Abstract
In humans up to 75% of newly generated B cells and about 30% of mature B cells exhibit some degree of autoreactivity1. Yet, how B cells establish and maintain tolerance in the face of autoantigen exposure during and after development is not certain. Studies of model BCR transgenic systems have highlighted the critical role played by functional unresponsiveness or ‘anergy’2,3. Unlike T cells, evidence suggests that receptor editing and anergy, rather than deletion, account for much of B cell tolerance4,5. However, it remains unclear whether the mature diverse B cell repertoire of mice contains anergic autoreactive B cells, and if so, whether antigen was encountered during or after their development. By taking advantage of a reporter mouse in which B cell antigen receptor (BCR) signaling rapidly and robustly induces GFP expression under the control of the Nur77 regulatory region, antigen-dependent and – independent BCR signaling events in vivo during B cell maturation were visualized. Here we show that B cells encounter antigen during development in the spleen, and that this antigen exposure in turn tunes the responsiveness of BCR signaling in B cells at least partly by down-modulating expression of surface IgM but not IgD BCRs, and by modifying basal calcium levels. By contrast, no analogous process occurs in naive mature T cells. Our data demonstrate not only that autoreactive B cells persist in the mature repertoire, but that functional unresponsiveness or ‘anergy’ exists in the mature B cell repertoire along a continuum, a fact that has long been suspected, but never yet shown. These results have important implications for understanding how tolerance in T and B cells is differently imposed, and how these processes might go awry in disease.
Moran et al., recently generated a novel reporter of antigen receptor (AgR) signaling to examine developmental checkpoints during thymic development6. They took advantage of the dynamic expression pattern of the orphan nuclear hormone receptor Nur77, which is rapidly induced in response to negative selection and TCR stimulation, to develop a GFP reporter BAC Tg line of mice7. Interestingly, Nur77 is also an immediate early gene that is rapidly transcriptionally upregulated in response to BCR signaling8. To visualize AgR signaling in vivo, we obtained independently generated reporter mice from the Gensat consortium in which eGFP protein expression is under the control of the Nr4a1/Nur77 regulatory region9 . Founders harbored two distinct insertion sites driving ‘high’ or ‘low’ GFP expression. These were independently backcrossed to the C57BL/6 genetic background, yielding GFPHI and GFPLO lines.
Basal expression of GFP in peripheral CD4 and CD8 T cells was higher in both of the GFPHI and GFPLO lines compared to the reporter line described by Moran et al., (Figure S1A). Although basal GFP expression in B cells was significantly higher in the GFPHI line relative to the Moran et al. reporter, the GFPLO line failed to express GFP in B cells, suggesting an isolated positional effect. For this reason, all subsequent B cell studies have focused on the GFPHI reporter. Following stimulation of thymocytes and peripheral T cells with PMA and/or ionomycin, GFP expression was rapidly induced (Figure S1B; data not shown). In vitro stimulation of either the TCR with anti-CD3 or the BCR with anti-IgM also induced GFP expression in a dose-dependent manner (Figure 1A, S1C; data not shown). GFPHI mice were crossed to the IgHEL BCR transgenic line (MD4, which recognizes hen egg lysozyme (HEL)) to generate mice with a monoclonal BCR repertoire. Such ‘MD4-GFP’ mice exhibited dose-dependent GFP induction following treatment with HEL in vitro (Figure 1B, S1D).
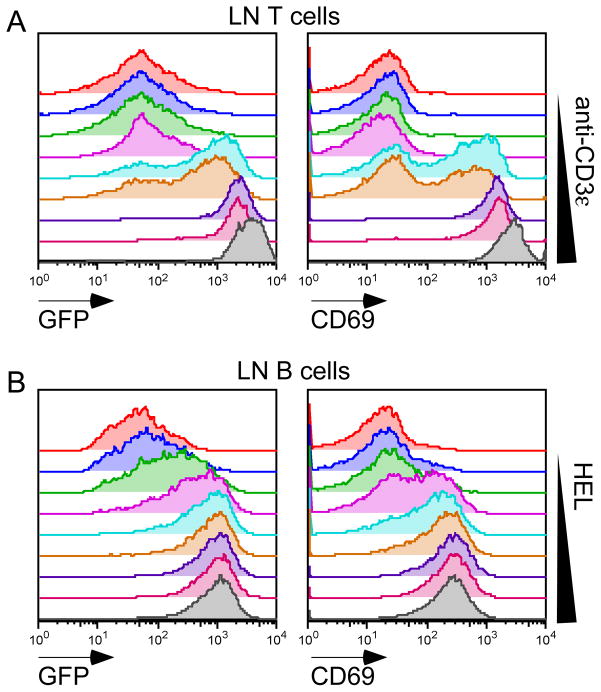
Figure 1. Nur77-GFP Bac Tg reporter is responsive to antigen receptor signaling in vitro.
