, Hardy MA. Immunological considerations in composite tissue transplantation: overview. Microsurgery. 2002;20:412–9. doi: 10.1002/1098-2752(2000)20:8<412::aid-micr12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807–18. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 35.Vicari-Christensen M, Repper S, Basile S, et al. Tacrolimus: review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners' understanding and offer strategies for education patients and promoting adherence. Prog Transplant. 2009;19:277–84. doi: 10.1177/152692480901900315. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro R, Fung JJ, Jain AB, et al. The side effects of FK506 in humans. Transplant Proc. 1999;264:63–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Sratta RJ for the FK/MMF Multi-Center Study Group. Simultaneous use of tacrolimus and mycophenolate mofetil in combined pancreas-kidney recipients: a multicenter report. Transplant Proc. 1997;29:654–5. doi: 10.1016/s0041-1345(96)00383-1. [DOI] [PubMed] [Google Scholar]
- 38.Clavijo-Alvarez JA, Hamad GG, Taieb A, et al. Pharmacologic approaches to composite tissue allograft. J Hand Surg. 2007;32:104–18. doi: 10.1016/j.jhsa.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer IR, Araki R, Ford ML. Paradoxical aspects of rapamycin immunobiology in transplantation. Minireview Am J Transplant. 2011;11:654–9. doi: 10.1111/j.1600-6143.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKane W, Kanganas C, Preston R, et al. Treatment of calcineurin inhibitor toxicity by dose reduction plus introduction of mycophenolate mofetil. Transplant Proc. 2001;33:1224–5. doi: 10.1016/s0041-1345(00)02396-4. [DOI] [PubMed] [Google Scholar]
- 41.Landin L, Cavadas PC, Rodriguez-Perez JC, et al. Improvement in renal function after late conversion of sirolimus-based immunosuppression in composite tissue allotransplantation. Transplantation. 2010;90:691–2. doi: 10.1097/TP.0b013e3181ebf7ae. [DOI] [PubMed] [Google Scholar]
- 42.Nevins TE, Matas AJ. Medication noncompliance: another iceberg's tip. Transplantation. 2004;77(5):776–8. doi: 10.1097/01.tp.0000110409.71847.6f. [DOI] [PubMed] [Google Scholar]
- 43.Baumeister S, Kleist C, Dohler B, et al. Risk of allogeneic hand transplantation. Microsurgery. 2004;24:98–103. doi: 10.1002/micr.20003. [DOI] [PubMed] [Google Scholar]
- 44.Golshayan D, Pascual M. Tolerance-inducing immunosuppressive strategies in clinical transplantation: an overview. Drugs. 2008;68:2113–30. doi: 10.2165/00003495-200868150-00004. [DOI] [PubMed] [Google Scholar]
- 45.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–24. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 46.Ramsamooj R, Llull R, Black KS, et al. Composite tissue allografts in rats: IV. Graft-versus-host disease in recipients of vascularized bone marrow transplants. Plast Reconstr Surg. 1999;104(5):1365–71. doi: 10.1097/00006534-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Ciancio G, Millar J, Garcia-Morales RO, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant recipients. Transplantation. 2001;71:827–35. doi: 10.1097/00007890-200104150-00002. [DOI] [PubMed] [Google Scholar]
- 48.Hivelin M, Siemionow M, Grimbert P, et al. Extracorporeal photopheresis: from solid organs to face transplantation. Transpl Immunol. 2009;21(3):117–28. doi: 10.1016/j.trim.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Kuo YR, Goto S, Shih HS, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769–77. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 50.Popp FC, Renner P, Eggenhofer E, et al. Mesenchymal stem cells as immunomodulators after liver transplantation. Liver Transpl. 2009;15:1192–98. doi: 10.1002/lt.21862. [DOI] [PubMed] [Google Scholar]
