Abstract
Objective
We previously demonstrated that altered zinc homeostasis is an important feature of pediatric sepsis, thus raising the possibility of zinc supplementation as a therapeutic strategy in sepsis. Herein we tested the hypothesis that prophylactic zinc supplementation would be beneficial in a murine model of peritoneal sepsis.
Design
Murine model of sepsis (intraperitoneal fecal slurry injection).
Setting
Basic science research laboratory.
Patients/Subjects
C57BL/6 male mice.
Interventions
Intraperitoneal fecal slurry injection, with or without zinc supplementation (10 mg/kg of intraperitoneal zinc gluconate for 3 days prior to CLP).
Measurements
Survival over 3 days following CLP, markers of inflammation, bacterial load studies, and immuno-phenotyping studies.
Results
Zinc-supplemented mice demonstrated a significant survival advantage compared to control (non-supplemented) mice. Zinc-supplemented mice also demonstrated moderate reductions of inflammation and immune activation. The survival advantage primarily correlated with reduced in vivo bacterial load in zinc-supplemented mice, compared to controls. In addition, peritoneal macrophages harvested from zinc-supplemented mice demonstrated a significantly enhanced phagocytosis capacity for E. coli and S. aureus, compared to peritoneal macrophages harvested from control mice.
Conclusion
Prophylactic zinc supplementation reduces bacterial load and is beneficial in a murine model of peritoneal sepsis.
INTRODUCTION
Zinc is an essential trace element for normal functioning of the innate and adaptive immune systems (1,2), and there has recently been interest surrounding zinc supplementation as a therapeutic strategy for critically ill patients (3,4). Sepsis continues to be a major source of morbidity and mortality in critically ill patients with few therapeutic options beyond antibiotics, intensive care unit-based organ support, and vaccines (5-7).
Using genome-wide expression profiling, we first demonstrated that pediatric septic shock is characterized by wide-spread and persistent repression of genes that are either directly involved in zinc homeostasis, or are directly affected by alterations of zinc homeostasis (8). This observation has been validated in multiple follow-up studies (9-14), and we have also demonstrated that serum zinc levels are abnormally low in non-survivors of pediatric septic shock (8). Collectively, these clinical data indirectly suggest that zinc supplementation may be a valid therapeutic strategy in sepsis (15). Multiple revisions of a protocol testing zinc supplementation after induction of peritoneal-induced sepsis failed to show either benefit or harm (Wong et al. unpublished data). In the experiments described herein we tested the hypothesis that prophylactic zinc supplementation is beneficial in mice subsequently subjected to a model of peritonitis-induced sepsis.
MATERIALS AND METHODS
Prophylactic zinc supplementation and peritonitis model
All aspects of this study complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23 revised 1996) and met approval of the local Institutional Animal Care and Use Committee. Male C57BL/6 mice (Harlan, Denver, CO), 4 to 5 weeks of age, were injected (intraperitoneal) with 10 mg/kg of zinc gluconate every 24 hours, for 3 days prior to induction of peritonitis. Intraperitoneal zinc injections continued every 24 hours after the induction of peritonitis. Control mice received an equal volume of intraperitoneal normal saline using the same temporal sequence. Mice were housed either 4 or 2 mice per cage, with an equal number of mice in each cage assigned to zinc and control groups in order to control for possible variations in shipments and environment.
Peritonitis was induced by intraperitoneal injection of a fecal-slurry as previously described (16,17). Fecal contents were harvested from the cecum of adult donor mice by a midline laparotomy immediately after carbon dioxide asphyxiation. Fecal material was weighed and suspended in sterile water with 5% dextrose at a final concentration of 80 mg/ml. The fecal-slurry was then injected into the peritoneum of control and zinc-supplemented mice at a dose of 1.5 mg/gm. Mice were subsequently observed for 72 hours (survival studies), or sacrificed at 24 hours after induction of peritonitis to conduct assays corresponding to the various experimental readouts.