(A) Histograms represent GFP and CD69 expression of GFPLO Tg lymph node (LN) T cells treated with varying doses of plate-bound anti-CD3ε for 16 hours (0.00625-6.4μg/ml in a four-fold dilution series).
(B) Histograms represent GFP and CD69 expression of IgHEL Tg / GFPHI Tg LN B cells treated with varying doses of hen egg lysozyme (HEL) for 16 hours (0.125–16 ng/ml in a two-fold dilution series).
Data are representative of at least three independent experiments.
To define which AgR-induced biochemical pathways were required to drive GFP expression, we treated anti-CD3 and anti-IgM- stimulated lymphocytes with a range of small molecule inhibitors in vitro. These experiments revealed a nearly complete dependence on Src family kinases (SFKs) in T cells and Syk kinase in B cells (Figure S1E, F). In B cells, GFP expression was partially dependent upon the PKC, calcineurin, MAPK, and PI3K pathways, while in T cells, GFP expression most clearly required PKCs (Figure S1E, F).
To define whether signals other than AgR ligation were sufficient to drive GFP expression in B cells, we treated GFPHI B cells in vitro with various stimuli. TLR4 and TLR9 ligands, along with anti-CD40, could drive GFP expression in B cells, but this effect was considerably less robust than anti-IgM stimulation (Figure S1G). Importantly, B cell activating factor (BAFF) treatment with doses as high as 200 ng/ml, sufficient to induce prolonged B cell survival, failed to induce GFP reporter expression in B cells (Figure S1G).
The reporter responded to TCR-dependent signaling in vivo, as revealed by GFP expression at TCR-dependent checkpoints during thymic development. Signaling by the preTCR, comprised of a recombined TCRβ chain and the invariant preTα chain, drives developing thymocytes to transit the beta-selection checkpoint. We observed abrupt upregulation of GFP expression at the “double negative” DN3b stage of development, precisely at the beta-selection checkpoint transition (Figure S2A).
Upon successful transit through the beta-selection checkpoint, DN thymocytes upregulate the CD4 and CD8 coreceptors, and recombine the TCRα chain to express a mature αβTCR. These cells then undergo TCR-dependent positive or negative selection. We observed marked GFP upregulation in post-selection CD69HI TCRβHI “double positive” DP thymocytes (Figure S2B), as did Moran et al6.
It has been speculated that at the border of positive and negative selection, SP4 thymocytes can be rescued from death by adopting the regulatory T cell fate. Indeed, CD25+ SP4 thymocytes expressed much higher GFP levels than conventional SP4 thymocytes suggesting strong TCR signaling favors the Treg fate, in agreement with results of Moran et al., (Figure S2C)6.
We reported that titration of CD45 expression in an allelic series of mice regulates TCR signaling during thymic development10. We crossed the GFPHI reporter onto a genetic background harboring two copies of the Lightning (L) CD45 allele, in which a point mutation in the extra-cellular domain leads to reduced surface expression of CD45 (15% of wild type in L/L mice)10. Both the fraction of high GFP-expressing cells and the average GFP content of post-selection DP thymocytes was markedly reduced in so-called L/L GFP mice (Figure S2D). This result suggests that the GFP reporter is indeed sensitive to genetic titration of TCR signal strength.
To identify analogous BCR-dependent signaling checkpoints during B cell development, we assessed successive stages of BM B cell development in GFPHI reporter mice (Figure 2A, S3A, S3B;11). We observed virtually no GFP expression except in the mature B cells that recirculate to the BM (Hardy fraction F; IgMloIgDhi), suggesting that GFP upregulation occurs sometime after the early BM stages of development despite evidence of the contribution of antigen encounter to deletion and receptor editing in the BM12.
