Abstract
Since publication of the first randomized controlled trial describing rapid antidepressant effects of ketamine, several reports have confirmed the potential utility of this dissociative anesthetic medication for treatment of major depressive episodes, including those associated with bipolar disorder and resistant to other medications and electroconvulsive therapy. These reports have generated several questions with respect to who might respond to ketamine, how, and for how long. To start answering these questions. We used PubMed.gov and ClinicalTrials.gov to perform a systematic review of all available published data on the antidepressant effects of ketamine and of all recently completed, ongoing, and planned studies. To date, 163 patients, primarily with treatment-resistant depression, have participated in case studies, open-label investigations, or controlled trials. All controlled trials have used a within-subject, crossover design with an inactive placebo as the control. Ketamine administration has usually involved an anaesthesiologist infusing a single, subanesthetic, intravenous dose, and required hospitalization for at least 24 hours postinfusion. Response rates in the open-label investigations and controlled trials have ranged from 25% to 85% at 24 hours postinfusion and from 14% to 70% at 72 hours postinfusion. Although adverse effects have generally been mild, some patients have experienced brief changes in blood pressure, heart rate, or respiratory rate. Risk–benefit analyses support further research of ketamine for individuals with severe mood disorders. However, given the paucity of randomized controlled trials, lack of an active placebo, limited data on long-term outcomes, and potential risks, ketamine administration is not recommended outside of the hospital setting.
Keywords: Antidepressant, bipolar disorder, glutamate, ketamine, major depressive disorder, treatment-resistant depression
It has been more than a decade since ketamine, an anesthetic medication, was first reported to have therapeutic effects in major depressive disorder (MDD) (1). The randomized controlled trial (RCT) provided evidence that a single, intravenous (IV), subanesthetic dose of ketamine may relieve depressive symptoms within hours. This added to preclinical work suggesting ketamine's antidepressant properties and was particularly exciting because it generated an avenue for developing novel treatments for MDD patients who had not previously responded to pharmacotherapy. At the administered dose, ketamine primarily acts as an antagonist of the N-methyl-D-aspartate (NMDA) receptor and thus targets the excitatory amino acid neurotransmitter, glutamate. In contrast, most approved antidepressant medications primarily target the brain monoamine systems (2). Only a minority of patients responds to a first trial with one of these medications, and some patients do not show any signifi-cant antidepressant response even after multiple trials. This latter group is said to have treatment-resistant depression (TRD) (3). The second ketamine RCT was conducted specifically in TRD patients, including some individuals who had not responded to electroconvulsive therapy (ECT) (4). Again, IV ketamine had rapid, but transient, antidepressant effects.
These data have generated several questions. First, how might the antidepressant response to ketamine be maintained? In both RCTs, ketamine's effects peaked at 24 hours postinfusion and lasted, in a minority of patients, for several weeks at most. Second, are modes of ketamine administration other than IV equally efficacious? Although the onset of antidepressant action may be more rapid, RCTs on IV administration of conventional antidepressants do not support increased efficacy over oral administration (5). Third, might it be possible to predict who will respond to ketamine? The antidepressant effects of conventional medications can be predicted from certain patient variables (6). Fourth, might ketamine prove useful in actively suicidal patients? In both initial RCTs, these patients were excluded from participation, but ketamine still reduced suicidal ideation. A final question to be addressed pertains to the use of ketamine for ECT anesthesia (7). Might ECT efficacy improve when ketamine is used rather than another anesthetic medication?
To address these questions, we reviewed all currently available patient data on the antidepressant effects of ketamine. We first present the results of our systematic review. We then discuss these results in the context of the questions posed in the previous paragraph. Importantly, we do not evaluate in detail the mostly preclinical literature on brain glutamate function and depression. Reviews on this topic are available elsewhere (8–11).
Methods and Materials
In January 2012, we searched PubMed.gov using the terms “ketamine” AND (“major depressive disorder” OR “bipolar disorder” OR “affective disorder”). We included studies in which patients met clinical criteria for a mood disorder, received ketamine to study its antidepressant effects, and were assessed clinically for at least 230 min (~4 hours) postketamine. If articles cited additional studies with these criteria, then these were also included. Table 1 provides an overview of included studies.
Table 1.