- 51.Prabhune KA, Gorantla VS, Maldonado C, et al. Mixed allogeneic chimerism and tolerance to composite tissue allografts. Microsurgery. 2000;20:441–7. doi: 10.1002/1098-2752(2000)20:8<441::aid-micr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 52.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6(10):577–83. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 53.Siemionow MZ, Kulahci Y, Bozkurt M. Composite tissue allotransplantation. Plast Reconstr Surg. 2009;124:e327–39. doi: 10.1097/PRS.0b013e3181bf8413. [DOI] [PubMed] [Google Scholar]
- 54.Schneeberger S, Lucchina S, Lanzetta M, et al. Cytomegalovirus-related complications in human hand transplantation. Transplantation. 2005;80:441–7. doi: 10.1097/01.tp.0000168454.68139.0a. [DOI] [PubMed] [Google Scholar]
- 55.Lautenschlager I. CMV infection, diagnosis and antiviral strategies after liver transplantation. Transpl Int. 2009;22:1031–40. doi: 10.1111/j.1432-2277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 56.Gordon CR, Siemionow M, Papay F, et al. The world's experience with facial transplantation: What have we learned thus far? Ann Plast Surg. 2009;63:572–8. doi: 10.1097/SAP.0b013e3181ba5245. [DOI] [PubMed] [Google Scholar]
- 57.Luan FL, Stuckey LJ, Park JM, et al. Six month prophylaxis is cost-effective in transplant patients at high risk of cytomegalovirus infection. J Am Soc Nephrol. 2009;20:2449–58. doi: 10.1681/ASN.2008111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danzinger-Isakov L, Kumar D. AST infectious diseases community of practice. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9:S258–62. doi: 10.1111/j.1600-6143.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 59.Husain S, Alexander BD, Munoz P, et al. Opportunistic mycelial fungal infections in organ transplant recipients: Emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–9. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 60.Okie S. Brave New face. N Engl J Med. 2006;354(9):889–94. doi: 10.1056/NEJMp068005. [DOI] [PubMed] [Google Scholar]
- 61.Siemionow MZ, Papay F, Alam D, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009;374(9685):203–9. doi: 10.1016/S0140-6736(09)61155-7. [DOI] [PubMed] [Google Scholar]
- 62.Lantieri L, Meningaud JP, Grimbert P, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1 year follow-up study. Lancet. 2008;372:639–45. doi: 10.1016/S0140-6736(08)61277-5. [DOI] [PubMed] [Google Scholar]
- 63.Brill S, Clarke A, Veale D, et al. Psychological management amd body image issues in facial transplantation. Body image. 2006;3(1):1–15. doi: 10.1016/j.bodyim.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.O'Carroll R, Couston M, Cossar J, et al. Psychological outcome and quality of life following liver transplantation: A prospective, national, single-center study. Liver Transplantation. 2003;9(7):712–20. doi: 10.1053/jlts.2003.50138. [DOI] [PubMed] [Google Scholar]
- 65.Wiggins O, Barker J. Patient selection for facial transplantation III: Ethical considerations. Int J Surg. 2004;2(2):118–9. doi: 10.1016/S1743-9191(06)60062-9. [DOI] [PubMed] [Google Scholar]
- 66.Lantieri L, Hivelin M, Audard V, et al. Feasibility, reproducibility, risks and benefits of face transplantation: A prospective study of outcomes. Am J Transplant. 2011;11:367–78. doi: 10.1111/j.1600-6143.2010.03406.x. [DOI] [PubMed] [Google Scholar]
- 67.Barker JH, Brown CS, Cunningham M, et al. Ethical considerations in human facial tissue allotransplantation. Ann Plast Surg. 2008;60:103–9. doi: 10.1097/SAP.0b013e31804bdf42. [DOI] [PubMed] [Google Scholar]
