Abstract
Aim
Gamma-aminobutyric acid (GABA) is a multifunctional molecule with various physiological effects throughout the body. The regulation of GABA receptor (GABAR) plays a key role in reducing the damage mediated by oxidative stress (OS). Extended hepatectomy causes fatal OS-induced injury in the liver remnant. We aimed to investigate the effect of a GABAR agonist in extended hepatectomy.
Methods
Saline or a GABAR agonist (43.56 nmol/g body weight of muscimol) was given intravenously at 4 h preoperatively. C57BL/6 mice were divided into three groups: laparotomy only, 90% hepatectomy with saline and 90% hepatectomy with a GABAR agonist. Liver samples were obtained at 6 h after surgery.
Results
Survival curves were prolonged by the GABAR agonist. Histopathological findings and biochemical profiles showed that the GABAR agonist reduced liver damage. Immunohistological assessment demonstrated that the GABAR agonist prevented apoptotic induction. As shown by 4-hydroxynonenal, which reflects OS-induced damage, 90% hepatectomy caused OS and the GABAR agonist reduced OS. We measured ATM, H2AX, Akt and free radical scavenging enzymes since they may be affected by GABAR regulation, and found that Akt was greatly decreased after 90% hepatectomy, but it recovered with the GABAR agonist.
Conclusions
GABAR is activated by a specific agonist in the liver in vivo. This activation reduces OS-madiated damage after extended hepatectomy in vivo, and the mechanism via an Akt-dependent pathway may be a key.
Keywords: γ-aminobutyric acid receptor, oxidative stress, free radicals, liver, Akt
INTRODUCTION
Oxygen is required for cell survival. However, it also poses a potential hazard via reactive oxygen species (ROS) and reactive nitrogen species (RNS), with the modification of macromolecules (lipids, proteins and nucleic acids) and alteration of their biological functions.1-3 Therefore, ROS/RNS were initially considered as harmful products of the normal aerobic metabolism process. Currently, there is growing evidence that controlled ROS/RNS production also plays a physiological role, especially in regulating cell signaling to involve cell proliferation, differentiation and apoptosis.1-3 Oxidative stress (OS) is currently defined as an imbalance between the production of ROS/RNS and the antioxidant capacity of the cell.1-3 These antioxidants ensure a defense against ROS/RNS-induced damage to lipids, proteins and deoxyribonucleic acid (DNA).2
Gamma-aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the mammalian brain, and almost all research that has focused on GABA or the regulation of GABA receptor (GABAR) has only been in the brain.4-8 Currently, GABA is considered to be a multifunctional molecule with various physiological effects throughout the body.9,10 Some studies have focused on the effects of regulation of GABAR in other fields, such as stem cell proliferation, renal function and metabolism.5,11 It is also evident that the liver contains GABA and its transporter,10 and hepatic GABAR has already been detected.12 However, in the liver, the functions of GABA and the effects of GABAR regulation are not well understood.
Many studies on the brain have demonstrated that GABA and/or the regulation of GABAR have positive/negative effects on brain damage caused by many factors, such as metabolic failure, ischemia, anoxia and toxic injury.5,7,8 Additionally, the availability of GABA/GABAR in the brain has been clinically investigated in patients with hepatic encephalopathy.13 It has been found that GABAR regulation has beneficial effects against OS mediated by free radicals (FR),5,7 and that these effects can be mainly explained by prevention of the response to DNA damage,5,14,15 cell survival pathways16,17 or the FR scavenging system.18,19
Liver resection is considered the standard treatment for primary malignant tumors and liver metastases. Currently, advanced surgical techniques for hepatectomy and technical improvements in the preoperative evaluation of liver function have resulted in a decline in perioperative morbidity and mortality. Extended hepatectomy has the advantage of high curability, but increases morbidity and mortality compared with more limited resections.20 The volume of the remnant liver is correlated with perioperative morbidity and mortality.20 Shear stress induced by hepatectomy and subsequent portal hypertension triggers a liver regeneration cascade, but also causes fatal damage in the liver remnant by FR-mediated OS.21-23
This study aimed to investigate the effect of GABAR activation on postoperative liver function by using an extended hepatectomy model in the mouse, and we discuss possible pathways.
MATERIALS AND METHODS
Animals
C57BL/6 mice (males, 12-16 weeks of age, approximately 30 g body weight) were used (C57BL/6NHsd; Harlan Laboratories, Indianapolis, IN). Mice were housed in a specific pathogen-free condition with 12-h light/dark cycles, food and water. All experimental protocols were approved by the ethical committee of the Mayo Clinic (Protocol No. IACUC 33307 and 24907) in accordance with The National Institute of Health Guide for the Care and Use of Laboratory Animals.
Intravenous injection of a GABAR agonist
The dose of 8.49 μg/g body weight (43.56 nmol/g body weight) of GABAA receptor agonist (muscimol 5 mg, 195.01 g/mol; G-019, Sigma-Aldrich Co., St. Louis, MO) was used. Four hours before surgery, a total volume of 1.0 ml was intravenously injected from the tail vein by using a 30-gauge needle.
Study design
Mice were divided into three groups: (i) laparotomy only with saline (normal saline, 1.0 ml, i.v.); (ii) 90% hepatectomy with saline (normal saline, 1.0 ml, i.v.); and (iii) 90% hepatectomy with a GABAR agonist (1.0 ml of muscimol, i.v.). In the survival study, 10 mice in each group were observed until 24 h after surgery.
Focal and/or patchy necrosis is an important finding after hepatectomy24-27. Progressive necrosis was confirmed from the early postoperative period,24,25,27 and we collected all samples at 6 h after 90% hepatectomy. There were eight mice in each group.
At first, we evaluated the liver damage after 90% hepatectomy in each group. Next, we confirmed the OS induction by lipoperoxidation, in each group. Previous studies focused on ataxia-telangiectasia mutated kinase (ATM)/H2AX, H2AX, Akt and antioxidant enzymes as possible pathways, and have demonstrated that these possible pathways are a reasonable explanation for the positive effects of GABAR regulation against OS in the brain.5,16,17. Finally, we investigated these pathways to clarify the possible explanation for the effect of GABAR regulation on OS in the liver remnant after extended hepatectomy.
Extended hepatectomy and postoperative care
Comprehensive details of the surgical procedures for murine hepatectomy and postoperative care in our institution have been previously described.28 In brief, the right middle segment remained in our 90% hepatectomy. Each mouse was cared for separately after surgery, and the body temperature was maintained by a heating pad. Postoperative observation was performed every 1 h after surgery, and 0.3 ml of warm lactate Ringer’s solution was routinely administered every 2 h after surgery. No surgical complications were confirmed in each case at sampling autopsy.
Biochemical assay and coagulation profile
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (T-Bil) levels, and the international normalized ratio of prothrombin time (PT-INR) were measured. Serum AST, ALT and T-Bil levels were assessed by commercial kits (SGOT, SGPT and total bilirubin reagent, respectively; Biotron, Hemet, CA). The microplate reader (Spectra Max M5e; Molecular Devices, Sunnyvale, CA, USA) was set at 540 nm for wavelength measurement. The PT-INR in plasma was measured by the i-STAT System (Abbott, Princeton, NJ).
Histopathological and immunohistological assessment
Liver tissue was fixed in 10% neutral-buffered formalin, embedded in paraffin, and sliced into 4-μm sections. The morphological characteristics and graft injury scores were assessed after hematoxylin-eosin (H-E) staining. The liver damage score (point) has previously been described elsewhere.29 The scores were counted in 10 fields (x100) in each slide, and then these scores were averaged for each slide.