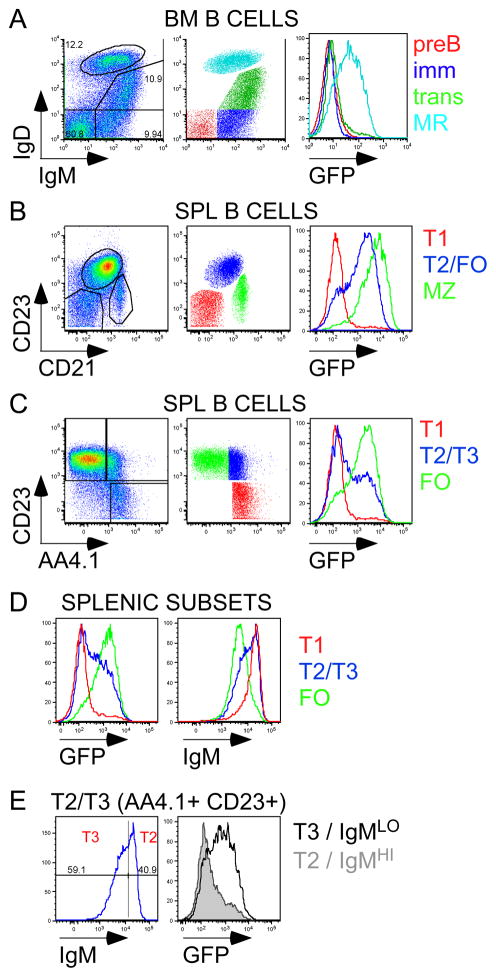
Figure 2. Expression of Nur77-GFP Bac Tg reporter is up-regulated at specific checkpoints during B cell development.
(A) Left: Plot of GFPHI Tg BM CD19+ B cells stained for IgM and IgD to identify pre-B, immature, transitional, and mature recirculating subsets (counter-clockwise from bottom left corner). Middle: BM subsets are color-coded. Right: overlaid histograms representing GFP expression in these subsets.
(B) Left: Plot of GFPHI Tg Splenic CD19+ B cells stained for CD23 and CD21 expression to identify NF/T1 (CD23-CD21−), T2/FO (CD23+CD21int), and MZ (CD21hi) subsets. Middle: Splenic B cell subsets are color-coded. Right: overlaid histograms represent GFP expression in these subsets.
(C) Left: Plot of GFPHI Tg Splenic CD19+ B cells, excluding MZ compartment, stained for CD23 and AA4.1 expression to identify T1 (AA4.1+CD23−), T2/3 (AA4.1+CD23+), and FO (AA4.1-CD23+) subsets. Middle: Splenic B cell subsets are color-coded. Right: overlaid histograms represent GFP expression in these subsets.
(D) Overlaid histograms represent GFP (left) and IgM (right) expression in T1, T2/3, and FO subsets as identified in 2C.
(E) Left: T2/3 (AA4.1+CD23+) B cell subset subdivided by IgM expression into T2 (IgMhi) and T3 (IgMlo) stages. Right: overlaid histograms represent GFP expression T2 and T3 subsets.
All data are representative of at least three independent experiments.
Splenic B cell development, which follows maturation in the BM, is subdivided into successive transitional stages13–15. We observed a bimodal distribution of GFP expression among splenic B cells and found that early transitional B cells (T1) are largely GFP-negative but later transitional stages (T2/T3) contained a large proportion of GFP-positive B cells (Figure 2B, C). Mature follicular B cells were mostly GFP positive and exhibited a broad distribution of GFP expression (Figure 2C). Importantly, a similar pattern of GFP expression, albeit much lower levels, was evident in an independently generated GFP reporter (Figure S3C;6). GFP expression across these splenic developmental stages inversely correlated with surface IgM expression (Figure 2D). Transitional B cell stages previously have been subdivided into T2 and T3 stages on the basis of surface IgM down-regulation (Figure 2E;15). We observed that GFP upregulation appears to occur at precisely this transition between T2 and T3 stages (Figure 2E, S3D,E).
Interestingly, in vitro BCR stimulation of BM and splenic B cell subsets resulted in GFP upregulation to differing extents. Minimal GFP upregulation was seen in BM immature and transitional stages, but robust upregulation was evident in splenic T1, T2, and follicular B cells (Figure S4). This argues that splenic, but not BM subsets, have the capacity to upregulate GFP.
