Abstract
Mice with a mutation in the Clock gene (ClockΔ19) have a number of behavioral phenotypes that suggest alterations in dopaminergic transmission. These include hyperactivity, increased exploratory behavior, and increased reward value for drugs of abuse. However, the complex changes in dopaminergic transmission that underlie the behavioral abnormalities in these mice remain unclear. Here we find that a loss of CLOCK function increases dopamine release and turnover in striatum as indicated by increased levels of metabolites HVA and DOPAC, and enhances sensitivity to dopamine receptor antagonists. Interestingly, this enlarged dopaminergic tone results in downstream changes in dopamine receptor (DR) levels with a surprising augmentation of both D1- and D2-type DR protein, but a significant shift in the ratio of D1:D2 receptors in favor of D2 receptor signaling. These effects have functional consequences for both behavior and intracellular signaling, with alterations in locomotor responses to both D1-type and D2-type specific agonists and a blunted response to cAMP activation in the ClockΔ 19 mutants. Taken together, these studies further elucidate the abnormalities in dopaminergic transmission that underlie mood, activity, and addictive behaviors.
Introduction
The circadian rhythm, evolution’s innate timekeeping system, plays a central role in the regulation of the normal physiology and behavior of nearly every living organism (Ko and Takahashi, 2006). The desynchronization of one’s “clock” in the form of genetic mutations, disruptions in sleep/wake cycle, or dysregulation of circulating hormones is a shared feature of numerous health problems including many psychiatric diseases (Barnard and Nolan, 2008; Takahashi et al., 2008). Numerous studies have found alterations in physiological rhythms including sleep/wake activity, body temperature, blood pressure, and hormones in major depression and bipolar disorder (BD) (Atkinson, 1975; Linkowski et al., 1994; Linkowski et al., 1987). Indeed, the cycling nature of BD (including seasonal variations in mood states) led to the first postulations that there was a circadian component to the pathology of the disease (Cassidy and Carroll, 2002; McClung, 2007; Sayer et al., 1991). More recently, human genetics studies have identified single nucleotide polymorphisms (SNPs) and haplotypes in various circadian genes that associate with psychiatric disorders. For example, cryptochrome 1 (Cry1) and neuronal PAS domain-containing protein 2 (Npas2) have a statistically significant association with major depression while circadian locomotor output cycles kaput (Clock) and vasoactive intestinal peptide (Vip) are associated with BD (Soria et al., 2010). Finally, many of the common treatments for these conditions including mood stabilizing agents and antidepressants appear to alter or synchronize the internal clock (Possidente et al., 1992; Welsh and Moore-Ede, 1990).
The circadian clock is set by a core loop of proteins that usually cycle over a period of approximately 24 hours. Essential elements of this core loop include the transcription factors CLOCK and brain and muscle Arnt-like protein-1 (BMAL1) which heterodimerize and bind to E-box elements within a number of genes regulating their transcription (Ko and Takahashi, 2006; Takahashi et al., 2008). The CLOCK-BMAL1 dimer positively regulates the Period (Per) and Cryptochrome (Cry) genes. The PER and CRY proteins themselves can form a complex, and upon re-entry into the nucleus inhibit their own transcription by repressing the function of CLOCK-BMAL1 in a negative feedback loop (Ko and Takahashi, 2006). In addition to this core loop, there are a number of other proteins implicated in regulating the timing mechanism through diverse modifications (Cardone et al., 2005; Grimaldi et al., 2009; Katada and Sassone-Corsi, 2010; Tataroglu and Schafmeier, 2010). Though the master pacemaker lies within the suprachiasmatic nucleus (SCN) of the hypothalamus, virtually every cell in the body possesses an auxiliary clock which can be synchronized to the SCN or in some cases oscillate semi-autonomously (Ko and Takahashi, 2006).
Mounting evidence supports a role for the regulation of diverse neurotransmitter systems by the circadian clock. Dopamine and other neurotransmitters implicated in mood disorders have diurnal rhythms with regard to their levels, and the activity and expression of their receptors or enzymes associated with their metabolism (Akhisaroglu et al., 2005; Ozaki et al., 1993; Wirz-Justice, 1987). Mice with a mutation in the Clock gene (ClockΔ 19 mutants) display changes in dopaminergic transmission consistent with an overall increase in dopaminergic activity (Dzirasa et al., 2010; McClung et al., 2005). Moreover, these mice have a behavioral phenotype that closely models human bipolar mania including disrupted circadian rhythms, hyperactivity, decreased depression-related behavior, lowered levels of anxiety, and increased preference for multiple drugs of abuse (Gekakis et al., 1998; King et al., 1997; McClung et al., 2005; Roybal et al., 2007). Aberrant monoamine function has been proposed to contribute to the pathology of many psychiatric diseases partially because drugs that act on their transporters or receptors are effective treatments (Barchas, 1999). As a result, numerous studies have examined the association between dopamine signaling and psychiatric disease. For example, a recent study provided evidence for an interaction between the catechol-O-methyltransferase (COMT) Val158Met allele and the DRD3 Ser9Gly genotypes in bipolar I disorder (Lee et al., 2011). Interestingly, ClockΔ19 mice have a defect in the ability of neurons in limbic circuits of the brain to synchronize as animals are performing behavioral tasks which likely contributes to their overall manic-like profile (Dzirasa et al., 2010; Dzirasa et al., 2011). It is possible that this lack of synchronization in striatal regions is due to changes in dopamine receptor function in response to an altered dopaminergic tone. Thus, in this study, we examined the influence of the ClockΔ 19 mutation on dopaminergic transmission in the striatum.
Materials and Methods
Animals
ClockΔ 19 mutant mice were created by N-ethyl-N-nitrosurea mutagenesis and produce a dominant-negative CLOCK protein defective in transcriptional activation activity as described (King et al., 1997). For all experiments using ClockΔ 19 mutants, adult male mutant (Clock/Clock) and wild type (+/+; WT) littermate controls on a mixed BALBc/C57BL/6J background were group housed in sets of 2–4 per cage on a 12/12-h light dark cycle (lights on at 6:00 a.m. = Zeitgeber time (ZT) 0, lights off at 6:00 p.m. = ZT 12) with food and water provided ad libitum. All animal use was conducted in accordance with the National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committees of UT Southwestern Medical Center.
