Abstract
Betulinic acid is a natural product possessing abundant and favourable biological activity, including anti-cancer, anti-malarial, anti-inflammatory and anti-HIV properties, while causing minimal toxicity to unaffected cells. The full biological potency of betulinic acid cannot be fully unlocked, however, for a number of reasons, a primary one being its limited solubility in aqueous and biologically pertinent organic media. Aiming to improve the water solubility of betulinic acid without disrupting its structurally related bioactivity, we have prepared different ionic derivatives of betulinic acid. Inhibition bioassays on HIV-1 protease-catalysed peptide hydrolysis indicate significantly improved performance resulting from converting the betulinic acid to organic salt form. Indeed, for one particular cholinium-based derivative, its water solubility is improved more than 100 times and the half maximal inhibitory concentration (IC50) value (22 μg mL−1) was one-third that of wide-type betulinic acid (60 μg mL−1). These encouraging results advise that additional studies of ionic betulinic acid derivatives as a therapeutic solution against HIV-1 infection are warranted.
Keywords: Betulinic acid, anti-HIV, ionic liquid, HIV-1 protease, derivative
Introduction
Betulinic acid (3β-hydroxy-lup-20(29)-en-28-oic acid) (1 in Scheme 1) is a pentacyclic lupane-type triterpene. This compound and its derivatives have exhibited a variety of biological properties such as anti-HIV-1 (human immunodefficiency virus type-1), anti-cancer, anti-bacterial, anti-malarial, anti-inflammatory and anthelmintic activities1–3. Several mechanisms have been offered as to how betulinic acid and its derivatives are able to act as HIV-1 anti-virals4. The first mechanism involves the inhibition of HIV-1 replication and maturation5–8. Some major derivatives of betulinic acid investigated for this mechanism include dihydrobetulinic acid5,6, 3-alkylamido-3-deoxy-betulinic acid derivatives8, and 3-O-(3′,3′-dimethylsuccinyl) betulinic acid9,10. In particular, the last derivative showed a high activity against HIV-induced cytopathic effects in HIV-1-infected MT-4 cells, with the likely mechanism being that this derivative might be involved in the step(s) of virion assembly and/or budding of virions but does not appear to inhibit HIV-1 protease activity9; another group10 also suggested that the last derivative exhibited no inhibition on the HIV-1 protease in vitro nor interference with virus assembly or release, but it acts at a late stage of the virus life cycle: it specifically delays the cleavage of Gag between the capsid (CA) and p2, causing the deferred formation of mature viral core and minimised HIV-1 infectivity. By a second mechanism, betulinic acid derivatives block viral infection at a post-binding, envelope-dependent step during the fusion of the virus to the cell membrane, which can be determined from viral binding and syncytium formation11–16. These derivatives include ω-undecanoic amides of betulinic acid11,12, ω-aminoalkanoic acid derivatives13, betulinic acid derivative RPR10361114,16 and IC9564 (4S-[8-(28-betuliniyl) aminooctanoylamino]-3R-hydroxy-6-methylheptanoic acid)15. A third mechanism of anti-HIV activity of pentacyclic triterpenes is based upon the inhibitory activity against HIV-1 reverse transcriptase17. Since reverse transcriptase is required for early proviral DNA synthesis, inhibition of the reverse transcriptase-catalysed polymerisation of DNA from viral RNA inhibits the viral replication. Reverse transcriptases can be virus-specific and are considered viable chemotherapeutic targets. However, a number of studies have also suggested that betulinic acid and its derivatives may show no inhibition towards HIV-1 reverse transcriptase6,11,12. Yet another mechanism is the inhibition of HIV-1 protease by betulinic acid (half maximal inhibitory concentration, IC50 = 9 μM18 or 2.5 μM19) and other triterpenes, although some derivatives (e.g. 3-O-(3′,3′-dimethylsuccinyl) betulinic acid9 and ω-aminoalkanoic acid derivatives11,12) apparently failed to inhibit the activity of HIV-1 protease. The HIV-1 protease consists of two identical monomers which contribute to the construction of only one active site. It is a retroviral aspartyl protease that catalyses the cleavage of newly synthesized polyproteins to produce the mature protein components of an infectious HIV virion; hence inhibition of HIV-1 protease effectively disrupts the virus’ ability to replicate and infect additional cells. For this reason, several inhibitors of HIV-1 protease have been developed as chemotherapeutic agents for treating AIDS, including saquinavir, indinavir, tipranavir and darunavir20,21. The mechanism of HIV-1 protease inhibition by betulinic acid and derivatives is still not well understood because these compounds may exhibit other inhibition mechanisms as discussed above. Quéré et al.19 suggested that triterpenes including betulinic acid could inhibit the protease dimerisation, a prerequisite for protease activation; computer modelling showed that several triterpenes could dock into the hydrophobic interface site of the relaxed monomer.
Scheme 1.