The induction of apoptosis was measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) (ApopTag Peroxidase In Situ Apoptosis Detection Kit, S7100, Chemicon International, Inc., Billerica, MA) and cysteine aspartic acid protease (caspase)-3 immunostaining (cleaved caspase-3 [Asp175] antibody, 9661S, Cell Signaling Technology, Inc., Danvers, MA). TUNEL-positive nuclei were stained brown and negative nuclei were counterstained light blue. Caspase-3-positive nuclei were stained brown and negative nuclei were counterstained blue. Slides were scanned with an automated high-throughput scanning system (Scanscope XT, Aperio Technologies, Inc., Vista, CA). To quantify the immunohistological findings, positive-stained nuclei were counted by Aperio Imagescope software (Aperio Technologies, Inc.). All nuclei were classified into four color intensity levels, and the higher two levels were considered as positive. The ratio of positive-stained nuclei to all nuclei was calculated and the mean ratio per mm2 was determined.
Western blot analysis
Primary antibodies for 4-hydroxynonenal (4-HNE) (4-hydroxynonenal antibody, ab46545; Abcam Inc., Cambridge, MA), ATM (phospho-[Ser/Thr] ATM/ATR substrate [4F7] rabbit mAb, Cell Signaling Technology, Inc., 2909), phosphorylated histone H2AX (γH2AX) (phospho-histone H2A.X [Ser139] antibody, 2577, Cell signaling Technology, Inc.), Akt (phospho-Akt [Ser473] [193H12] rabbit mAb, 4058, Cell Signaling Technology, Inc.), superoxide dismutase (SOD) 1 (SOD1) (Cu/Zn superoxide dismutase, LS-B2907; LifeSpan BioSciences, Inc., Seattle, WA), SOD 2 (SOD2) (Mn superoxide dismutase [SOD2], LS-C62194; LifeSpan BioSciences, Inc.) and catalase (catalase, LS-B2554; LifeSpan BioSciences, Inc.) were used. Mouse liver samples were collected, homogenized, and centrifuged at high speed for 10 min at 4°C. The supernatant was then collected and used for BCA protein determination (BCA Protein Assay Reagent, Thermo Fisher Scientific, Rockford, IL) and western blot analysis. Forty micrograms of protein were run on 4-20% tris-glycine gels (Invitrogen, Carlsbad, CA) and transferred onto a 0.45 μm nitrocellulose membrane (Bio-Rad, Life Science Research, Hercules, CA). The membranes were then blocked with 5% nonfat milk made up in Tris-buffered saline solution. After blocking the membranes, they were incubated at 4°C over night with the primary antibody. The next day, the membranes were washed three times for 10 min with Tris-buffered saline solution and then incubated with the peroxidase-conjugated secondary for 1 h, shaking, at room temperature. After incubation, the membranes were once again washed three times for 10 min with Tris-buffered saline solution and then developed using chemiluminescence (Amersham, Bioscience, Piscataway, NJ). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a control. Signals were quantified by using ImageQuant 5.0 software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
The results are presented as mean ± standard deviation. The Student t test was used for the comparison of unpaired continuous variables between groups. Survival curves were constructed by the Kaplan-Meier method (the log-rank test). Statistical calculations were performed using SPSS Software Version 16.0 (SPSS Inc., Chicago, IL). A p value <0.05 was considered statistically significant.
RESULTS
Survival curves
The survival after 90% hepatectomy was poorer than that after laparotomy (p <0.0001). Survival after 90% hepatectomy with the GABAR agonist was prolonged compared with that after 90% hepatectomy with saline (p = 0.0392).
Liver parenchymal injury
Histopathological findings are visualized by H-E staining. Inflammatory cell infiltration, vacuolization, hepatocyte ballooning and necrosis were confirmed after 90% hepatectomy.
Changes in liver damage scores are evaluated. This score was significantly lower with laparotomy than that with 90% hepatectomy with saline (0.1 ± 0.0 vs. 5.9 ± 0.7 points, p <0.0001), and it was significantly higher with 90% hepatectomy with saline than that with the GABAR agonist (5.9 ± 0.7 vs. 4.8 ± 1.0 points, p = 0.0241).
Biochemical and coagulation profiles
Changes in AST, ALT, and T-Bil levels and PT-INR are measured. There was a significant difference in AST levels between laparotomy and 90% hepatectomy with saline (56.1 ± 17.1 vs. 567.8 ± 111.2 U/L, p <0.0001), as well as in ALT levels (51.9 ± 13.5 vs. 486.0 ± 55.7 U/L, p <0.0001), but there were no significant differences in T-Bil levels (0.50 ± 0.19 vs. 0.64 ± 0.22 mg/dL, p = 0.2012) and PT-INR (0.98 ± 0.07 vs. 1.00 ± 0.07, p = 0.6515).
There was a significant difference in AST levels between 90% hepatectomy with saline and that with the GABAR agonist (567.8 ± 111.2 vs. 442.0 ± 100.3 U/L, p = 0.0331), but there were no differences in ALT levels (486.0 ± 55.7 vs. 424.6 ± 69.1 U/L, p = 0.0703), T-Bil levels (0.64 ± 0.22 vs. 0.66 ± 0.24 mg/dL, p = 0.7455) and PT-INR (1.00 ± 0.07 vs. 0.99 ± 0.09, p = 0.9041).
Apoptotic induction
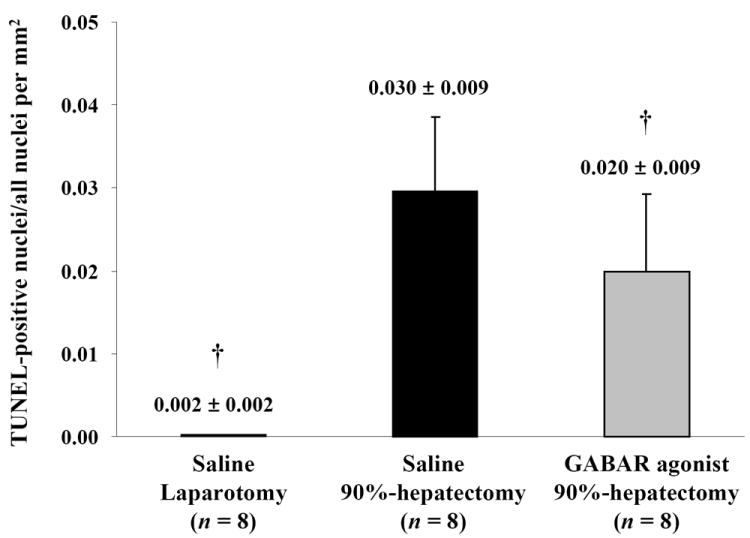
TUNEL immunostaining results are shown in Fig. 1A-C. The ratio of positive-stained nuclei in TUNEL immunostaining showed significant differences between laparotomy and 90% hepatectomy with saline (p <0.0001) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0465) (Fig. 1D).
