Abstract
Background
Phosphodiesterase type IV (PDE4), an important component of the cyclic adenosine monophosphate (cAMP) cascade, selectively metabolizes cAMP in the brain to the inactive monophosphate. Basic studies suggest that PDE4 mediates the effects of several antidepressants. This study sought to quantify the binding of 11C-(R)-rolipram, a PDE4 inhibitor, as an indirect measure of this enzyme’s activity in the brain of individuals with major depressive disorder (MDD) compared with healthy control subjects.
Methods
11C-(R)-Rolipram brain positron emission tomography scans were performed in 28 unmedicated MDD subjects and 25 age- and gender-matched healthy control subjects. Patients were moderately depressed and about one half were treatment-naive. 11C-(R)-Rolipram binding in the brain was measured using arterial 11C-(R)-rolipram levels to correct for the influence of cerebral blood flow.
Results
Major depressive disorder subjects showed a widespread, approximately 20% reduction in 11C-(R)-rolipram binding (p = .002), which was not caused by different volumes of gray matter. Decreased rolipram binding of similar magnitudes was observed in most brain areas. Rolipram binding did not correlate with the severity of depressive or anxiety symptoms.
Conclusions
This study is the first to demonstrate that brain levels of PDE4, a critical enzyme that regulates cAMP, are decreased in unmedicated individuals with MDD in vivo. These results are in line with human postmortem and rodent studies demonstrating downregulation of the cAMP cascade in MDD and support the hypothesis that agents such as PDE4 inhibitors, which increase activity within the cAMP cascade, may have antidepressant effects.
Keywords: cAMP, compartmental analysis, in vivo imaging, second messenger, unipolar depression, unmedicated
The second messenger cyclic adenosine monophosphate (cAMP) has been implicated in both the pathophysiology and treatment of major depressive disorder (MDD). This hypothesis posits that decreased signaling through the cAMP cascade is associated with depression (1–5) and that diverse antidepressant treatments increase signal transduction of the cAMP cascade as a common mechanism of action (6). Cyclic nucleotide phosphodiesterase type IV (PDE4) selectively metabolizes cAMP in the brain to the inactive monophosphate (7) and is, therefore, an important component of the cAMP cascade. Phosphodiesterase type IV may also be useful as an in vivo biomarker to assess the link between cAMP and MDD, given that PDE4 can be imaged using positron emission tomography (PET), and is also a potential target of antidepressants.
Several weeks of use are typically necessary before antidepressants manifest their therapeutic effects. This time lag is in line with decreased signaling through the cAMP cascade reported in human postmortem studies (1–5) and with antidepressant-induced increases in gene expression downstream of the cAMP cascade in rodents (6) (e.g., increases in both brain-derived neurotrophic factor [BDNF] and a transcription factor regulating the expression of BDNF, cAMP response element binding protein [CREB]). Several postmortem brain studies in patients with MDD or depressed suicide victims found that multiple markers of the cAMP cascade were decreased, including adenylyl cyclase (1,2), cAMP-dependent protein kinase A (PKA) (3), CREB (4), and BDNF (5). In contrast, rodent experiments have consistently found that chronic, but not acute, administration of various pharmacologic classes of antidepressants—including selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, and tricyclic antidepressants—upregulate those components of the cAMP cascade including PDE4 (6). One postmortem brain study found that unmedicated patients with MDD had lower CREB levels than control subjects, while medicated MDD patients had CREB levels similar to those of control subjects (4).
The enzymatic activity of PDE4 is regulated by PKA via a feedback mechanism. That is, high concentrations of cAMP stimulate PKA to phosphorylate PDE4, thereby increasing its enzymatic activity and returning the concentration of cAMP to steady state (7). Consistent with this feedback mechanism, both in vitro and in vivo studies indicated that PDE4 levels or its enzyme activity parallel the activity of the cAMP cascade (8–10). In particular, chronically enhanced or diminished noradrenergic neurotransmission altered PDE4 levels to higher and lower levels, respectively, in the same direction as the corresponding activity of noradrenergic neurotransmission (8).
This study sought to use 11C-(R)-rolipram PET imaging to compare the density and enzymatic activity of PDE4 between unmedicated patients with MDD and healthy control subjects. Phosphorylation of PDE4 not only increases its enzymatic activity but also increases the potency (affinity) of rolipram to inhibit PDE4 (11). Previous studies from our laboratory confirmed that, in rats, the in vivo binding of 11C-(R)-rolipram reflected the phosphorylation status of PDE4 (12). In that study, activation and deactivation of PKA, which regulates the activity of PDE4, increased and decreased rolipram binding, respectively. Therefore, in vivo binding of 11C-(R)-rolipram measures both the density of the enzyme and its activity, as reflected by the affinity of rolipram to bind to PDE4. Within the framework of the cAMP hypothesis of MDD, we predicted that unmedicated individuals with MDD would have decreased cAMP cascade activity and therefore decreased binding of 11C-(R)-rolipram in the brain.