To determine whether the amount of GFP expression in unstimulated B cells reflected BCR signal strength/antigen exposure, we took advantage of our previously characterized allelic series of CD45-expressing mice10,16. In these animals, CD45 expression is genetically varied across a broad range and correlates with BCR signal strength16. L/L mice expressing reduced surface expression of CD45 exhibit impaired BCR signal transduction. H/- mice express a normally splicing CD45 Tg superimposed on endogenous wild type CD45 to produce an animal with supraphysiologic CD45 expression. B cells from these mice exhibit enhanced BCR signal strength. Upon crossing the Nur77-GFP reporter mouse to the CD45 allelic series, we noted that GFP expression at the T1 stage was unaffected, while increasing CD45 expression resulted in a higher proportion of GFP positive B cells at the T2 stage (Figure 3A, S5A). Importantly, the distribution of GFP expression in this compartment remained bimodal, further supporting the notion that a discrete signaling event occurs at this stage, the threshold of which is regulated by CD45 and BCR signal strength. GFP expression in follicular mature B cells was markedly reduced in L/L mice, consistent with a reduction in BCR signal strength, but was minimally altered in H/- mice with higher CD45 expression (Figure 3A, S5B). However, modulation of GFP expression by CD45 was much more apparent in the MZ compartment, suggesting their exquisite sensitivity to BCR signal strength (Figure 3A, S5B).
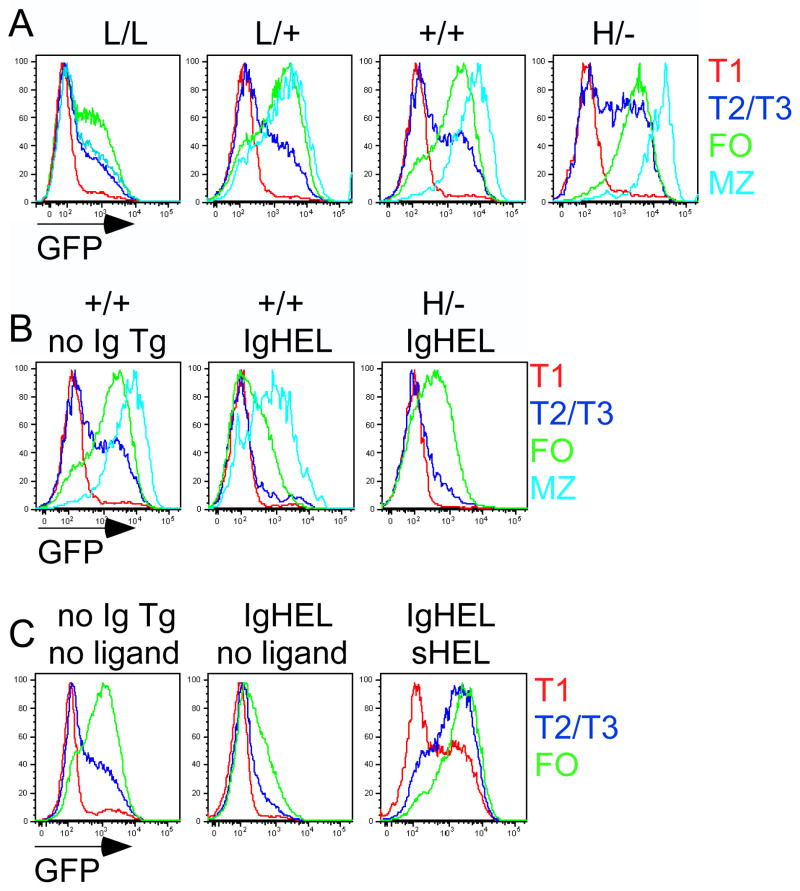
Figure 3. Expression of Nur77-GFP Bac Tg reporter is sensitive to genetic modulation of BCR signal strength and antigen.
(A) CD45 allelic series (low to high CD45 expression: L/L, L/+, +/+, H/−) GFPHI Tg splenic B cells were stained to identify B cell subsets as in Figure 2B, C. Overlaid histograms represent GFP expression in splenic subsets as gated in S5A.