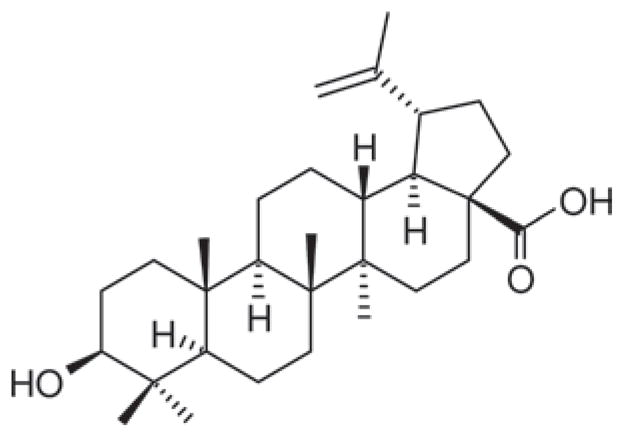
Betulinic acid (1).
Due to the mutation of HIV in response to most chemotherapeutic drugs, there is a continual demand for the development of novel anti-HIV compounds, particularly agents that are at once inexpensive and minimally toxic. A major impediment to unleashing the anti-HIV potency of betulinic acid lies in its extremely poor solubility in aqueous solutions and, to a lesser extent, in common organic solvents such as alcohols, ethers and esters. The solubility of betulinic acid in water is in fact only about 0.02 μg mL−1 at room temperature22. Its solubility in common organic solvents at 25°C is also fairly low; e.g. 1% (w/v) in ethanol and 5% (w/v) in DMSO23. A limited number of derivatives of betulinic acid were reported to yield improved water solubility and biological activity compared to unmodified betulinic acid1,3,24. Building on this early progress using lessons gained from our experience with ionic liquids23,25, it is anticipated that novel ionic derivatives of betulinic acid may exhibit further improved water solubility and anti-HIV activity. Of particular relevance, betulinic acid coupled with amino acids at the C-28 carboxylic acid position has been reported to exhibit improved water solubility and toxicity against a cultured human melanoma (MEL-2) cell line26. In the current study, we initially prepared a glycinyl conjugate of betulinic acid for subsequent modification, as this form showed the highest water solubility and cytotoxicity against cancerous cells among the amino acid derivatives of betulinic acid studied earlier26.
Recently, the Rogers group27–29 introduced a new concept to eliminate the problematic polymorphism of pharmaceutical drugs by converting them into stable liquid forms, so-called “ionic liquid active pharmaceutical ingredients” (IL-API). In its simplest manifestation, the idea is to transform the API into ionic form followed by exchange of the counter ion (metathesis) with a bulky organic counter ion that lends itself towards rendering the salt proper (ion pair) a liquid. Interestingly, the counter ion need not merely serve to frustrate ionic packing (preventing crystallisation) but can furthermore be selected to display desired biological functions complementary to the API. For example, sodium ibuprofen (an anti-inflammatory) can be paired with didecyldimethylammonium bromide (anti-bacterial and anti-inflammatory) to engender the IL-API didecyldimethylammonium ibuprofenate which retains dual biological roles30. Motivated by this molecular flexibility, we forecast that betulinic acid derivatives can be designed not only to achieve excellent aqueous solubility and improved anti-HIV activity, but also to show secondary biological function introduced via the counter ion. In a preliminary study, we evaluate a number of ionic betulinic acid derivatives for their ability to inhibit HIV-1 protease activity, while other possible inhibition routes remain a topic of current investigation in our laboratories.
Methods
Materials
The following materials were supplied by Sigma-Aldrich: betulinic acid (90%), glycine methyl ester hydrochloride, N,N′-dicyclohexylcarbodiimide (DCC), 4-(dimethylamino)pyridine (DMAP), choline chloride, benzalkonium chloride and Amberlyst® A26 hydroxide form. The chromogenic peptide (Ac-Arg-Lys-Ile-Leu-Phe(4-NO2)-Leu-Asp-Gly-NH2, product no. 22089, MW 1047.21) was acquired from AnaSpec (Fremont CA, USA). The HIV-1 protease, recombinant E. coli (10,000 U,† product no. 382136), from Calbiochem® was provided by EMD Chemicals, Inc (Gibbstown, NJ, USA).