Methods and Materials
Participants
Two groups were studied: 1) healthy control subjects with no personal history of a major psychiatric or neurological disorder and no first-degree relative with a mood or psychotic disorder (n = 25), and 2) patients who met DSM-IV criteria for MDD, in a current major depressive episode, without psychotic features (n = 28) (Table 1). Subjects were between 18 and 55 years of age and in good physical health, as determined by medical history, physical examination, blood labs, electrocardiogram, and urinalysis. Volunteers were excluded if they had a history of alcohol or substance abuse within the past year or a lifetime history of alcohol or substance dependence. All participants underwent a urine drug screen for amphetamines, benzodiazepines, cocaine metabolites, opiates, and cannabinoids on the day of the 11C-(R)-rolipram PET scan. Participants were not allowed to ingest caffeine after midnight on the day before the PET scan. In addition, herbal remedies or use of over-the-counter medications with known central nervous system effects were not permitted during the study.
Table 1.
Demographic and Clinical Characteristics of the Study Samples
| Control Subjects (n = 25) |
MDD (n = 28) | |
|---|---|---|
| Proportion Female (n) | 36% (9) | 32% (9) |
| Age | 37 ± 11 | 36 ± 11 |
| Depression and Anxiety Ratings | ||
| MADRS | .8 ± 1.6 | 30 ± 6 |
| HDRS-17 | .6 ± 1.0 | 20 ± 6 |
| HAM-A | .6 ± 1.0 | 18 ± 7 |
| Age of Onset | NA | 17 ± 9 |
| Duration of Current Episode (Months) | NA | 76 ± 123 |
| Treatment Naive | NA | 13 |
| Length of Time Medication Free (Months) [Range] | NA | 28 ± 40 [.8–120] |
| Current Comorbid Anxiety Disorders | 0 | 13 |
| Subjects with Lifetime History of Suicide Attempts (n) | 0 | 3 |
| Prior Exposure to Antipsychotic Agent (n) | 0 | 1 |
| Lifetime History of Substance Abuse (n) | 0 | 2 |
| Proportion of Current Cigarette Smokers (n) | 24% (6) | 32% (9)a |
Values are mean ± SD.
HAM-A, Hamilton Rating Scale for Anxiety; HDRS-17, Hamilton Rating Scale for Depression (17 item); MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder.
Includes three social smokers. Six of the nine subjects smoked cigarettes daily.
Diagnoses for MDD patients were established by an unstructured interview with a psychiatrist and the Structured Clinical Interview for DSM-IV (13). Major depressive disorder patients with serious suicidal ideation or psychosis were excluded from the study, but those with certain secondary anxiety disorders (generalized anxiety disorder, panic disorder, social phobia, anxiety disorder not otherwise specified, agoraphobia without panic disorder) were allowed to participate. In addition, we included patients with a remote history of posttraumatic stress disorder or obsessive-compulsive disorder but excluded subjects if these disorders were active at the time of enrollment. Patients with MDD were required to have a score of ≥20 on the Montgomery-Åsberg Depression Rating Scale (MADRS) (14) at the time of the 11C-(R)-rolipram PET scan. Major depressive disorder patients were required to be free from psychotropic medications for at least 2 weeks (6 weeks for fluoxetine) before the PET scan.
Data Acquisition
Evaluation of Symptom Severity
Severity of depressive and anxiety symptoms was assessed for both control subjects and MDD patients using the MADRS, the 17-item Hamilton Rating Scale for Depression (HDRS-17) (15), and the Hamilton Rating Scale for Anxiety (HAM-A) (16).
Brain Imaging
Positron emission tomography, magnetic resonance imaging, and data processing were conducted as previously described (17). Please see the Supplemental Methods in Supplement 1 for further details.
After intravenously administering 11C-(R)-rolipram, PET images were acquired for 90 minutes. To calculate 11C-(R)-rolipram binding in the brain, which is not influenced by cerebral blood flow or peripheral clearance, unmetabolized arterial 11C-(R)-rolipram levels were measured for 90 minutes. Because only free 11C-(R)-rolipram enters the brain, plasma free fraction (fP) of 11C-(R)-rolipram was measured using arterial plasma in each scan. High-resolution anatomical magnetic resonance imaging scans were performed for each subject, except one MDD patient, to analyze PET data after transforming into the single standard space (Montreal Neurological Institute space) to eliminate intersubject variability in shape and size of the brain (see Supplemental Methods in Supplement 1 for details).