(B) CD45+/+ GFPHI Tg and H/- (high CD45) GFPHI Tg splenic B cells with unrestricted (IgHEL-) or restricted (IgHEL+) repertoire in the absence of sHEL antigen were analyzed as in 3A. Overlaid histograms represent GFP expression in splenic subsets as gated in S5C.
(C) CD45+/+ GFPHI Tg splenic B cells with unrestricted (IgHEL-) or restricted (IgHEL+) repertoire in the presence or absence of sHEL antigen were analyzed as in 3A. Overlaid histograms represent GFP expression in splenic subsets as gated in S5D. All animals in Figure 3 were generated through genetic crosses. All data are representative of at least five independent experiments.
Since Nur77-GFP expression is regulated by modulation of BCR signal strength (Figure 3A, S5B), we hypothesized that endogenous antigen exposure might drive BCR signaling during maturation of wild type B cells with a diverse repertoire. To explore this possibility, we took advantage of the MD4/ML5 system in which mice with a monoclonal IgHEL BCR Tg can be studied in the presence or absence of soluble ligand (sHEL)2. In the Nur77-GFP reporter mice with the IgHEL BCR Tg restricted repertoire in the absence of antigen, we observed a marked reduction in GFP in splenic B cells (Figure 3B, S5B, C). Importantly, the bimodal distribution of GFP expression observed in the context of a wild type repertoire was lost in this context. Further increasing CD45 expression in the context of such a restricted repertoire to increase tonic BCR signaling resulted in increasing GFP expression, but again only in a unimodal rather than bimodal distribution (Figure 3B, S5C). Finally, introduction of sHEL ligand by crossing ML5 (sHEL Tg) mice to IgHEL Tg reporter mice resulted in increased GFP expression as expected, and remarkably reconstituted bimodal GFP expression in the transitional splenic stages of development (Figure 3C, S5D, S6). These data suggest that normal B cell development is characterized by a wide range of antigen experience, and that Nur77-driven GFP distribution in follicular mature B cells serves as a marker of such exposure.
To determine whether antigen recognition during splenic B cell development had functional consequences on signaling, we selectively gated on the extremes of GFP expression. We observed that high GFP-expressing B cells had dampened S6 kinase phosphorylation and calcium entry relative to low GFP-expressing B cells in response to IgM ligation (Figure S7A, 4A). Moreover, we observed that basal calcium levels were elevated in high-GFP expressing B cells, reminiscent of anergic B cells identified in various model BCR transgenic systems17,18. Dampened inducible signaling and increased basal calcium were not isolated properties of very high GFP-expressing B cells, but rather appeared to represent continuous functional properties across the entire spectrum of GFP expression of mature follicular B cells (Figure 4A). Furthermore, restricting the BCR repertoire in the absence of ligand ablated differences in functional responsiveness, but not in basal calcium (Figure S7B). Importantly, neither inducible calcium responses, nor basal calcium levels correlated with GFP expression in naive CD25-CD4+ T cells, suggesting that only in B cells does antigen exposure tune functional responsiveness (Figure S8A).
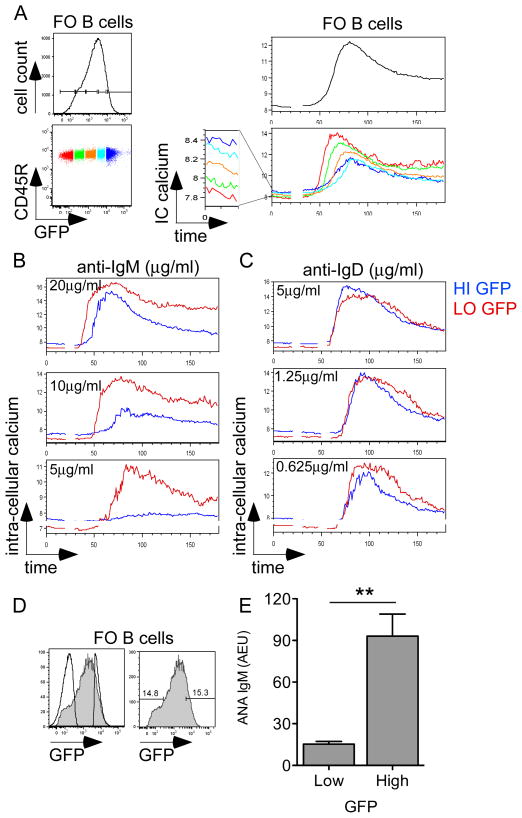
Figure 4. GFP expression predicts functional responsiveness and autoreactivity of B cells.