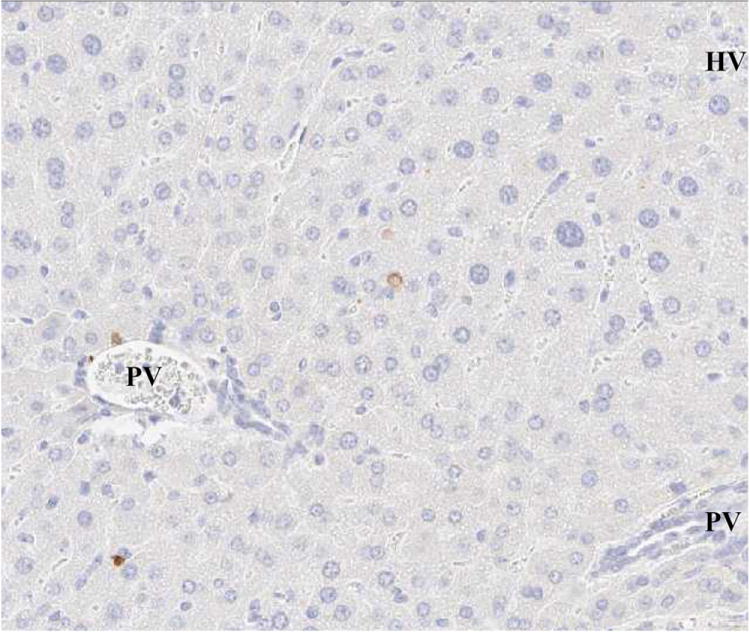
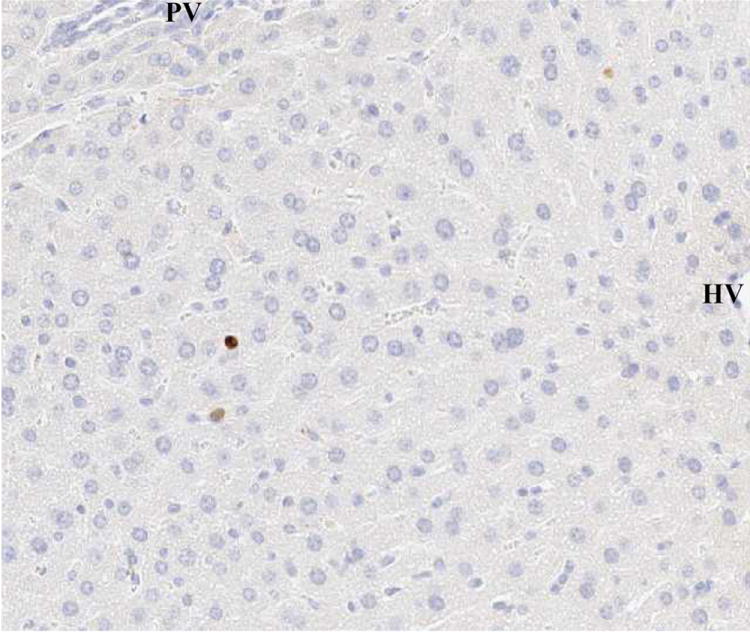
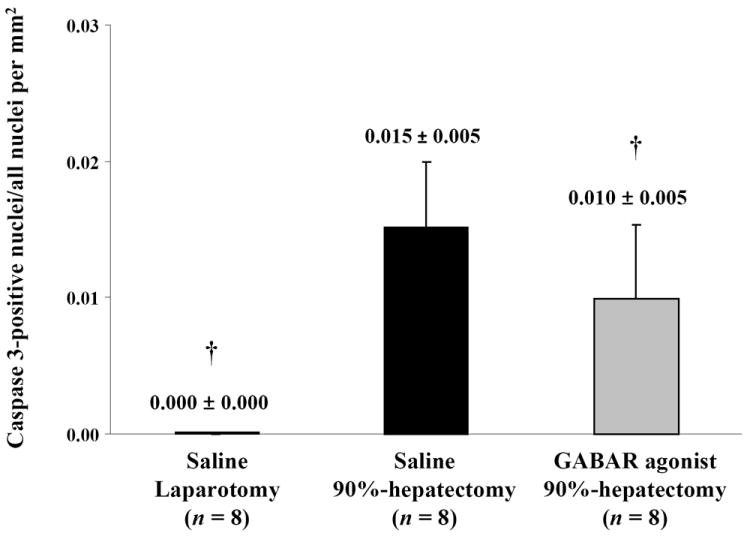
Figure 1. Immunohistological assessment by TUNEL and caspase-3 staining.
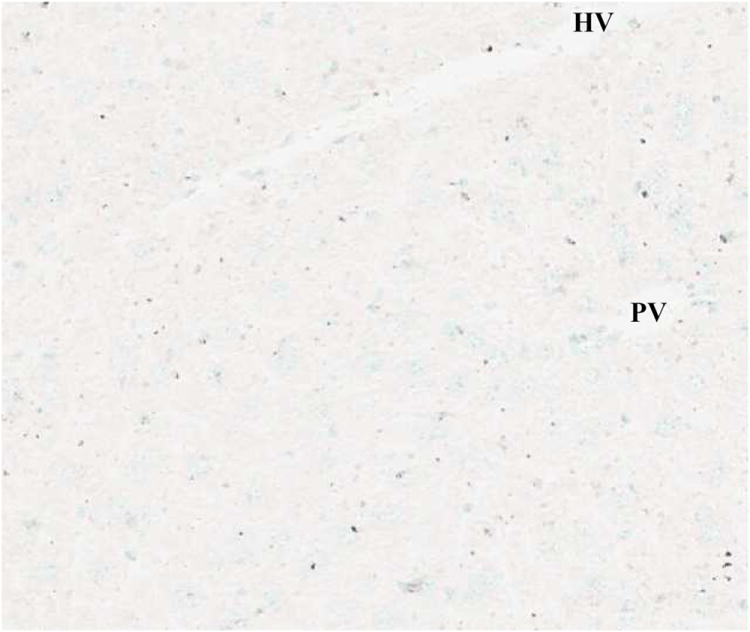
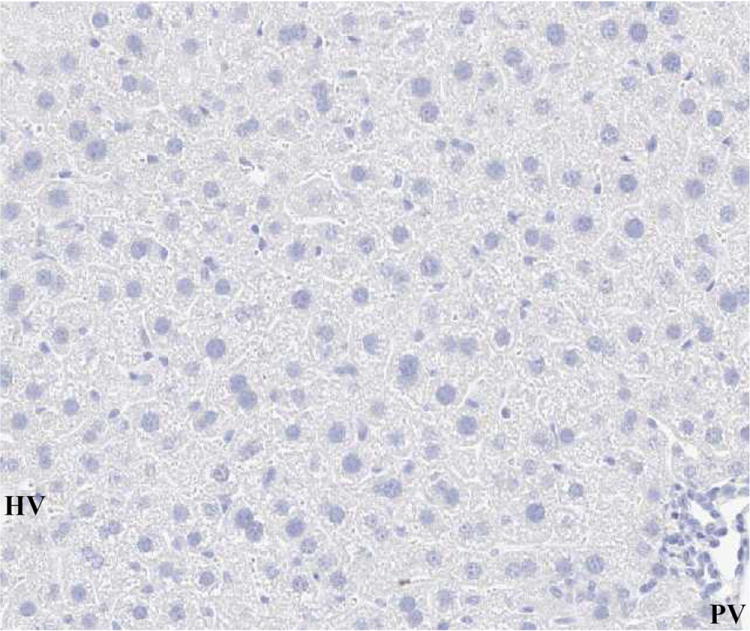
(A) Laparotomy with saline (TUNEL, x100).