Measurement of myeloperoxidase activity
Myeloperoxidase was measured as an indication of neutrophil infiltration in lung tissue, as previously described (18). Briefly, whole lung tissue was homogenized and myeloperoxidase activity was assessed using spectrophotometry and defined as the quantity of enzyme degrading 1 μmol hydrogen peroxide/min at 37°C, expressed in units per 100 mg of tissue.
Measurement of serum cytokines and chemokines
Serum levels of KC, interleukin (IL)-6, IL-1β, IL-10, tumor necrosis factor (TNF)-α, and macrophage inflammatory protein-1 (MIP-1) were analyzed using a Luminex multiplex system (Luminex Corporation, Austin, TX) according to instructions from the manufacturer.
In vivo bacterial load
Twenty-four hours after induction of peritonitis mice were sacrificed with carbon dioxide asphyxiation, and blood and peritoneal fluid were harvested and subjected to serial log-fold dilutions using sterile normal saline. Lungs and spleens were also collected 24 hours after induction of peritonitis and 100 mg of tissue was suspended in 1 ml of sterile normal saline and homogenized. Homogenized samples were briefly centrifuged and the resulting supernatants were subjected to serial log-fold dilutions in normal saline. The dilutions from all samples were then plated onto blood agar plates (Becton Dickinson, Franklin Lakes, NJ) and incubated for 24 hours in a bacterial culture incubator. After the 24 hour incubation period, bacterial culture plates were manually counted by a technician blinded to the experimental conditions.
Ex vivo phagocytosis assay
After 3 days of the zinc supplementation protocol, zinc-supplemented and control mice were sacrificed with carbon dioxide asphyxiation and peritoneal macrophages were harvested via midline laparotomy and peritoneal lavage with 5 ml of sterile saline. Cell suspensions were subsequently centrifuged and the resulting pellets were re-suspended in cell culture media (DMEM with 10% fetal calf serum; Gibco BRL, Carlsbad, CA). Cells were then counted and re-suspended for plating onto 96-well tissue culture plates (Becton Dickinson) at a concentration of 2 × 105 cells per well. Tissue culture plates were then incubated overnight in a tissue culture incubator at 37° C and room air/5% CO2.
After the 24 incubation period, phagocytosis of E. coli or S. aureus was measured using the Vybrant™ Phagocytosis Assay Kit (Molecular Probes, Inc., Eugene, OR) according the manufacturer’s instructions. Briefly, cells were incubated with either fluorescein-labeled E. coli or S. aureus for 2 hours at 37° C. After this incubation period the culture media was removed and any remaining extracellular fluorescein activity was quenched by one minute incubation in trypan blue. The trypan blue suspension was then removed and intracellular fluorescein activity was measured in a fluorescence 96-well plate reader (Promega, Madison, WI) at 480 nm excitation/520 nm emission as an indicator of phagocytosis.
Immuno-phenotyping studies
Splenic single-cell suspensions were prepared using standard procedures (19). The macrophage MHC II and CD11b expression was determined ex vivo. Additional splenocytes were incubated for 24 hours with plate-bound anti-CD3 and CD28. After isolation, CD69 expression on CD4 and CD8 naive (CD44 low / CD62L high), effector (CD44 high / CD62L high) and memory (CD44 high / CD62L low) cells was determined after analysis using an LSR II flow cytometer and FACS DIVA software (BD Pharmingen).
The FACS antibodies were conjugated with fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll, and allophycocianin-labeled antibodies. Specifically, the following anti-mouse antibodies specific towards the following antigens were purchased from BD Pharmingen (San Diego, CA): CD11b (clone: M1/70), MHC-II (clone: M1/70), CD4 (clone: RM4-5), CD8 (clone: 53-6.7), CD62L (clone: MEL-14). CD44 (clone: IM7), and CD69 (clone: H1.2F3). The following anti-mouse antibody against F4/80 (clone: A3-1) was purchased from AbD Serotec (Raleigh, NC).