- 68.Wallace CG, Wei FC. The current status, evolution and future of facial reconstruction. Chang Gung Med J. 2008;31(5):441–7. [PubMed] [Google Scholar]
- 69.Tanner PB, Mobley SR. External auricular and facial prosthetics: a collaborative effort of the reconstructive surgeon and anaplastologist. Facial Plast Surg Clin North Am. 2006;14:137–45. doi: 10.1016/j.fsc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Nerem RM. Tissue engineering: the hope, the hype, and the future. Tissue Eng. 2006;12:1143–50. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- 71.Neumeister MW, Wu T, Chambers C. Vascularized tissue-engineered ears. Plast Reconstr Surg. 2006;117:116–22. doi: 10.1097/01.prs.0000195071.01699.ce. [DOI] [PubMed] [Google Scholar]
- 72.Barret JP, Gavalda J, Bueno J, et al. Full face transplant: the first case report. Ann Surg. 2011;254(2):252–6. doi: 10.1097/SLA.0b013e318226a607. [DOI] [PubMed] [Google Scholar]
- 73.Bueno J, Barret JP, Serracanta J, et al. Logistics and strategy of multiorgan procurement involving total face allograft. Am J Transplant. 2011;11(5):1091–7. doi: 10.1111/j.1600-6143.2011.03489.x. [DOI] [PubMed] [Google Scholar]
- 74.Yi C, Guo S. Facial transplantation: lessons so far. Lancet. 2009;374(9685):177–8. doi: 10.1016/S0140-6736(09)61292-7. [DOI] [PubMed] [Google Scholar]
- 75.Pomahac B, Pribaz J, Eriksson E, et al. Restoration of facial form and function after severe disfigurement from burn injury by a composite facial allograft. Am J Transplant. 2011;11:386–93. doi: 10.1111/j.1600-6143.2010.03368.x. [DOI] [PubMed] [Google Scholar]
- 76.Pomahac B, Lengele B, Ridgway EB, et al. Vascular considerations in composite midfacial allotransplantation. Plast Reconstr Surg. 2010;125:517–22. doi: 10.1097/PRS.0b013e3181c82e6f. [DOI] [PubMed] [Google Scholar]
- 77.Ryan CM, Schoenfeld DA, Thorpe WE, et al. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338:362–6. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 78.Dissanaike S, Rahimi M. Epidemiology of burn injuries: highlighting cultural and sociodemographic aspects. Int Rev Psychiatry. 2009;21(6):505–11. doi: 10.3109/09540260903340865. [DOI] [PubMed] [Google Scholar]
- 79.Atiyeh BS, Costagliola M, Hayek SN. Burn prevention mechanisms and outcomes: Pitfalls, failures and successes. Burns. 2009;35:181–93. doi: 10.1016/j.burns.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Brussselaers N, Monstrey S, Snoeij T, et al. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity and mortality. Crit Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarke A, Butler PE. Facial transplantation: adding to the reconstructive options after severe facial injury and disease. Expert Opin Biol Ther. 2005;5(12):1539–46. doi: 10.1517/14712598.5.12.1539. [DOI] [PubMed] [Google Scholar]
- 82.Furr LA, Wiggins O, Cunningham M, et al. Psychosocial implications of disfigurement and the future of human face transplantation. Plast Reconstr Surg. 2007;120:559–65. doi: 10.1097/01.prs.0000267584.66732.e5. [DOI] [PubMed] [Google Scholar]
- 83.MacGregor FC. Facial disfigurement: problems and management of social interaction and implications for mental health. Aesthetic Plast Surg. 1990;14(4):249–57. doi: 10.1007/BF01578358. [DOI] [PubMed] [Google Scholar]
- 84.Siemionow M, Gatherwright J, Diohan R, et al. Cost analysis of conventional facial reconstruction procedures followed by face transplantation. Am J Transplant. 2011;11(2):379–85. doi: 10.1111/j.1600-6143.2010.03373.x. [DOI] [PubMed] [Google Scholar]