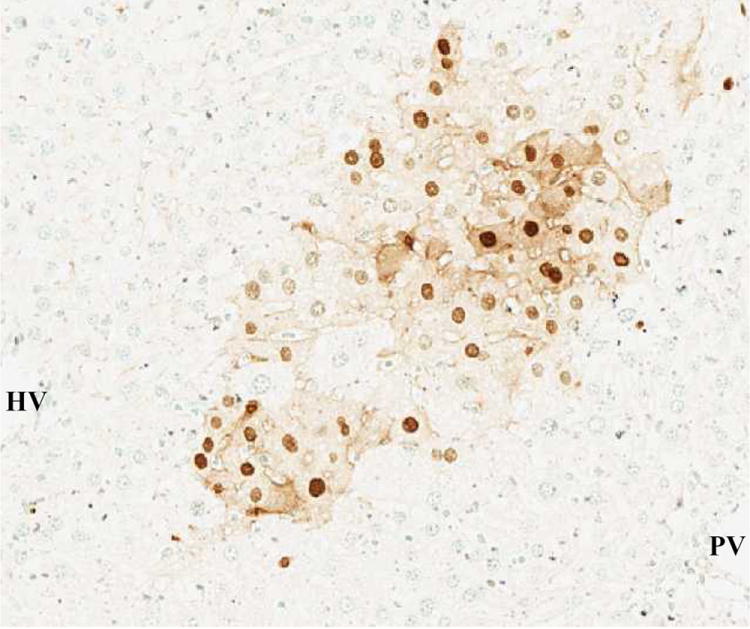
(B) 90% hepatectomy with saline (TUNEL, x100).
TUNEL-positive nuclei were stained brown and negative nuclei were counterstained light blue.
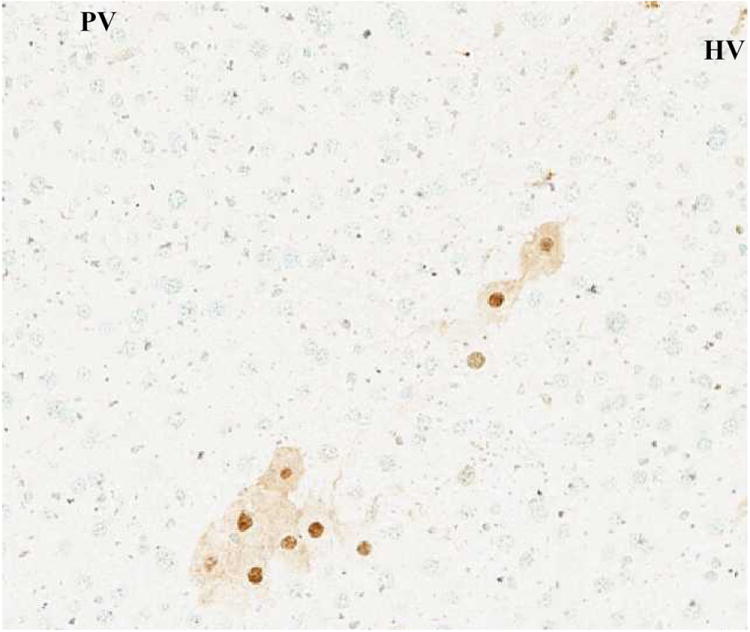
(C) 90% hepatectomy with GABAR agonist (TUNEL, x100).
(D) Changes in the ratio of TUNEL-positive nuclei (†p <0.05).
These results suggested that the GABAR agonist decreased the ratio of TUNEL-positive cells.
(E) Laparotomy with saline (caspase-3, x100).
(F) 90% hepatectomy with saline (caspase-3, x100).
Caspase-3-positive nuclei were stained brown and negative nuclei were counterstained blue.
(G) 90% hepatectomy with GABAR agonist (caspase-3, x100).
(H) Changes in the ratio of caspase-3-positive nuclei (†p <0.05).
These results indicated that the GABAR agonist decreased the ratio of caspase-3-positive cells.
Abbreviations: cysteine aspartic acid protease, caspase-3; γ-aminobutyric acid receptor, GABAR; hepatic vein, HV; not significant (p ≥ 0.05), NS; portal vein, PV; terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling. TUNEL.
The results of caspase-3 immunostaining are shown in Fig. 1E-G. The ratio of positive-stained nuclei with caspase-3 immunostaining showed significant differences between laparotomy and 90% hepatectomy with saline (p <0.0001) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0492) (Fig. 1H).
Lipoperoxidation
We investigated 4-HNE to confirm OS-induced damage (Fig. 2A). Normalized 4-HNE (the ratio of 4-HNE/GAPDH) showed significant differences between laparotomy and 90% hepatectomy with saline (p = 0.0032) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0210) (Fig. 2B).
Figure 2. Western blot analysis of HNE.
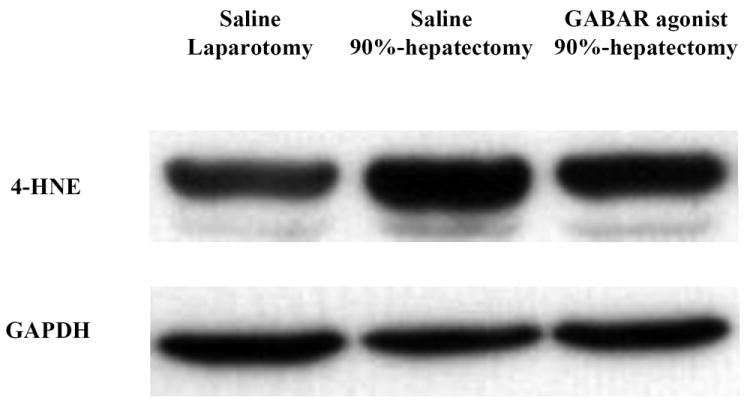
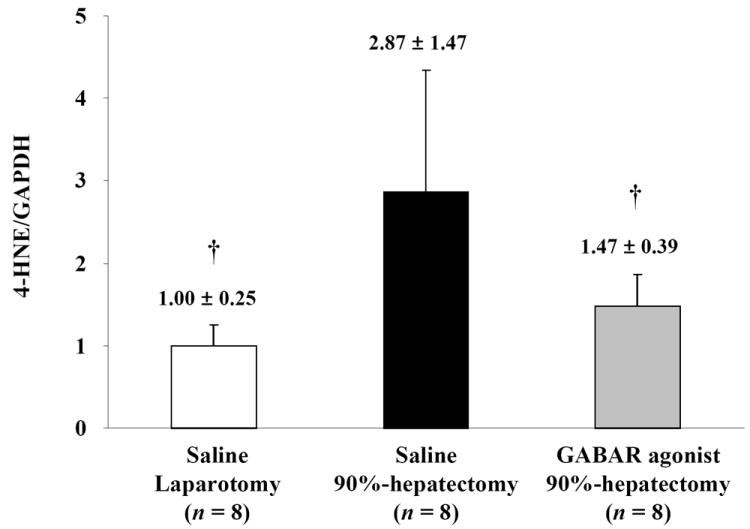
(A) Changes in the intensity of 4-HNE and GAPDH.
(B) Changes in quantitative HNE/GAPDH (†p <0.05).
Ninety percent hepatectomy resulted in lipoperoxidation and the GABAR agonist reduced lipoperoxidation.
Abbreviations: 4-hydroxynonenal, 4-HNE; γ-aminobutyric acid receptor, GABAR; glyceraldehyde-3-phosphate dehydrogenase, GAPDH.
Response to and repair of DNA damage
To evaluate the pathway for the response against OS, ATM and H2AX were investigated (Fig. 3A). Although normalized ATM (the ratio of ATM/GAPDH) showed a significant difference between laparotomy and 90% hepatectomy with saline (p = 0.0313), there was no significant difference between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0633) (Fig. 3B).
Figure 3. Western blot analyses of ATM and H2AX.