(A) Left: GFPHI Tg FO (CD23+AA4.1-) splenic B cells were subdivided into color-coded bins on the basis of GFP expression. Right: GFPHI Tg splenic B cells were loaded with Indo-1 dye and stimulated with 10μg/ml anti-IgM. Ratio-metric assessment of intra-cellular calcium was carried out by flow cytometry. Upper right panel represents calcium entry in total FO splenic B cells. Lower right panel represents basal and inducible intra-cellular calcium in GFP-specific bins.
(B, C) Intra-cellular calcium entry was assessed in GFPHI Tg FO splenic B cells upon anti-IgM (B) or anti-IgD (C) stimulation at varying doses. High and low GFP-expressing gates are overlaid.
Data in 4A-C are representative of at least three independent experiments.
(D) Overlaid histograms represent pre- and post-sort follicular mature CD23+ AA4.1- B cells as gated in S9. 15% lowest and highest GFP fractions from the follicular mature B cell compartment were selected for sorting.
(E) High and low-expressing GFP B cells sorted as described in S9A, 4C were stimulated in vitro with LPS for 4 days. Supernatants were subjected to ANA IgM ELISA and total IgM ELISA. Graph represents quantification of ANA IgM normalized to total IgM from 4 independent sorting experiments +/− SEM. Significance was assessed via unpaired t-test. ** = p<0.005
Mature B cells express two isotypes of the BCR, IgM and IgD. We wanted to determine whether the functional responsiveness in GFP B cells was modulated in response to stimulation through the IgD BCR in the same manner as it is to the IgM BCR. We found that this was not the case (Figure 4B, C); responsiveness to IgM BCR stimulation was markedly blunted in cells with high GFP expression, while IgD responsiveness remained intact. Stimulation with anti-kappa antibodies to ligate both surface IgM and IgD resembled isolated IgD stimulation (Figure S8B). By simultaneously staining for surface IgM expression with a non-stimulatory monovalent Fab fragment and assessing calcium responses in GFP B cells, we show that differences in surface IgM expression largely accounted for the functional differences at different levels of GFP expression (Figure S8C). However, basal calcium differences were independent of surface IgM expression (Figure S8D).
Additional characteristics of monoclonal Tg anergic B cells include failure to upregulate activation markers in response to various stimuli3. We stimulated sorted high and low GFP-expressing B cells and observed that activation marker upregulation in response to IgM stimulation is impaired in high GFP-expressing B cells (Figure S9, S10). Importantly, responses to LPS and CD40 were unaffected, as was in vitro survival in the presence or absence of BAFF (data not shown).
Finally, to determine directly whether the BCR repertoire of mature B cells with high GFP expression and impaired functional responses was indeed autoreactive, sorted high and low GFP-expressing B cells were polyclonally stimulated in vitro with LPS, and secreted antibody was assessed for anti-nuclear antibody (ANA) reactivity. (Figure S9, 4D, E). Importantly, neither cell proliferation, nor antibody secretion following LPS stimulation were impaired in high GFP B cells (data not shown). We found a significant increase in ANA reactivity suggesting auto- or poly-reactivity in the repertoire of such naturally occurring anergic B cells (Figure 4D, E).
The human B cell repertoire is characterized by a high prevalence of polyreactive/autoreactive BCRs1,19. Anergy or functional unresponsiveness may serve to keep such autoreactive clones in check3. Array data have revealed that wild type B cells exhibit an intermediate phenotype between antigen-naive and anergic B cells, suggesting the possible presence of anergic B cells in the wild type mature repertoire20,21. It has been recently argued that the so-called T3 splenic subset may in fact represent sequestered anergic B cells rather than an intermediate developmental stage22,23. However, it has been uncertain how prevalent anergy is in the normal mature B cell repertoire21. We show that there is a continuum of anergy or unresponsiveness to anti-IgM stimulation in the mature B cell compartment, and that this responsiveness is, in turn, tuned by developmental antigen recognition.
