Abstract
Blue light, particularly in the wavelength range of 405–470 nm, has attracted increasing attention due to its intrinsic antimicrobial effect without the addition of exogenous photosensitizers. In addition, it is commonly accepted that blue light is much less detrimental to mammalian cells than ultraviolet irradiation, which is another light-based antimicrobial approach being investigated. In this review, we discussed the blue light sensing systems in microbial cells, antimicrobial efficacy of blue light, the mechanism of antimicrobial effect of blue light, the effects of blue light on mammalian cells, and the effects of blue light on wound healing. It has been reported that blue light can regulate multi-cellular behavior involving cell-to-cell communication via blue light receptors in bacteria, and inhibit biofilm formation and subsequently potentiate light inactivation. At higher radiant exposures, blue light exhibits a broad-spectrum antimicrobial effect against both Gram-positive and Gram-negative bacteria. Blue light therapy is a clinically accepted approach for Propionibacterium acnes infections. Clinical trials have also been conducted to investigate the use of blue light for Helicobacter pylori stomach infections and have shown promising results. Studies on blue light inactivation of important wound pathogenic bacteria, including Staphylococcus aureus and Pseudomonas aeruginosa have also been reported. The mechanism of blue light inactivation of P. acnes, H. pylori, and some oral bacteria is the photo-excitation of intracellular porphyrins and the subsequent production of cytotoxic reactive oxygen species. Although it may be the case that the mechanism of blue light inactivation of wound pathogens (e.g., S. aureus, P. aeruginosa) is the same as that of P. acnes, this hypothesis has not been rigorously tested. Limited and discordant results have been reported regarding the effects of blue light on mammalian cells and wound healing. Under certain wavelengths and radiant exposures, blue light may cause cell dysfunction by the photo-excitation of blue light sensitive chromophores, including flavins and cytochromes, within mitochondria or/and peroxisomes. Further studies should be performed to optimize the optical parameters (e.g., wavelength, radiant exposure) to ensure effective and safe blue light therapies for infectious disease. In addition, studies are also needed to verify the lack of development of microbial resistance to blue light.
Keywords: Blue light, Infectious disease, Drug resistance, Intracellular porphyrins, Reactive oxygen species, Wound healing, Microbial signaling
1. Introduction
The rapidly increasing emergence of antibiotic resistance may be leading to the end of a period that has extended over the past 50 years termed "the antibiotic era" (Nordmann et al., 2011). Bacteria replicate very rapidly and a mutation that helps a microbe survive in the presence of an antibiotic drug will quickly become predominant throughout the microbial population. Recently, the New Delhi metallo-β-lactamase (NDM-1) (Nordmann et al., 2011; Park, 2010), that confers resistance to penicillins, cephalosporins and in most cases to carbapenems, has been found in the United States (Mochon et al., 2011; Savard et al., 2011). The inappropriate prescription of antibiotics for viral diseases, the failure of some patients to complete their treatment regimen, and overuse of antibiotics in livestock feedstuffs also exacerbate the problem. Many physicians are concerned that several bacterial infections soon may become untreatable. In addition to its adverse effects on public health, antibiotic resistance contributes to higher health care costs (Filice et al., 2010; Mauldin et al., 2010). Treating resistant infections often requires the use of more expensive or more toxic drugs and can result in longer hospital stays for infected patients. As a result, a major research effort has been led to find alternative antimicrobial approaches to which, it is hypothesized, bacteria will not be easily able to develop resistance.
As a non-pharmacological technique, light-based antimicrobial therapies, including photodynamic therapy (PDT) (Dai et al., 2011a; Dai et al., 2009a; Dai et al., 2009b; Dai et al., 2010; Hamblin and Hasan, 2004) and ultraviolet C (UVC) irradiation therapy (Dai et al., 2012a; Dai et al., 2011b; Dai et al., 2012b; Taylor et al., 1993; Thai et al., 2005), have been extensively investigated as alternatives to traditional antibiotics. Advantages of light-based antimicrobial therapies include equal killing effectiveness regardless of antibiotic resistance. However, one major disadvantage of PDT as a two-part (dye + light) combination approach is the challenge of introducing photosensitizers into certain bacteria (Wainwright, 1998) and into infected tissues and less than perfect selectivity of many photosensitizers for microbial cells over host tissue. The use of UVC irradiation, on the other hand, has different limitations due to its detrimental effects on mammalian cells and possible damage to host tissue (Dai et al., 2012b).
Another novel light-based approach, blue light therapy, is attracting increasing attention due to its intrinsic antimicrobial effect without the addition of exogenous photosensitizers. In addition, it is accepted that blue light is much less detrimental to mammalian cells than ultraviolet irradiation (Kleinpenning et al., 2010). The mechanism of the antimicrobial effect of blue light is still not fully understood. The commonly accepted hypothesis is that blue light excites endogenous intracellular porphyrins, and this photon absorption then leads to energy transfer and ultimately, the production of highly cytotoxic reactive oxygen species (ROS) – most notably singlet oxygen (1O2) (Ashkenazi et al., 2003; Hamblin et al., 2005; Maclean et al., 2008b) in a similar manner to PDT.
In this review, we will discuss blue light microbial signaling as well as its implications for antimicrobial therapies, the broad antimicrobial efficacy of blue light, the mechanism of the antimicrobial effect of blue light, the effects of blue light on mammalian cells, and the effects of blue light on wound healing. To the best of our knowledge, this is the first review on blue light therapy for infectious diseases.
2. Blue light signaling in microbial cells and implications for light inactivation of bacteria
2.1. Blue light regulates biofilm formation and pathogeneses of bacteria
New exciting discoveries have been made concerning how microbial physiology changes in response to changes in environmental light exposure. Until recently, these responses were regarded as specialized adaptations involving a small sub-group of phototrophic bacteria. However, the genomes of many photosynthetic and chemotrophic bacteria, which are not known to have photophysiological responses to light, also encode photoreceptor proteins. The discovery of those photoreceptors has triggered the formulation of new concepts that not only phototrophic bacteria but also photosynthetic and chemotrophic microbes can sense light (Gomelsky, 2010). There is supportive evidence that a lifestyle choice of some bacteria between the motile single-cellular planktonic state and the multicellular surface-attached community state (biofilm) is photo-regulated by a range of mechanisms including bacterial two-component systems, the second messenger cyclic di-GMP system, and direct interactions of photoreceptors with transcription factors (Purcell et al., 2007; van der Horst et al., 2009). It was also observed that light plays an important role in an important decision that some pathogenic bacteria have to make between environmental and pathogenic lifestyles (Mussi, 2010; Ondrusch and Kreft, 2011; Swartz et al., 2007). Blue light has been identified as a key factor that can affect all these physiological responses.