Statistical Analysis
All statistical analyses were conducted using SigmaStat for Windows Version 3.0 (SyStat Software, San Jose, CA). Survival analysis was conducted using a Kaplan-Meier survival curve statistics. Normally distributed data were analyzed by Student’s t test and depicted as means ± standard error of the mean. Non-normally distributed data were analyzed by Rank Sum Test and depicted as box-whisker plots. Bacterial colony count data were log transformed prior to analysis. For all statistical tests a p value < 0.05 was considered significant.
RESULTS
Prophylactic zinc supplementation confers a survival advantage in mice subjected to peritonitis
In these experiments we determined the effect of prophylactic zinc supplementation on survival after intraperitoneal fecal-slurry injection. Control mice (not supplemented with zinc) and zinc-supplemented mice received intraperitoneal fecal-slurry injections and survival was monitored over a 72 hour period. As shown in Figure 1, prophylactic zinc supplementation conferred a significant survival advantage over the 72 hour observation period.
Figure 1.
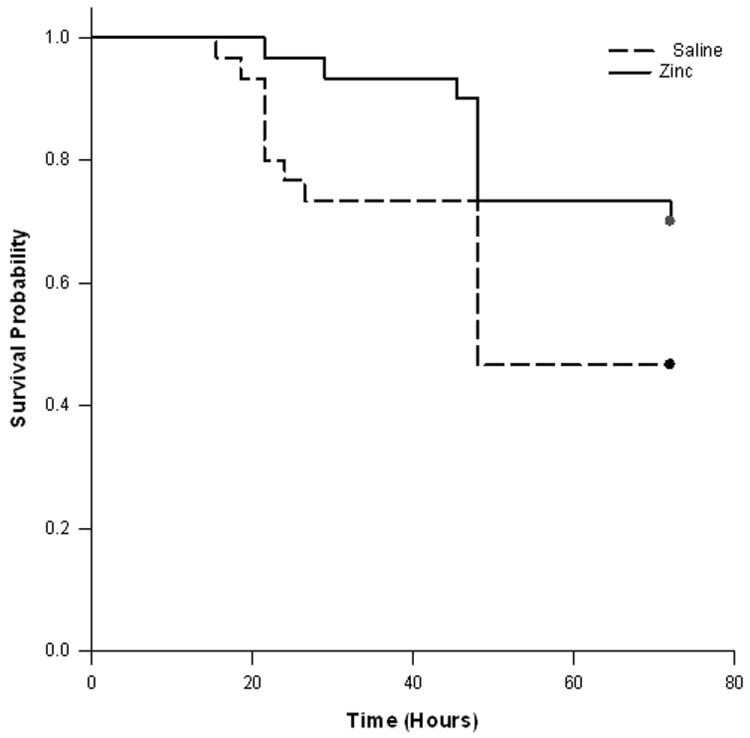
Survival analysis for control and zinc-supplemented animals. Both groups were subjected to intra-peritoneal fecal-slurry challenge and survival was monitored over 72 hours. The survival curves were significantly different (p < 0.05) based on Kaplan-Meier Survival Analysis (n = 30 animals per group).
Prophylactic zinc supplementation attenuates lung inflammation and systemic KC levels
Since an excessive inflammatory response is thought to play a role in the pathophysiology of sepsis, and the lung is a primary target organ in sepsis, in these experiments we measured neutrophil infiltration (by MPO assay) in the lungs of mice 24 hours after fecal-slurry challenge. As shown in Figure 2, lung MPO activity was significantly attenuated in the lungs of zinc-supplemented mice, compared to control mice. We also measured serum KC levels 24 hours after fecal-slurry challenge. As shown in Figure 3, serum KC levels were significantly lower in zinc-supplemented mice, compared to control mice. Other serum cytokines/chemokines (i.e. IL-10, IL-1β, IL-6, MIP-1, and TNFα) and lung NF-κB activity were not significantly different between the two experimental groups (data not shown). These data demonstrate that prophylactic zinc supplementation modestly reduces local and systemic inflammation in mice subjected to fecal-slurry peritonitis.
Figure 2.