It has long been observed that marked IgM downregulation is seen in BCR transgenic systems in the presence of either antigen or enhanced BCR signal strength2,16,19,24–26. IgD, by contrast, remains relatively unmodulated in these systems. Here, we show that in the wild type B cell repertoire, IgM downregulation correlates with the extent of antigen recognition during development and accounts for dampened B cell responses to anti-IgM stimulation. By contrast, IgD expression and responses are intact. We suggest that this constitutes a general mechanism to modulate BCR signaling in autoreactive B cells, but permit them to persist as a pool of extended Ab specificity for purposes of protective immunity. Indeed, we demonstrate an increased proportion of ANA-reactive BCR specificities in high GFP expressing B cells, suggesting that these cells are auto- or poly-reactive. It is tempting to speculate that this large reservoir of dormant autoreactive B cells in the mature BCR repertoire may serve as the source of pathogenic autoantibodies that characterize rheumatic diseases such as systemic lupus erythematosus.
Online Materials and Methods
Mice
The CD45 allelic series including Lightning (L/L) and H/H, H/- (HE) mice have been previously described10,16,27, as have IgHEL (MD4) and sHEL (ML5) mice2. Nur77-eGFP BAC Tg mice were obtained from the Gensat consortium9 . Nur77-GFP reporter mice described in Moran et al. were generously supplied by the Hogquist lab28. All strains were backcrossed to C57BL/6 genetic background at least six generations. Mice were used for all functional and biochemical experiments at age 5–9 wk. All mice were housed in a specific pathogen-free facility at University of California, San Francisco in accordance with the University’s Animal Care Committee and National Institutes of Health guidelines.
Abs and other reagents
The following antibodies were used: antibodies to murine CD1d, CD4, CD5, CD8, CD11b, CD11c, CD19, CD21, CD23, CD24, CD25, CD43, CD44, CD69, CD93 (AA4.1), BP-1, IgD, IgM, pNK, γδTCR, TCRβ were conjugated to FITC, PE, PerCP-Cy5.5, PE-Cy5.5, PE-Cy7, Pacific blue, APC, or Alexa 647 for FACS staining (eBiosciences or BD Biosciences); phospho-S6 kinase Alexa 488 (2F9) antibody for intracellular staining; unconjugated CD3ε (2C11) antibody (Harlan); goat anti-armenian hamster Ig (H+L), goat anti-mouse IgM Fab’2 for stimulation and Fab fragment coupled to Alexa 647 for surface staining (Jackson Immunoresearch); biotinylated anti-IgD (BD); streptavidin (Sigma); Mouse IgM-UNLB, mouse IgH+L-UNLB, goat anti-mouse IgM biotin, and streptavidin-HRP for ELISA, and goat anti-mouse kappa for stimulation (Southern Biotech). Inhibitors and stimuli include: ionomycin 1μM, PKC inhibitors: Go-6983 40 nM, Ro-32-0432 40 nM, Bay 61-3606 10 μM, CSA 20μM, PP2 20μM, Ly-294002 20 nM (Calbiochem); PMA 0.02μg/ml, CHX 10μg/ml, LPS 50μg/ml, HEL (Sigma); U0126 10μM (Cell signaling); CpG 2μM (Invivogen); anti-CD40 1 μg/ml (BD); BAFF 200 ng/ml (R&D).
Flow cytometry and data analysis
Cells were stained with antibodies of the indicated specificities and analyzed on a FACSCalibur or Fortessa (BD Biosciences) as described previously30. Data analysis was performed using FlowJo v8.8.4 (Treestar). Statistical analysis and graphs were generated using Prism v4c (GraphPad Software).
In vitro lymphocyte stimulation (+/− inhibitor)
Single cell suspensions of lymphocytes were plated at a concentration of 1.5x106 cells/ml in complete DMEM and were incubated in the presence of various stimuli and/or inhibitors at doses described above for 16 hours. Assays were performed as previously described29.
Calcium measurements
Assays were performed as previously described29 except Indo-1 dye (Invitrogen) was used to load cells, and a UV laser on the BD Fortessa was used for detection. Prior to stimulation and analysis, splenocytes were surface stained for expression of CD23 and AA4.1 to identify B cell subsets. Where noted, cells were also pre-stained with anti-IgM Fab fragments to identify surface IgM expression without inducing BCR stimulation. Stimulation was carried out using either varying doses of anti-IgM Fab’2, anti-kappa Ab, or biotinylated anti-IgD followed by streptavidin cross-linking (15 μg/ml), or varying doses of anti-CD3ε followed by goat anti-armenian hamster Ig cross-linking (50 μg/ml).