Overview of Published Studies of the Antidepressant Effects of Ketamine in Mood Disorder Patients to Date
| Patients |
Ketamine Treatment |
Ketamine Treatment |
Antidepressant Response |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Diagnosis | Comorbidity | TRD | Delivery | Dose | Formulation | Placebo | Medication Status | No. of Doses | Measures | 4 h | 24 h | 72 h | Adverse Effects During Administration | |
| Case Studies (total N = 14 patients received ketamine) | |||||||||||||||
| Correll and Futter (21) | 2 | MDD | No | Yes | IV 5 days |
.27–.3 mg/kg/h | Racemic | — | Not reported | 1–3 (2.5–5 months) | BDI HDRS |
Not assessed | Unclear | Yes | “Heady” |
| Goforth and Holsinger (35) | 1 | MDD | Not reported | Yes | IM | 1.5 mg/kg | Racemic | — | On | 1 (during ECT) | MADRS | Not assessed | Unclear | Yes | Not reported |
| Liebrenz et al. (16, 17) | 1 | MDD | Current substance dependence | Yes | IV 50 min |
.5 mg/kg | Racemic | — | On | 2 (6 weeks) | BDI HDRS |
Not assessed | Yes (infusion 1 only) | Yes (infusion 1 only) | None |
| Kollmar et al. (58) | 1 | MDD | Not reported | Yes | IV 40 min |
.5 mg/kg | Racemic | — | Not reported | 2 (2 weeks) | BDI HDRS |
Not assessed | Yes | Unclear | Not reported |
| Stefanczyk-Sapieha et al. (59) | 1 | MDD | Metastatic prostate cancer | Unclear | IV 60 min |
.5 mg/kg | Racemic | — | On | 2 (10 days) | BDI HDRS |
No | No | No | Visual hallucination |
| Paul et al. (19) | 2 | MDD | No | Yes | IV 50 min |
.5 mg/kg | Racemic | — | On | 2 (1 week, planned) | BDI HDRS |
Not assessed | Yes (n = 1) | Yes (n = 1) | Dizzy Unintentional crying |
| .25 mg/kg | S-enantiomer | No | No | Fatigue “Muzzy” |
|||||||||||
| Bjerre and Fontenay (12) | 1 | BD | Not reported | Not reported | IV 60 min |
.5 mg/kg | S-enantiomer | — | Not reported | 1 | Unclear | No | No | No | ? |
| Messer et al. (60) | 2 | MDD | No | Yes | IV 40 min |
.5 mg/kg | Racemic | Saline (n = 1) | Unclear | 2–6 (2–6 days, planned) | BDI HDRS |
Not assessed | Yes | Yes | “Intoxicated” |
| Denk et al. (20) | 1 | MDD | Not reported | Yes | IV 40 min |
.25 mg/kg | S-enantiomer | — | Not reported | 1 | BDI MADRS |
Unclear | Unclear | No | Dissociation |
| Glue et al. (13) | 2 | BD | Not reported | Yes | IM | .5–1 mg/kg | Racemic | — | Not reported | 3 | MADRS | Not reported | Yes (1.0 mg/kg only) | Not reported | Dissociation Lightheaded Sedation |
| Open-Label Investigations (total N = 83 patients received ketamine, most not repeatedly) | |||||||||||||||
| Machado-Vieira et al. (23) | 23a | MDD | Not reported | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | BDI HDRS MADRS |
Yes (48%) | Randomization to riluzole or placebo at 4–6 h postketamine | Not reported | |
| Phelps et al. (22) | 26a | MDD | Not reported | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | BDI HDRS MADRS |
Yes (43%) | Not reported | ||
| DiazGranados et al. (24) | 33a | MDD | Not reported | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | BDI HDRS MADRS |
Yes (??%) | Perceptual disturbance | ||
| Ibrahim et al. (25) | 40a | MDD | Not reported | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | MADRS | Yes (??%) | Dissociation | ||
| Ibrahim et al. (26) | 42a | MDD | Current anxiety disorder (52%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | BDI HDRS MADRS |
Yes (40%) | Perceptual disturbances, drowsiness, confusion, cardiovascular changes | ||
| Salvadore et al. (28) | 11b | MDD | Current anxiety disorder (73%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | MADRS | Yes (45%) | Not assessed | Not reported | |
| Salvadore et al. (27) | 15b | MDD | Current anxiety disorder (60%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | MADRS | Yes (40%) | Not reported | ||
| Salvadore et al. (29) | 14b | MDD | Not reported | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | MADRS | Yes (15%) | Not reported | ||
| Mathew et al. (32) | 26c | MDD | Current anxiety disorder (77%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 1 | QIDS MADRS |
Yes (62%) | Yes (66%) | Yes (54%) | Dizzy Numb Sleepy |
| aan het Rot et al. (33) | 9c | MDD | Current anxiety disorder (70%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | — | Off | 6 (2–3 days, planned) | QIDS MADRS |
Yesd (100%) | Not assessed | Yes (70%) | Feeling strange Dissociation Blood pressure and heart rate changes Headache Fatigue |
| Controlled Trials (total N = 66 patients received ketamine) | |||||||||||||||
| Berman et al. (1) | 8 | MDD or BD | Current anxiety disorder (13%) | Not reported | IV 40 min |
.5 mg/kg | Racemic | Saline (randomized order) | Off | 1 | BDI HDRS |
Yes (13%)d | Yes (25%)d | Yes (50%)d | Positive symptoms |
| Zarate et al. (4) | 17 | MDD | Lifetime anxiety disorder (65%) | ≥2 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | Saline (randomized order) | Off | 1 | BDI HDRS |
Yes (56%) | Yes (71%) | Yes (35%) | Blood pressure changes Confusion Dizziness |
| DiazGranados et al. (14) | 17 | BD | Lifetime anxiety disorder (35%) | ≥1 failed ATHF trial + prospective lithium/valproate trial) | IV 40 min |
.5 mg/kg | Racemic | Saline (randomized order) | On | 1 | BDI HDRS MADRS |
Yes (61%) | Yes (41%) | Yes (24%) | Dissociation Feeling strange Blood pressure and heart rate changes |
| Valentine et al. (31) | 10 | MDD | Current anxiety disorder (20%) | Not reported | IV 40 min |
.5 mg/kg | Racemic | Saline (fixed order) | Off | 1 | BDI HDRS |
No (0%)d | Yes (40%)d | Yes (30%)d | Dissociation Blood pressure changes |
| Zarate et al. (15) | 14 | BD | Lifetime anxiety disorder (73%) | ≥1 failed ATHF trial + prospective lithium/valproate trial) | IV 40 min |
.5 mg/kg | Racemic | Saline (randomized order | On | 1 | BDI HDRS MADRS |
Yes (64%) | Yes (43%) | Yes (14%) | Woozy/drowsy Cognitive impairment Anxiety Nausea Dizziness Blurred vision Headaches |
N represents the number of patients who actually received ketamine. Across studies, antidepressant responses were defined as a >50% reduction in scores on at least one depression measure (at 4 h, 24 h, and 72 h postketamine).