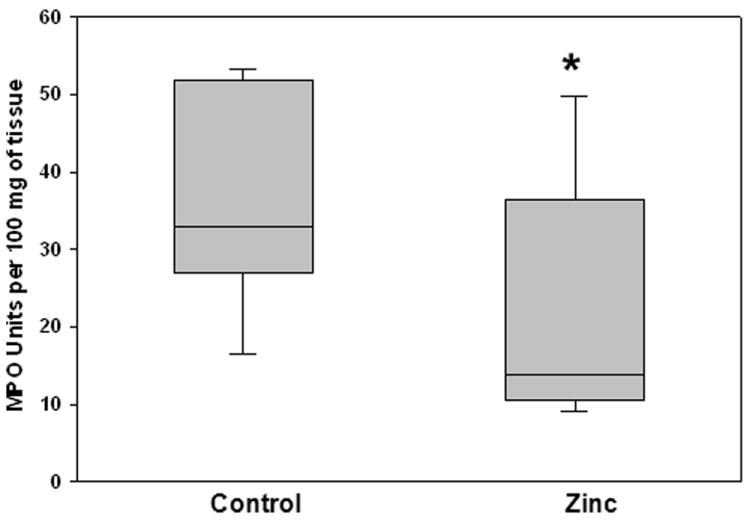
Whole lung myeloperoxidase (MPO) activity in control and zinc-supplemented animals 24 hours after fecal-slurry challenge. Data are expressed as the medians and interquartile ranges (* p < 0.05 versus control; Mann-Whitney Rank Sum Test; n = 10 animals per group).
Figure 3.
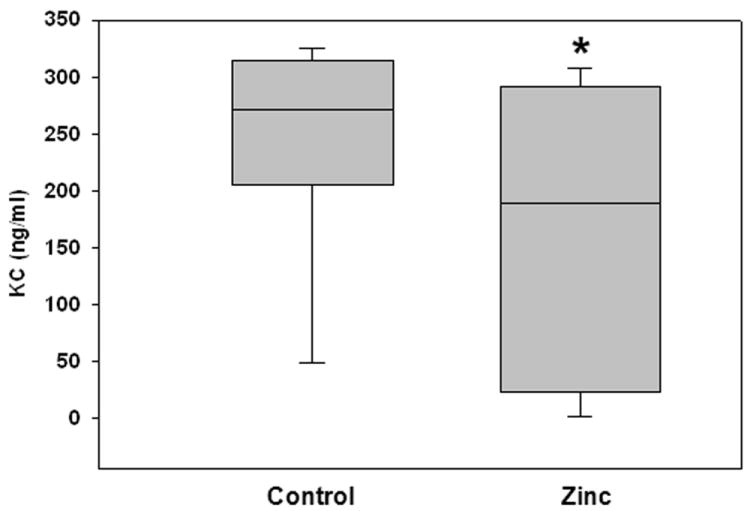
Serum levels of KC in control and zinc-supplemented animals 24 hours after fecal-slurry challenge. Data are expressed as the medians and interquartile ranges (* p < 0.05 versus control; Mann-Whitney Rank Sum Test; n = 10 animals per group).
Prophylactic zinc supplementation reduces in vivo bacterial load
Since attenuation of the inflammatory response cannot fully account for the survival advantage observed in zinc-supplemented mice, we next measured in vivo clearance of bacteria in peritoneal fluid, blood, lung, and spleen 24 hours after fecal-slurry challenge. As shown in Figure 4, colony forming units of bacteria in the blood and spleen homogenates were significantly reduced in the zinc-supplemented mice, compared to the control mice (please note that data are plotted in a log-scale). These data indicate that prophylactic zinc supplementation reduces bacterial load in mice subjected to fecal-slurry peritonitis.
Figure 4.