Drugs
(−)-Quinpirole hydrochloride (LY 171,555), R(+)-SKF 81297 hydrobromide, S(−)-Raclopride (+)tartrate salt, R(+)-SCH 23390 hydrochloride, and forskolin were purchased from Sigma-Aldrich. Cocaine hydrochloride was provided by the National Institute on Drug Abuse (NIDA).
Neurochemistry
Dorsal striatum dopamine measurements were performed as described previously (Goldberg et al., 2003). Briefly, fresh brains were dissected from adult animals and 1 mm coronal slices taken using a mouse brain matrix. Tissue punches of the dorsal striatum 1 mm in diameter were placed in 1.5 ml microcentrifuge tubes, weighed, and frozen on dry ice. The samples were stored at −80°C until further processing. The tissue punches were homogenized by sonication in 49 volumes of 0.1 N perchloric acid and 0.2 mM sodium bisulfite. The homogenate was centrifuged at 20.000 g for 10 min at 4°C to pellet the debris. Twenty-five microliters of the resulting homogenate was loaded into an autosampler connected to a high-performance liquid chromatography instrument with an electrochemical detector (ESA CoulArray with Model 5014B Microdialysis Cell) to measure the levels of dopamine and dopamine metabolites homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC). Neurotransmitter levels were normalized to tissue weight.
Locomotor activity
Mice were individually placed in automated locomotor activity chambers equipped with infrared photobeams (San Diego Instruments) and measurements began immediately. Fine and ambulatory motor activity of the animals was continuously measured with the data collected in 5-min bins. Locomotor activity dose-response studies were performed in a randomized Latin-square design. Mice were moved to the behavioral testing room 1 h prior to the habituation period each day. The first hour of each locomotor activity session was measured as baseline activity. After this time, the animals received an injection of vehicle or the indicated dose of each drug and locomotor activity was measured for an additional hour. Locomotor activity data is reported as percent change in activity, while raw beam break data is shown in Supplementary Figure 1.
Quantitative immunoblotting
Animals were sacrificed by cervical dislocation, their brains removed, and 1 mm slices prepared using a brain block. Fresh dorsal striatum tissue punches were dissected and frozen on dry ice for later processing. The tissue punches were thawed and homogenized by sonication in RIPA buffer (Pierce) containing protease inhibitors and phosphatase inhibitor cocktails I and II (Sigma-Aldrich). A DC protein assay (Biorad) was performed on these total homogenates to ascertain protein concentrations. Proteins were boiled for 10 min at 90°C in loading buffer with DTT. Total protein fractions were separated by 12% SDS-PAGE and immunoblotted using antibodies specific for D1 (1:2000, GeneTex) or D2 (1:1000, Millipore) DRs, or glyceraldehyde 3-phospate dehydrogenase (GADPH) (1:10000, Fitzgerald). Horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) were used in combination with the Supersignal West Dura system (Promega) to detect the signal on film. Densitometry was conducted using NIH IMAGE software. Data were expressed as a ratio to the corresponding GADPH signal to control for potential discrepancies in protein loading.
Acute slice pharmacology and quantitative immunoblotting
Preparation and incubation of striatal slices was performed as reported previously (Hamada et al., 2004). Wild type and ClockΔ 19 mutant mice were sacrificed by decapitation. Their brains were rapidly dissected and placed in ice-cold oxygenated Krebs buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 10 mM D-glucose, 1.5 mM CaCl2, 1.5 mM MgSO4, 1.25 mM KH2PO4). The striatum was microdissected from 350 μm coronal sections prepared on a vibratome. Individual slices were placed in polypropylene incubation tubes with 2 ml of fresh Krebs buffer containing 10 μg/ml adenosine deaminase (Sigma) and pre-incubated at 30°C with continuous oxygenation with 95% O2/5% CO2 for 60 min. The Krebs buffer was replaced with fresh solution after the first 30 min. Slices were then treated with Krebs buffer alone, or buffer containing forskolin (10 μM) for 5 min. After the treatments, slices were transferred to eppendorf tubes, quickly frozen on dry ice, and stored at −80°C until processed for immunoblotting. The samples were sonicated in boiling lysis buffer (1% SDS with 50 mM sodium fluoride) and boiled for an additional 10 min. The protein concentration of each sample was determined by the DC protein assay (Biorad). Aliquots of sample were combined in Laemmli SDS sample buffer (Bio-Rad), and boiled at 95°C for 5 min. Equal amounts of protein (~75 μg) were loaded onto 10% polyacrylamide gels and separated by SDS/PAGE electrophoresis at 150 V for 5 h. Proteins were transferred overnight at 4°C onto Immobilon PVDF membranes (Millipore,) at 35V in 1XTG buffer. Membranes were incubated overnight at 4°C with the following primary antibodies diluted in blocking buffer: mouse anti-GluR1 (1:3000), and rabbit anti-phospho-GluR1 ser845 (1:2000) from Millipore Corporation, mouse anti-p44/42 MAPK (ERK1/2) (1:1000), and rabbit phospho-p44/42 (Thr202/Tyr204) (1:2000) from Cell Signaling Technology, and tubulin as loading control (Epitomics). Blots were probed simultaneously for total and phosphorylation state-specific antibodies because infrared IR-Dye 680 conjugated goat anti-rabbit and IR-Dye 800 conjugated goat anti-mouse secondary (1:10,000, LI-COR Biosciences) antibodies were used for two-color detection. Blots were scanned using the Odyssey Infrared Imaging System (LI-COR Biosciences) interfaced to a PC running Odyssey 2.1 software for quantification. Proteins bands were quantified using an integrated intensity and mean band background subtraction method. These values were then expressed as a ratio to the corresponding tubulin integrated intensity to control for potential discrepancies in amount of protein loaded among samples. Phospho-protein levels were normalized to total protein values.