ATHF, Antidepressant Treatment History Form; BD, bipolar disorder; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; IM, intramuscular; IV, intravenous; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; QIDS, Quick Inventory of Depressive Symptoms; TRD, treatment-resistant depression.
These are partially overlapping groups of patients.
These are partially overlapping groups of patients.
The group of 9 patients was recruited from the single-dose responders in the original group of 26 patients.
Previously unpublished data obtained from study investigators.
We also searched ClinicalTrials.gov for studies that were recently completed but still unpublished, currently recruiting participants, or not yet enrolling. Table 2 again provides an overview.
Table 2.
Overview of Planned and Ongoing Ketamine for Depression Studies Listed on ClinicalTrials.gov
| Patients |
Ketamine Treatment |
Outcome Measures |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Psychiatric Diagnosis | TRD | Delivery method | Dose | Formulation | Placebo | No. of Administrations | Antidepressant Effects | Other | |
| Open Label Investigations | |||||||||
| Yale University/New Haven Hospital | “Depression” | Not required | IV 1–2 min |
.2 mg/kg | Racemic | — | 1 | MADRS | Suicide ideation |
| University Hospital Geneva | MDD | ≥2 unsuccessful medication trials | IV | .5 mg/kg | Racemic | — | 1 | MADRS | Adverse events Brain function |
| Controlled Trials | |||||||||
| Baylor College of Medicine/Mount Sinal School of Medicine | MDD | ≥3 failed ATHF trials | IV 40 min |
.5 mg/kg | Racemic | Midazolam (parallel arm) | 1 | MADRS QIDSa |
Adverse eventsa Brain functiona Neuropsychological functiona |
| Mount Sinai School of Medicine | MDD | ≥1 failed ATHF trial | IN | ≤50 mg | Racemic | Saline (randomized order) | 1 | MADRS | Adverse events |
| AstraZenica/University of Manchester/University of Oxford | MDD | Not required | IV | Unknown | Racemic | Saline (parallel arm) | 1 | MADRS | Brain function Behavioral tasks Pharmacokinetics |
| Washington University School of Medicine/University of Miami | MDD | “at PI discretion” | IV | .27 mg/kg + .00225 mg/kg/min | Racemic | Saline (randomized order) | 2 (with gabapentin or placebo PO before infusion) | HDRS | Adverse events |
| National Institutes of Mental Health (substudy 4) | MDD or BD | ≥1 failed ATHF trial | IV | Unknown | Racemic | Saline (randomized order) | 1 | Unknown | Brain function |
| University of New South Wales/Northside Clinic Wesley Hospitals | MDD or BD | Not required | IV | .1–.4 mg/kg | Racemic | Saline (parallel arm) | Up to 8 (weekly) | Unknown | Psychiatric symptoms Dissociative symptoms Cognitive impairment |
| Juvenile Bipolar Research Foundation | BD | Yes | IN | 10–20 mg | Racemic | Flat tonic water (parallel arm) | 4 | Unknown | Aggression Manic symptoms OCD symptoms |
| Ketamine During ECT | |||||||||
| Massachusetts General Hospital | MDD | Yes (ECT eligible) | IV | .5 mg/kg | Racemic | Saline (parallel arm) | 3 (with ECT) | HDRS | Brain function Cognitive impairment |
| University of New South Wales/Northside Clinic Wesley Hospitals | MDD or BD | Yes (ECT eligible) | IV | .25–.5 mg/kg | Racemic | Saline (parallel arm) | 3–6 (with ECT) | Depression rating scales | Cognitive impairment |
| Mayo Clinic | “Depression” | Yes (ECT eligible) | Unknown | Unknown | Racemic | Methohexital (parallel arm) | Several (with ECT) | “Hospital Anxiety and Depression Scale” | Unknown |
| University of Aberdeen/Royal Carnhill Hospital | “Depression” | Yes (ECT eligible) | IV | Unknown | Racemic | Propofol (parallel arm) | At least 4 (with ECT) | HDRS MADRS |
Cognitive impairment |
For all studies listed here, study status on ClinicalTrials.gov is “Completed,” “Enrolling by Invitation,” “Recruiting,” or “Not Yet Recruiting,” and results have not yet been published.
ATHF, Antidepressant Treatment History Form; BD, bipolar disorder; ECT, electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale; IN, intranasal; IV, intravenous; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; PO, per oral; QIDS, Quick Inventory of Depressive Symptoms; TRD, treatment-resistant depression.
Information available from study investigators.