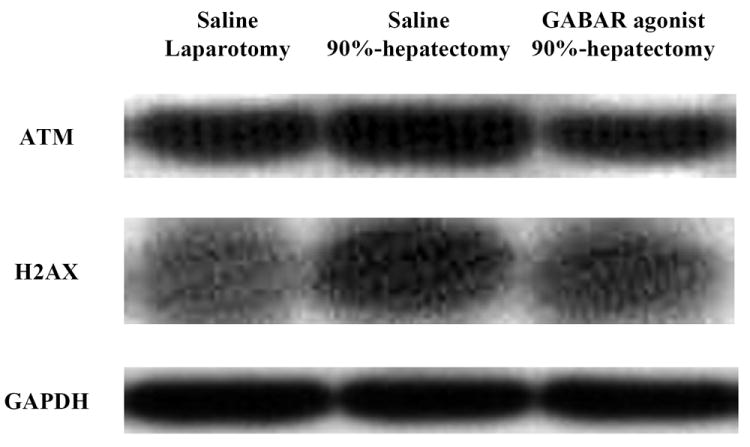
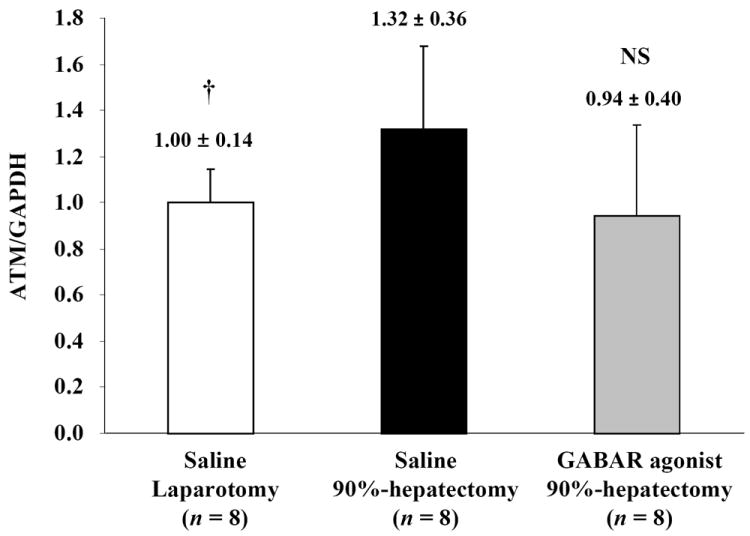
(A) Changes in the intensity of ATM, H2AX, Akt and GAPDH.
(B) Changes in quantitative ATM/GAPDH (†p <0.05).
Notably, there were no differences in ATM between 90% hepatectomy with saline and that with the GABAR agonist.
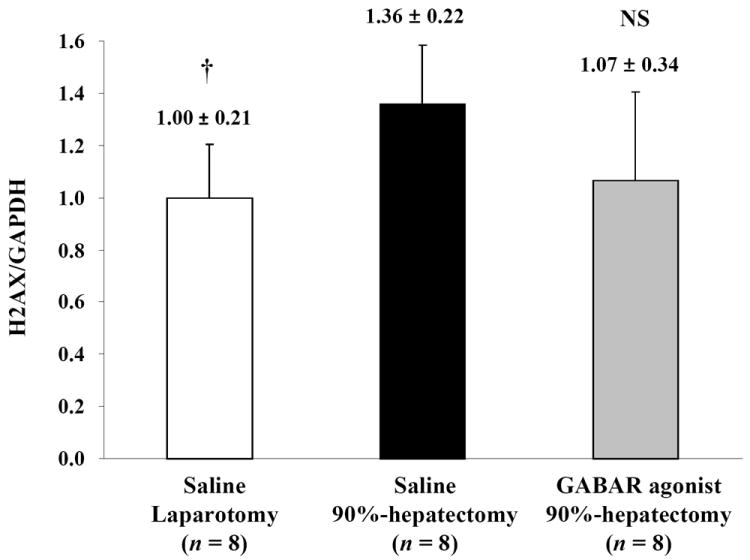
(C) Changes in quantitative H2AX/GAPDH (†p <0.05).
Notably, there were no differences in H2AX between 90% hepatectomy with saline and that with the GABAR agonist.
Abbreviations: ataxia-telangiectasia mutated kinase, ATM; γ-aminobutyric acid receptor, GABAR; glyceraldehyde-3-phosphate dehydrogenase, GAPDH; not significant (p ≥ 0.05), NS.
Normalized H2AX (the ratio of H2AX/GAPDH) showed a significant difference between laparotomy and 90% hepatectomy with saline (p = 0.0050), but there was no significant difference between 90% hepatectomy with saline and that with GABAR agonist (p = 0.0625) (Fig. 3C).
Promotion of cell survival
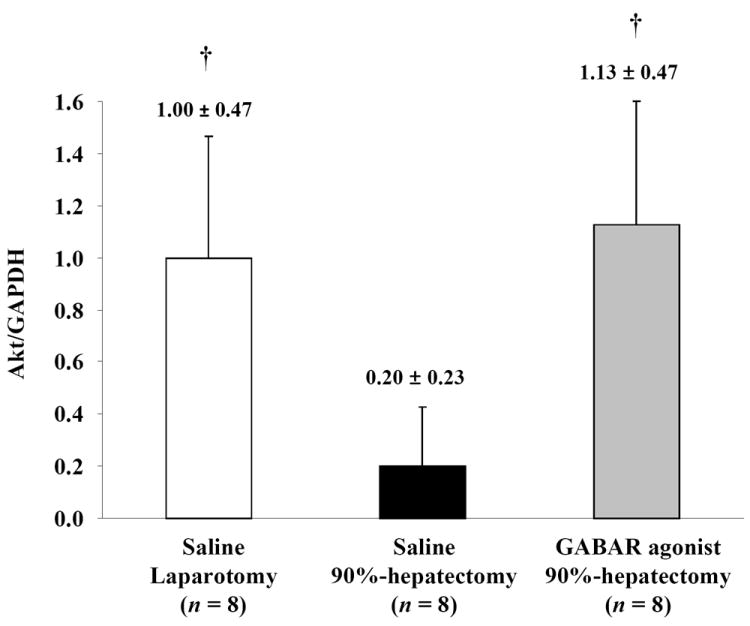
Akt was investigated to determine the survival pathway (Fig. 4A). Normalized Akt (the ratio of Akt/GAPDH) showed significant differences between laparotomy and 90% hepatectomy with saline (p = 0.0006) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0002) (Fig. 4B).
Figure 4. Western blot analysis of Akt.
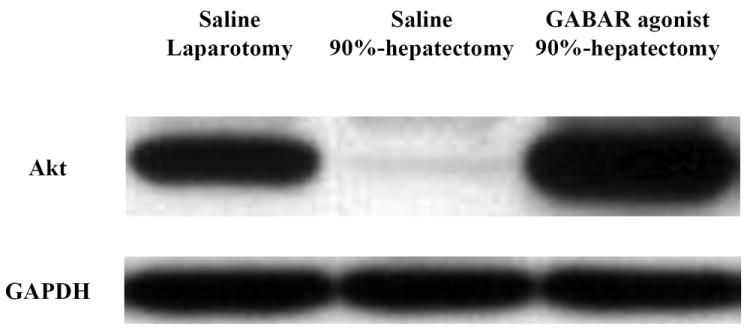
(A) Changes in the intensity of Akt and GAPDH.
(B) Changes in quantitative Akt/GAPDH (†p <0.05).
Notably, there was a considerable difference in Akt between 90% hepatectomy with saline and that with the GABAR agonist.