Statistics
All data were analyzed using an unpaired student’s t-test or an ANOVA followed by a Bonferonni post-hoc test, unless otherwise specified. Analyses over time were conducted using a repeated two-way ANOVA followed by a Bonferonni post-hoc test to control for multiple comparisons. Analyses were conducted using the GraphPad Prism 5 statistical software for Windows. All data are presented as means ± standard error of the mean with p< 0.05 considered statistically significant. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Results
Dopaminergic transmission is altered in the striatum of ClockΔ19 mice
Since the locomotor hyperactivity of the ClockΔ19 mice (McClung et al., 2005; Roybal et al., 2007) suggests an increase in dopaminergic signaling in dorsal striatum, we decided to measure levels of dopamine and metabolites in this region. To determine if there were significant changes in dopamine transmission in the striatum, we performed HPLC on tissue preparations from ClockΔ19 mice compared to controls. Here we found no significant difference in total dopamine levels between wild type and ClockΔ 19 mutants. However, when we looked at dopamine metabolites and dopamine turnover, significant differences emerged. There was an increase in DOPAC (T(22) = 2.983; p < 0.01) and HVA (T(23) = 4.858; p < 0.0001) in the mutant mice. When dopamine turnover was assessed, we found no changes in the ratio of DOPAC/DA but there was a significant enhancement in the HVA/DA ratio (T(23) = 3.486; p < 0.01). These changes in the levels of dopamine metabolites reflect alterations in dopamine neurotransmission within the dorsal striatum. As both the intraneuronal metabolite DOPAC and the extraneuronal metabolite HVA are augmented, it can be inferred that there is an increase in dopamine synthesis, release, and turnover although there is no net change in total dopamine levels
ClockΔ19 mice have an altered response to dopamine receptor antagonists
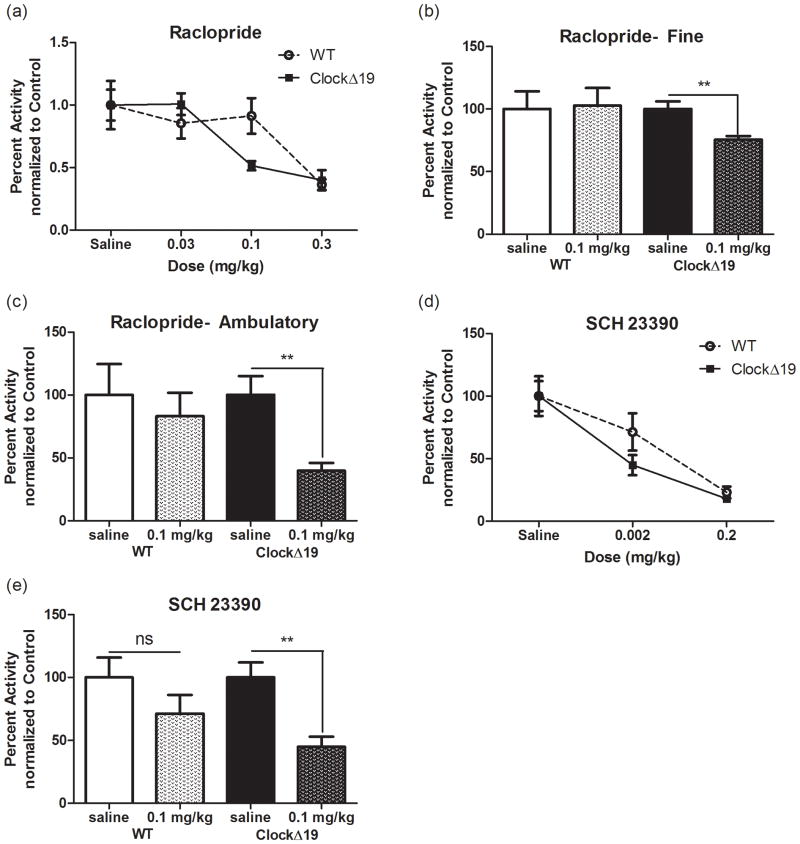
To explore the functional consequences of elevated striatal dopamine levels and metabolism in the ClockΔ19 mice, we evaluated the locomotor response to dopamine receptor antagonists. Locomotion in response to these agents provides information about levels of the endogenous ligand, dopamine, in the extracellular space. We assessed the ability of the D2-type antagonist raclopride to inhibit locomotor activity across a limited range of doses from 0.03 to 0.3 mg/kg (Figure 1A-C). Raclopride is a selective D2-type antagonist with the highest affinity for D2 (KI = 1.8 nM) and D3 (KI = 2.4 nM) receptors and minimal or negiligible affinity for D4 and D1 receptors (KI = 2400 and 18000 nM, respectively). As has been reported previously, the Clock 19 mutants display hyperactivity in response to a novel environment. Therefore data were normalized within genotype to saline controls in order to directly compare changes in percent activity in response to the drug (Roybal et al., 2007). We found a significant effect of dose (F(3,40) = 11.81; p < 0.0001) but no effect of genotype and no interaction (Figure 1A). However, closer evaluation of the dose-response curves suggested that there might be a leftward shift in responding to raclopride indicating a possible increase in sensitivity to the drug specifically at the 0.1 mg/kg dose. When a separate analysis of fine and ambulatory movement was performed at this dose, we discovered that the ClockΔ 19 mutants displayed a significant decrease in percent activity in both fine (Figure 1B, T(9) = 3.393; p < 0.01) and ambulatory (Figure 1C, T(10) = 3.75; p < 0.01) movements compared to saline controls. Furthermore, this effect was not detected in the wild type animals suggesting increased sensitivity in the ClockΔ 19 mutants. We observed a similar increase in sensitivity when we administered the D1/D5 antagonist SCH 23390 (D1 KI = 0.2 nM; D5 KI = 0.3 nM). We found a significant effect of dose (F(2,43) = 21.96; p < 0.0001) but no effect of genotype and no interaction (Figure 1D), but once again there was a left-ward shift in the dose-response curve of the ClockΔ 19 mutants. At the lower 0.002 mg/kg dose, the activity of the wild type mice was not significantly attenuated while the ClockΔ 19 mutant mice displayed a 56% reduction in total locomotor activity (Figure 1E) (T(15) = 3.481, p = 0.0033). Taken together, these results are consistent with an increase in functional dopamine receptor protein and/or an increase in extracellular dopamine.
Figure 1.
Enhanced locomotor response to DA antagonists. (a) Mean ± SEM for percent change in locomotor activity for 60 min following an i.p. injection of raclopride. There was a main effect of dose (F(3,40) = 11.81; p < 0.0001) by two-way ANOVA. (b) and (c) Percent activity at the 0.1 mg/kg dose of SCH 23390 separated into fine (b) and ambulatory (c). motor activity. Student’s t-test found differences in fine (T(9) = 3.393; p = 0.008) and ambulatory (T(10) = 3.75; p = 0.0038) components of locomotion in ClockΔ 19 mutants. (d) Mean ± SEM for percent change in locomotor activity for 60 min following an i.p. injection of SCH 23390. There was a main effect of dose (F(2,43) = 21.96; p < 0.0001) by two-way ANOVA. (e) Student’s t-test showed that activity was attenuated in ClockΔ 19 mutants (T(15) = 3.481; p = 0.0033) but not controls at a 0.002 mg/kg dose. N = 7–10 per group.