Results
Patient and Treatment Details
Although most publications to date report on (adult) MDD patients, some case studies (12,13) and two recent RCTs (14,15) focused on depressed patients with bipolar disorder (BD). Patients with current comorbidity other than anxiety disorders have generally been excluded. One exception is a case of an MDD patient with polysubstance dependence (16,17). Although most studies have determined the degree of treatment-resistance using the Antidepressant History Treatment Form (3) or Thase-Rush criteria (18), the minimum required level of TRD has varied. In case reports, the TRD criteria have generally not been specified, but ketamine was never administered in treatment-naive patients.
Ketamine is usually given as a racemic mixture. In some cases, only the S-enantiomer was given (12,19,20). Most studies have used a dose of .5 mg/kg, administered via IV infusion over 40 to 60 min. Two cases received ketamine at .27 to .3 mg/kg/hour for 5 days (21). Two other cases received ketamine at .5 to 1 mg/kg via intramuscular (IM) administration (13). The number of ketamine administrations has varied from 1 to 3 (ad hoc) or 6 (conform study design).
Antidepressant Responses to Ketamine
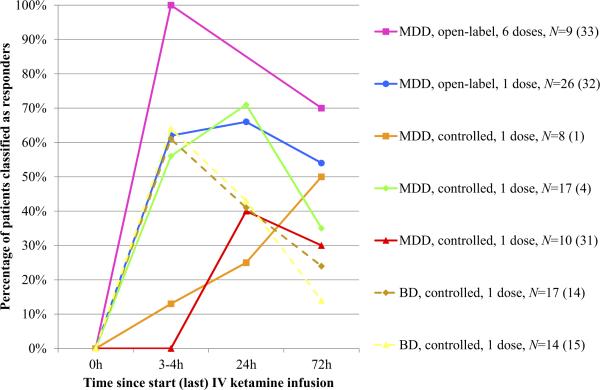
Table 1 lists ketamine's antidepressant effects 24 hours postin-fusion (acute response) and 72 hours postinfusion (sustained response) for each study. We defined a response as 50% or greater reduction on at least one of the depression measures. These data were unavailable for only two studies, one in which patients were randomized to riluzole or placebo 4 to 6 hours after ketamine (22–26) and one that focused exclusively on neural correlates of ketamine's antidepressant effects approximately 4 hours postinfusion (27–29). Figure 1 summarizes the antidepressant response rates to ketamine at 3 to 4 hours, 24 hours, and 72 hours postinfusion for the two open-label investigations and five controlled trials in which these data were obtained (this was not done in two additional open-label investigations, discussed later). At 72 hours postinfusion, antidepressant response rates have varied from 14% to 70%. Following the recommendations of Sackett et al. (30), the number needed to treat (NNT) with respect to achieving a significant anti-depressant response at this time point was calculated for all controlled trials, as were the corresponding 95% confidence intervals (CIs). For the study by Berman et al. (1), the NNT was three (95% confidence interval [CI]: 1 to infinity). For the studies by Zarate et al. (4) and Valentine et al. (31), the NNT was 5 (95% CI: 2 to infinity). For the study by DiazGranados et al. (14), the NNT was 4 (95% CI: 2–51). For the study by Zarate et al. (15), the NNT was 7 (95% CI: 3 to infinity). See Table S1 in Supplement 1 for details.
Figure 1.
Antidepressant responses to ketamine at 3 to 4 hours, 24 hours, and 72 hours postinfusion across all open-label investigations and controlled trials to date (N = 101). The 2010 study by aan het Rot et al. (33) involved six ketamine infusions; the data visualized here represent antidepressant responses after the last infusion. Studies in which more patients classified as sustained (>72 hours) responders are listed higher in the figure legend. BD, bipolar disorder (dashed lines); IV, intravenous; MDD, major depressive disorder (continuous lines).
There have been three controlled trials in addition to the initial two RCTs (1,4). In one trial, all patients first received saline and then were crossed over to ketamine a week later (31). The lack of treatment blinding does not seem to have inflated ketamine's antidepressant effects because the percentage of sustained responders in this study was not higher than in the initial RCTs (1,4). The two most recent trials focused on depressed BD patients receiving lithium or valproate (14,15). It should be noted that BD patients receiving valproate were less likely to respond to ketamine and to complete the study. This may have confounded the results. Furthermore, the BD patients in these two studies had failed more antidepressant trials than the MDD patients in the previous studies, as well as a prospective trial of a mood stabilizer, and therefore were possibly more resistant to ketamine. Rates of anxiety disorders and ECT unresponsiveness were also higher. Nonetheless, ketamine's effects in BD should be evaluated in light of the limited treatment options for bipolar depression.
Additional open-label investigations have been performed in relatively large numbers of patients. However, one study did not measure ketamine's effects beyond approximately 4 hours postin-fusion (29). Another study added riluzole or placebo at this time point (26); although riluzole maintenance treatment was no better than placebo, 27% of patients had not relapsed 4 weeks postketamine. In a third study, TRD patients considered ketamine responders at 72 hours postinfusion were then randomized to riluzole or placebo (32). Some of these patients, after having relapsed, subsequently participated in a fourth study, in which 24-hour responders to a first ketamine dose received five additional doses over 10 days (33). This decreased their relapse rate (Figure 1); one patient remained depression-free for several months (34).