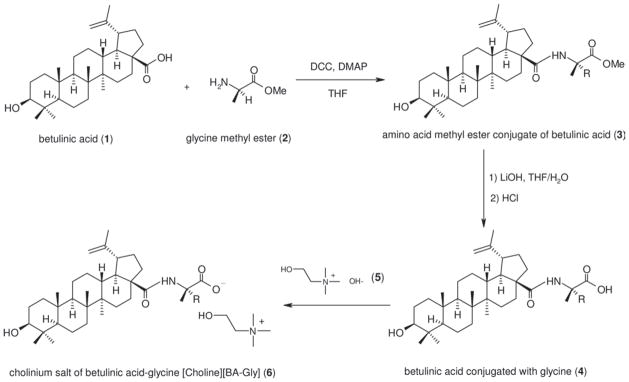
Preparation of ionic salts of betulinic acid conjugated with glycine
The early steps of the synthesis (synthesis of 4 in Scheme 2) are based upon a modification of a literature protocol26. Initially, betulinic acid (1, 200 mg, 0.44 mmol), glycine methyl ester (2, 100 mg, 0.80 mmol) and triethylamine (81 mg, 0.80 mmol) were dissolved in 50 mL anhydrous tetrahydrofuran (THF) at room temperature. To this solution, DCC (108 mg, 0.52 mmol) and DMAP (30 mg, 0.25 mmol) were added and the reaction mixture stirred at room temperature under nitrogen for 48 h. After completion of the reaction, the precipitate (dicyclohexylurea byproduct) was filtered off and the filtrate evaporated under vacuum to remove THF. The crude product was dissolved in 100 mL of ether/ethyl acetate (2:1, v/v) and extracted with 100 mL of water to remove the excess water-soluble carbodiimide reagent and any remaining dicyclohexylurea. The organic layer was further extracted with 1.0 N HCl (2 × 100 mL) to remove DMAP31, saturated NaHCO3 solution (1 × 100 mL), and finally with water (1 × 100 mL)32,33. The purified organic layer was dried over Na2SO4. After filtration, ethyl acetate was evaporated under vacuum to yield the glycine methyl ester conjugate of betulinic acid (3) (230 mg, 99% yield).
Scheme 2.
General steps for preparing a cholinium salt of betulinic acid conjugated with an amino acid (6, [Choline][BA-Gly]).
The methyl ester (3, 200 mg, 0.38 mmol) was dissolved in 100 mL of THF/H2O (4:1, v/v) solution, followed by the addition of LiOH (45 mg, 1.88 mmol). The reaction mixture was stirred at room temperature for 4 h under N2. At the end of the reaction, THF was evaporated under vacuum and the product obtained was dissolved in 200 mL of ethyl acetate, followed by washing with water, 0.1 M HCl and water again. The organic layer was then dried with Na2SO4, and after filtration the solvent was removed under vacuum to yield glycine conjugate of betulinic acid (4) (168 mg, 86% yield).
Choline hydroxide was prepared from choline chloride following an anion-exchange column approach used previously34,35. The glycinylated betulinic acid (4, 150 mg) was dissolved in 50 mL of THF/H2O (4:1, v/v), followed by the addition of a five-fold molar excess of choline hydroxide (5). The reaction mixture was stirred at room temperature for 24 h. After this time, THF was removed by rotary evaporation. The crude product was extracted into 100 mL of ethyl acetate, and washed with distilled water (2 × 100 mL) to remove the excess choline hydroxide. The organic layer was dried and ethyl acetate removed as above to give the cholinium salt of betulinic acid-glycine ([Choline][BA-Gly], 6) (130 mg, 72% yield), representing a 61% overall product yield for Scheme 2.
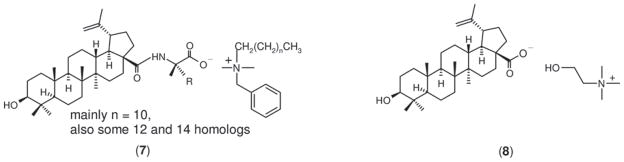
Following the above reaction strategies, we similarly prepared the benzalkonium salt of glycinylated betulinic acid ([Bzk][BA-Gly], 7; as ionic mixtures, see Scheme 3) (470 mg product from 209 mg of betulinic acid) as well as cholinium betulinate ([Choline][BA], 8) in 64% yield (120 mg) starting from 153 mg of betulinic acid.
Scheme 3.
Structures of benzalkonium salt of betulinic acid-glycine (7, [Bzk][BA-Gly]), and cholinium salt of betulinic acid (8, [Choline][BA]).
[Choline][BA-Gly] (6), m.p. 205°C–210°C. 1H NMR (300 MHz, DMSO-d6), δ (ppm) = 0.61 (3H, s), 0.73 (2H, s), 0.83 (4H, s), 0.89 (2H, s), 0.99 (2H, m), 1.02 (5H, m), 1.05–1.06 (3H, m), 1.10 (2H, m), 1.14–1.16 (3H, m), 1.20 (4H, m), 1.24 (4H, m), 1.28 (3H, m), 1.32 (3H, s), 1.66–1.71 (8H, m), 4.23–4.25 (1H, m), 4.52 (1H, m) and 5.53–5.56 (4H, m). 13C NMR (DMSO-d6), δ (ppm) = 14.9, 16.3, 16.5, 18.5, 19.5, 25.0, 25.9, 27.7, 28.7, 31.0, 33.9, 37.2, 48.1, 77.3, 157.1 and 177.8.