ClockΔ19 mice have an altered behavioral response to a D2R-type agonist
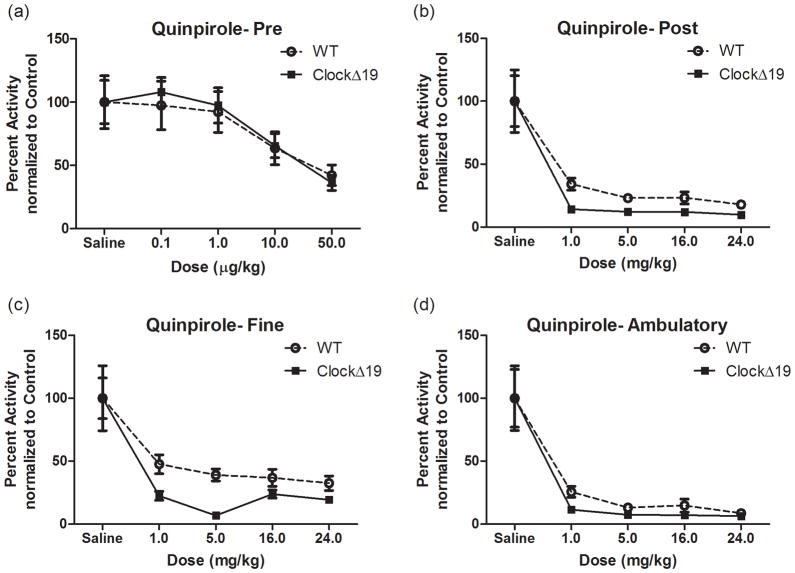
Given that ClockΔ19 mice have altered dopaminergic transmission, we evaluated the influence of dopamine receptor agonists on their locomotor responses. Low doses of D2-type agonists act preferentially on dopamine neurons (Shi et al., 1997). Therefore we assayed D2-type auto-receptor function by administering ip injections of the D2-type agonist quinpirole-HCl at doses ranging from 0.1 to 50 μg/kg (Figure 2). As expected, there was a highly significant main effect of quinpirole dose (F(4,93) = 7.31; p < 0.0001) (Figure 2A). However, we saw no main effect of genotype on the total locomotor response to quinpirole at any of these lower doses and no interaction (Figure 2A). We then repeated the quinpirole locomotor dose response study with higher doses ranging from 1 to 24 mg/kg to ascertain whether there were any differences in postsynaptic responses (FigureB-D). Once again, we observed a significant main effect of quinpirole dose on total locomotor activity (F(4,83) = 25.89; p < 0.0001) with no effect of genotype and no interaction. However, when total activity was subdivided into fine versus ambulatory motor behavior, there was a significant main effect of genotype (F(1,87) = 7.73; p < 0.01) and dose (F(4,87) = 19.27; p < 0.0001) for fine motor movements (Figure 2C) with only an effect of quinpirole dose for ambulations (F(4,85) = 25.95; p < 0.0001) (Figure 2D). This suggests that the ClockΔ19 mice are more sensitive to the locomotor inhibiting effects of quinpirole as demonstrated by the potentiation of the decrease in levels of fine motor movements produced in response to postsynaptic D2-type receptor stimulation. As fine motor movements are defined by repeated crossings of the same beam, a possible interpretation of this data is a decrease in stereotyped behavior. Altogether, these data suggest a specific potentiation of postsynaptic D2-type receptors in the ClockΔ 19 mutants.
Figure 2.
Locomotor dose response to D2 agonist. (a-d) Mean ± SEM for percent change in locomotor activity for 60 min following an i.p. injection of quinpirole. (a)There was a main effect of quinpirole dose (F(4,93) = 7.31; p < 0.0001) with no genotype effect at the lower doses tested. N = 9–14 per group. (b)There was a main effect of quinpirole dose (F(4,93) = 25.89; p < 0.0001) with no effect of genotype at the higher doses tested. Subdividing this data into fine (c) and ambulatory (d) motor movements revealed a significant effect of genotype (F(1,87) = 7.73; p = 0.0067) and dose (F(4,87) = 19.27; p < 0.0001) for fine motor movements in (c) with only an effect of quinpirole dose for ambulations in (d) (F(4,85) = 25.95; p < 0.0001).
ClockΔ19 mice are insensitive to the locomotor stimulating effects of a D1-type agonist
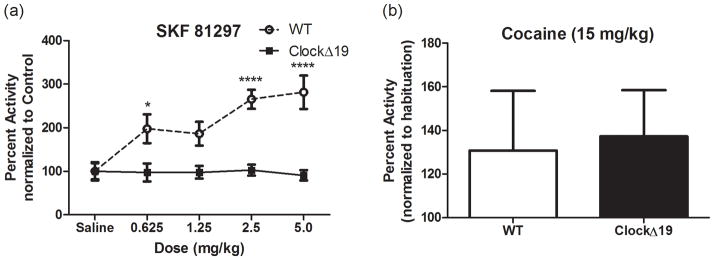
We next assayed the locomotor response to the highly selective full D1/D5 agonist SKF 81297 which consistently elevates locomotor activity in wild type animals (Desai et al., 2005). Analysis by two-way ANOVA revealed a significant genotype × dose interaction (F(4,40) = 4.97; p = 0.0024) with a main effect on both genotype (F(1,40) = 53.45; p < 0.0001) and dose (F(4,40) = 4.55; p < 0.01) (Figure 3A). Bonferroni post-hoc analysis showed a significant increase in total percent activity in the wild type mice over the ClockΔ 19 mutants at the 0.625, 2.5 and 5.0 mg/kg doses (p < 0.05, p < 0.0001 and p < 0.0001, respectively). To ensure that the lack of locomotor activation was not a function of reaching a ceiling effect, we challenged a set of wild type and ClockΔ 19 mutant animals with a 15 mg/kg dose of the psychostimulant cocaine-HCl. We observed a similar 30% increase in total locomotion in both wild type and mutant mice (Figure 3B). These data suggest a significant perturbation of D1-type functional receptors in the ClockΔ 19 mutants.
Figure 3.