Calculation of 11C-(R)-Rolipram Binding in Brain
11C-(R)-Rolipram binding levels were measured by compartmental modeling as total distribution volume (VT/fP) (18) in 10 large preselected regions covering most brain areas: frontal, parietal, lateral temporal, occipital, medial temporal, and anterior cingulate cortices; caudate; putamen; thalamus; and cerebellum. Right- and left-side data were combined for each region. Two additional supplementary analyses were performed. First, to investigate possible changes in rolipram binding in small regions, VT/fP was also calculated in each volume element (i.e., voxel) of the images by Logan plot (19), and parametric images were created where each voxel value was VT/fP. These parametric images were analyzed using Statistical Parametric Mapping (SPM) version 2005 (SPM5) (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). Second, to eliminate the influence of individual differences in gray matter volume, partial volume correction (20) was applied to the parametric images using magnetic resonance images segmented to gray and white matter. Rolipram binding levels in the 10 regions were subsequently measured (see Supplemental Methods in Supplement 1 for details).
Statistical Analyses
Age and body weight were compared between the control and MDD groups using a two-sample t test. Gender and percentage of cigarette smokers were compared between the two groups using Fisher’s exact test. Injection activity, specific activity, and mass dose (see Supplemental Methods in Supplement 1), and clearance from plasma of 11C-(R)-rolipram were compared between groups using a Mann-Whitney U test, because a Shapiro-Wilk normality test indicated nonparametric distribution of the data. VT/fP in the 10 brain regions of control subjects and MDD patients was compared using repeated measures, two-way analysis of variance with regions as the within-subjects (repeating) factor. The repeated measures, two-way analysis of variance was also performed to study the effect of gender, cigarette smoking, prior medication, and current comorbid anxiety disorders. The correlation between each of the MADRS, HDRS-17, and HAM-A ratings and VT/fP in the 10 brain regions of MDD patients was investigated using Spearman’s bivariate correlation. VT/fP in each voxel from control subjects and MDD patients was compared using SPM5 two-sample t test. The relationship between each of the MADRS, HDRS-17, and HAM-A ratings and VT/fP in each voxel of MDD patients was studied using an SPM5 regression model. All of the SPM analyses were performed with and without global normalization of proportional scaling. In the analyses that used binding in the 10 regions, p < .05 was considered statistically significant. In the SPM analyses, a false discovery rate corrected to p < .05 was considered statistically significant. One-sided tests to both directions were applied in the SPM analyses and two-sided tests were applied in the other analyses. The statistical analyses were performed using IBM SPSS Statistics 19 (Armonk, New York) for data from the 10 regions or SPM5 for voxel data. Radioactivity was reported as the standard uptake value (SUV), which normalizes for injected activity and body weight:
SUV = (activity per g of tissue/injected activity) × (body weight in g)
Standard uptake value of 1 equals the average radioactivity in the entire body immediately after injection. Results are shown as mean ± SD.
Results