An example of blue light induced regulation of biofilm and blue light inactivated pathogenesis involves the opportunistic human pathogen Acinetobacter baumannii (Mussi, 2010). Based on spectral, genetic, and functional studies, the investigators found that A. baumannii ATCC 17978 senses and responds to blue light through a process that depends on the expression of a functional blue-light-sensing A (bslA) gene, which codes for the production of a BLUF (blue light sensing using flavin)-containing photoreceptor and is the only photo receptor identified in the A. baumannii. Motility, biofilm formation, and pellicles were observed only when bacterial cells were incubated in the dark. The effect of blue light on A. baumannii virulence was tested using a filamentous strain of Candida albicans, which served as a model of A. baumannii interactions with human alveolar epithelial cells (i.e. C. albicans filaments were used as a model of human alveolar epithelial cells in the study). It was observed that, the killing of C. albicans filaments by A. baumannii was increased by more than 100 times when C. albicans were co-cultured with A. baumannii in the presence of blue light compared to in the absence of blue light (Mussi, 2010).
2.2. Blue light sensing receptor families: blue revival
Losi and Gartner (Losi, 2011) have referred to a “blue revival” defined by the identification and molecular characterization of long sought after-plant blue light receptors, employing flavins as chromophores - chiefly classified as cryptochromes and phototropins. The latter photo receptors are the first-discovered members of the so-called light, oxygen, voltage (LOV)-protein family, largely spread among bacteria, fungi, archaea, and plants. Many sequenced microbial genomes during the last few years have added BLUF to the family of blue light receptors, and have led to intense "genome mining" efforts, which have highlighted the intriguing wealth of blue light sensing in prokaryotes (Kanazawa, 2010; Tang, 2010; Tschowri, 2009). In addition to LOV and BLUF, a third member of “blue revival” list is the photoactive yellow protein (PYP). Functional analysis of PYP in the heterotrophic deep sea bacterium Idiomarina loihiensis revealed 1) an unexpected functional versatility in the PYP family of photoreceptors; 2) that light suppressed biofilm formation and determined the bacterial living preferences (van der Horst, 2009). Similar studies have been performed in fungi (Rodriguez-Romero, 2010) and cyanobacteria (Pisciotta, 2010; Rockwell, 2011).
2.3. Summary
Blue light can be sensed by numerous bacteria and can induce physiological responses elicited by the blue light receptors. As a result of this, blue light can regulate bacterial motility, suppress biofilm formation, and subsequently potentiate light inactivation of bacteria. On the other hand, the presence of blue light may also activate or increase bacterial virulence. To better understand how blue light can affect bacterial virulence and biofilms, further studies are needed to answer the following questions; to what extent blue light affects biofilm formation and dispersal, and through what mechanism blue light can activate virulence factors and modulate pathogenesis, etc. (Gomelsky, 2010).
3. Bactericidal efficacy of blue light
3.1. In vitro studies
3.1.1. Blue light inactivation of Propionibacterium acnes
P. acnes is a gram-positive bacterium held to be (partly) responsible for acne symptoms and the antimicrobial resistance of P. acnes has been a worldwide problem (Eady et al., 2003). In an in vitro study carried out by Kawada et al (Kawada et al., 2002), five P. acnes strains isolated from randomly-selected acne patients were used to assess the antimicrobial efficacy of blue light (407–420 nm). Bacterial suspensions were exposed to blue light for 60 min at a distance of 25 cm with an irradiance of 90 mW/cm2 (i.e., total radiant exposure 324 J/cm2 at the lamp aperture). P. acnes viability was decreased by 15.7% and 24.4%, respectively, immediately and at 60 min after the irradiation.
In another study, Ashkenazi et al (Ashkenazi et al., 2003) used an intense blue light lamp (407–420 nm) to inactivate P. acnes. Cultures in the test tubes were subjected to illumination by placing the test tubes horizontally in order to obtain maximal exposure to the blue light. Some cultures were illuminated again after 24 h and some were even illuminated three times, after an additional 24 h. The lamp was located 10 cm above the horizontal test tube and produced 20 mW/cm2 homogeneous illumination at the culture tube surface. Two ventilators are also located near the lamp on both sides in order to prevent any heating of the illuminated samples. The viability of 24 h cultures grown anaerobically in liquid medium was reduced by less than 2-log10 units (99%) when illuminated once with a light dose of 75 J/cm2. Better photo inactivation effects were obtained when cultures were illuminated twice or three times consecutively with a light dose of 75 J/cm2 and an interval of 24 h between illuminations. The viability of the culture under these conditions decreased by 4-log10 units (99.99%) after two illuminations and by 5-log10 units (99.999%) after three illuminations. X-ray microanalysis and transmission electron microscopy revealed structural damages to membranes in the illuminated P. acnes.
3.1.2. Blue light inactivation of Helicobacter pylori
H. pylori is a gram-negative microaerophilic bacterium which selectively colonizes the mucus layer of the human stomach and may cause peptic ulcer and adenocarcinoma. A study using a 405-nm diode laser for inactivation of H. pylori in vitro was carried out by Hamblin et al (Hamblin et al., 2005). The laser provided a 2-cm-diameter spot with an irradiance of 100 mW/cm2 on the surface of the bacterial suspension. Two hundred (200) µL of bacterial suspension was added to each well of a hanging drop slide that was placed on a black background to avoid reflectance of light. All seven strains tested, including strain ATCC 700824 that expresses virulence factors and a multi antibiotic resistant strain, were killed at least 99.9% by 20 J/cm2 of light exposure, suggesting that 405-nm light might be applied to inactivate antibiotic-resistant strains.
3.1.3. Blue light inactivation of oral bacteria
Fukui et al (Fukui et al., 2008) used a light source with the wavelength ranging from 400 to 700 nm to determine the most effective wavelength for inhibiting Porphyromonas gingivalis, a bacterium responsible for periodontal disease. It was reported that growth of P. gingivalis irradiated at 400 nm and 410 nm was significantly suppressed compared with a non-irradiated control, whereas wavelengths of 430 nm and longer produced no significant inhibition.
Therefore, Fukui et al (Fukui et al., 2008) subsequently tested the efficacy of blue light against P. gingivalis by using a light-emitting device equipped with monochromatic wavelength of 405 nm,. Two hundred (200) µL of suspension was applied to each well of 96-well culture plates. An exposure to 15 J/cm2 (measured at the lamp aperture) blue light produced significant inhibition of P. gingivalis (more than 75% inhibition compared with the non-irradiated control) under all irradiation conditions of 50 mW/cm2 for 300 s, 200 mW/cm2 for 75 s, or 400 mW/cm2 for 38 s.
Soukos et al (Soukos et al., 2005) found that broadband light (380–520 nm) can kill oral bacteria in culture medium and in dental plaque samples obtained from human subjects with chronic periodontitis. In culture medium, Prevotella intermedia and Prevotella nigrescens were completely killed (>5-log10-cycle reduction) by exposure to 4.2 J/cm2 light. Prevotella melaninogenica was reduced by 70% by exposure to 4.2 J/cm2 (P<0.008) and completely killed by exposure to 21 J/cm2. The viability reduction values of P. gingivalis were 22.75% (P<0.001), 87.45% (P<0.00002), and 98.52% (P<0.000001) after exposure to 4.2, 21, and 42 J/cm2 light, respectively.