Locomotor dose response to D1 agonist (a) Two-way ANOVA revealed a significant genotype × dose interaction (F(4,40) = 4.97; p = 0.0024) with a main effect of genotype (F(1,40) = 53.45; p < 0.0001) and dose (F(4,40) = 4.55; p =0.004). Bonferroni post-hoc analysis showed a significant difference between the means at the 0.625 (*p <0.05), 2.5 (****p < 0.0001) and 5.0 mg/kg (****p < 0.0001) doses. (b) Wild type and ClockΔ 19 mutant mice showed a modest 30% increase in locomotor activity to this dose of cocaine.
ClockΔ19 mice have an altered D1/D2 ratio in striatum
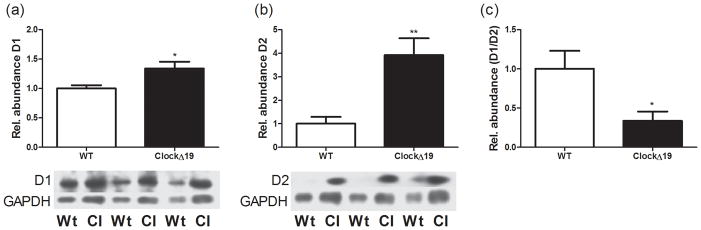
Since the results from the locomotor experiments with D1-type and D2-type receptor agonists and antagonists demonstrated a functional change in dopamine receptor activity, we next set out to determine whether there were changes in dopamine receptor protein levels in dorsal striatum. We looked specifically at D1 and D2 receptor protein levels because they are the most abundant subtypes in striatum. Surprisingly, evaluation of total protein levels revealed a small but significant increase in total D1 receptor levels in the ClockΔ19 mice (T(8) = 2.739; p = 0.0255) (Figure 4A). These data were acquired during the same time of the day in which the locomotor activity assays were performed. However, when we measured D2 protein levels we found a much more robust increase (T(8) = 3.775; p = 0.0054) (Figure 4B) which results in a dramatic decrease in the D1/D2 ratio in striatum (T(8) = 2.553; p = 0.034) (Figure 4C). Thus, the altered response to dopaminergic drugs is associated with an improper D1/D2 receptor composition in striatum.
Figure 4.
Dopamine receptor protein levels. (a) Total D1DR protein levels in striatum are increased in ClockΔ 19 mutants (T(8) = 2.739; p = 0.0255). (b) Total D2DR protein levels in striatum are increased in ClockΔ 19 mutants (T(8) = 3.775; p = 0.0054) (c) The ratio of D1DR protein to D2DR protein is significantly altered in the ClockΔ 19 mutants (T(8) = 2.553; p = 0.034). N= 4–5 animals per genotype. All values were normalized to GAPDH.
D1/D2 receptor signaling is altered in the ClockΔ19 mice
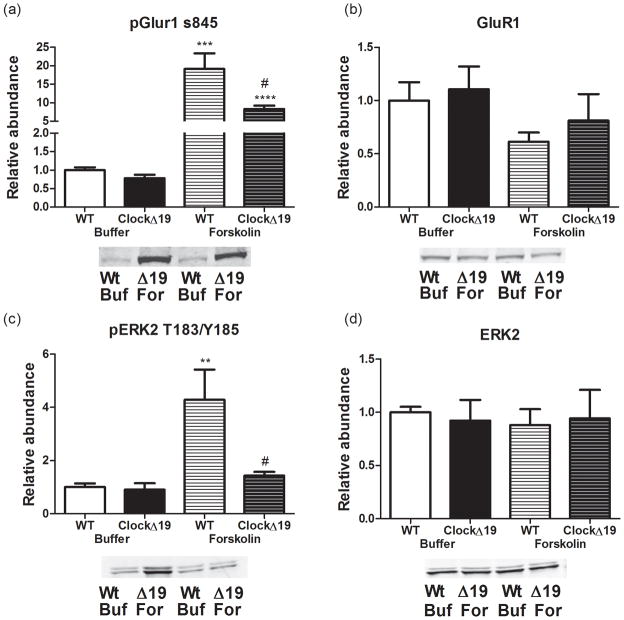
To further define the effects of the ClockΔ19 mice altered D1/D2 protein ratio on striatal dopamine signaling, we evaluated the responsiveness of the cAMP/PKA cascade to forskolin, a potent adenylate cyclase activator. D1-type DRs are coupled to Gαs and their activation results in sharp elevations in intracellular cAMP, thereby activating PKA, whereas the Gαi-coupled D2 dopamine receptors antagonize this pathway (Herve, 2011). We assessed PKA-dependent phosphorylation of the GluR1 subunit of the AMPA receptor (Snyder et al., 2000) and the MEK2-dependent activation of ERK2 protein kinase (Vossler et al., 1997) following treatment with forskolin, as two important downstream effectors of dopamine signaling. Treatment of striatal slices with forskolin increased PKA-dependent phosphorylation of GluR1 at Ser845 in wild type (Figure 5A, T(13) = 5.359, p = 0.0001) and ClockΔ 19 mice (T(12) = 11.05, p < 0.0001) without affecting total levels of GluR1 (Figure 5B). However the response in wild type mice was much larger than that seen in the ClockΔ19 mice (T(9) = 2.288, p = 0.0479). A similar effect was observed for phospho-ERK2-T183/Y185. Forskolin caused an increase phosphorylation of ERK2 in the wild type mice (T(13) = 3.527, p = 0.0037), but this response was attenuated and did not reach significance in the ClockΔ 19 mutants (Figure 5C). Thus, there was a loss of cAMP/PKA signaling, presumably via the Raf- and Rap1-dependent pathway (Vossler et al., 1997) in the ClockΔ 19 mutants compared to wild type controls (T(9) = 2.24, p = 0.05). The drug treatments had no effect on total ERK2 levels in either genotype (Figure 5D) and there were no baseline differences in total ERK2 (Figure 5D) or phospho-ERK2 between genotypes (Figure 5C). Thus the increase in striatal dopamine and DR levels in the ClockΔ19 mutant mice corresponded with marked attenuation in downstream cAMP/PKA signaling for these two pathways.
Figure 5.