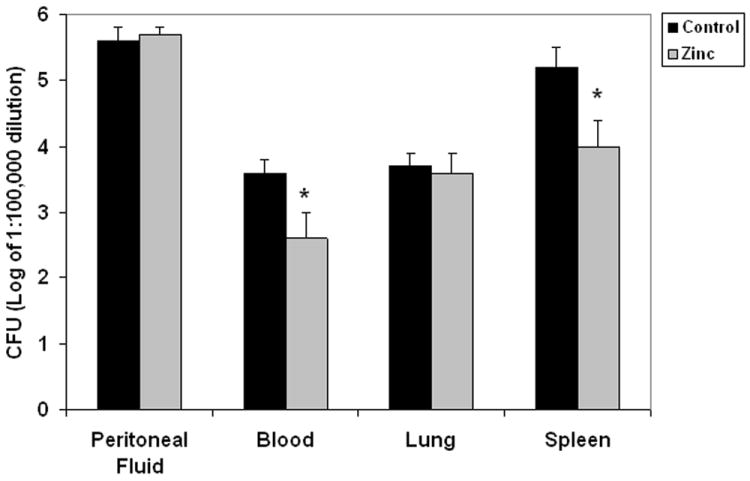
Bacterial colony counts from peritoneal fluid, blood, lung homogenates, and spleen homogenates in control and zinc- supplemented animals 24 hours after fecal-slurry challenge. Data are expressed as the mean log10 of colony forming units ± SEM (* p < 0.05 versus control; Mann-Whitney Rank Sum Test; n = 10 animals per group). Note that differences are in log scale.
Prophylactic zinc supplementation enhances ex vivo phagocytosis
Having demonstrated that prophylactic zinc supplementation reduces in vivo bacterial load, we next measured ex vivo phagocytosis of E. coli and S. aureus by peritoneal macrophages harvested from zinc-supplemented and control mice. As shown in Figure 5, peritoneal macrophages from zinc-supplemented mice had a substantially greater phagocytosis capacity for both E. coli and S. aureus, compared to peritoneal macrophages from control mice.
Figure 5.
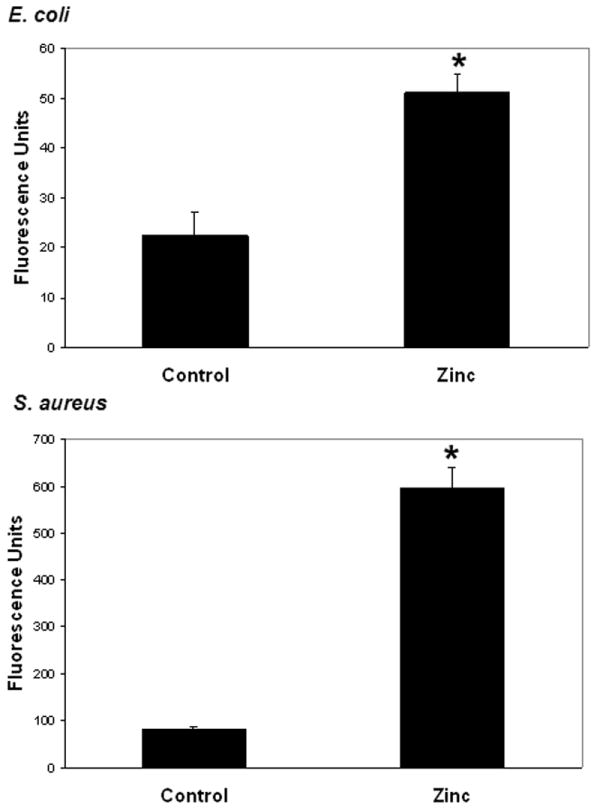
Ex vivo phagocytosis of fluorescein -labeled E. coli or S. aureus by peritoneal macrophages derived from control and zinc-supplemented animals. Data are expressed as the mean intracellular fluorescence units ± SEM (* p < 0.05 versus control; Student’s t test; n = 5 animals per group).