Although it is difficult to draw any conclusions from the case studies listed in Table 1, some observations are worth mentioning. First, although sustained antidepressant responses have been observed in most cases in which racemic ketamine was given, these have not been observed with S-ketamine alone (12,19,20). Second, IM administration was effective at 1.0 mg/kg in one study and, in combination with ECT, at 1.5 mg/kg in another study (13,35). Third, in agreement with observations from open-label investigations and controlled trials (Table 1), adverse events during ketamine administration were generally mild and did not persist beyond the infusion.
Ongoing Studies
Table 2 lists two open-label investigations and seven RCTs currently posted on ClinicalTrials.gov that have been completed but not yet published results or that are (almost) recruiting participants. Notably, TRD status is not always required, S-ketamine (or R-ketamine) is not being investigated, and two studies involve intranasal (IN) ketamine administration. In some studies, the IV dose is lower than that used previously, which may increase insight in the dose at which ketamine has optimal antidepressant effects and minimal psychotomimetic effects. Most studies still administer a single dose. One exception is a placebo-controlled between-groups repeated-dose IV ketamine study in a mixed group of MDD and BD patients. Rather than using saline as the control, one study uses a subanesthetic dose of midazolam, a commonly used benzodiazepine anesthetic mediation.
Table 2 also lists four RCTs in which ketamine is combined with ECT. In two studies, ketamine is added to the regular anesthetic medication used during ECT. In two other studies, ketamine is directly compared with a standard anesthetic medication and therefore administered at an equipotent anesthetic dose.
Discussion
Ketamine's rapid antidepressant effects are currently supported by data from 163 patients (Table 1). Most studies have enrolled patients considered resistant to approved antidepressant medications. This is important because TRD has been associated with high patient burden and suffering, disproportionate clinician involvement, and escalating mental health and general medical costs (36,37). Because the goal of administering ketamine to TRD patients is to generate a novel treatment option, the direction to take is to address the questions generated by the studies conducted thus far.
For the controlled trials conducted so far, NNTs at 72 hours postinfusion ranged from 3 to 5 in MDD (1,4,31) and from 4 to 7 in BD (14,15). In comparison, in (non-TRD) primary care patients, NNTs for conventional antidepressants to induce a clinical response after 6 to 8 weeks of treatment range from 7 to 16 (38). Although this appears to suggest superior antidepressant efficacy of ketamine, because of the small numbers of patients included in the five ketamine trials, the NNTs are not significant. Moreover, a comparison across very different patient groups and treatment regimens may be inappropriate. The clinical significance of the antidepressant response to ketamine in these trials awaits further study in more naturalistic settings.
Maintenance of Antidepressant Responses to Ketamine
Few studies have systematically followed patients beyond 72 hours postketamine. It is unclear why many patients showing a response at 24 hours postketamine relapse less than 48 hours later (Figure 1). The clinical variables predicting quick relapse, like those predicting response (discussed later), should be a focus of future studies. It is also unclear why some patients maintain their response for several weeks. Studies that have attempted to maintain ketamine's effects after a single dose have done so in one of two ways. Riluzole was previously suggested to have potential antidepressant properties (39–41). It was administered orally only to sustained ketamine responders in one study (32), and to all participating MDD patients 4 to 6 hours after receiving an IV ketamine infusion in another study (26). Unfortunately, in both studies, riluzole failed to provide any benefit over placebo in maintaining response to a single ketamine dose.
In another study, patients acutely responding to an initial IV ketamine dose (all of whom had previously participated in the single-dose open-label investigation detailed in Mathew et al. [32]) received five additional doses over a period of 10 days (33). All first-dose responders maintained their response throughout the trial. When they were subsequently followed naturalistically, all remained depression-free longer than in the single-dose study. Overall the procedures were deemed safe and well tolerated. An RCT taking a similar approach is currently underway (Table 2).
Despite the reasonable risk–benefit ratio, however, there are disadvantages to continuing patients on thrice-weekly or even weekly IV ketamine infusions. A long-term treatment that does not require hospital visits is preferable. One option might be to start patients receiving a single ketamine dose on an approved monoaminergic antidepressant or mood stabilizing medication. Even though patients may have previously not responded to this medication, it might still help maintain an acute response to ketamine. This type of approach, although practical, remains speculative at this point and future studies are clearly needed.
Exploring Other Delivery Modes of Ketamine
Another option is to consider alternative ketamine administration routes. Oral administration is the easiest to implement, which may be especially relevant to maintenance treatment. It is not currently being pursued as a treatment option, unlike IN ketamine (Table 2), which may have similar advantages and has previously been shown to benefit analgesic-refractory chronic pain patients at a dose comparable to that used in most IV ketamine studies (42). IM administration has also been suggested recently (13).
Oral, IN, and IM administration have potential disadvantages as well. Especially with oral but also with IM administration (43), ketamine's bioavailability will be relatively low and also more variable across patients. The same may be true about IN administration. Therefore, although these alternative modes of administration may help maintain an acute response to IV ketamine, they may not work as well when used during the initial dosing. The rapid pharmacologic action associated with IV administration may at least partially explain why studies conducted so far have shown a fast onset of antidepressant action. Previous research on monoaminergic antidepressants also suggests that the onset of antidepressant action may be more rapid with IV than with oral administration (5). Unlike with IV administration, with other modes of administration ketamine might only be efficacious in TRD after several doses. Conducting a study consisting of a single IV ketamine dose followed by additional doses given otherwise is one possible new study design.