D1 dopamine receptor signaling. Effects of treatment of striatal slices with forskolin (For) (10 μM, 5 min) or KREB’s buffer (Buf). Representative blots with quantification. N = 3 animals per genotype with 2–3 biological replicates per n. (a) Treatment of striatal slices with forskolin increased p-GluR1 Ser845 in wild type (T(13) = 5.359; ***p = 0.0001) and ClockΔ 19 mice (T(12) = 11.05; ****p < 0.0001), but the induction in the mutant mice was significantly weaker than that observed in controls (T(9) = 2.288; #p = 0.05). (b) Treatments did not affect total GluR1 levels. (c)Treatment of striatal slices with forskolin increased phospho-ERK2 T183/Y185 in wild type mice (T(13) = 3.524; **p = 0.0037) but not in ClockΔ 19 animals resulting in a significant difference between genotypes with this treatment (T(9) = 2.24; #p = 0.05). (d)Treatments did not affect total levels of ERK2.
Discussion
Our results show that loss of function of the circadian transcription factor CLOCK results in robust changes in dopaminergic transmission and dopamine receptor function. Mice with the dominant-negative ClockΔ 19 mutation display altered responses to dopamine receptor antagonists, suggesting increased dopaminergic tone. This is consistent with the increased dopamine cell firing and bursting previously reported in these mutants (Coque et al., 2011; McClung et al., 2005). Interestingly, these mice have an increase in total tissue dopamine content but no change in metabolite levels in the nucleus accumbens (Coque et al., 2011). In the dorsal striatum we detected no change in total dopamine levels, but rather an increase in DOPAC which could indicate greater monoamine oxidase A (MAO-A) enzymatic activity. This is interesting since previous studies found that the circadian proteins BMAL1, NPAS2 and PER2 can regulate the transcription of MAO-A (Hampp et al., 2008). However the DOPAC/dopamine ratio was not significantly different between genotypes because the mutants displayed a parallel trend toward augmented dopamine levels. It should be noted that the measures of dopamine and metabolites were taken under basal home cage conditions, a circumstance under which the locomotor hyperactivity phenotype of the mutants is less pronounced. It is possible that a significant difference in dopamine levels may have been observed if tissue was taken after some manipulation like exposure to a novel environment. One might hypothesize that neurochemical differences would be enhanced just as the locomotor behavior phenotype is exaggerated, a hypothesis that warrants further examination in future studies. Both total levels of HVA and the HVA/DOPAC ratio were increased in the mutants suggestive of an overall increase in transmitter release and extracellular dopamine, which is consistent with the observation of increased sensitivity to D1-type and D2-type receptor antagonists. The difference in dopamine transmission between the ventral and dorsal striatum could be due to the fact that the rate limiting enzyme in dopamine synthesis, tyrosine hydroxylase is increased in the ClockΔ19 mice selectively in the VTA and not in the substantia nigra (SN) (McClung et al., 2005). Future studies will determine if there are differences in dopaminergic activity in the SN as seen in the VTA.
The ClockΔ 19 mutants failed to produce a locomotor activating response to a D1-type agonist. However they show a normal locomotor response to cocaine. This suggests that D1- type receptor signaling is impaired in the ClockΔ19 mice while D2-type responses might be heightened. The change in D1-type receptor activity could represent an attempt of the circuit to compensate for the increased dopaminergic tone in these mice. We did not see a decrease in D1 receptor protein, and in fact detected a slight increase in protein in the striatum. Thus this absence of response to the agonist is not due to a lack of protein transcription or translation. It is possible that there are differences in receptor affinity which underlie the difference in response as both D1 and D2 DRs have been shown to occupy high and low affinity states (Richfield et al., 1989). Additionally, it is becoming increasingly clear that DR heteromers represent a biologically relevant subpopulation within the brain with unique pharmacological and signal transduction profiles (Hasbi et al., 2011). A change in the proportion of D1-D2 heteromers might be expected to change the profile of the response to individual DR agonists. Interestingly, the D1-D2 heteromer has already been implicated in two psychiatric disorders, addiction and schizophrenia (Hasbi et al., 2011). Similarly, there may be changes in the fraction of synaptic versus extra-synaptic receptors, accounting for discrepancy between D1R protein levels and activity. Curiously, a lack of response to a D1 agonist is similarly seen in both D1R knockout mice and in mice in which the D1R is overexpressed (Dracheva et al., 1999; Xu et al., 1994). Studies involving independently generated D1R knockout mice have generated conflicting results with reports of no change in horizontal locomotor activity or slight locomotor depression (Drago et al., 1994; Smith et al., 1998) and other reports of hyperactivity (Xu et al., 1994). This discrepancy can likely be attributed to multiple factors ranging from differences in targeting strategy, background strain, and experimental conditions. D1 receptors are prominent not only in striatum but also in prefrontal cortex and these receptors may have opposing roles in the regulation of locomotor activity (Wilkinson, 1997), therefore future studies will be necessary to assess changes in cortical dopamine transmission in the ClockΔ19 mice.
It is likely that a change in the D1/D2 ratio in striatum underlies the reduced response to a D1-type agonist. Indeed we find a robust increase in D2 receptor expression in the ClockΔ19 mice and an increased response to a D2-type agonist. Moreover, our pharmacological studies performed ex vivo with acute slices demonstrated deficiencies in PKA signaling cascades in the mutants. For instance, while forskolin stimulated GluR1 Ser845 phosphorylation in both wild types and mutants, the increase was four-fold higher in the wild type. These results point towards a possible increase in antagonism of cAMP stimulation by D2-type receptors. Moreover, the signaling link from PKA to ERK2 appeared to be almost completely missing in the ClockΔ19 mice. Increased D2 receptor signaling has been implicated in bipolar disorder. Analysis of post-mortem brain samples of the pre-frontal cortex from diseased brains and healthy controls found DRD2 expression increased in bipolar patients and decreased in schizophrenics relative to controls (Zhan et al., 2011). Moreover, data collected from in vivo imaging studies of D2 receptor occupancy within the basal ganglia have reported an increase in binding in bipolar psychotic patients (Nikolaus et al., 2009; Wong et al., 1997).
There have been a number of interesting studies which suggest that in general, activation of D1-expressing neurons (i.e. the “direct pathway”) of adult animals enhances the response to drugs of abuse while activation of D2-expressing neurons (i.e. the “indirect pathway”) antagonizes the response (Reviewed in Lobo and Nestler, 2011). The ClockΔ19 mice have an increase in the response to drugs of abuse, thus these results may seem to be contradictory to the majority of the literature. However, it is likely that these effects are due to the increased dopaminergic activity and represent an attempt of the system to compensate. Furthermore, mice with a knockout of the D2 receptor have a reduction in the response to most drugs of abuse, thus alterations that occur throughout development may also be important. Future studies with selective effects on specific neuronal populations within the ClockΔ19 mice will have to be performed to better understand the exact role of the direct and indirect striatal pathways in the overall manic-like phenotype of these mice.