Feuerstein et al (Feuerstein et al., 2004) tested the effect of blue light at wavelengths of 400–500 nm on the viability of oral bacterial, including P. gingivalis, Fusobacterium nucleatum, Streptococcus mutans and Enterococcus faecalis. Bacteria in suspension or on agar plates were exposed to three photo-curing light sources, quartz-tungsten-halogen lamp, light-emitting diode and plasma-arc, at the irradiances between 260 and 1300 mW/cm2 for up to 3 min. The results showed the minimal inhibitory dose for P. gingivalis and F. nucleatum was 16–62 J/cm2, a value significantly lower than that for S. mutans and E. faecalis (159–212 J/cm2). The authors suggested that blue light induces a phototoxic effect mainly on Gram-negative periodontal pathogens.
3.1.4. Blue light inactivation of S. aureus, P. aeruginosa and other bacteria
By using a 405-nm superluminous diode light source, Enwemeka et al (Enwemeka et al., 2008) investigated the effect of blue light on two strains of methicillin-resistant S. aureus (MRSA): USA 300 strain of community-acquired MRSA and IS853 strain of hospital-acquired MRSA. Each strain was separately diluted to a cell count of 5×106 colony forming units (CFU)/mL in 0.9% normal saline. Then, the bacteria were volumetrically streaked onto round 35-mm petri plates with tryptic soy agar and exposed to blue light. The light aperture was placed at a standard distance of 1–2 mm perpendicularly above each open plate with the irradiance of 100 mW/cm2 at the lamp aperture. Maximum eradication of the USA-300 (92.1%) and the IS-853 colonies (93.5%) was achieved within 9.2 and 8.4 min of irradiation, respectively.
In another study carried out by the same group (Enwemeka et al., 2009), the authors reported the effect of a different wavelength of blue light (470 nm) on the same two strains of MRSA: USA 300 and IS-853. The irradiance at the lamp aperture was 30 mW/cm2 and the lamp was place at 1–2 mm perpendicularly above each open plate with bacterial culture. Both strains lost nearly 30% CFU with as little as 3 J/cm2 of light exposure. As much as 90.4% of the US-300 and the IS-853 colonies, respectively, were killed with an exposure of 55 J/cm2.
An in vitro study to evaluate the antimicrobial effect of 405 and 470 nm blue light on S. aureus and P. aeruginosa was carried out by Guffey et al (Guffey and Wilborn, 2006). Bacterial suspensions were firstly inoculated on agar plates and then the plates were irradiated with super luminous diode probes with peak emission at 405 or 470 nm. Irradiation was timed to yield 1, 3, 5, 10, and 15 J/cm2 exposures with two agar plates for each light exposure. With 405-nm light, the maximum CFU reduction of 95.1% was achieved for P. aeruginosa at the light exposure of 10 J/cm2 and 87.9% for S. aureus at 15 J/cm2. With 470-nm light, the maximum CFU reduction of 96.5% was achieved for P. aeruginosa at the light exposure of 5 J/cm2 and 62.0% for S. aureus at 15 J/cm2. Interestingly, the authors reported lower CFU reductions at higher light exposures for P. aeruginosa at both 405 and 470 nm (e.g., at 470 nm, 96.5% CFU reduction at 5 J/cm2 but only 39.3% CFU reduction at 15 J/cm2), which were not observed in other studies. One possible reason might be the small number of replicates used in the study (only two plates for each light exposure) and large standard deviations were seen with the data.
Maclean et al (Maclean et al., 2008a) screened the visible light spectrum by using a xenon broad white-light source with a selection of filters and found that blue light of wavelength between 400–420 nm was bactericidal to S. aureus, including MRSA. The maximum inactivation occurred at 405±5 nm. A 2.4-log10 CFU reduction was achieved when the bacterial suspension (2 mL volume in each well of a 12-well micro-plate, giving a liquid depth of 7.2 mm) exposed to a light exposure of 23.5 J/cm2 at 405±5 nm.
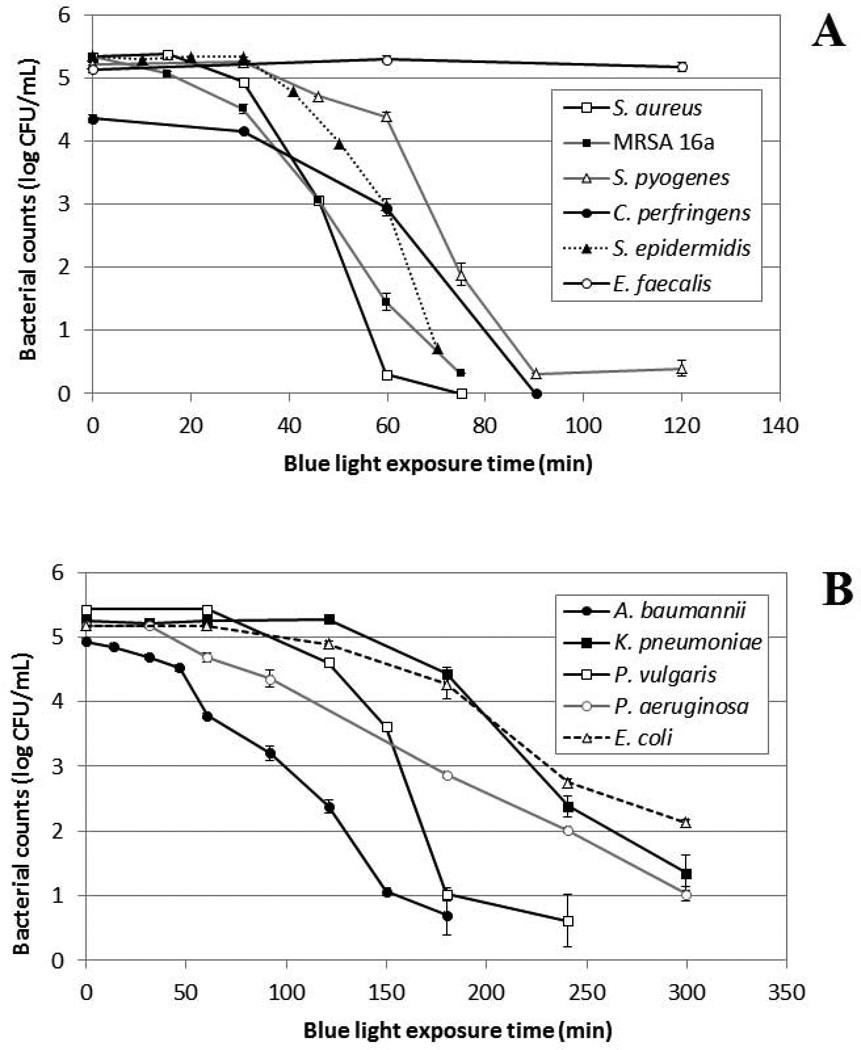
As a result, in a later study, Maclean et al (Maclean et al., 2009) tested the bactericidal effect of 405-nm light from a LED array on selected medically important bacteria, including Gram-positive S. aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Enterococcus faecalis, and Clostridium perfringens, and Gram-negative A. baumannii, P. aeruginosa, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. A 2-mL volume of bacterial suspension with a depth of 7 mm, stirred by a mini magnetic bar, was held within one well of a 12-well micro plate. The LED array was then placed on top of the well with bacterial suspension. The irradiance at the surface of the suspension was around 10 mW/cm2. The experimental arrangement was such that no build-up of heat occurred around the LED or was transmitted to the bacterial suspension. The results showed that both Gram-positive and Gram-negative species were successfully inactivated, with the general trend showing Gram-positive species to be more susceptible than Gram-negative bacteria (Fig. 1). Detailed investigation of the bactericidal effect of the blue-light on S. aureus suspensions, for a range of different cell densities of 103–109 CFU/mL, demonstrated that 405-nm LED array illumination could cause complete inactivation at high cell densities: inactivation levels corresponding to a 9-log10-unit reduction were achieved.