Abbreviations: γ-aminobutyric acid receptor, GABAR; glyceraldehyde-3-phosphate dehydrogenase, GAPDH.
The activities of antioxidant enzymes
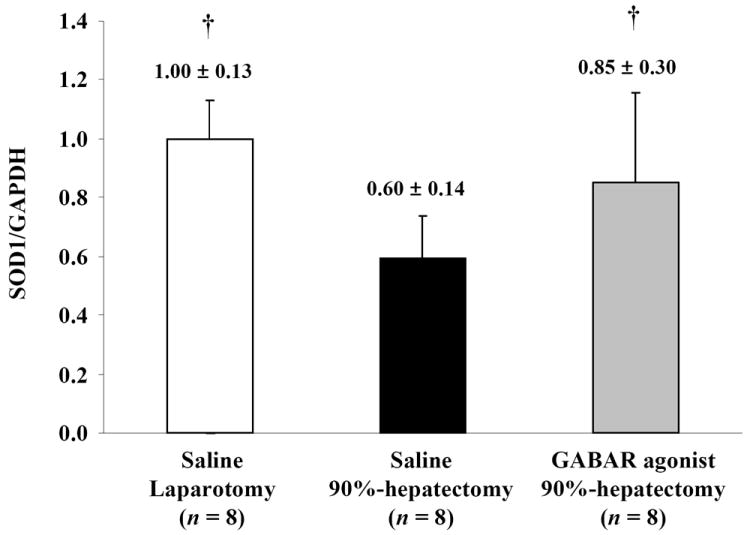
To verify the FR scavenging system, SOD1, SOD2 and catalase were investigated (Fig. 5A). Normalized SOD1 (the ratio of SOD1/GAPDH) showed significant differences between laparotomy and 90% hepatectomy with saline (p <0.0001) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.0475) (Fig. 5B).
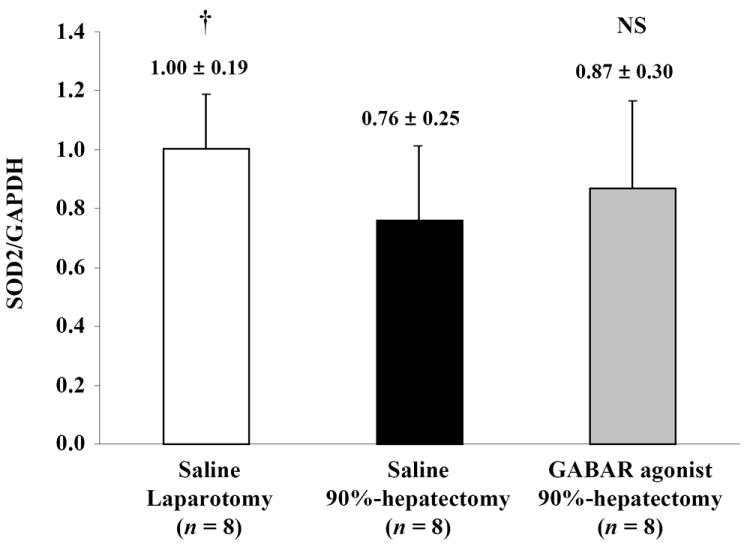
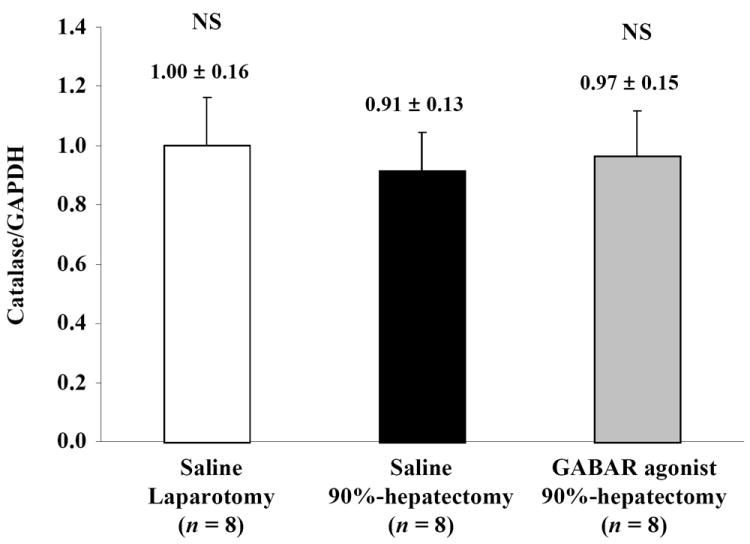
Figure 5. Western blot analyses of SOD1, SOD2 and catalase.
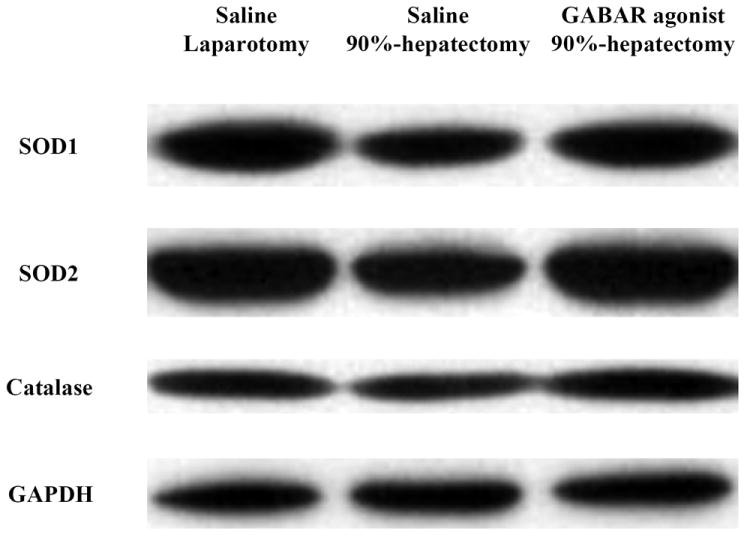
(A) Changes in the intensity of SOD1, SOD2, catalase and GAPDH.
(B) Changes in quantitative SOD1/GAPDH (†p <0.05).
These results showed that SOD1 was decreased with 90% hepatectomy with saline compared with laparotomy. Additionally, there was a large difference in SOD1 between 90% hepatectomy with saline and that with the GABAR agonist.
(C) Changes in quantitative SOD2/GAPDH (†p <0.05).
These results showed that SOD2 was decreased with 90% hepatectomy with saline compared with laparotomy. Additionally, there was no difference in SOD2 between 90% hepatectomy with saline and that with the GABAR agonist.
(D) Changes in quantitative catalase/GAPDH.
There were no differences in catalase, between laparotomy and 90% hepatectomy with saline, and between 90% hepatectomy with saline and that with the GABAR agonist, respectively.
Abbreviations: γ-aminobutyric acid receptor, GABAR; glyceraldehyde-3-phosphate dehydrogenase, GAPDH; not significant (p ≥0.05), NS; superoxide dismutase, SOD.
Although normalized SOD2 (the ratio of SOD2/GAPDH) showed a significant difference between laparotomy and 90% hepatectomy with saline (p = 0.0482), there was no significant difference between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.4451) (Fig. 5C).
For normalized catalase (the ratio of catalase/GAPDH), there was no significant difference between laparotomy and 90% hepatectomy with saline (p = 0.2585) and between 90% hepatectomy with saline and that with the GABAR agonist (p = 0.4876) (Fig. 5D).