Predictors of Response
Before administering ketamine to any depressed patient, it would be useful to have insight into the likelihood that the intervention will indeed be beneficial. On the one hand, ketamine's antidepressant effects have been predicted by certain brain functions, such as neural activity in response to seeing fearful faces or during a working memory task (27–29). However, while these group-level findings are interesting in the context of existing biological models of depression, they might be difficult to implement in clinical practice at the level of individual patients. On the other hand, it has been reported that having a family history of alcoholism may increase the probability of having a significant antidepressant response at approximately 4 hours postketamine from 18% to 67% (22), and having previously received ECT (without success) may result in a smaller reduction in depression scores at this time point (25). Of course it is difficult to draw any firm conclusions from these two studies. Nevertheless, it is hoped that they encourage the investigation of other clinical variables and their predictive value over longer periods.
Potential Efficacy of Ketamine in Acutely Suicidal Patients
There are clinical conditions in which a rapid onset of therapeutic action can be extremely critical. One common situation arises when a patient is acutely suicidal. Although such patients were excluded from both initial ketamine RCTs, ketamine rapidly reduced suicide ideation scores on the depression rating scale used (1,4). This has since been replicated in two open-label investigations of MDD patients and in one RCT of BD patients (15,24,44). In the latter study, suicidality was reduced for 3 days postinfusion. Moreover, suicidality may not only decrease at an explicit or conscious cognitive level but also at an implicit or subconscious level, as measured by an Implicit Association Test (44). Overall, the results to date suggest that IV ketamine might prove useful in emergency room settings, where acutely suicidal depressed patients often present (45). This is currently being investigated in at least one study (Table 2).
ECT and Ketamine Anesthesia
One of the most promising aspects of ketamine is that even patients resistant to ECT might benefit from it (4,25). The reverse, whether patients who do not respond to subanesthetic IV ketamine doses might still benefit from ECT, is not known. However, ketamine is occasionally given for ECT anesthesia. More commonly used agents include methohexital, propofol, and thiopental.
One case report detailed an MDD patient who received ketamine anesthesia during ECT and who showed a sustained antidepressant response after a single seizure (35). This was unusual because ECT customarily requires at least six treatments and because the patient had not shown such a rapid response after previous ECT. Two uncontrolled studies have since shown that depression scores may decrease faster during ECT in patients given ketamine than in patients given propofol or thiopental (46,47). Furthermore, at least two RCTs of ketamine versus another anesthetic agent during ECT are underway (Table 2). Ketamine can decrease seizure threshold and increase seizure duration (7). Perhaps this may help explain why it might increase the efficacy of ECT.
Rather than replace a routine anesthetic agent with ketamine during ECT, two ongoing RCTs will add (a subanesthetic IV dose of) ketamine. Once all four ketamine/ECT RCTs are completed, it will be interesting to see which of the two approaches has a better risk–benefit profile. Furthermore, these studies are important because they include neuropsychological measures. Ketamine acutely impairs multiple cognitive functions (48), and cognitive deficits have been observed in high-dose (but not low-dose) recreational ketamine users (49). This has raised questions about the viability of ketamine as a treatment option for TRD.
It is important to study ketamine's neuropsychological effects in more detail, but doing so in MDD patients is difficult because ketamine's antidepressant effects will likely contribute to a reduction of the cognitive impairments seen in MDD, thereby obscuring any adverse neuropsychological effects. This difficulty may be circumvented by studying IV ketamine as an adjunctive therapy to ECT. Preliminary results from one study listed in Table 2 suggest that although subanesthetic IV doses of ketamine may indeed enhance the antidepressant effects of ECT, they do not appear to alter neuropsychological outcomes (50).
Ketamine's Effects on Neurobiology
Ketamine's antidepressant effects have mostly been explained in terms of its effects on the glutamate system. There is a large body of preclinical literature on brain glutamate function and depression (8–10). One study has looked at ketamine's effects on brain levels of glutamate in depressed patients, using proton magnetic resonance spectroscopy (31). Unexpectedly, neither the acute nor the sustained antidepressant effects of ketamine were associated with significant changes in brain glutamate levels measured at 3 hours and 2 days postketamine. Perhaps events downstream from post-synaptic glutamate receptors had already taken place at these times.
In this context, a recent review on preclinical ketamine studies is relevant (51). It is currently thought that ketamine inhibition of NMDA receptors (paradoxically) leads to glutamate release and subsequent activation of other glutamate receptors, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Included in the cascade of postsynaptic events at the AMPA receptor level are activation of brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin (mTOR). MDD has been associated with low BDNF levels as well as with low mTOR expression (52,53). Ketamine has been found to increase mTOR phosphorylation but not BDNF levels (20,23). Synaptogenesis in response to mTOR activation is hypothesized to contribute to ketamine's acute and sustained antidepressant effects (54). However, several inconsistencies remain. In healthy volunteers, ketamine does not appear to affect brain glutamate levels even during IV infusion (55). In mice, ketamine does not appear to activate mTOR signaling (56). Clearly more work is needed to delineate the biological mechanism underlying ketamine's antidepressant effects.