Fig. 1.
Blue light (405-nm) inactivation of medically important bacteria. (A) Gram-positive bacteria; (B) Gram-negative bacteria. Irradiance: 10 mW/cm2. (The graphs have been redrawn based on the data presented in reference (Maclean et al., 2009))
Lipovsky et al (Lipovsky et al., 2010) tested the efficacy of blue light in killing wound pathogenic bacteria S. aureus and E. coli. Light sources were LED arrays at 415 and 455 nm with the irradiance of 100 mW/cm2 at the surface of target. Bacteria were exposed to the blue light in two different cultures: suspension and culture on agar plate. The authors observed an increase in cell count when S. aureus was illuminated with 415 nm at a light exposure of 30 J/cm2. A maximal reduction in viability, 90%, was measured for S. aureus after illumination with 415 nm light at 120 J/cm2. Illumination at 455 nm was less effective for killing S. aureus. Only 50% reduction was obtained following an exposure of 120 J/cm2. E. coli was much more susceptible to blue light. An almost complete reduction occurred after an exposure of 30 J/cm2 at 415 nm, and a total reduction of between 98% and 99.9% was achieved following a light exposure of 60 J/cm2. Illumination with 455 nm resulted in dose-dependent cytotoxicity, with the highest reduction in cell count, of 90%, observed after an exposure of 120 J/cm2. Unlike S. aureus, neither 415 nm nor 455 nm enhanced proliferation of E. coli at any light exposure. The results of blue light illumination (415 or 455) on plates were similar to those obtained for suspensions of S. aureus or E. coli.
3.1.5. Summary
It has been found that blue light can mediate a broad-spectrum antimicrobial effect, including both Gram-negative (Feuerstein et al., 2005; Guffey and Wilborn, 2006; Hamblin et al., 2005; Lipovsky et al., 2010; Maclean et al., 2009; Soukos et al., 2005) and Gram-positive bacteria (Ashkenazi et al., 2003; Enwemeka et al., 2009; Enwemeka et al., 2008; Feuerstein et al., 2005; Guffey and Wilborn, 2006; Kawada et al., 2002; Lipovsky et al., 2010; Maclean et al., 2008a, 2009). Studies were carried out to screen the visible spectrum and determine most effective antimicrobial wavelength (Fukui et al., 2008; Maclean et al., 2008a). While the wavelength range of 402–420 nm has been reported to be the most effective range of spectrum, 455 and 470 nm have also been found to be of antimicrobial potential for some bacterial species (e.g., S. aureus). It would be of great interest to elucidate the mechanisms underlying antimicrobial effect of blue light, for example to identify the intracellular photosensitizing chromophores in different bacterial species, so optimal therapeutic wavelength can be determined in a bacterial species dependent manner. The antimicrobial efficacy of blue light varies significantly between different studies. This might be due to the different experimental conditions in different studies, including light sources (wavelength spectrum, laser or LED, etc), light exposures, bacterial species and strains, culture conditions (cultures in liquid media or on agar plates), etc. In some studies (Enwemeka et al., 2009; Enwemeka et al., 2008; Fukui et al., 2008; Kawada et al., 2002), only the light exposures or irradiances at the lamp apertures were reported and these parameters on the surfaces of cultures were not provided. As a result, it is difficult to quantitatively compare the outcomes of these studies with other studies. The studies on the in vitro antimicrobial effect of blue light are summarized in Table 1.
Table 1.
Summary of blue light inactivation of bacteria in vitro
| Light source | Radiant exposure | Bacterial species/strains | Inactivation efficacy | Ref. |
|---|---|---|---|---|
| 407–420 nm metal halide lamp | 324 J/cm2 at lamp aperture; lamp-target distance: 25 cm. | P. acnes | 15.7% reduction in CFU immediately after irradiation; 24.4% reduction 60 min after irradiation. | (Kawada et al., 2002) |
| 407–420 nm intense light lamp | 75–225 J/cm2 | P. acnes | 2 log10 at 75 J/cm2, 4 log10 at 150 J/cm2, and 5 log10 at 225 J/cm2. | (Ashkenazi et al., 2003) |
| 405-nm diode laser | 20 J/cm2 | H. pylori | >99.9% | (Hamblin et al., 2005) |
| 405 nm light-emitting device | 15 J/cm2 at lamp aperture | P. gingivalis | >75% | (Fukui et al., 2008) |
| 380–520 nm broadband light | 4.2–42 J/cm2 | P. gingivalis, P. intermedia, P. nigrescens, P. melaninogenica, and S. constellatus | P. intermedia and P. nigrescens >5 log10 at 4.2 J/cm2; P. melaninogenica >5 log10 at 21 J/cm2; P. gingivalis 1.83 log10 at 42 J/cm2. | (Soukos et al., 2005) |
| 400–500 nm light lamps used for dental restoration | Irradiance between 260 and 1300 mW/cm2 for up to 3 min | P. gingivalis, F. nucleatum, S. mutans, and E. faecalis | The minimal inhibitory dose for P. gingivalis and F. nucleatum was 16–62 J/cm2, for S. mutans and S. faecalis was 159–212 J/cm2. | (Feuerstein et al., 2004) |
| 405-nm superluminous diode light | 50.4–55.2 J/cm2 at lamp aperture; lamp-target distance: 1–2 mm | MRSA USA 300; MRSA IS-853 | 92.1% for USA 300; 93.5 for IS-853 | (Enwemeka et al., 2008) |
| 470-nm superluminous diode light | 55 J/cm2 at lamp aperture; lamptarget distance: 1–2 mm | MRSA USA 300; MRSA IS-853 | 90.4% for both strains | (Enwemeka et al., 2009) |
| 405 and 470 nm light | 15 J/cm2 | S. aureus, P. aeruginosa | S. aureus 90% at 405 nm, 62% at 470 nm; P. aeruginosa 95.1% at 405 nm, 96.5% at 470 nm. | (Guffey and Wilborn, 2006) |
| 405 nm light | 23.5 J/cm2 | S. aureus | 2.4-log10 | (Maclean et al., 2008a) |
| 405-nm LED | 36–216 J/cm2 for gram-positive; 108–180 J/cm2 for gram-negative. | S. aureus, S. epidermidis, S. pyogenes, E. faecalis, C. perfringens, A. baumannii, P. aeruginosa, E. coli, P. vulgaris, and K.pneumoniae | 2.6–5.0 log10 for gram positive; 3.1–4.7 log10 for gram negative. | (Maclean et al., 2009) |
| 415 and 455 nm LED | 60–120 J/cm2 | S. aureus, E. coli. 51 | S. aureus 90% at 415 nm; 50% at 455 nm. E. coli 100% at 415 nm; 98–99% at 455 nm. | (Lipovsky et al., 2010) |
3.2. Animal studies
Fan et al (Fan et al., 2012) developed an animal model of acne by intradermal injection of P. acnes in rat auricular tissue. Female Sprague-Dawley rats aged 3–5 weeks, weight 100–120 g, were used. The investigators then tested the efficacy of intense pulsed blue light (420 nm) on the treatment of acne in the rat model. Levels of tumor necrosis factor alpha (TNF-α) and matrix metalloproteinase 2 (MMP-2), markers of inflammation implicated in acne, were assessed by immunohistochemistry and quantitative polymerase chain reaction (PCR). Results indicated that treatment with intense pulsed blue light led to marked improvement after 6 bi-weekly treatments. TNF-α and MMP-2 levels correlated with the extent of acneiform activity were reduced by treatment with blue light.