Prophylactic zinc supplementation mildly suppresses components of the adaptive immune system
Having demonstrated that prophylactic zinc supplementation reduces in vivo bacterial load and enhances ex vivo bacterial phagocytosis, we next assessed the effect of prophylactic zinc supplementation on the adaptive immune system. Blood samples and spleens were obtained from both control mice and zinc-supplemented mice for immune phenotyping studies. There were no significant differences in the number and types of white blood cells in the blood samples of both groups (data not shown). There were no significant differences in the number of splenic T cells, splenic B cells, splenic macrophages, and splenic dendritic cells in both groups (data not shown). As shown in Figure 6, CD11b and MHC II expression was slightly, but significantly lower in the zinc-supplemented mice compared to control mice. As shown in Figure 7, there was a slight, but significant decrease in the percentage of naïve and effector CD4 cells in zinc-supplemented mice, compared to controls, 24 hours after ex vivo stimulation with platebound anti-CD3 antibody, a T cell specific mitogen. There was also a slight, but significant decrease in the percentage of CD8 memory cells in the zinc-supplemented mice, compared to controls, 24 hours after a similar ex vivo stimulation. These data suggest that zinc supplementation has a mild suppressive effect on the adaptive immune system.
Figure 6.
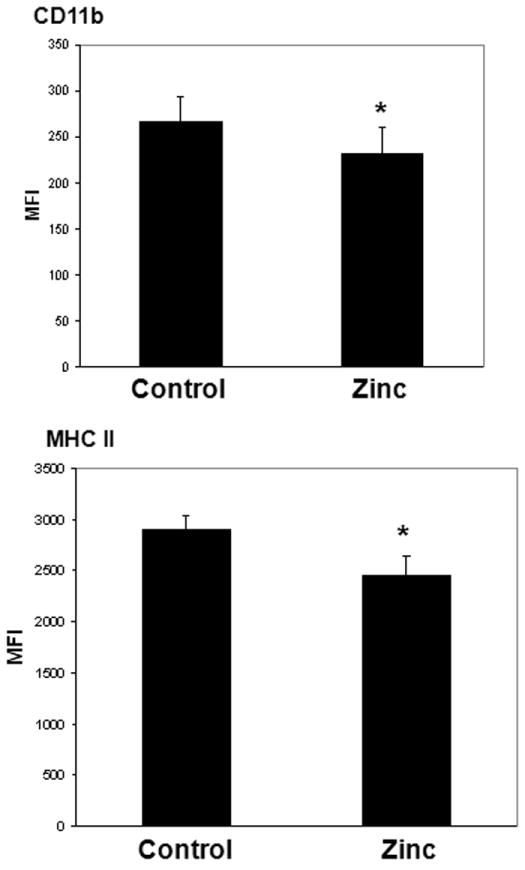
CD11b and MHC II expression on splenic macrophages derived from control and zinc-supplemented animals. Data are expressed as mean fluorescence intensity (MFI) ± S.E.M. (* p < 0.05 versus control; Student’s t Test; n = 6 animals per group). The number and percentage of splenic macrophages were not significantly different between the two groups (data not shown).
Figure 7.
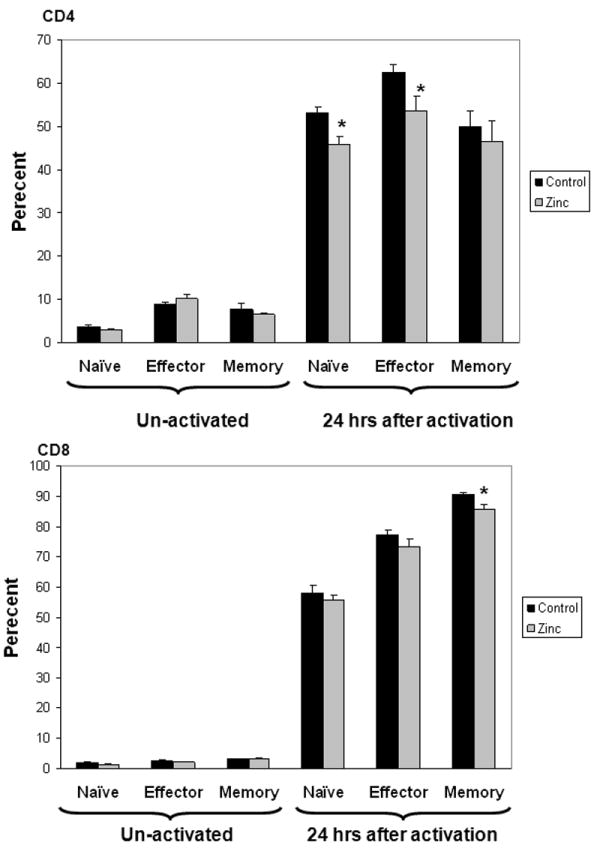
Naïve, effector, and memory CD4 and CD8 splenic T lymphocytes, respectively, before and after 24 hours of ex vivo activation in control and zinc-supplemented animals. Data are expressed as percent ± S.E.M. (*p < 0.05 versus control; Student’s t test, n = 6 animals per group). The total number and total percentage of splenic T lymphocytes were not significantly different between the two groups (data not shown).