Conclusion: Where Do We Go from Here?
In a recent review of IV ketamine for TRD (57), the authors conclude that further studies are necessary because of 1) the limited numbers of patients studied, 2) interstudy variations in ketamine treatment methods, 3) the short duration of outcome assessments, and 4) ketamine's unknown long-term adverse effects. We agree, but by systematically reviewing all published, ongoing, and planned ketamine studies in MDD and BD to date (Tables 1 and 2) and by explicitly discussing several important clinical questions that are only starting to be answered, we aimed to guide researchers interested in the therapeutic potential of ketamine in their design of future studies.
Specifically, we recommend studying several maintenance treatment options. With repeated ketamine dosing, IN or IM administration might be more feasible than IV administration (33). Oral administration of ketamine, another glutamate medication such as riluzole, or even a traditional antidepressant medication could be considered for maintenance treatment once patients have responded to an initial IV ketamine dose.
Furthermore, we recommend studying larger groups of patients so predictors of response can be scrutinized. The data on familial alcoholism and ECT resistance (22,25) are encouraging, but the sample sizes were small, and both studies were limited to the impact of these clinical variables on depression reductions approximately 4 hours postketamine rather than after days or weeks. The impact of other clinical variables such as anxiety, trauma, and personality disorder comorbidity also deserves attention.
In terms of who might benefit from ketamine, the data obtained in suicidal patients (24,44) and in patients receiving ECT (46,47) are especially encouraging. Acutely suicidal patients may receive ketamine in emergency room settings. ECT has long been considered the last-resort treatment option for patients with TRD, at least partially because of concerns about cognitive side effects. For these patients in particular, ketamine seems to provide an attractive alternative. Moreover, even if ketamine does not prove useful as a stand-alone antidepressant medication, it appears to speed up the antidepressant effects of ECT (46,47). Importantly, ketamine might do so without the deleterious cognitive effects frequently observed with ECT (50). Ketamine/ECT studies may be more suitable for disentangling ketamine's direct effects on cognitive functions from its indirect effects on these functions (through depression reduction) than ketamine RCTs such as those listed in Figure 1. We encourage the inclusion of neuropsychological measures in all ketamine for depression studies but point out that the unbiased measurement of ketamine's cognitive effects is difficult.
We conclude with two additional comments. First, we recommend considering an active placebo when designing new RCTs. IV saline is inactive and therefore easily distinguishable from IV ketamine. One ongoing RCT instead uses IV midazolam, an anesthetic medication with acute subjective properties similar to those of IV ketamine yet presumably without ketamine's antidepressant effects (Table 2). The use of an active placebo will facilitate adequate blinding of both patients and clinical raters.
Second, we point out to clinicians enthused by ketamine's potential as a rapid antidepressant agent for TRD that all studies at the National Institute of Mental Health and the Mount Sinai School of Medicine (26,32,33) took place in close collaboration with anesthesiologists, and with patients hospitalized for at least 24 hours post-ketamine. This was done to ensure adequate monitoring of vital functions and prudent risk management. Patients with unstable medical illnesses were excluded. Because some patients experienced brief hyper- or hypotensive episodes, tachycardia or brady-cardia, and bradypnea (33), we do not recommend clinicians administering ketamine outside a hospital setting, or even inside a hospital setting when clinicians do not have appropriate training in managing the medical complications that might arise with ketamine. In the United States (but perhaps not elsewhere), there is no regulatory mandate for clinicians to obtain permission from a local medical ethics board for administering ketamine for depression. However, there need to be clear hospital guidelines for the use of anesthetic medication for nonanesthetic use, and patients should provide written informed consent for the use of ketamine “off-label.”
With this caveat, ketamine has generally been well tolerated. When administered by trained clinicians in appropriate settings, it thus seems a reasonable treatment option for most TRD patients, with the possible exception of individuals with a history of addiction or psychosis (49). It remains to be seen if ketamine's rapid antidepressant effects can be maintained over longer periods, but researchers have begun to address this. Moreover, even if IV ketamine does not become a viable therapy for the treatment of TRD, the initial RCTs (1,4) and all studies that followed have helped pave the way for the development of new drugs for TRD patients. Given their personal distress and burden on society, this alone is noteworthy.
Supplementary Material
Acknowledgments
Dr. aan het Rot is currently supported by a Veni grant from the Netherlands Organization for Scientific Research. Dr. Mathew is currently supported by the Department of Veterans Affairs and Grant No. R01MH81870 from National Institute of Mental Health.
We thank Robert M. Berman, M.D., Gerald W. Valentine, M.D., and Lisa M. Roach, B.A., for providing the previously unpublished data provided in Figure 1.
Dr. aan het Rot reports no biomedical financial interests or potential conflicts of interest. Dr. Zarate is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Mathew has, in the past 24 months, received research funding or salary support from the Banner Family Fund, Brain and Behavior Fund, Bristol-Myers Squibb, Bround Foundation, Department of Veterans Affairs, Evotec, Johnson & Johnson, and the National Institute of Mental Health (Grant No. 5R01MH81870) and has received consulting or lecture fees from AstraZeneca, Cephalon, Inc., Roche, and Takeda. He has received study drug from Sanofi-Aventis, the manufacturer of riluzole (Rilutek). In addition, Drs. Charney and Mathew have been named as inventors on a use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the U.S. Food and Drug Administration for this indication, Dr. Charney and the Mount Sinai School of Medicine could benefit financially. Dr. Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine is approved for this use.