Twenty-five healthy control subjects and 28 individuals with MDD were studied; the demographic and clinical characteristics of the subjects are presented in Table 1. Patients were moderately depressed at the time of the scan, as assessed by the MADRS (score = 30 ± 6). The patients also showed the HDRS-17 of 20 ± 6 at the same time. About half of the patients were treatment-naive; the other half had been free from psychotropic medications for an average of 28 months. No statistically significant differences were noted for gender (p = .78), number of cigarette smokers (p = .56 by including three social smokers in the MDD group), or age (p = .90) between the MDD and control groups.
Brain Image and Blood Data
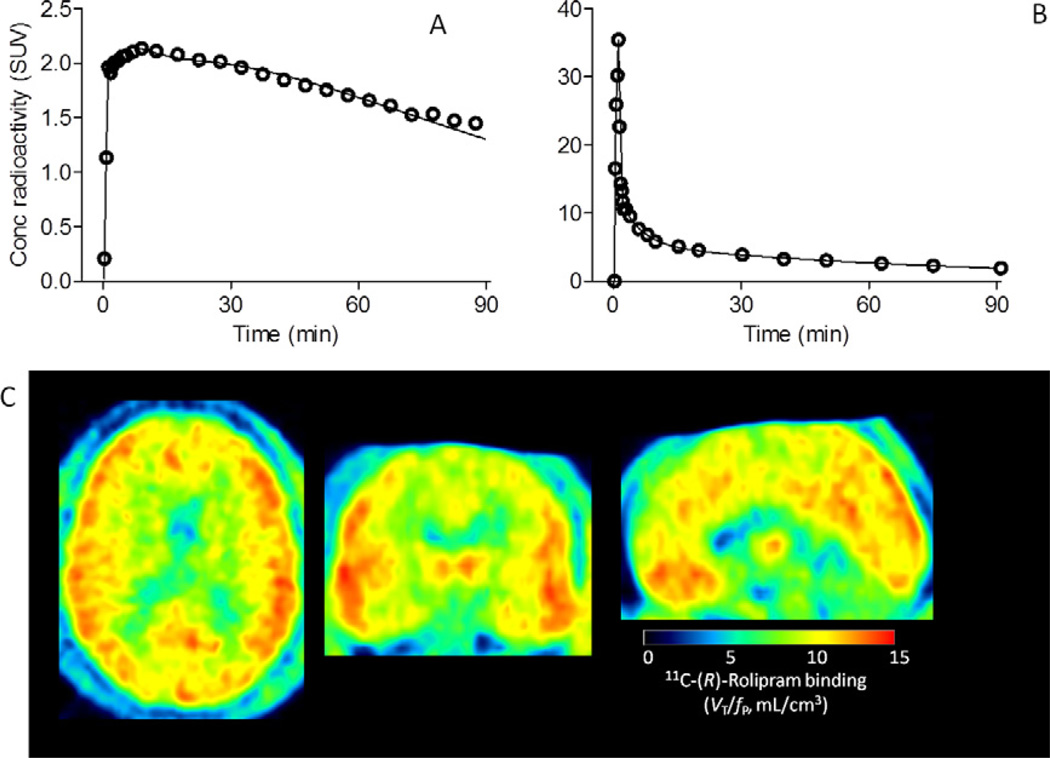
Injection activity, specific activity, and mass dose of 11C-(R)-rolipram did not differ between control subjects and individuals with MDD (p > .29). After intravenous injection of 11C-(R)-rolipram, brain activity peaked at ~2 SUV and decreased over time (Figure 1A). 11C-(R)-Rolipram in arterial plasma peaked at ~30 SUV at ~75 seconds and decreased quickly (Figure 1B). 11C-(R)-Rolipram binding in brain was measured by compartmental fitting (solid line in Figure 1A) using 11C-(R)-rolipram in arterial plasma as the input function. Basic brain PET quantification theory posits that 11C-(R)-rolipram binding measured as VT/fP, i.e., total distribution volume (VT) normalized to plasma free fraction of 11C-(R)-rolipram (fP), is independent of cerebral blood flow as well as of clearance of 11C-(R)-rolipram from plasma (21). For example, greater cerebral blood flow causes faster brain uptake and faster washout from brain. VT/fP calculated by compartmental modeling cancels the effect of cerebral blood flow because binding of 11C-(R)-rolipram to PDE4 is reversible, and VT/fP is calculated using brain activity of both the uptake (0 to ~10 minutes) and washout (~10 to 90 minutes) phases. VT/fP is independent of clearance of 11C-(R)-rolipram from plasma because VT/fP is brain activity normalized to plasma 11C-(R)-rolipram levels, i.e., the area under the curve of brain divided by the area under the curve of plasma from time zero to infinity. Normalization to fP is required because only free (i.e., unbound to plasma proteins) 11C-(R)-rolipram enters the brain.
Figure 1.
Positron emission tomography measurement of 11C-(R)-rolipram binding. To measure 11C-(R)-rolipram binding in brain as well as metabolism and clearance of the radioligand in plasma, both regional brain (A) and blood (B) data were acquired for 90 minutes; this included data before and well after the peak. Unmetabolized levels of 11C-(R)-rolipram (open symbols in [B]) were measured in arterial plasma in each scan by radio high-performance liquid chromatography. High-performance liquid chromatography separated the unmetabolized 11C-(R)-rolipram and radiometabolites and enabled measurement of the unmetabolized radioligand levels. Tri-exponential fitting (solid line in [B]) was performed for unmetabolized 11C-(R)-rolipram. From this tri-exponentially fitted curve and brain data (open symbols in [A]), an unconstrained two-compartment model provided the fitted curve (solid line in [A]) and gave total distribution volume (VT). VT was normalized to plasma free fraction (fP) measured by ultrafiltration. These VT/fP values in the 10 regions were compared between control subjects and subjects with major depressive disorder. In addition, 11C-(R)-rolipram binding levels (VT/fP) were calculated in each volume element (i.e., voxel) of the images by Logan plot (C) to compare groups in each voxel using Statistical Parametric Mapping (Figure S1 in Supplement 1). The representative data shown here were obtained from a 26-year old male patient with major depressive disorder. Conc, concentration; SUV, standard uptake value.