DISCUSSION
The main finding from this study is that prophylactic zinc supplementation confers a significant survival advantage in mice subjected to fecal-slurry peritonitis, a clinically relevant model of sepsis. The mechanism of the survival advantage appears to involve a reduction in bacterial load, as demonstrated by a decreased systemic bacterial burden in zinc-supplemented mice (log fold), as well as enhanced ex vivo phagocytosis by peritoneal macrophages obtained from zinc-supplemented mice. Prophylactic zinc supplementation also appears to modestly attenuate the host inflammatory/immune response to sepsis. Thus, it appears that prophylactic zinc supplementation in mice enhances the beneficial role of the inflammatory/immune response to sepsis (i.e. pathogen eradication), while at the same time attenuating potentially detrimental excessive inflammation.
Our data are directly supported by experimental data from the Knoell laboratory demonstrating that zinc deficiency leads to increased mortality, inflammation, and organ injury in experimental sepsis (20,21). The Knoell laboratory also demonstrated that restoration of zinc, in animals previously rendered zinc deficient, partially reverses this phenotype through a mechanism that may involve decreased activity of the pro-inflammatory transcription factor, NF-κB (20,21). Our data are indirectly supported by previous studies demonstrating that zinc deficiency leads to loss of T cells and B cells via apoptosis (22), and by the demonstration that Toll-like receptor signaling is dependent on zinc homeostasis (23). Conversely, a previous study by Krones et al., involving a porcine model of endotoxemia, demonstrated that zinc administration was deleterious (24). However, it is difficult to compare this study, with our current study, because the study by Krones et al. involved endotoxin administration (as opposed bacterial peritonitis) and used a much higher dose of zinc administered simultaneously with endotoxin.
Clinical data also exist supporting a role for prophylactic zinc supplementation as a therapeutic strategy in infection-related disease processes. In clinical states associated with immune suppression, such as sickle cell disease, human immunodeficiency virus (HIV) infection, Down syndrome, and in the elderly, zinc supplementation has been shown to restore NK cell activity, lymphocyte production, mitogen responses, wound healing, and resistance to infection (25). In clinical trials involving children with acute gastroenteritis in developing countries, both duration and severity of diarrhea were significantly decreased by zinc supplementation (26,27). Additionally, zinc supplementation in children living in rural communities significantly decreased the incidence of both diarrhea and acute lower respiratory tract infections (27). Finally, a recent randomized controlled trial tested the ability of zinc supplementation, in addition to other pharmaconutrients, to reduce the rate of nosocomial infection in critically ill children (28). There was no efficacy in the overall study population, but a secondary analysis of only the immune-compromised patients demonstrated a reduction in nosocomial infections.
The experimental protocol used in the current study could be criticized as not having clinical relevance because zinc supplementation was provided before the sepsis challenge. Indeed, during initial experiments we observed that administration of zinc after the sepsis challenge conferred neither a survival advantage nor a survival disadvantage. However, sepsis is commonly a secondary complication for patients initially admitted to the intensive care unit for non-infectious conditions, and the current strategies being contemplated for zinc supplementation in critically ill patients are centered on prophylactic zinc supplementation as a means to prevent sepsis and/or to reduce the untoward effects of sepsis (28). Accordingly, the protocol that we used mimics this strategy of prophylactic zinc supplementation.