[Bzk][BA-Gly] (7), m.p. 175°C–180°C. 1H NMR (300 MHz, DMSO-d6), δ (ppm) = 0.62 (3H, s), 0.73 (3H, s), 0.84 (8H, m), 0.88 (3H, s), 1.26 (27H, m), 1.51–1.60 (9H, m), 1.66–1.74 (7H, m), 2.06–2.14 (4H, m), 2.25–2.42 (3H, m), 2.47 (6H, m), 2.91 (7H, m), 3.17–3.23 (6H, m), 4.23 (2H, t), 4.48–4.50 (3H, m), 4.63 (1H, s), 5.70–5.73 (1H, d) and 7.50 (5H, s). 13C NMR (DMSO-d6), δ (ppm) = 14.5, 14.9, 16.3, 16.5, 18.5, 19.5, 21.0, 22.3, 22.6, 25.0, 25.7, 25.9, 26.4, 27.7, 28.0, 28.6, 29.1, 29.3, 29.6, 30.8, 31.8, 32.7, 33.9, 34.5, 34.7, 37.3, 38.0, 38.8, 39.2, 42.5, 47.2, 48.0, 49.2, 49.7, 50.5, 55.5, 56.1, 61.9, 64.0, 66.8, 68.8, 77.3, 97.7, 109.9, 128.7, 129.5, 130.8, 133.5, 151.3, 157.2, 176.2 and 178.1.
[Choline][BA] (8), m.p. 260°C. 1H NMR (300 MHz, DMSO-d6), δ (ppm) = 0.61 (3H, s), 0.73 (3H, s), 0.83 (3H, s), 0.87 (3H, s), 1.18 (4H, m), 1.27 (3H, s), 1.37–1.40 (4H, m), 1.59 (3H, s), 1.71–1.77 (2H, m), 2.07–2.11 (1H, d), 2.47 (3H, p), 3.09 (5H, s), 4.48 (1H, m) and 4.61 (1H, m). 13C NMR (DMSO-d6), δ (ppm) = 14.9, 16.3, 16.5, 18.5, 19.6, 21.1, 25.8, 27.7, 28.6, 29.9, 31.0, 33.2, 34.6, 37.3, 37.6, 37.9, 38.8, 39.0, 42.6, 47.3, 49.4, 50.6, 53.7, 55.5, 55.6, 56.3, 67.7, 77.3, 109.6, 151.8 and 178.2.
Aqueous solubility of betulinic acid analogues
Starting with 50 μL of DMSO solution containing 5 mg mL−1 of a particular betulinic acid derivative, distilled water was added in 50 μL increments in order to visually observe a turbid solution. Each time a clear solution resulted, additional water was added in 50 μL steps. The relative water solubility was finally expressed in the dilution steps required to achieve clear solution (i.e. 2x, 3x).
Inhibition of HIV-1 protease activity
To a reaction mixture containing 0.17 mM of the chromogenic peptide substrate Ac-Arg-Lys-Ile-Leu-Phe (4-NO2)-Leu-Asp-Gly-NH2 in acetate buffer (0.1 M, pH 4.7, I = 0.2 M)‡ was added 20 μL of a DMSO solution of the desired betulinic acid analogue and 20 μL of HIV-1 protease solution (100 U). The reaction mixture (1.0 mL) was incubated at 37°C, with aliquots periodically withdrawn for high-performance liquid chromatography (HPLC) analysis. A control reaction was carried out under the same conditions, but without the addition of the betulinic acid analogue. The rate of peptide hydrolysis was periodically monitored by a LC-20AT Shimadzu HPLC equipped with a Phenomenex® Kinetex C18 column (100 mm × 4.6 mm, particle size: 2.6 μm). The HPLC column was eluted with a gradient of acetonitrile (15%–25% in 15 min) in 0.2% (v/v) acetic acid at a flow rate of 1.0 mL min−1. The detection wavelength was 280 nm. The initial reaction rate was calculated from the decrease in the peptide substrate peak area in the HPLC chromatograms. All reactions were run in triplicate.
Results and discussion
Preparation of ionic derivatives of betulinic acid
Three ionic derivatives of betulinic acid were successfully prepared, including cholinium and benzalkonium salts of glycinylated betulinic acid (6 and 7, respectively) and a cholinium salt of betulinic acid (8). Derivatives 6 and 7 were prepared following steps illustrated in Scheme 2, whereas derivative 8 was prepared by simple acid–base neutralisation reaction between betulinic acid and cholinium hydroxide. The purpose of conjugating a water-soluble amino acid (i.e. glycine) at the C-28 carboxylic acid position of betulinic acid is mainly to improve the water solubility, with the possibility to enhance the biological activity as well26. The grafting of glycine methyl ester to betulinic acid via amide linkage was based on classical carbodiimide coupling chemistry. This was followed by base-catalysed hydrolysis to generate 426,32,38. Glycine-conjugated betulinic acid (4) was further reacted with cholinium hydroxide or benzalkonium hydroxide to produce the corresponding salts of betulinic acid as shown in step 3 of Scheme 2. There are two motivations for preparing these salts: (i) to further improve the water solubility of betulinic acid by forming more polar ionic compounds and (ii) to introduce secondary biological functionality through the presence of the counter ion. Taking 7 as an example, benzalkonium compounds are known for having anti-bacterial properties27. In the case of cholinium compounds such as 6, many have been shown to be non-toxic, and choline chloride is even an essential micronutrient for human health39.