Preliminary experiments conducted in our laboratory have shown that blue light can be effective in mouse models of bacterial infection in wounds and burns (Dai et al, manuscripts in preparation). Using bioluminescent bacteria and a low light imaging camera (Demidova et al., 2005) we have been able to demonstrate that 415-nm LED illumination can reduce the bacterial burden by up to 3 log10-units in a light dose dependent manner in both S. aureus and P. aeruginosa infections. In the case of the virulent P. aeruginosa blue light therapy of localized infections can prevent mice dying from systemic infections.
3.3. Clinical studies
3.3.1. Treatment of acne vulgaris
Acne vulgaris is one of the most common disorders for which patients seek dermatologic care (Kim and Armstrong, 2011). The disease burden of acne ranges from facial scarring to social, psychological, and emotional distress as well as self-perception of poor health. Penicillin, which does not suppress P. acnes, is found to be clinically ineffective in the treatment of acne (Tan and Tan, 2005).
An open label clinical trial on the treatment of acne vulgaris using a metal halide lamp (407–420 nm) was carried out by Kawada et al (Kawada et al., 2002). Thirty (30) patients with mild to moderate acne lesions involving the face and/or the back and/or the chest participated in this study. Each patient received treatments twice a week up to 5 weeks. Blue light therapy achieved a marked reduction of comedones, papules, pustules, and comedones + papules + pustules by 45.5, 59.3, 46.8, and 51.2% at 3 weeks, as well as by 57.8, 69.3, 73.3, and 64.0% at 5 weeks, respectively. Assessment of efficacy by the investigators showed that 77% of the patients were improved by week 5. By week 5, 40% of the patients showed marked improvement or clearance of their acne lesions.
A similar study was carried out by Noborio et al (Noborio et al., 2007) using an intense blue light source (405–420 nm). In this study, the treatment dose was 4 J/cm2 per pulse and each area received six pulses of light. After the first pulse was delivered, the hand piece was moved to the next area and the face was covered in a circular manner six times. The treatment was performed once or twice a week until the patient had satisfactory results. Of the 10 patients, eight showed an improvement with this phototherapy, and the mean treatment time was 12.4 treatments. The reduced acne severity score reflected the apparent clinical improvement in inflammatory acne, such as a decrease in the number of prominent pustules.
In a prospective study, Ammad et al (Ammad et al., 2008) evaluated the use of intense blue light within the spectral range of 415–425 nm (peak 420 nm) in the treatment of acne vulgaris. Twenty one (21) patients with mild to moderate facial acne were treated. All patients were given 14-min treatment sessions twice a week for 4 weeks. Significant improvement was achieved in the Leeds Acne Grade (P = 0.001). The inflammatory (P = 0.001) and noninflammatory (P = 0.06) lesion counts also improved significantly. A similar change was noted in the Dermatology Life Quality Index (DLQI) (P = 0.001); a degree of significance was also achieved in the patients' and the investigators' visual analog scale (VAS) scores (P = 0.01 and P = 0.001, respectively). P. acnes CFU counts failed to show a significant decrease at the end of the treatment and remained almost constant (P = 0.660). In conclusion, the investigators believed that blue light does appear to have some role in the management of acne and may be beneficial for the treatment of a select group of mild to moderate acne patients.
Omi et al (Omi et al., 2004) reported a study on the use of 420-nm intense light for the treatment of acne. A total of 28 adult healthy volunteers with facial acne were treated with a total of eight serial biweekly 15-min treatment sessions. Clinical counts of acne, as well as moisture, sebum and pH measurements were taken between each session. Nine of the 28 patients were followed for 2–3 months after the last treatment. Detection of bacteria in acne pustules was analyzed by culture and by PCR. Ultrastructural changes were examined in eight patients after four sessions of the light therapy. Overall, there was a 64.7% improvement in acne lesions. There were no bacterial changes before or after the therapy, although damaged P. acnes were observed at the ultrastructural level.
Wheeland and Dhawan (Wheeland and Dhawan, 2011) evaluated the efficacy and tolerability of treating mild-to-moderate facial acne using a hand-held LED blue light (410 nm) device in conjunction with a proprietary foam cleanser and skin rebuilding serum. Treatment were performed twice daily for eight weeks, plus the cleanser before treatments and the serum after each evening treatment. Among 33 subjects aged 25– 45 years old, 28 completed. In a 3 cm × 5 cm target area receiving a daily dose of 29 J/cm2, treatment was associated with significant reductions from baseline in the inflammatory lesion count from week 1 onward (P <0.01) and in the non-inflammatory lesion count from week 4 onward (P <0.05). The number of flares was significantly reduced from baseline from week 2 onward (P <0.05), and flare severity and flare redness were significantly reduced from baseline from week 4 onward (P <0.01 and P <0.05, respectively). At week 8, more than 90 percent of subjects reported improvements in their skin's overall appearance, clarity, radiance, tone, texture and smoothness.
A similar study was performed by Gold et al (Gold et al., 2009). Twenty one (21) patients with mild-to-moderate facial acne were treated with self-applied, blue light LED (414 nm) once daily for six minutes for a period of eight weeks. During the study period, the total number of comedones on the face had significantly reduced for the assessment at Day 7 (P<0.019) and at Day 28 (P<0.001). The total number of open comedones (blackheads) on the face during the treatment period was reduced significantly (P<0.02) for assessment at treatment Day 7 (P<0.005) and for the assessment at Day 28. The total number of closed comedones (whiteheads) on the face during the treatment period, was reduced significantly (P<0.007) for the assessment at Day 28. The total number of papules during treatment had reduced significantly for assessment at Day 7 (P<0.048) and Day 28 (P<0.005). The total number of pustules during treatment had reduced, but this difference was not statistically significant.