DISCUSSION
Although GABA was initially thought to be confined to the central nervous system, it is currently considered to be a multifunctional molecule with various physiological effects throughout the body.9,10 The liver contains GABA and its transporter,10 and hepatic GABAR has been detected.12 However, the function of GABA and/or GABAR in the liver is unknown. Our histopathological, biochemical and immunohistological results showed that 90% hepatectomy resulted in apoptotic induction and subsequent liver damage, and that GABAR activation by a specific agonist reduced this damage and apoptotic induction in vivo; however, the behavior of endogenous GABA is still unclear.
OS, which causes DNA damage and subsequent apoptosis, is an imbalance between FR production and antioxidant defenses.1-3 From the view point of FR production, ROS/RNS can attack and damage a variety of critical biological molecules, including lipids, essential cellular proteins and DNA.1-3 Products of lipid peroxidation can be easily detected in biological fluids and tissues, and can reliably and rapidly reflect the sensitive and specific signals of lipid peroxidation that occur in vivo.30,31 The compound 4-HNE is an end product of lipoperoxidation with antiproliferative and proapoptotic properties.30,31 Our results of 4-HNE showed that OS occurred after 90% hepatectomy, and GABAR activation by a specific agonist reduced OS in vivo.
As described above, ROS/RNS has been suggested as a major contributing factor for DNA damage in progression of OS. As a sensor of DNA damage responses, the protein kinase ATM can be initiated through rapid intermolecular autophosphorylation induced by DNA damage,14,32 phosphorylate various proteins, and subsequently amplify the responses to DNA damage.14 This DNA damage-inducible kinase activates H2AX.5 Histone H2AX is a variant histone that represents approximately 10% of the total H2A histone proteins in normal human fibroblasts.33 H2AX is required for cell cycle arrest and DNA repair following double-stranded DNA breaks.5,33 DNA damage results in the rapid phosphorylation of H2AX by ATM and ATR (ATM and Rad3-related kinase).5,34,35 Within minutes following DNA damage, H2AX is phosphorylated at the sites of the DNA damage.5,36 This very early event in the DNA-damage response is required for the recruitment of a multitude of DNA-damage response proteins. Therefore, histone H2AX is activated by ATM after DNA damage.5 The ATM/H2AX signaling pathway is important in the response to and repair of DNA damage induced by OS,5,15 and our results of ATM and H2AX clearly showed that OS after 90% hepatectomy caused DNA-damage signaling and triggered a subsequent DNA-repair process. In previous studies of brain research, it has been suggested that GABAR regulation by specific agonists or antagonists affects the prevention of DNA damage due to OS via the ATM/H2AX pathway in vivo and in vitro,5,14,15 although H2AX may be related to an unknown pathway.5 However, our results suggested that the preventive effects on shear stress-induced OS after 90% hepatectomy by GABAR activation did not depend on the ATM/H2AX pathway.
Akt also plays a critical role in controlling apoptosis,32,37,38 and promotes cell survival to prohibit apoptosis.38-42 Apoptotic machinery is inhibited by the activation of Akt.37,43,44 Akt is an integral component of the anti-apoptotic process related to the activation of phosphatidylinositol-3 kinase (PI3K),17 and is downstream from ATM.38,45 Akt may have an unknown pathway.37 Our results clearly showed that 90% hepatectomy caused OS and greatly decreased Akt, and suggested that a subsequent apoptotic process was triggered. With regard to GABAR regulation of OS in the brain, Akt has also been suggested as a possible pathway.5,16,46 Studies on the brain have suggested that GABAR regulation by specific agonists or antagonists affect the prevention of apoptotic induction due to OS via the Akt pathway in vivo and in vitro.16,17,46 Our results suggested that the promotion of cell survival via the Akt pathway was strongly disturbed in OS after 90% hepatectomy, and we speculated that this preventive effect of GABAR regulation against OS may depend on Akt pathway.
As described above, OS is an imbalance between FR production and antioxidant defenses. With regard to these defenses, FR scavenging enzymes, such as SOD and catalase, also play an important role in reducing DNA damage and subsequent apoptosis.2,3,47 Cells are normally able to defend themselves against OS-induced damage through this scavenging system.3,47 Therefore, antioxidant enzymes reduce OS, and these effects have also been reported in GABAR regulation.18,19 However, our results revealed that this scavenging system did not appear to be triggered, although OS after 90% hepatectomy decreased SOD1 and SOD2, but not catalase, compared with laparotomy. The GABAR agonist caused an increase in only SOD1. Our findings of the behavior of SOD1, SOD2 and catalase in vivo are not sufficient evidence that GABAR regulation affects the FR scavenging system. One possible explanation for these responses after 90% hepatectomy and this discrepancy between studies in the behavior of SOD1, SOD2 and catalase may be that these scavenging systems failed to stimulate some reactive molecules, which evaded the detoxification process and damaged potential targets because of the considerable damage after 90% hepatectomy, even though FR scavenging enzymes can cope with huge amounts of ROS.48 As a result, this may present a dilemma when seeking to determine causal relationships and better insight into the intricacies of stress signaling in vivo.
In the brain research field, studies on GABAR regulation show conflicting data. Some studies have reported that GABAR activation by specific agonists was successful, and others have shown that GABAR antagonists had positive effects against OS.5-7 Previous studies have also focused on ATR/ATM/H2AX, ATM/H2AX, H2AX, Akt and antioxidant enzymes as possible pathways and an explanation for the positive effects of GABAR agonists against OS in the brain.5,16,17 We consider that these previous studies showed that the regulation of GABAR by either agonists or antagonists affected OS via these pathways in the brain.5,6,8,49 Although some previous studies have shown that GABAR regulation has some effects on tissue throughout the body, as well as GABA behavior in hepatocytes,10,50 caution should be shown when interpreting the direct effects of GABAR regulation on hepatocytes; more detailed studies including experiments in vitro are still required.
In conclusion, GABAR activation by a specific agonist in vivo can be achieved in the liver, and this activation appears to reduce OS-mediated damage after extended hepatectomy in vivo, and the mechanism via an Akt-dependent pathway may be a key. More detailed studies are required in this area of research.
Acknowledgments
We are very grateful to Dickson W. Dennis, Monica Castanedes-Casey, Virginia R. Phillips, Linda G. Rousseau and Melissa E. Murray (Department of Neuroscience, Mayo Clinic Florida, Jacksonville, FL, USA) for their diagnostic and technical support for histopathological and immunohistological assessment.
Financial Support: This work was partially supported by grants to J.H. Nguyen from the Deason Foundation, Sandra and Eugene Davenport, Mayo Clinic CD CRT-II, and from the National Institutes of Health (R01NS051646-01A2), as well as a grant to T. Hori from the Uehara Memorial Foundation (No. 200940051, Tokyo, 171-0033, Japan).
Footnotes
Contribution of colleagues: J.H. Nguyen designed the study. L.B. Gardner and T. Hori performed the research. T. Hori performed the surgery and wrote the paper. F. Chen, A.M.T. Baine and T. Hata assisted in the research. J.H. Nguyen and S. Uemoto supervised the research.
All authors have no financial conflict of interest.