Inhibition of HIV-1 protease by ionic derivatives of betulinic acid
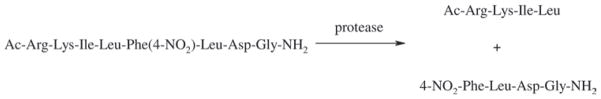
To evaluate the effectiveness of our new ionic derivatives of betulinic acid (6–8) in inhibiting HIV-1 protease activity, we selected the enzymatic hydrolysis of a chromogenic peptide (Ac-Arg-Lys-Ile-Leu-Phe(4-NO2)-Leu-Asp-Gly-NH2) for our bioassay, as shown in Scheme 4. This method has evolved into a reliable and mature technique for the following reasons: (i) the peptide substrates are typically designed to be water-soluble, so the hydrolysis reaction can be performed in aqueous buffer with little addition of and interference from organic cosolvents36,40; (ii) HIV-1 protease only cleaves the two hydrophobic amino acid residues of substrates occupying the S1-S1′ sites, thus the reactant and products can be easily detected and quantified41 and (iii) this reaction can be monitored by a number of means, such as UV–vis absorption spectroscopy36,41, fluorescence spectroscopy37 and HPLC analysis41,42.
Scheme 4.
HIV-1 protease-catalysed cleavage of oligopeptide substrate (see similar reactions in refs. 36,41,42).
It has long been known that the addition of NaCl to this assay reaction improves the catalytic activity of protease due to non-selective salting-out of hydrophobic substrates onto the enzyme surface36,43. Our experimental data in Figure 1 are in line with this trend: a higher HIV-1 protease activity was observed as the ionic strength increased from 0.05 to 0.50 M. However, we also observed that at I = 0.5 M the substrate became insoluble in the acetate buffer due to the strong salting-out effect, and the use of 5% (v/v) of DMSO was necessary for the complete dissolution of peptide. Since 5% DMSO is toxic for cultured cells, to minimise the deleterious effects of DMSO, we performed the remaining enzymatic assays in a pH 4.7 acetate buffer system at a buffer molarity of 0.1 M (I = 0.20 M), where the peptide remained fully soluble in the aqueous solution.
Figure 1.
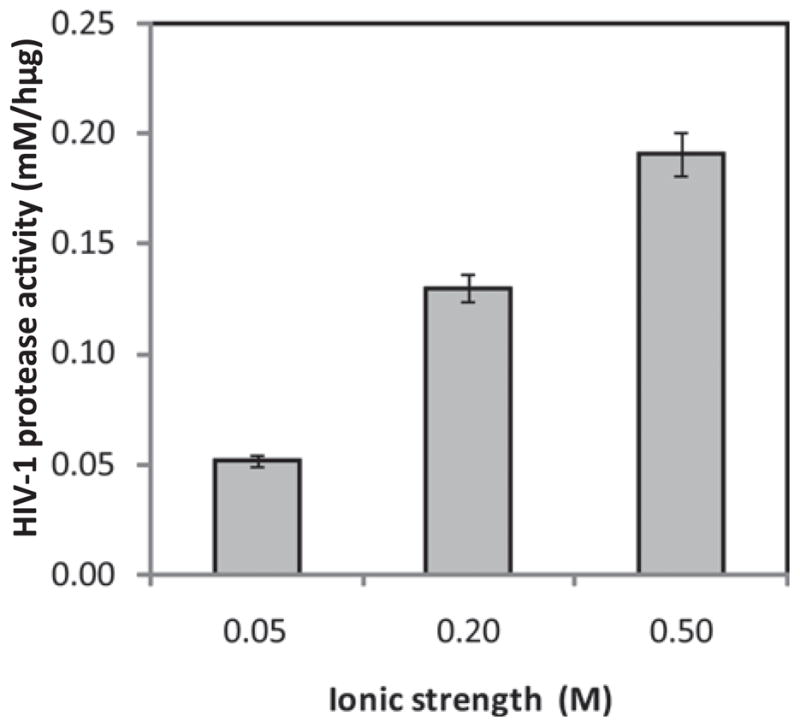
Effect of buffer ionic strength (I) on HIV-1 protease activity (conditions: 0.17 mM substrate in 1.0 mL of 0.1 M acetate buffer (pH 4.7); 100 U HIV-1 protease; NaCl added to adjust I; assay conducted at 37°C).