Widespread Decrease of 11C-(R)-Rolipram Binding in MDD
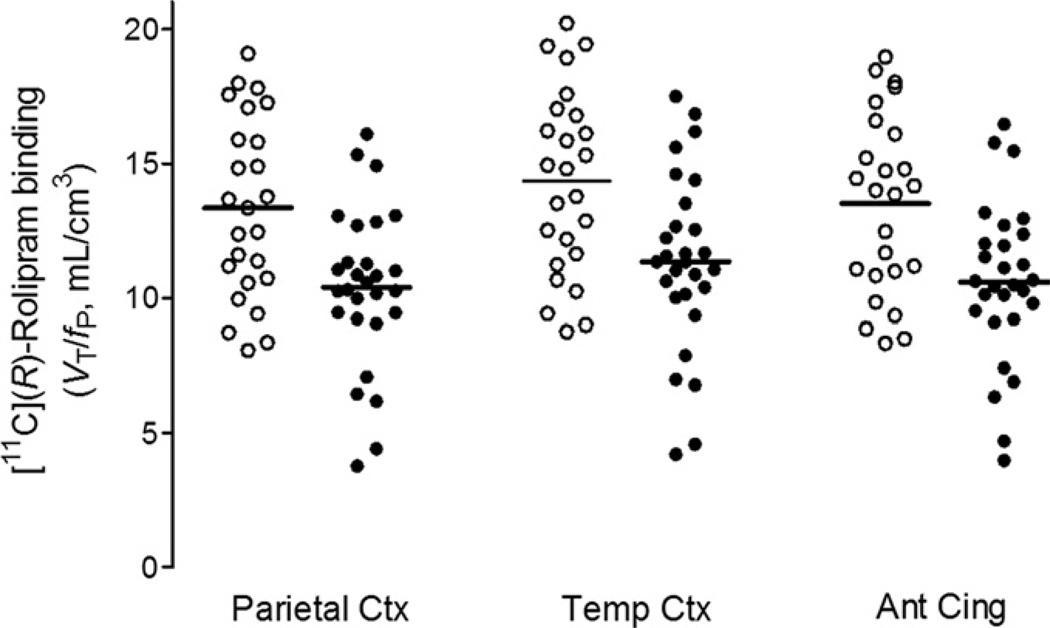
Both the analysis based on large brain regions as well as that based on data from each voxel showed a widespread decrease of 11C-(R)-rolipram binding measured as VT/fP in the MDD group. Rolipram binding in the 10 large preselected regions across brain (frontal, parietal, lateral temporal, occipital, medial temporal, and anterior cingulate cortices; caudate; putamen; thalamus; and cerebellum) showed widespread and significant decreases in the MDD group (Figure 2; VT/fP across the 10 regions; control subjects: 13.4 ± 3.3 mL/g, MDD: 10.5 ± 3.0 mL/g, F = 11.07, df = 1, 51, p = .002 for group difference in the 10 regions analyzed using repeated measures, two-way analysis of variance with regions as the within-subjects [repeating] factor). The magnitude of the decrease was similar across the 10 brain regions, ranging from 20% (frontal cortex and caudate) to 24% (cerebellum), with an average decrease of 22%. Statistical parametric mapping analysis with no global normalization, i.e., without adjusting average values between the two groups, performed on parametric images of VT/fP similarly detected a widespread and significant decrease of VT/fP in most brain. The results of SPM further support those obtained for the brain data drawn from the preselected regions (Figure S1 in Supplement 1, false discovery rate corrected p ranged from .004 to .009 in most brain areas).
Figure 2.
11C-(R)-Rolipram binding levels in healthy control subjects (open symbols) and patients with major depressive disorder (closed symbols) measured as total distribution volume, VT/fP, by unconstrained two-compartment model using brain data in large regions. Major depressive disorder patients showed a widespread and almost uniform decrease of 20% to 24% across 10 brain regions (p = .002). The figure shows results from 3 of the 10 regions. Bars indicate group means. Ant, anterior; Cing, cingulate; Ctx, cortex; fP, plasma free fraction; Temp, temporal; VT, total distribution volume.
Because the control and MDD groups were matched for age, gender, and cigarette smoking, the significant decrease in 11C-(R)-rolipram binding was unlikely to have been caused by group differences in these three factors; indeed, additional analyses using data from the 10 regions confirmed this. Rolipram binding was not correlated with age in either group. In control subjects, rolipram binding did not differ between female subjects and male subjects. However, in the MDD group, there was a marginally nonsignificant trend for female subjects to have lower binding levels than male subjects (VT/fP across the 10 regions; female subjects: 9.0 ± 3.8, male subjects: 11.3 ± 2.4, p = .053, F = 4.10, df = 1, 26). To further explore whether gender affected rolipram binding between groups, VT/fP across the 10 regions was compared using repeated measures, two-way analysis of variance and selecting gender as a covariate. The overall binding decrease in the MDD group remained highly significant when controlling for gender (p = .001; F = 12.09, df = 1, 50).
In control subjects, rolipram binding did not differ between cigarette smokers and nonsmokers. However, in the MDD group, smokers—including social smokers—had significantly lower binding levels than nonsmokers (VT/fP across the 10 regions; MDD smokers: 8.5 ± 2.6 mL/g, MDD nonsmokers: 11.5 ± 2.8 mL/g, F = 7.85, df = 1,26, p = .009). VT/fP across the 10 regions was compared using repeated measures, two-way analysis of variance and selecting cigarette smoking as a covariate. The overall binding decrease in the MDD group remained highly significant when controlling for cigarette smoking (p = .002; F = 10.68, df = 1, 50), and a significant interaction was noted between group and smoking (p = .01; F = 7.23, df = 1, 50), suggesting that smoking had different effects on the control and MDD groups.