References
- 1.Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–40. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 3.Turan B. Role of antioxidants in redox regulation of diabetic cardiovascular complications. Curr Pharm Biotechnol. 2010;11:819–36. doi: 10.2174/138920110793262123. [DOI] [PubMed] [Google Scholar]
- 4.Pamenter ME, Hogg DW, Ormond J, Shin DS, Woodin MA, Buck LT. Endogenous GABAA and GABAB receptor-mediated electrical suppression is critical to neuronal anoxia tolerance. Proc Natl Acad Sci USA. 2011;108:11274–9. doi: 10.1073/pnas.1102429108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–4. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira JM, Burnett AL, Rameau GA. Activity-dependent regulation of surface glucose transporter-3. J Neurosci. 2011;31:1991–9. doi: 10.1523/JNEUROSCI.1850-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol. 2011;300:818–26. doi: 10.1152/ajpregu.00426.2010. [DOI] [PubMed] [Google Scholar]
- 8.Kurt S, Crook JM, Ohl FW, Scheich H, Schulze H. Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex. Hear Res. 2006;212:224–35. doi: 10.1016/j.heares.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Gong Y, Zhang M, Cui L, Minuk GY. Sequence and chromosomal assignment of a human novel cDNA: similarity to gamma-aminobutyric acid transporter. Can J Physiol Pharmacol. 2001;79:977–84. [PubMed] [Google Scholar]
- 10.Norikura T, Kojima-Yuasa A, Opare Kennedy D, Matsui-Yuasa I. Protective effect of gamma-aminobutyric acid (GABA) against cytotoxicity of ethanol in isolated rat hepatocytes involves modulations in cellular polyamine levels. Amino Acids. 2007;32:419–23. doi: 10.1007/s00726-006-0381-3. [DOI] [PubMed] [Google Scholar]
- 11.Chu XY, Liang Y, Cai X, Cuevas-Licea K, Rippley RK, Kassahun K, et al. Metabolism and renal elimination of gaboxadol in humans: role of UDP-glucuronosyltransferases and transporters. Pharm Res. 2009;26:459–68. doi: 10.1007/s11095-008-9799-5. [DOI] [PubMed] [Google Scholar]
- 12.Biju MP, Pyroja S, Rajeshkumar NV, Paulose CS. Hepatic GABAA receptor functional regulation during rat liver cell proliferation. Hepatol Res. 2001;21:136–46. doi: 10.1016/s1386-6346(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht J, Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–46. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 14.Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA. 2004;101:8809–14. doi: 10.1073/pnas.0403062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andang M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci USA. 2011;108:5837–42. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Li C, Yin XH, Zhang GY. Additive neuroprotection of GABA A and GABA B receptor agonists in cerebral ischemic injury via PI-3K/Akt pathway inhibiting the ASK1-JNK cascade. Neuropharmacology. 2008;54:1029–40. doi: 10.1016/j.neuropharm.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Kang JS, Ki YW, Lee SH, Lee SJ, Lee KS, et al. Akt/GSK3beta signaling is involved in fipronil-induced apoptotic cell death of human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2011;202:133–41. doi: 10.1016/j.toxlet.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Tejada S, Roca C, Sureda A, Rial RV, Gamundi A, Esteban S. Antioxidant response analysis in the brain after pilocarpine treatments. Brain Res Bull. 2006;69:587–92. doi: 10.1016/j.brainresbull.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Li Y, Yan G, Bu Q, Lv L, Yang Y, et al. Protective Role of Taurine Against Morphine-Induced Neurotoxicity in C6 Cells via Inhibition of Oxidative Stress. Neurotox Res. 2011 doi: 10.1007/s12640-011-9247-x. in press. [DOI] [PubMed] [Google Scholar]
- 20.Bachellier P, Rosso E, Pessaux P, Oussoultzoglou E, Nobili C, Panaro F, et al. Risk factors for liver failure and mortality after hepatectomy associated with portal vein resection. Ann Surg. 2011;253:173–9. doi: 10.1097/SLA.0b013e3181f193ba. [DOI] [PubMed] [Google Scholar]
- 21.Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453–64. doi: 10.1006/niox.2001.0373. [DOI] [PubMed] [Google Scholar]
- 22.Schoen Smith JM, Lautt WW. The role of prostaglandins in triggering the liver regeneration cascade. Nitric Oxide. 2005;13:111–7. doi: 10.1016/j.niox.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortensen KE, Conley LN, Hedegaard J, Kalstad T, Sorensen P, Bendixen C, et al. Regenerative response in the pig liver remnant varies with the degree of resection and rise in portal pressure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G819–30. doi: 10.1152/ajpgi.00179.2007. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–70. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 25.Rudich N, Zamir G, Pappo O, Shlomai Z, Faroja M, Weiss ID, et al. Focal liver necrosis appears early after partial hepatectomy and is dependent on T cells and antigen delivery from the gut. Liver Int. 2009;29:1273–84. doi: 10.1111/j.1478-3231.2009.02048.x. [DOI] [PubMed] [Google Scholar]
- 26.Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46:802–12. doi: 10.1002/hep.21728. [DOI] [PubMed] [Google Scholar]
- 27.Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–9. doi: 10.1016/s0039-6060(97)90283-x. [DOI] [PubMed] [Google Scholar]
- 28.Hori T, Ohashi N, Chen F, Baine A, Gardner L, Hata T, et al. Simple and reproducible hepatectomy in the mouse using the clip technique. World J Gastroenterol. 2012 doi: 10.3748/wjg.v18.i22.2767. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori T, Uemoto S, Zhao X, Chen F, Baine A, Gardner L, et al. Surgical guide including innovative techniques for orthotopic liver transplantation in the rat: Key techniques and pitfalls in whole and split liver grafts. Annals of Gastroenterology. 2010;13:270–95. [Google Scholar]
- 30.Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, et al. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008;45:111–8. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voulgaridou GP, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res. 2011;711:13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009;27:1987–98. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Adamski R, Chen J. Focus on histone variant H2AX: to be or not to be. FEBS Lett. 2010;584:3717–24. doi: 10.1016/j.febslet.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 35.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 36.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, et al. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through an Akt dependent mechanism. J Biol Chem. 2011 doi: 10.1074/jbc.M111.245985. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez Y, Amran D, de Blas E, Aller P. Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4) Protein kinase modulation and reactive oxygen species generation. Biochem Pharmacol. 2009;77:384–96. doi: 10.1016/j.bcp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–7. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 40.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 41.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–36. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 42.Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, et al. The survival pathways phosphatidylinositol-3 kinase (PI3-K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49(1):204–14. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- 43.Kim D, Chung J. Akt: versatile mediator of cell survival and beyond. J Biochem Mol Biol. 2002;35:106–15. doi: 10.5483/bmbrep.2002.35.1.106. [DOI] [PubMed] [Google Scholar]
- 44.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 45.Khalil A, Morgan RN, Adams BR, Golding SE, Dever SM, Rosenberg E, et al. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–91. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cesetti T, Fila T, Obernier K, Bengtson CP, Li Y, Mandl C, et al. GABAA Receptor Signaling Induces Osmotic Swelling and Cell Cycle Activation of Neonatal Prominin+ Precursors. Stem Cells. 2011;29(2):307–19. doi: 10.1002/stem.573. [DOI] [PubMed] [Google Scholar]
- 47.Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–52. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 48.Maulik N, Das DK. Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim Biophys Acta. 2008;1780:1368–82. doi: 10.1016/j.bbagen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Goodchild AK, Van Deurzen BT, Sun QJ, Chalmers J, Pilowsky PM. Spinal GABA(A) receptors do not mediate the sympathetic baroreceptor reflex in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R320–31. doi: 10.1152/ajpregu.2000.279.1.R320. [DOI] [PubMed] [Google Scholar]
- 50.Skvorak KJ, Hager EJ, Arning E, Bottiglieri T, Paul HS, Strom SC, et al. Hepatocyte transplantation (HTx) corrects selected neurometabolic abnormalities in murine intermediate maple syrup urine disease (iMSUD) Biochim Biophys Acta. 2009;1792:1004–10. doi: 10.1016/j.bbadis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