Intracellular phospho-S6 kinase staining
Staining and stimulation performed as previously described16.
B cell sorting and stimulation
GFP high and low-expressing B cells were sorted using a Moflo cell sorter. Splenic and LN cells were pooled, stained for CD23 and AA4.1 as well as DAPI to identify CD23+ AA4.1- mature B cells. Highest and lowest 15% GFP B cells were retrieved and were incubated with varying stimuli. Sorted cells were plated at a concentration of 1.5x 106 cells/ml in complete DMEM and were stimulated with anti-IgM Fab’2 at varying doses for 16 hours. Cells were then stained for CD69 expression in order to assess activation marker upregulation. Alternatively, sorted cells were incubated with 10 μg/ml LPS at a concentration of 6x106 cells/ml in complete DMEM media in order to drive polyclonal antibody secretion. Supernatants were then harvested and subjected to ELISA assays.
ELISA assays
ELISA to detect total IgM was performed as previously described30. ANA ELISA kit obtained from Inova, Inc. was used per manufacturer’s instructions. Biotinylated anti-mouse IgM (1:5000) and streptavidin-HRP conjugate (1:4000) were used for detection in both assays for signal amplification (Southern Biotech), and slow kinetic TMB (Sigma) was used as substrate. Molecular devices SpectraMax and SoftMax Pro software were used to read plates. ANA IgM quantification was normalized to total IgM for each sample.
Supplementary Material
Acknowledgments
We thank Al Roque for assisting with animal husbandry. We thank Zhi-en Wang and Jonathan Paw for help with cell sorting. This work was supported by the Rosalind Russell Medical Research Foundation Bechtel Award (to J.Z.), an American College of Rheumatology REF Rheumatology Investigator Award (to J.Z.), an Arthritis National Research Foundation grant (to J.Z.), and National Institutes of Health Grants K08 AR059723 (to J.Z.) as well as the Howard Hughes Medical Institute (to AW).
Footnotes
Author contribution: J.Z. and A.W. designed research; J.Z. and R.P. performed research; J.Z. and R.P. analyzed data; and J.Z. and A.W. wrote the paper.
References
- 1.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 3.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang J, et al. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med. 1997;186:1513–1522. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halverson R, Torres R, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 6.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine. 2011 doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109 (Suppl):S57–66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 8.Mittelstadt PR, DeFranco AL. Induction of early response genes by cross-linking membrane Ig on B lymphocytes. J Immunol. 1993;150:4822–4832. [PubMed] [Google Scholar]
- 9.The Gene Expression Nervous System Atlas (GENSAT) Project, NINDS Contract # N01NS02331 to The Rockefeller University (New York, NY).
- 10.Zikherman J, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 13.Loder F, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends Immunol. 2003;24:343–349. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 15.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 16.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci U S A. 2011;109:E3–12. doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke M, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunol Rev. 2010;237:249–263. doi: 10.1111/j.1600-065X.2010.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duty JA, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glynne R, et al. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature. 2000;403:672–676. doi: 10.1038/35001102. [DOI] [PubMed] [Google Scholar]
- 21.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 22.Merrell K, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Teague BN, et al. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol. 2007;178:7511–7515. doi: 10.4049/jimmunol.178.12.7511. [DOI] [PubMed] [Google Scholar]
- 24.Cornall RJ, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 25.Benschop RJ, et al. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 26.Fields ML, Erikson J. The regulation of lupus-associated autoantibodies: immunoglobulin transgenic models. Curr Opin Immunol. 2003;15:709–717. doi: 10.1016/j.coi.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Virts EL, Diago O, Raschke WC. A CD45 minigene restores regulated isoform expression and immune function in CD45-deficient mice: therapeutic implications for human CD45-null severe combined immunodeficiency. Blood. 2003;101:849–855. doi: 10.1182/blood-2002-07-1969. [DOI] [PubMed] [Google Scholar]
- 28.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zikherman J, et al. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. The Journal of Immunology. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23 :635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.