In the absence of HIV-1 protease, no hydrolysis of the substrate peptide was observed during the assay, confirming that the reaction is predominantly driven by the enzyme. The uninhibited enzyme reaction was initiated by the injection of 100 U of protease into the substrate solution, with the observed initial reaction rate (in units of mM h−1 μg−1 enzyme) at 37°C reflecting the native (100%) protease activity. Relative to this baseline, the inhibition studies were conducted by adding 20 μL of a DMSO solution of betulinic acid or one of its ionic derivative into the reaction mixture to achieve a final concentration of 10, 25, 50 or 100 μg mL−1. The corresponding reaction rates were compared with that of the betulinic acid-free control reaction, and the degree of inhibition was calculated in terms of the half maximal inhibitory concentration (IC50). As shown in Table 1, pepstatin A, a known natural inhibitor of aspartyl proteases44, gave an IC50 value of 29 μg mL−1 (i.e. 44 μM), which is consistent with the published value for acetyl pepstatin42,45. In contrast, betulinic acid (1) itself is associated with a rather high IC50 of 60 μg mL−1 (130 μM), presumably due to its poor solubility in aqueous media. In our experience, once the DMSO solution of betulinic acid is injected into the acetate buffer, betulinic acid begins to precipitate, causing the mixture to become cloudy. On the other hand, the ionic derivatives of betulinic acid (6–8) showed much improved water solubility (Table 1); in particular, [Choline][BA] (8) was at least 100 times more soluble in water than the virgin betulinic acid. Linked to this improved water solubility, these ionic derivatives were associated with lower IC50 values, especially in the cases of 7 (IC50= 28 μg mL−1 or 37 μM) and 8 (IC50 = 22 μg mL−1 or 40 μM), which gave results better than pepstatin A. These highly encouraging results suggest that suitable ionic derivatives of betulinic acid will display vastly improved aqueous solubility over the natural parent compound with the capability of liberating its latent biological activity in the inhibition of HIV-1 protease. We suspect that this positive outcome will further stimulate the investigation of additional biological functions of these ionic derivatives of betulinic acid, as well as the exploration of ionic forms of other potential drug candidates.
Table 1.
Inhibition of HIV-1 protease activity (IC50) by betulinic acid and derivativesa.
| IC50
|
Water solubility | |||
|---|---|---|---|---|
| HIV-1 protease inhibitor | μM | μg mL−1 | ||
| Pepstatin A | 29 | 44 | ||
| 1 | Betulinic acid | 60 | 130 | ≪ 2x, 0.02 μg mL−1 at 25°C (see ref. 22) |
| 6 | [Choline][BA-Gly] | 54 | 86 | 3x |
| 7 | [Bzk][BA-Gly] | 28 | 37 b | 2x |
| 8 | [Choline][BA] | 22 | 40 | > 100x |
Note:
values and water In general, the percentage error of IC50 solubility were about 5%;
Estimated using the molar mass of main homologue of benzalkonium salt shown in Scheme 3.
The exact mechanism of HIV-1 protease inhibition by betulinic acid and derivatives is not yet known. The computer modelling has suggested that betulinic acid and other similar triterpenes have the suitable molecular size to be fitted into the hydrophobic interface site of the relaxed monomer, which inhibits the protease dimerisation19. In addition to hydrophobic interactions, hydrogen-bonding is also a possible interaction between HIV-1 protease and hydroxyl/carboxyl groups in triterpene scaffold46. As the main objective of this study is to prepare more water-soluble derivatives of betulinic acid and screen their inhibitory effect against HIV-1 protease, further studies on other inhibition mechanisms using both biological assays and computer modelling are being carried in our laboratories and will be reported later.
Conclusions
Using straightforward coupling chemistry, we have prepared three new ionic derivatives of betulinic acid which display significantly improved water solubility. These compounds give lower IC50 values towards HIV-1 protease than the native betulinic acid, particularly for 7 and 8, which show IC50 values roughly two and three times lower than that of betulinic acid, respectively. These preliminary data suggest the high potential of ionic derivatives of betulinic acid in future treatment of HIV/AIDS. Concurrent studies in our laboratories include the investigation of alternate potential inhibition mechanisms of these new derivatives, such as the inhibition of HIV-1 reverse transcriptase and the blocking of viral entry into the cell, and these results will be reported in due course.
Footnotes
One unit (U) is defined as 1 ng of purified protein; one unit will hydrolyse 1.0 pmole of peptide substrate (SQNYPIVQ) per minute at 25°C in pH 4.7 buffer.
Although it has been reported that high ionic strength (I) may facilitate the binding between a peptide substrate and HIV-1 protease (see refs. 36,37), we observed that the water-soluble substrate can be “salted-out” by the addition of sufficiently concentrated NaCl; e.g. for I = 0.5 M.
Declaration of interest
The author (HZ) acknowledges the support provided by the National Institutes of Health-RIMI grant (5P20MD003941).