In conclusion, we have demonstrated that prophylactic zinc supplementation confers a significant survival advantage in mice subjected to sepsis. The mechanism of protection appears to primarily involve a reduction in ongoing bacterial load. While the mechanism by which zinc reduces ongoing bacterial remains to be elucidated, the data nonetheless support the concept that prophylactic zinc supplementation may be an effective therapeutic strategy in critically ill patients at risk for developing sepsis.
Acknowledgments
Supported by NIH grants T32GM008478 (J.E.N.) and R01-GM064619 (H.R.W.).
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 2.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Cvijanovich NZ, King JC, Flori HR, Gildengorin G, Wong HR. Zinc homeostasis in pediatric critical illness. Pediatr Crit Care Med. 2009;10:29–34. doi: 10.1097/PCC.0b013e31819371ce. [DOI] [PubMed] [Google Scholar]
- 4.Heyland DK, Jones N, Cvijanovich NZ, Wong H. Zinc supplementation in critically ill patients: a key pharmaconutrient? JPEN J Parenter Enteral Nutr. 2008;32:509–519. doi: 10.1177/0148607108322402. [DOI] [PubMed] [Google Scholar]
- 5.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornell TT, Wynn J, Shanley TP, Wheeler DS, Wong HR. Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics. 2010;125:1248–1258. doi: 10.1542/peds.2009-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler DS, Zingarelli B, Wheeler WJ, Wong HR. Novel pharmacologic approaches to the management of sepsis: targeting the host inflammatory response. Recent Pat Inflamm Allergy Drug Discov. 2009;3:96–112. doi: 10.2174/187221309788489779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong HR, Freishtat RJ, Monaco M, Odoms K, Shanley TP. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Pediatr Crit Care Med. 2010;11:349–355. doi: 10.1097/PCC.0b013e3181c519b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong HR, Cvijanovich N, Allen GL, Lin R, Anas N, Meyer K, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37:1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanley TP, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, et al. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med. 2007;13:495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cvijanovich N, Shanley TP, Lin R, Allen GL, Thomas NJ, Checchia P, et al. Validating the genomic signature of pediatric septic shock. Physiol Genomics. 2008;34:127–134. doi: 10.1152/physiolgenomics.00025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak JS, Wong HR. Novel therapeutic agents in pediatric sepsis -- zinc. The Open Inflammation Journal. 2011;4:107–111. doi: 10.2174/1875041901104010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 18.Solan PD, Piraino G, Hake PW, Denenberg A, O’Connor M, Lentsch A, et al. Liver X receptor alpha activation with the synthetic ligand T0901317 reduces lung injury and inflammation after hemorrhage and resuscitation via inhibition of the nuclear factor kappaB pathway. Shock. 2011;35:367–374. doi: 10.1097/SHK.0b013e3181f7d742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 20.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, et al. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–1388. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King LE, Osati-Ashtiani F, Fraker PJ. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J Nutr. 2002;132:974–979. doi: 10.1093/jn/132.5.974. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 24.Krones C, Klosterhalfen B, Fackeldey V, Junge K, Rosch R, Schwab R, Stumpf M, Klinge U, Schumpelick V. Deleterious effect of zinc in a pig model of acute endotoxemia. J Invest Surg. 2004;17:249–256. doi: 10.1080/08941930490502817. [DOI] [PubMed] [Google Scholar]
- 25.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 26.Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics. 1999;103:e42. doi: 10.1542/peds.103.4.e42. [DOI] [PubMed] [Google Scholar]
- 27.Ruel MT, Rivera JA, Santizo MC, Lonnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics. 1997;99:808–813. doi: 10.1542/peds.99.6.808. [DOI] [PubMed] [Google Scholar]
- 28.Carcillo JA, Dean JM, Holubkov R, Berger J, Meert KL, Anand KJ, Zimmerman J, Newth CJ, Harrison R, Burr J, Willson CC, Nicholson C. The randomized comparative pediatric critical illness stress-induced immune suppression (CRISIS) prevention trial. Pediatr Crit Care Med. Nov 10; doi: 10.1097/PCC.0b013e31823896ae. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