Prior use of antidepressant medication also did not affect the significant decrease in rolipram binding observed in individuals with MDD. Specifically, MDD patients with and without prior antidepressant use did not differ significantly with respect to rolipram binding levels (p = .47). In addition, in MDD patients previously treated with antidepressants, no correlation was observed between the length of the medication-free period before PET and current rolipram binding levels (p > .53 in any of the 10 regions). Rolipram binding did not differ between MDD subjects with and without current comorbid anxiety disorders (p = .17; F = 1.95, df = 1, 26).
The Magnitude of the Reduction in 11C-(R)-Rolipram Binding Was Similar Across Regions
Statistical parametric mapping analysis with global normalization showed that the magnitude of the decrement in 11C-(R)-rolipram binding in MDD was similar across brain regions. This analysis is capable of detecting differing magnitudes of group differences across regions. If a group difference is detected after global normalization, the difference is caused by differing magnitudes in the group difference obtained across regions. This SPM analysis with global normalization detected no significant difference between control subjects and MDD patients (false discovery rate corrected p > .70).
Decreased 11C-(R)-Rolipram Binding Was Not Due to Decreased Gray Matter Volume
Because the decrements in rolipram binding observed in MDD could conceivably have been caused by reduced gray matter volume (though not by decreased PDE4 per gray matter volume), we also analyzed VT/fP parametric maps after correcting for gray matter volume, i.e., partial volume correction followed by measuring VT/fP in the 10 regions. As with the data obtained without partial volume correction, the results of the partial volume corrected analysis showed widespread and significant decreases in rolipram binding in the 10 regions in the MDD patients versus the control subjects, with an average decrease of 15% (Figure S2 in Supplement 1; VT/fP across the 10 regions; control subjects: 14.5 ± 3.1 mL/g, MDD: 12.4 ± 3.8 mL/g, F = 7.81, df = 1, 50, p = .007).
11C-(R)-Rolipram Binding and Symptom Severity
No significant correlation was found between rolipram binding and severity of depressive or anxiety symptoms as assessed by three different rating instruments—the MADRS, the HDRS-17, and the HAM-A. Neither rolipram binding measured in the 10 brain regions nor each voxel showed significant correlation (p > .22).
Global Decrease of 11C-(R)-Rolipram Binding Was Independent of Efflux Transporters at the Blood-Brain Barrier
We investigated whether decreased access of the radioligand to the brain of individuals with MDD versus control subjects may have resulted in the observed decrease in 11C-(R)-rolipram binding in MDD. Decreased access of (R)-rolipram to the brain may be caused by several factors—including faster plasma clearance of the radioligand in patients compared with healthy subjects, a decrease in cerebral blood flow in patients, and fP of the radioligand—all of which were controlled in our analysis. However, decreased access of the radioligand to the brain of individuals with MDD compared with control subjects could also be caused by increased function of efflux transporters, such as permeability-glycoprotein (P-gp) at the blood-brain barrier. A PET study using 11C-verapamil reported that a small sample of individuals with MDD and bipolar disorder who were receiving various medications had decreased uptake of 11C-verapamil in some brain regions (22). To explore whether P-gp function may have confounded our study, we investigated whether (R)-rolipram is a substrate for P-gp, the most prevalent efflux transporter at the blood-brain barrier. Using knockout mice, we determined that (R)-rolipram was not a P-gp substrate (Figure S3 in Supplement 1).
Discussion
As hypothesized, the present study detected widespread and significantly lower levels of PDE4 in unmedicated individuals with MDD, as assessed by measuring binding of 11C-(R)-rolipram, a PET ligand that selectively binds to PDE4. Notably, the finding of lower levels of PDE4 is in line with decreased cAMP cascade activity, as suggested by previous human postmortem and rodent studies (6).
This study was the first to explore PDE4, an important component of the cAMP cascade, in living unmedicated individuals with MDD. Most other studies of brain molecular imaging to date—including those conducted in MDD patients—have primarily focused on receptors and transporters of a specific neurotransmitter (e.g., serotonin, dopamine). In contrast, the current study investigated a component of a major postreceptor signal transduction pathway in living patients with MDD and found a significant decrease in rolipram binding to PDE4 relative to healthy control subjects. The decrease echoes results of many postmortem studies of patients with MDD, as well as suicide victims, which reported decreased adenylate cyclase type IV and forskolin-stimulated cAMP formation (1,2), PKA (3), CREB (4), and BDNF (5). Furthermore, studies of rodents that were chronically administered antidepressants have consistently shown increases in rolipram binding and PDE4 levels (8,23,24), as well as in other components of the cAMP cascade (e.g., coupling of stimulatory G protein to adenylate cyclase, PKA, CREB, and BDNF) (6).