Footnotes
Supplementary material cited in this article is available online.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- 3.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl. 16):10–17. [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: A review. Depress Anxiety. 2004;19:1–9. doi: 10.1002/da.10135. [DOI] [PubMed] [Google Scholar]
- 6.Dodd S, Berk M. Predictors of antidepressant response: A selective review. Int J Psychiatry Clin Pract. 2004;8:91–100. doi: 10.1080/13651500410005423. [DOI] [PubMed] [Google Scholar]
- 7.Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, 3rd, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003;15:27–34. doi: 10.1176/jnp.15.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist. 2009;15:525–539. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Murrough JW. Ketamine as a novel antidepressant: From synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjerre J, Fontenay C. Ketamin ved melankolsk depression. Ugeskr Læger. 2010;172:460–461. [PubMed] [Google Scholar]
- 13.Glue P, Gulati A, Le Nedelec M, Duffull S. Dose- and exposure-response to ketamine in depression. Biol Psychiatry. 2011;70:e9–10. doi: 10.1016/j.biopsych.2010.11.031. author reply e11–12. [DOI] [PubMed] [Google Scholar]
- 14.DiazGranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, DiazGranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebrenz M, Borgeat A, Leisinger R, Stohler R. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Weekly. 2007;137:234–236. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- 17.Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J Biol Psychiatry. 2009;10:1–4. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- 18.Thase ME, Rush AJ. When at first you don't succeed: Sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23–29. [PubMed] [Google Scholar]
- 19.Paul R, Schaaff N, Padberg F, Moller HJ, Frodl T. Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: Report of two cases. World J Biol Psychiatry. 2009;10:241–244. doi: 10.1080/15622970701714370. [DOI] [PubMed] [Google Scholar]
- 20.Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck CW. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiatry. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- 21.Correll GE, Futter GE. Two case studies of patients with major depressive disorder given low-dose (subanesthetic) ketamine infusions. Pain Med. 2006;7:92–95. doi: 10.1111/j.1526-4637.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 22.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: Results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression [published online ahead of print November 11]. Int J Neuropsychopharmacol. 2011 doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed. Churchill Livingstone; New York: 2000. [Google Scholar]
- 31.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res Neuroimaging. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: A pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 34.Murrough JW, Perez AM, Mathew SJ, Charney DS. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry. 2011;72:414–415. doi: 10.4088/JCP.10l06447blu. [DOI] [PubMed] [Google Scholar]
- 35.Goforth HW, Holsinger T. Rapid relief of severe major depressive disorder by use of preoperative ketamine and electroconvulsive therapy. J ECT. 2007;23:23–25. doi: 10.1097/01.yct.0000263257.44539.23. [DOI] [PubMed] [Google Scholar]
- 36.Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(suppl 8):17–25. [PubMed] [Google Scholar]
- 37.Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Syst Rev. 2009;3:CD007954. doi: 10.1002/14651858.CD007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 40.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carr DB, Goudas LC, Denman WT, Brookoff D, Staats PS, Brennen L, et al. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: a randomized, double-blind, placebo-controlled, crossover study. Pain. 2004;108:17–27. doi: 10.1016/j.pain.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Murrough JW, Gallo JM, Collins KA, aan het Rot M, Charney DS. Reply to: Dose- and exposure-response to ketamine in depression. Biol Psychiatry. 2010;70:e11–e12. [Google Scholar]
- 44.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: Comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 47.Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: A retrospective study. Eur Arch Psychiatry Clin Neurosci. 2011;261:575–582. doi: 10.1007/s00406-011-0205-7. [DOI] [PubMed] [Google Scholar]
- 48.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 49.Morgan CJA, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: A 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 50.Loo C, Sainsbury K, Martin D, MacPherson R. Synergistic antidepressant effects with ketamine and ECT. J ECT. 2009;25:150. [Google Scholar]
- 51.Duman RS, Li N, Liu R-J, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Post RM. Role of BDNF in bipolar and unipolar disorder: Clinical and theoretical implications. J Psychiatr Res. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor MJ, Tiangga ER, NÃ Mhuircheartaigh Ri, Cowen P. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: A proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Covvey JR, Crawford AN, Lowe DK. Intravenous ketamine for treatment-resistant major depressive disorder. Ann Pharmacother. 2012;46:117–123. doi: 10.1345/aph.1Q371. [DOI] [PubMed] [Google Scholar]
- 58.Kollmar R, Markovic K, Thurauf N, Schmitt H, Kornhuber J. Ketamine followed by memantine for the treatment of major depressions. Aust N Z J Psychiatry. 2008;42:170. doi: 10.1080/00048670701787628. [DOI] [PubMed] [Google Scholar]
- 59.Stefanczyk-Sapieha L, Oneschuk D, Demas M. Intravenous ketamine “burst” for refractory depression in a patient with advanced cancer. J Palliative Med. 2008;11:1268–1271. doi: 10.1089/jpm.2008.9828. [DOI] [PubMed] [Google Scholar]
- 60.Messer M, Haller IV, Larson P, Pattison-Crisotomo J, Gessert CE. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.