- 85.Washington KM, Zanoun RR, Cadogan KA, et al. Composite tissue allotransplantation for the reconstruction of congenital craniofacial defects. Transplant Proc. 2009;41:523–7. doi: 10.1016/j.transproceed.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 86.Kung TA, Gosain AK. Pediatric facial burns. J Craniofac Surg. 2008;19(4):951–9. doi: 10.1097/SCS.0b013e318175f42f. [DOI] [PubMed] [Google Scholar]
- 87.LaRosa C, Baluarte HJ, Meyers KE. Outcomes in pediatric solid-organ transplantation. Pediatr Transplant. 2011;15(2):128–41. doi: 10.1111/j.1399-3046.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 88.McGrouther DA. Facial disfigurement. BMJ. 1997;314(7086):991. doi: 10.1136/bmj.314.7086.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Partridge J. Changing faces: The challenge of facial disfigurement. Penguin; London, UK: 1990. [Google Scholar]
- 90.Schilder P. The image and appearance of the human body. London, England: Kegan Paul, Trench, Trubner & Co, Ltd; 1935. [Google Scholar]
- 91.Slade PD. What is body image? Behave Res Ther. 1994;32:497–502. doi: 10.1016/0005-7967(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 92.Sainsbury DC. Body image and facial burns. Adv Skin Wound Care. 2009;22(1):39–44. doi: 10.1097/01.ASW.0000343717.36405.40. [DOI] [PubMed] [Google Scholar]
- 93.Price B. Body image: Nursing concepts and care. New York, NY: Prentice Hall; 1990. [Google Scholar]
- 94.Rumsey Y, Harcourt D. Body image and disfigurement: issues and interventions. Body Image. 2004;1(1):83–97. doi: 10.1016/S1740-1445(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 95.Noyes R, Jr, Frye SJ, Slymen DJ, et al. Stressful life events and burn injuries. J Trauma. 1979;19:141–4. doi: 10.1097/00005373-197903000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Bradbury E. Understanding the problems. In: Lansdown R, Rumsey N, Bradbury E, editors. Visibly different: Coping with disfigurement. London, England: Butterworth. Heineman; 1999. [Google Scholar]
- 97.Kubler-Ross E. On death and dying. London, England: Tavistock Publications; 1969. [Google Scholar]
- 98.Levine E, Degutis L, Pruzinsky T, et al. Quality of life and facial trauma: psychological and body image effects. Ann Plast Surg. 2005;54:502–10. doi: 10.1097/01.sap.0000155282.48465.94. [DOI] [PubMed] [Google Scholar]
- 99.Newell R. Altered body image: a fear-avoidance model of psycho-social difficulties following disfigurement. J Adv Nurs. 1999;30:1230–8. doi: 10.1046/j.1365-2648.1999.01185.x. [DOI] [PubMed] [Google Scholar]
- 100.Rumsey N, Clarke A, Musa M. Altered body image: the psychosocial needs of patients. Br J Community Nurs. 2002;7:563–6. doi: 10.12968/bjcn.2002.7.11.10886. [DOI] [PubMed] [Google Scholar]
- 101.Thompson A, Kent G. Adjusting to disfigurement: processes involved in dealing with being visible different. Clin Psychol Rev. 2001;21:663–82. doi: 10.1016/s0272-7358(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 102.Cobb S. Presidential address-1976. Social support as a moderator of life stress. Psychosom Med. 1976;38:300–14. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Sinno HH, Thibaudeau S, Duggal A, et al. Utility scores for facial disfigurement requiring facial transplantation (outcomes article) Plast Reconstr Surg. 2010;126(2):443–9. doi: 10.1097/PRS.0b013e3181e094fa. [DOI] [PubMed] [Google Scholar]
- 104.Barker JH, Vossen M, Banis JC., Jr The technical, immunological and ethical feasibility of face transplantation. Int J Surg. 2004;11:RA41–7. [Google Scholar]
- 105.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8(7):1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. [DOI] [PubMed] [Google Scholar]