In another study, Morton et al (Morton et al., 2005) utilized a blue LED light source (409–419 nm) in 30 subjects with mild to moderate facial acne. Over 4 weeks, patients received eight 10- or 20-min light treatments at 40 mW/cm2. An overall effect on inflammatory counts was observed at week 5, and a statistically significant decrease in inflamed counts was detected at the week 8 assessments, which continued to week 12.
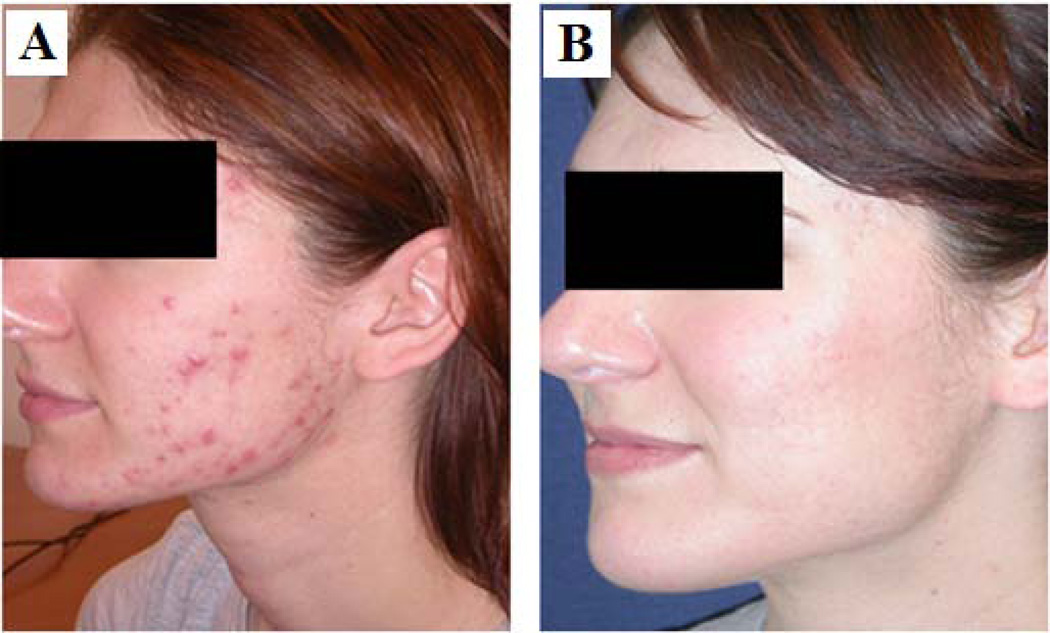
Clinical pictures (before and after pictures) of a representative patient treated at Wellman Center for Photomedicine, Massachusetts General Hospital with blue light and showing marked improvement are shown in Figure 2.
Fig. 2.
A case of a female acne patient treated at Wellman Center for Photomedicine with marked clinical improvement. A) before, and B) after blue light (407–420 nm) treatment.
3.3.2. Treatment of H. pylori infection in human stomach
H. pylori colonizes the mucus layer of the human stomach and duodenum, causes chronic gastritis, gastric ulcer, and is a risk factor for gastric adenocarcinoma. There is an over 20% failure rate in antibiotic therapy (Gisbert and Pajares, 2002; Suzuki et al., 2010), which is increasingly due to antibiotic resistance (Megraud and Lamouliatte, 2003).
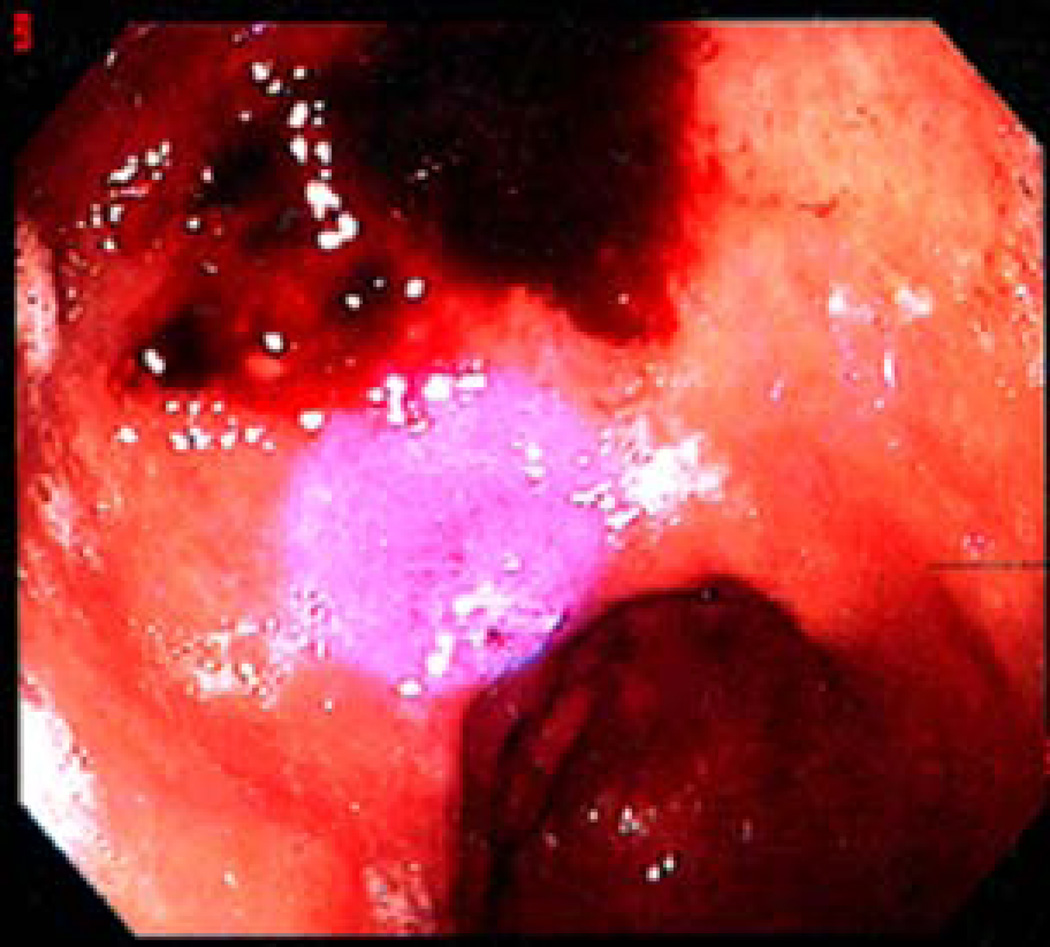
A pilot clinical study was carried by Ganz et al (Ganz et al., 2005) in 10 patients (aged between 26 and 75) using blue light at 405 nm to eradicate H. pylori in regions of the gastric antrum. Light was delivered from a diode laser via a flexible optical fiber, which was passed through the biopsy channel of the endoscope and gave a 1-cm diameter spot on the gastric mucosa (Figure 3). Weighed biopsies were taken from treated and non-treated control spots in the gastric antrum and colonies quantitatively cultured. It was found that, after an exposure of 40.5 J/cm2, the mean reduction in H. pylori colonies per gram tissue between treated and control spots was 91% (P<0.0001). Some patients had reductions approaching 99%.