It is increasing clear that the coupling of diverse neuronal populations throughout the brain is involved in encoding higher order cognitive processes. Indeed, previously we found that the ClockΔ19 mice have serious defects in the ability of limbic regions of the brain to synchronize activity as the mice explore certain anxiety-related environments (Dzirasa et al., 2010; Dzirasa et al., 2011). The changes in striatal dopamine transmission reported here including altered dopamine turnover and D1/D2 ratios in the ClockΔ 19 mutants likely contributes to the defects in proper synchronization that is necessary for the animal to make important decisions regarding their level of exploratory behavior. The nigrostriatal dopamine system and the dorsal striatum have traditionally been studied in terms of sensorimotor function while the mesolimbic dopamine system and the nucleus accumbens has been investigated for its role in motivation and addiction. However, just as the anatomical boundaries between these brain regions are imprecise so too are the functional distinctions. There is significant overlap in terms of contributions to brain stimulation reward, habitual behavior, reward prediction and reinforcement of memory consolidation (Roy A, 2009). Accordingly, it would not be surprising if the alterations in dopaminergic transmission reported here result in disrupted circuit level activity, and contribute significantly to the ClockΔ 19 mutant phenotype beyond the observed hyperactivity. Future studies will more specifically determine the role of altered striatal dopaminergic signaling in neuronal synchronization and related behaviors.
While we can ascribe these dopamine-related biochemical and behavioral abnormailities to a disruption in CLOCK function, the underlying mechanisms remain undetermined. CLOCK acting as a transcription factor may directly regulate diurnal expression of dopamine receptor subtypes or other dopamine related genes. Alternatively, CLOCK may indirectly influence dopamine transmission by acting on other upstream targets. In addition to their behavioral abnormalities, the ClockΔ 19 mutants have a well-described phenotype of metabolic syndrome, which includes disruptions in expression of factors associated with feeding and energy balance including insulin, leptin and ghrelin (Turek et al., 2005). Importantly, these peripheral hormones and peptides can influence the central dopaminergic system and directly impact mood and reward-related behaviors (Fulton et al., 2006; Hommel et al., 2006; Jiang et al., 2006; Williams et al., 2007). Future studies will be needed to parcel out the effects of these metabolic disturbances on the dopamine-related phenotype of the ClockΔ 19 mutants.
In summary, normal CLOCK function is involved in the regulation of dopamine transmission in the striatum. Loss of CLOCK function increases dopamine release and turnover in the striatum as indicated by increased levels of metabolites HVA and DOPAC and the increased sensitivity to dopamine receptor antagonists. Moreover, this increased dopaminergic tone results in altered levels of dopamine receptor protein with a surprising increase in D1DR and D2DR protein levels but a significant shift in the ratio of D1 to D2 receptors in favor of D2DR. Finally, these protein changes lead to functional consequences at both the level of behavior and cellular signaling with alterations in locomotor responses to both D1 and D2-type specific agonists, and a blunted activation of cAMP signaling in the ClockΔ 19 mutants. Taken together, these studies further define the defects in dopaminergic transmission that result from a nonfunctional CLOCK protein, and help elucidate the changes in dopaminergic transmission which are responsible for the regulation of activity, reward, and mood.
Supplementary Material
Table 1.
| Dopamine | HVA | DOPAC | HVA/DA | DOPAC/DA | |
|---|---|---|---|---|---|
| WT | 129.19 ± 4.50 | 12.49 ± 0.31 | 7.25 ± 0.40 | 9.37 ± 0.26 | 5.69 ± 0.39 |
| ClockΔ 19 | 138.66 ± 4.78 | 15.55 ± 0.53**** | 8.78 ± 0.32** | 11.35 ± 0.49** | 6.24 ± 0.25 |
Levels of dopamine (DA), HVA and DOPAC were measured in dorsal striatum tissue by HPLC. Values represent means pM/mg tissue ± standard errors. Dopamine levels were not significantly changed in the ClockΔ 19 mice compared with wild type controls (T(23) = 1.435, p = 0.1646). There were significant differences in the metabolites and metabolite ratios. HVA, DOPAC and the ratio of HVA/DA were increased in the ClockΔ 19 mice (**p<0.01, ***p<0.001, and ****p<0.0001, n = 12–13 animals per group).
Acknowledgments
We thank Dr. Joseph Takahashi at UT Southwestern for providing the CLOCK mutant mice. This work was funded by National Institutes of Health grants to J.A.B (MH79710, MH083711, DA016672, DA033485) and C.A.M. (MH082876, MH090683, DA023988). Dr. McClung has received research funding from Pfizer and GlaxoSmithKline on unrelated projects. Dr. McClung has also received honoraria from Pfizer, GlaxoSmithKline, Servier, and Johnson & Johnson. All other authors have nothing to disclose.