References
- 1.Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 2.Krasutsky PA. Birch bark research and development. Nat Prod Rep. 2006;23:919–942. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- 3.Baglin I, Mitaine-Offer AC, Nour M, Tan K, Cavé C, Lacaille-Dubois MA. A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents. Mini Rev Med Chem. 2003;3:525–539. doi: 10.2174/1389557033487917. [DOI] [PubMed] [Google Scholar]
- 4.Aiken C, Chen CH. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med. 2005;11:31–36. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, et al. Anti-AIDS agents, 11, Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 6.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwada Y, Wang HK, Nagao T, Kitanaka S, Yasuda I, Fujioka T, et al. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod. 1998;61:1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 8.Kashiwada Y, Chiyo J, Ikeshiro Y, Nagao T, Okabe H, Cosentino LM, et al. Synthesis and anti-HIV activity of 3-alkylamido-3-deoxy-betulinic acid derivatives. Chem Pharm Bull. 2000;48:1387–1390. doi: 10.1248/cpb.48.1387. [DOI] [PubMed] [Google Scholar]
- 9.Kanamoto T, Kashiwada Y, Kanbara K, Gotoh K, Yoshimori M, Goto T, et al. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob Agents Chemother. 2001;45:1225–1230. doi: 10.1128/AAC.45.4.1225-1230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Yuan X, Dismuke D, Forshey BM, Lundquist C, Lee KH, et al. Small-molecule inhibition of human immunodeficiency virus type 1 replication by specific targeting of the final step of virion maturation. J Virol. 2004;78:922–929. doi: 10.1128/JVI.78.2.922-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayaux JF, Bousseau A, Pauwels R, Huet T, Hénin Y, Dereu N, et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc Natl Acad Sci usa. 1994;91:3564–3568. doi: 10.1073/pnas.91.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evers M, Poujade C, Soler F, Ribeill Y, James C, Lelièvre Y, et al. Betulinic acid derivatives: a new class of human immunodeficiency virus type 1 specific inhibitors with a new mode of action. J Med Chem. 1996;39:1056–1068. doi: 10.1021/jm950670t. [DOI] [PubMed] [Google Scholar]
- 13.Soler F, Poujade C, Evers M, Carry JC, Hénin Y, Bousseau A, et al. Betulinic acid derivatives: a new class of specific inhibitors of human immunodeficiency virus type 1 entry. J Med Chem. 1996;39:1069–1083. doi: 10.1021/jm950669u. [DOI] [PubMed] [Google Scholar]
- 14.Labrosse B, Pleskoff O, Sol N, Jones C, Hénin Y, Alizon M. Resistance to a drug blocking human immunodeficiency virus type 1 entry (RPR103611) is conferred by mutations in gp41. J Virol. 1997;71:8230–8236. doi: 10.1128/jvi.71.11.8230-8236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz-Smith SL, Sun IC, Jin L, Matthews TJ, Lee KH, Chen CH. Role of human immunodeficiency virus (HIV) type 1 envelope in the anti-HIV activity of the betulinic acid derivative IC9564. Antimicrob Agents Chemother. 2001;45:60–66. doi: 10.1128/AAC.45.1.60-66.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bär S, Alizon M. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J Virol. 2004;78:811–820. doi: 10.1128/JVI.78.2.811-820.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pengsuparp T, Cai L, Fong HH, Kinghorn AD, Pezzuto JM, Wani MC, et al. Pentacyclic triterpenes derived from Maprounea africana are potent inhibitors of HIV-1 reverse transcriptase. J Nat Prod. 1994;57:415–418. doi: 10.1021/np50105a017. [DOI] [PubMed] [Google Scholar]
- 18.Ma C, Nakamura N, Miyashiro H, Hattori M, Shimotohno K. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem Pharm Bull. 1999;47:141–145. doi: 10.1248/cpb.47.141. [DOI] [PubMed] [Google Scholar]
- 19.Quéré L, Wenger T, Schramm HJ. Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem Biophys Res Commun. 1996;227:484–488. doi: 10.1006/bbrc.1996.1533. [DOI] [PubMed] [Google Scholar]
- 20.Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des. 2005;11:1805–1843. doi: 10.2174/1381612053764869. [DOI] [PubMed] [Google Scholar]
- 21.Mastrolorenzo A, Rusconi S, Scozzafava A, Barbaro G, Supuran CT. Inhibitors of HIV-1 protease: current state of the art 10 years after their introduction, From antiretroviral drugs to antifungal, antibacterial and antitumor agents based on aspartic protease inhibitors. Curr Med Chem. 2007;14:2734–2748. doi: 10.2174/092986707782360141. [DOI] [PubMed] [Google Scholar]
- 22.Jäger S, Winkler K, Pfüller U, Scheffler A. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta Med. 2007;73:157–162. doi: 10.1055/s-2007-967106. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Jones CL, Cowins JV. Lipase dissolution and stabilization in ether-functionalized ionic liquids. Green Chem. 2009;11:1128–1138. [Google Scholar]