Fig. 3.
Pilot clinical trial of blue light for gastric H. pylori infection (Ganz et al., 2005). Endoscopic intra-gastric photograph of a control site (post-biopsy with some visible blood), and the adjacent treatment site with the spot of laser light visible as a blue circle.
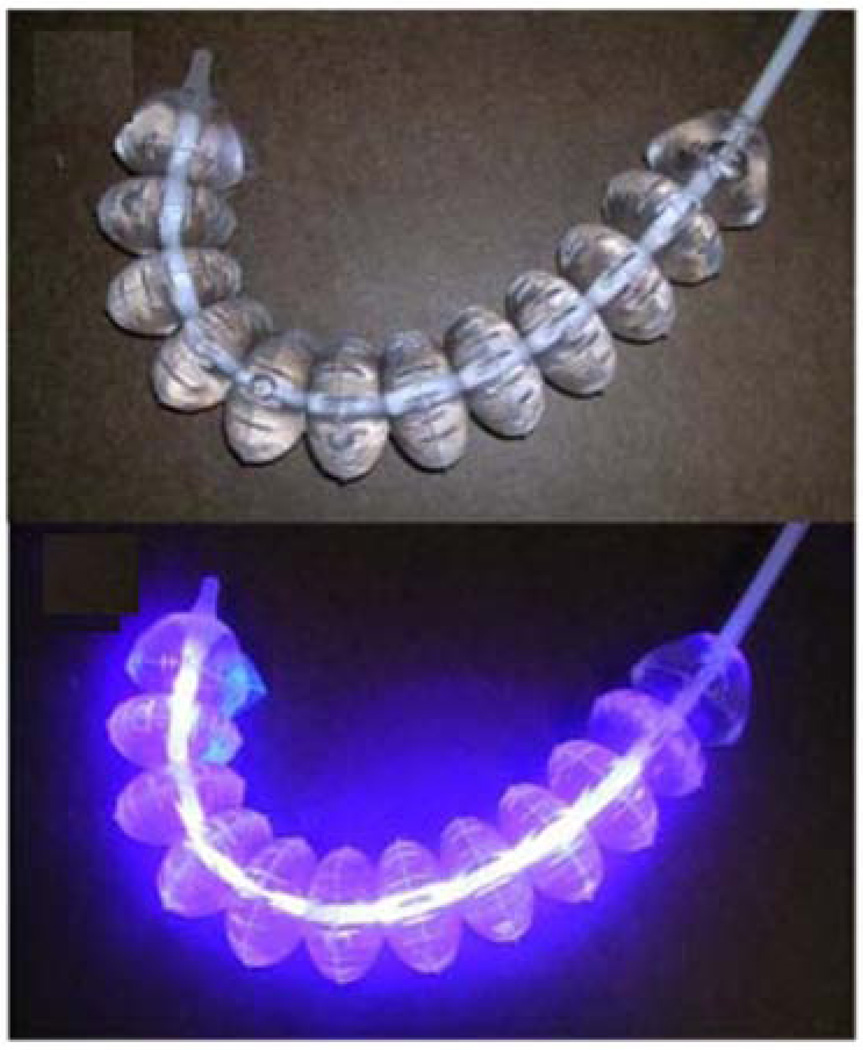
In a later study, Lembo et al (Lembo et al., 2009) conducted a prospective pilot trial using a novel light source consisting of laser diodes and diffusing fibers to deliver 408-nm illumination at escalating total fluences to the whole stomach. The light source included a catheter sheath enclosing a multi-segment balloon, which was inflated in the stomach to assist in the positioning of the light wand. The non-illuminated catheter is shown in Figure 4A and after illumination in Figure 4B. Eighteen (18) adults with H. pylori infection were treated at three U.S. academic endoscopy centers. A dose escalation study design was employed, in which five patients were exposed to light therapy for 15 min; five (5) patients were exposed for 30 min; seven were exposed for 45 min, and one was exposed for 60 min. Patients were evaluated at enrollment, at 5 days post-treatment, and again at 5 weeks (±7 days) post-treatment using a non-invasive urea breath test. Nine gastric biopsies were taken immediately pre-light treatment and at the conclusion of light treatment during the endoscopy procedure to allow quantitative microbiological culture and measure the level of H. pylori eradication achieved using the light therapy. Pre- and post-treatment biopsies were obtained from the following sites during endoscopy: antrum at 2, 3, and 4 cm from the pylorus; greater curvature of the corpus at 3, 4, and 5 cm proximal to the angulus; fundus and cardia at 2, 3, and 4 cm from the GE junction. The largest reduction in bacterial load was in the antrum (>97%), followed by body (>95%) and fundus (>86%). There was a correlation between log reduction and initial bacterial load in the antrum. There was no dose–response seen with increasing illumination times. The urea breath test results indicated that the bacteria repopulated in days following illumination.
Fig. 4.
Device for delivery of 408-nm illumination at escalating total fluences to the whole stomach. A) Light wand balloon before blue light illumination; B) light wand balloon illuminated by a 408-nm diode laser. Reprinted with permission from (Lembo et al., 2009).
Table 2 shows the summary of the clinical studies on blue light treatment for acne and H. pylori infections discussed in this review.
Table 2.