References Cited
- Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpirole-induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav. 2005;80:371–377. doi: 10.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Atkinson MK, Wolf DF., SR Autorhythmometry in manic-depressives. Chronobiologia. 1975;2:10. [PubMed] [Google Scholar]
- Barchas JDaA, Margaret . In: Basic Neurochemistry. Siegel GJA, Albers Bernard W, Fisher Wayne R, Uhler Stephen K, Michael D, editors. Philadelphia: Lippincnott-Rave; 1999. [Google Scholar]
- Barnard AR, Nolan PM. When Clocks Go Bad: Neurobehavioural Consequences of Disrupted Circadian Timing. PLoS Genet. 2008;4:e1000040. doi: 10.1371/journal.pgen.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. Circadian Clock Control by SUMOylation of BMAL1. Science. 2005;309:1390–1394. doi: 10.1126/science.1110689. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disorders. 2002;68:25–31. doi: 10.1016/s0165-0327(00)00325-6. [DOI] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, Sidor MM, Birnbaum SG, Graham A, Neve RL, et al. Specific Role of VTA Dopamine Neuronal Firing Rates and Morphology in the Reversal of Anxiety-Related, but not Depression-Related Behavior in the Clock[Delta]19 Mouse Model of Mania. Neuropsychopharmacol. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. A comparison of the locomotor stimulant effects of D1-like receptor agonists in mice. Pharmacol Biochem Behav. 2005;81:843–848. doi: 10.1016/j.pbb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Xu M, Kelley KA, Haroutunian V, Holstein GR, Haun S, Silverstein JH, Sealfon SC. Paradoxical Locomotor Behavior of Dopamine D1 Receptor Transgenic Mice. Exp Neurol. 1999;157:169–179. doi: 10.1006/exnr.1999.7037. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, McClung CA, Nicolelis MAL. Lithium Ameliorates Nucleus Accumbens Phase-Signaling Dysfunction in a Genetic Mouse Model of Mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, McGarity DL, Bhattacharya A, Kumar S, Takahashi JS, Dunson D, McClung CA, Nicolelis MAL. Impaired Limbic Gamma Oscillatory Synchrony during Anxiety-Related Behavior in a Genetic Mouse Model of Bipolar Mania. J Neurosci. 2011;31:6449–6456. doi: 10.1523/JNEUROSCI.6144-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon Ramon P, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin Regulation of the Mesoaccumbens Dopamine Pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK Protein in the Mammalian Circadian Mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, et al. Parkin-deficient Mice Exhibit Nigrostriatal Deficits but Not Loss of Dopaminergic Neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: The interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41:81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–1103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, et al. Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock Influence on Mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd B, George S. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain. 2011;4:26. doi: 10.1186/1756-6606-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D. Identification of a specific assembly of the G protein Golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5 doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin Receptor Signaling in Midbrain Dopamine Neurons Regulates Feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Jiang H, Betancourt L, Smith RG. Ghrelin Amplifies Dopamine Signaling by Cross Talk Involving Formation of Growth Hormone Secretagogue Receptor/Dopamine Receptor Subtype 1 Heterodimers. Molecular Endocrinology. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW, Takahashi JS. The Mouse Clock Mutation Behaves as an Antimorph and Maps Within the W(19H) Deletion, Distal of Kit. Genetics. 1997;146:1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chen SH, Huang SY, Tzeng NS, Chang YH, Wang CL, Lee IH, Yeh TL, Yang YK, et al. The COMT and DRD3 genes interacted in bipolar I but not bipolar II disorder. World J Biol Psychiatry. 2011;12:385–391. doi: 10.3109/15622975.2010.505298. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Onderbergen AV, Hubain P, Copinschi G, L’Hermite-Baleriaux M, Leclercq R, Brasseur M, Mendlewicz J, Cauter EV. The 24-Hour Profiles of Cortisol, Prolactin, and Growth Hormone Secretion in Mania. Arch Gen Psychiatry. 1994;51:616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Mendlewicz J, Kerkhofs M, Leclerq R, Golstein J, Brasseur M, Copinschi G, Cauter EV. 24-Hour Profiles of Adrenocorticotropin, Cortisol, and Growth Hormone in Major Depressive Illness: Effect of Antidepressant Treatment. J Clin Endocrinol Metab. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Frontiers in neuroanatomy. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Müller HW. In vivo imaging of synaptic function in the central nervous system: II. Mental and affective disorders. Behav Brain Res. 2009;204:32–66. doi: 10.1016/j.bbr.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Duncan WC, Jr, Johnson KA, Wehr TA. Diurnal variations of serotonin and dopamine levels in discrete brain regions of Syrian hamsters and their modification by chronic clorgyline treatment. Brain Res. 1993;627:41–48. doi: 10.1016/0006-8993(93)90746-a. [DOI] [PubMed] [Google Scholar]
- Possidente B, Lumia AR, McEldowney S, Rapp M. Fluoxetine shortens circadian period for wheel running activity in mice. Brain Res Bull. 1992;28:629–631. doi: 10.1016/0361-9230(92)90114-d. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neurosci. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Roy AW. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer HK, Marshall S, Mellsop GW. Mania and seasonality in the southern hemisphere. J Affect Disorders. 1991;23:151–156. doi: 10.1016/0165-0327(91)90027-p. [DOI] [PubMed] [Google Scholar]
- Shi WX, Smith PL, Pun CL, Millet B, Bunney BS. D1–D2 Interaction in Feedback Control of Midbrain Dopamine Neurons. J Neurosci. 1997;17:7988–7994. doi: 10.1523/JNEUROSCI.17-20-07988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Striplin CD, Geller AM, Mailman RB, Drago J, Lawler CP, Gallagher M. Behavioural assessment of mice lacking D1A dopamine receptors. Neurosci. 1998;86:135–146. doi: 10.1016/s0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, Gutierrez-Zotes A, Puigdemont D, Bayes M, Crespo JM, et al. Differential Association of Circadian Genes with Mood Disorders: CRY1 and NPAS2 are Associated with Unipolar Major Depression and CLOCK and VIP with Bipolar Disorder. Neuropsychopharmacol. 2010;35:1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O, Schafmeier T. Of switches and hourglasses: regulation of subcellular traffic in circadian clocks by phosphorylation. EMBO Rep. 2010;11:927–935. doi: 10.1038/embor.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Moore-Ede MC. Lithium lengthens circadian period in a diurnal primate, Saimiri sciureus. Biol Psychiatry. 1990;28:117–126. doi: 10.1016/0006-3223(90)90629-g. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS. The nature of interactions involving prefrontal and striatal dopamine systems. J Psychopharmacol. 1997;11:143–150. doi: 10.1177/026988119701100207. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, et al. Hypoinsulinemia Regulates Amphetamine-Induced Reverse Transport of Dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. Circadian rhythms in mammalian neurotransmitter receptors. Prog Neurobiol. 1987;29:219–259. doi: 10.1016/0301-0082(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Wong DF, Pearlson GD, Tune LE, Trevor Young L, Cidis Meltzer C, Dannals RF, Ravert HT, Reith J, Kuhar MJ, Gjedde A. Quantification of Neuroreceptors in the Living Human Brain: IV. Effect of Aging and Elevations of D2-like Receptors in Schizophrenia and Bipolar Illness. J Cereb Blood Flow Metab. 1997;17:331–342. doi: 10.1097/00004647-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Zhan L, Kerr JR, Lafuente MJ, Maclean A, Chibalina MV, Liu B, Burke B, Bevan S, Nasir J. Altered expression and coregulation of dopamine signalling genes in schizophrenia and bipolar disorder. Neuropathol Appl Neurobiol. 2011;37:206–219. doi: 10.1111/j.1365-2990.2010.01128.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.