- 24.Mukherjee R, Kumar V, Srivastava SK, Agarwal SK, Burman AC. Betulinic acid derivatives as anticancer agents: structure activity relationship. Anticancer Agents Med Chem. 2006;6:271–279. doi: 10.2174/187152006776930846. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 2008;10:696–705. [Google Scholar]
- 26.Jeong HJ, Chai HB, Park SY, Kim DS. Preparation of amino acid conjugates of betulinic acid with activity against human melanoma. Bioorg Med Chem Lett. 1999;9:1201–1204. doi: 10.1016/s0960-894x(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 27.Hough WL, Rogers RD. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull Chem Soc Jpn. 2007;80:2262–2269. [Google Scholar]
- 28.Stoimenovski J, MacFarlane DR, Bica K, Rogers RD. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm Res. 2010;27:521–526. doi: 10.1007/s11095-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 29.Bica K, Rijksen C, Nieuwenhuyzen M, Rogers RD. In search of pure liquid salt forms of aspirin: ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys Chem Chem Phys. 2010;12:2011–2017. doi: 10.1039/b923855g. [DOI] [PubMed] [Google Scholar]
- 30.Hough WL, Smiglak M, Rodríguez H, Swatloski RP, Spear SK, Daly DT, Pernak J, Grisel JE, Carliss RD, Soutullo MD, et al. The third evolution of ionic liquids: active pharmaceutical ingredients. New J Chem. 2007;31:1429–1436. [Google Scholar]
- 31.Ho PT, Ngu K-y. An effective synthesis of N-(9-fluorenylmethyloxycarbonyl) α-amino aldehydes from S-benzyl thioesters. J Org Chem. 1993;58:2313–2316. [Google Scholar]
- 32.Ammazzalorso A, Amoroso R, Bettoni G, De Filippis B, Giampietro L, Pierini M, Tricca ML. Asymmetric synthesis of (S)-ibuprofen by esterification with amides of (S)-lactic acid as chiral auxiliaries: experimental and theoretical results. Tetrahedron Lett. 2002;43:4325–4328. [Google Scholar]
- 33.Rodrigues PC, Roth T, Fiebig HH, Unger C, Mülhaupt R, Kratz F. Correlation of the acid-sensitivity of polyethylene glycol daunorubicin conjugates with their in vitro antiproliferative activity. Bioorg Med Chem. 2006;14:4110–4117. doi: 10.1016/j.bmc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Dinarès I, de Miguel CG, Ibáñez A, Mesquida N, Alcalde E. Imidazolium ionic liquids: A simple anion exchange protocol. Green Chem. 2009;11:1507–1510. [Google Scholar]
- 35.Zhao H, Baker GA, Holmes S. New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Org Biomol Chem. 2011;9:1908–1916. doi: 10.1039/c0ob01011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards AD, Phylip LH, Farmerie WG, Scarborough PE, Alvarez A, Dunn BM, et al. Sensitive, soluble chromogenic substrates for HIV-1 proteinase. j Biol Chem. 1990;265:7733–7736. [PubMed] [Google Scholar]
- 37.Porter DJ, Hanlon MH, Carter LH, 3rd, Danger DP, Furfine ES. Effectors of HIV-1 protease peptidolytic activity. Biochemistry. 2001;40:11131–11139. doi: 10.1021/bi010418p. [DOI] [PubMed] [Google Scholar]
- 38.Chinchilla R, Nájera C, Yus M, Heumann A. Kinetic resolution of racemic carboxylic acids with homochiral alcohols and dicyclohexylcarbodiimide. Tetrahedron: Asymmetry. 1991;2:101–104. [Google Scholar]
- 39.Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- 40.Dunn BM, Kammermann B, McCurry KR. The synthesis, purification, and evaluation of a chromophoric substrate for pepsin and other aspartyl proteases: design of a substrate based on subsite preferences. Anal Biochem. 1984;138:68–73. doi: 10.1016/0003-2697(84)90770-x. [DOI] [PubMed] [Google Scholar]
- 41.Dunn BM, Jimenez M, Parten BF, Valler MJ, Rolph CE, Kay J. A systematic series of synthetic chromophoric substrates for aspartic proteinases. Biochem J. 1986;237:899–906. doi: 10.1042/bj2370899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewtrakul S, Subhadhirasakul S, Rattanasuwan P. HIV-1 protease inhibitory effects of some selected plants in Caesalpiniaceae and Papilionaceae families. Songklanakarin J Sci Technol. 2003;25:509–514. [Google Scholar]
- 43.Wondrak EM, Louis JM, Oroszlan S. The effect of salt on the Michaelis Menten constant of the HIV-1 protease correlates with the Hofmeister series. FEBS Lett. 1991;280:344–346. doi: 10.1016/0014-5793(91)80327-y. [DOI] [PubMed] [Google Scholar]
- 44.Gruber A, Wheat JC, Kuhen KL, Looney DJ, Wong-Staal F. Differential effects of HIV-1 protease inhibitors on dendritic cell immunophenotype and function. J Biol Chem. 2001;276:47840–47843. doi: 10.1074/jbc.M105582200. [DOI] [PubMed] [Google Scholar]
- 45.Min BS, Bae KH, Kim YH, Miyashiro H, Hattori M, Shimotohno K. Screening of Korean plants against human immunodeficiency virus type 1 protease. Phytother Res. 1999;13:680–682. doi: 10.1002/(sici)1099-1573(199912)13:8<680::aid-ptr501>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Ohrngren P, Ekegren JK, Unge J, Unge T, Wallberg H, et al. Two-carbon-elongated HIV-1 protease inhibitors with a tertiary-alcohol-containing transition-state mimic. J Med Chem. 2008;51:1053–1057. doi: 10.1021/jm070680h. [DOI] [PubMed] [Google Scholar]