In conjunction with previous results from human postmortem and rodent studies, the results of the present investigation suggest that the use of PDE4 inhibitors to normalize the cAMP cascade activity is a putative novel treatment for depression. Rolipram—which was used as the imaging agent in this study—is a PDE4 inhibitor that increases signal transduction of the cAMP cascade by inhibiting metabolism of the second messenger cAMP. As part of a new pharmacologic class of agents, rolipram showed antidepressant effects in initial uncontrolled clinical studies; however, the ratio of efficacy to adverse reactions limited its clinical use (25,26). Furthermore, these initial open-label clinical trials may not have adequately explored rolipram’s potential as a novel antidepressant due to their small sample size. Modifying the prototype inhibitor rolipram may lead to the creation of a PDE4 inhibitor capable of antidepressant effects with fewer adverse reactions. Two modifications to rolipram could improve its clinical utility, namely: 1) improving selectivity among PDE4 subtypes, and 2) developing an allosteric modulator. Rolipram is an almost equipotent inhibitor of all four PDE4 subtypes (A, B, C, and D). Rodent studies have indicated that some of the four subtypes or splice variants of PDE4 play a greater role in rolipram’s antidepressant effects, while others are involved in its adverse reactions such as emesis and sedation (6,27,28). As a result, attempts are underway to develop an inhibitor selective for one of the PDE4 subtypes. Burgin et al. (29) recently reported a potent allosteric modulator selective for PDE4 subtype D that enhances cognition but has reduced potential to cause emesis in rodents (29). Allosteric modulators do not completely inhibit enzyme activity and are thus expected to have reduced potential to cause emesis while maintaining therapeutic effects.
Several strengths of our study design merit comment. First, to decrease the chance of type I error, we compared 25 to 28 subjects in each group, which is about twice as large as most brain molecular imaging studies. Second, we measured metabolite-corrected arterial input function and plasma free fraction of 11C-(R)-rolipram in each PET scan to measure rolipram binding not influenced by cerebral blood flow or plasma free fraction. Third, we controlled for several potentially confounding variables such as gender, cigarette smoking, prior use of medications, age, plasma clearance of the PET tracer, and the influence of P-gp on brain uptake of the PET tracer.
As regards the latter issue, we specifically examined several potential confounds that may have artifactually contributed to the overall decreased binding observed in individuals with MDD. First, even if plasma clearance of the radioligand had differed between patients and healthy subjects, such differences would not have caused this global difference in rolipram binding, because compartmental analysis corrects for different plasma clearance. Furthermore, the plasma clearance of 11C-(R)-rolipram was similar between MDD subjects (149 ± 69 mL/min) and control subjects (188 ± 101 mL/min; p = .25). Second, a global decrease could not have been due to an overall decrease in cerebral blood flow, because the compartmental method corrects for differences in flow between brain regions and between individuals based on measurement of both uptake and washout. Third, the fP of the radioligand is linearly related to the brain uptake of the radioligand, because only the free drug in plasma has access to the brain. We corrected for this effect by using VT normalized for (i.e., divided by) fP. Fourth, decreased access of the radioligand to the brain of patients compared with control subjects could be caused by greater function of efflux transporters at the blood-brain barrier, such as P-gp; to assess this possibility, we investigated whether rolipram was a substrate for P-gp using knockout mice and found that rolipram was not a P-gp substrate. As noted in the Results, none of these factors caused the differences in rolipram binding observed between control subjects and individuals with MDD.
Although 11C-(R)-rolipram binding was significantly lower in this group of unmedicated individuals with MDD than control subjects, binding levels did not correlate with the severity of depressive or anxiety symptoms. Two possible explanations for this finding exist. First, downregulation of the cAMP cascade may be a pervasive, underlying condition in MDD, and therefore, downregulation of this cascade must be accompanied by changes in certain receptors or transporters (e.g., the serotonin transporter) for depressive symptoms to manifest. Second, because MDD subjects are heterogeneous in various ways, the impact of the decrease in rolipram binding to the symptom severity differed among patients. A study in a more homogenous group may be required to detect significant correlation between rolipram binding and the severity of the symptoms. In fact, in this study, cigarette smoking and gender appeared to affect rolipram binding in MDD subjects, indicating the utility of controlling these factors.
In conclusion, this PET study is the first to find that PDE4, which is a component of the cAMP cascade as well as a potential target of antidepressant treatment, is downregulated in vivo in unmedicated individuals with MDD. The findings of the current study and those of previous human postmortem and rodent studies are in line with downregulation of the cAMP cascade in unmedicated patients with MDD, although the cause of the downregulation is not well known. These data support the hypothesis that drugs that stimulate the cAMP cascade may ultimately provide novel therapeutic agents for depression.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
We thank Sergio Bauza, Jose Franco Chaves, Anibal Cravchik, Nancy Diazgranados, Madeline Gupta, Lobna Ibrahim, Daniel Mathews, Earlian Smith-Jackson, and the staff of the Experimental Therapeutics and Pathophysiology Branch, National Institute of Mental Health, for recruitment and clinical care of subjects with major depressive disorder; Kacey Anderson, David Clark, Maria Ferraris Araneta, Michele Drevets, Holly Giessen, Robert Gladding, Gerald Hodges, Kimberly Jenko, William Kreisl, Cheng-Ta Li, Harushige Ozaki, Denise Rallis-Frutos, Barbara Scepura, Cheryl Wallisch, Joan Williams, and the staff of the Positron Emission Tomography Department, National Institutes of Health, for successful completion of positron emission tomography scans; Ioline Henter for outstanding editorial assistance; Dave Luckenbaugh and Steve Fromm for statistical analysis; and PMOD Technologies (Zurich, Switzerland) for providing its image analysis and modeling software.
Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression. Dr. Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government.
Dr. Drevets has served as a consultant for Johnson and Johnson, Myriad/RBM, and Eisai within the past two years.
Footnotes
Drs. Fujita, Hines, Zoghbi, Mallinger, Liow, Zhang, Pike, Innis, and Ms. Dickstein report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C. Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res. 1994;633:297–304. doi: 10.1016/0006-8993(94)91552-0. [DOI] [PubMed] [Google Scholar]
- 2.Reiach JS, Li PP, Warsh JJ, Kish SJ, Young LT. Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. J Affect Disord. 1999;56:141–151. doi: 10.1016/s0165-0327(99)00048-8. [DOI] [PubMed] [Google Scholar]
- 3.Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [3H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet. 1998;352:1754–1755. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- 5.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 6.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 7.Houslay MD, Sullivan M, Bolger GB. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. In: August TJ, Murad F, Anders MW, Coyle JT, editors. Advances in Pharmacology. London: Academic Press; 1998. pp. 225–342. [DOI] [PubMed] [Google Scholar]
- 8.Ye Y, Conti M, Houslay MD, Farooqui SM, Chen M, O’Donnell JM. Noradrenergic activity differentially regulates the expression of rolipram-sensitive, high-affinity cyclic AMP phosphodiesterase (PDE4) in rat brain. J Neurochem. 1997;69:2397–2404. doi: 10.1046/j.1471-4159.1997.69062397.x. [DOI] [PubMed] [Google Scholar]
- 9.Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154:82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukada H, Harada N, Ohba H, Nishiyama S, Kakiuchi T. Facilitation of dopaminergic neural transmission does not affect [11C]SCH23390 binding to the striatal D1 dopamine receptors, but the facilitation enhances phosphodiesterase type-IV activity through D1 receptors: PET studies in the conscious monkey brain. Synapse. 2001;42:258–265. doi: 10.1002/syn.10013. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann R, Wilkinson IR, McCallum JF, Engels P, Houslay MD. cAMP-specific phosphodiesterase HSPDE4D3 mutants which mimic activation and changes in rolipram inhibition triggered by protein kinase A phosphorylation of Ser-54: Generation of a molecular model. Biochem J. 1998;333:139–149. doi: 10.1042/bj3330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh T, Abe K, Hong J, Inoue O, Pike VW, Innis RB, Fujita M. Effects of cAMP-dependent protein kinase activator and inhibitor on in vivo rolipram binding to phosphodiesterase 4 in conscious rats. Synapse. 2010;64:172–176. doi: 10.1002/syn.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 14.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Zanotti-Fregonara P, Zoghbi SS, Liow JS, Luong E, Boellaard R, Gladding RL, et al. Kinetic analysis in human brain of [11C](R)-rolipram, a positron emission tomographic radioligand to image phosphodiesterase 4: A retest study and use of an image-derived input function. Neuroimage. 2011;54:1903–1909. doi: 10.1016/j.neuroimage.2010.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 19.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 20.Müller-Gätner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 21.Carson RE. Tracer kinetic modeling in PET. In: Valk PE, Bailey DL, Townsend DW, Maisey MN, editors. Positron Emission Tomography Basic Science and Clinical Practice. London: Springer-Verlag; 2003. [Google Scholar]
- 22.de Klerk OL, Willemsen AT, Roosink M, Bartels AL, Hendrikse NH, Bosker FJ, den Boer JA. Locally increased P-glycoprotein function in major depression: A PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood-brain barrier. Int J Neuropsychopharmacol. 2009;12:895–904. doi: 10.1017/S1461145709009894. [DOI] [PubMed] [Google Scholar]
- 23.Andersen PH, Klysner R, Geisler A. Cyclic AMP phosphodiesterase activity in rat brain following chronic treatment with lithium, imipramine, reserpine, and combinations of lithium with imipramine or reserpine. Acta Pharmacol Toxicol (Copenh) 1983;53:337–343. doi: 10.1111/j.1600-0773.1983.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Terwilliger R, Lane C, Mezes PS, Conti M, Duman RS. Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J Neurosci. 1999;19:610–618. doi: 10.1523/JNEUROSCI.19-02-00610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O’Donnell JM, Zhang HT. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.