Summary of blue light treatment of acne vulgaris and gastritis in human patients
| Acne vulgaris | ||||
| Light source | Treatment protocol |
No. of patients |
Outcome | Ref. |
| 407–420 nm halide lamp | Each patient received treatments twice a week up to 5 weeks. | 30 | 77% patients were improved. | (Kawada et al., 2002) |
| 405–420 nm intense light | On average 12.4 treatments; once a week. | 10 | 80% patients showed an improvement. | (Noborio et al., 2007) |
| 415–425 nm intense light | Two treatments a week for 4 weeks. | 21 | Significant improvement was achieved in the Leeds Acne Grade. The inflammatory and noninflammatory lesion counts also improved significantly. | (Ammad et al., 2008) |
| 420 nm intense light | They were treated with a total of eight serial biweekly treatment sessions. | 28 | There was a 64.7% improvement in acne lesions. | (Omi et al., 2004) |
| 410 nm LED | Twice daily for eight weeks. | 28 | At week 8, more than 90 percent of subjects reported improvements in their skin's overall appearance, clarity, radiance, tone, texture and smoothness. | (Wheeland and Dhawan, 2011) |
| 414 nm LED | Blue light was applied to the applicable area once daily for six minutes for a period of eight weeks. | 21 | The total number of comedones on the face had significantly reduced for the assessment at day 7 and at day 28. | (Gold et al., 2009) |
| 409–419 nm LED | Over 4 weeks, patients received eight 10- or 20-min light treatments at 40 W/cm2. | 30 | An overall effect on inflammatory counts was observed at week 5, and a statistically significant decrease in inflamed counts was detected at the week 8 assessments, which continued to week 12. | (Morton et al., 2005) |
| Gastritis | ||||
| Light source | Treatment protocol | No. of patients | Outcome | Ref. |
| 405 nm diode laser with a flexible optical fiber | One treatment with an exposure of 40.5 J/cm2. | 10 | The mean reduction in H. pylor colonies per gram tissue between treated and control spots was 91% (P<0.0001). Some patients had reductions approaching 99%. | (Ganz et al., 2005) |
| 408 nm diode laser with diffusing fibers | A dose escalation study design was employed, in which 5 patients were exposed to light therapy for 15 min; 5 patients were exposed for 30 min; 7 were exposed for 45 min, and one was exposed for 60 min. | 18 | The largest reduction in bacterial load was in the antrum (>97%), followed by body (>95%) and fundus (>86%). | (Lembo et al., 2009) |
3.3.3. Environmental decontamination of hospital isolation room
Maclean et al (Maclean et al., 2010) assessed the effectiveness of a high-intensity narrow spectrum light (HINS-light) for the reduction of environmental bacterial contamination on surfaces at various sites within a hospital isolation room. The HINS-light, installed as ceiling-mounted light sources, was generated from a matrix of LED which emitted 405 nm light with full-width half-maximum of 14 nm. The HINS-light was designed to irradiate a surface area of 10 m2, and the levels of light irradiance were set, on the basis of extensive laboratory experimentation, to be sufficient to cause significant inactivation of exposed bacteria within the room environment. When the room was unoccupied, use of HINS-light resulted in 90% reduction of surface bacterial levels and when the room was occupied by an MRSA-infected burns patient, reductions between 56% and 86% were achieved, with the highest reduction (86%) measured following an extended period of HINS light operation.
In addition, an on/off intervention study was carried out by the authors to compare the bacterial contamination levels on contact surfaces within an occupied isolation room, with and without the HINS-light EDS in operation (Maclean et al., 2010). The room was occupied by an MRSA-positive female patient with 35% TBSA burn from a house fire. The study was conducted over a 15 day period divided into three phases: a four-day pre-HINS phase; a five-day HINS-on phase; and a six-day post-HINS-off phase. Samples from 70 sites were collected twice during each of the three phases, with a minimum of two days between sampling. It was reported that surface bacterial levels were reduced by 62% by HINS-light EDS treatment and returned to pretreatment contamination levels two days after the system was switched off.
In general, these reductions of S. aureus and MRSA by HINS-light treatment were greater than the reductions achieved by normal infection control and cleaning activities alone. The authors concluded that HINS-light EDS, used as a supplementary procedure, can make a significant contribution to bacterial decontamination in clinical environments.
In a similar study carried out by the researchers from the same group, Bache et al (Bache et al., 2011) assessed the use of the HINS-light in two different burn unit environments: an isolation room housing burn inpatients and the burn outpatient clinic, through which several patients pass each day, so total decontamination of the room between patients is almost impossible to achieve. The light sources were installed in the ceilings of the inpatient isolation room and outpatient clinic room. Environmental samples were collected from inpatient isolation room and the outpatient clinic, and comparisons were then made between the bacterial contamination levels observed with and without use of the HINS-light. Over 1000 samples were taken. Inpatient studies, with sampling carried out at 08:00 am, demonstrated a significant reduction in the average number of bacterial colonies following HINS-light EDS use of between 27% and 75% (P < 0.05). There was more variation when samples were taken at times of increased activity in the room. Outpatient studies during clinics demonstrated a 61% efficacy in the reduction of bacterial contamination on surfaces throughout the room during the course of a clinic (P = 0.02).
3.3.4. Summary
Blue light, with the wavelength range of 405–425 nm, has been employed in clinic to treat mild to moderate acne vulgaris, of which P. acnes is the causative pathogen. While the overall outcomes in all the studies were shown to be positive, discrepancies were reported in the therapeutic efficacies of different studies (Ammad et al., 2008; Gold et al., 2009; Kawada et al., 2002; Morton et al., 2005; Noborio et al., 2007; Omi et al., 2004; Wheeland and Dhawan, 2011). It was likely that the discrepancies were due to the different light delivery protocols used in the studies, differences in the spectra of light sources, variable situations of patient populations, etc. Clinical trials (Ganz et al., 2005; Lembo et al., 2009) have also been conducted to investigate the therapeutic efficacy of blue light for the treatment of gastritis, of which H. pylori is the responsible pathogen. In general, the clinical use of blue light for infectious disease is still at the early stage of development, for example, the sample sizes of all published clinical studies are very limited (n<30), the target bacteria are mainly limited to P. acnes and H. pylori. Further studies with larger sample size are warranted to optimize the parameters of blue light therapy, and to investigate the use of blue light for infections caused by bacteria other than P. acnes and H. pylori. Blue light has also been used for the environmental decontamination of hospital isolation room, to inactivate nosocomial pathogens including MRSA (Bache et al., 2011; Maclean et al., 2010)
4. Mechanisms of antimicrobial effect of blue light
4.1. Blue light inactivation of bacteria is oxygen dependent
Maclean et al (Maclean et al., 2008b) investigated the role of oxygen in the visible light inactivation of S. aureus. A xenon broadband white-light source together with a 400-nm long-pass filter was used for the visible-light exposure of bacterial suspensions. Oxygen enhancement was achieved by flowing oxygen over the surface of the S. aureus suspension during light illumination and results demonstrated an increased rate of bacterial inactivation, with approximately 3.5 times less specific dose being required for inactivation compared to that for a non-enhanced control. Oxygen depletion was achieved through the addition of oxygen scavengers to the S. aureus suspension, and significantly reduced bacterial inactivation was observed in the presence of oxygen scavengers. The authors concluded that the nature of the mechanism occurring within the visible-light-exposed S. aureus is photodynamic inactivation through the photo-excitation of intracellular porphyrins.
In a similar study, Feuerstein et al (Feuerstein et al., 2005) elucidated the mechanism of blue light phototoxicity on P. gingivalis and F. nucleatum. Bacteria were exposed to blue light (1) under aerobic and anaerobic environments and (2) in the presence of scavengers of ROS. Phototoxicity was not observed when the bacteria were exposed to light under anaerobic conditions. Dimethyl thiourea, a hydroxyl radical scavenger, was effective in reducing phototoxicity (P<0. 05). These results support the assumption that the phototoxic effect of blue light on the periodontopathogenic bacteria is oxygen dependent and that hydroxyl radicals play an important role in this process.
4.2. Intracellular porphyrins have been identified in bacteria susceptible to blue light inactivation
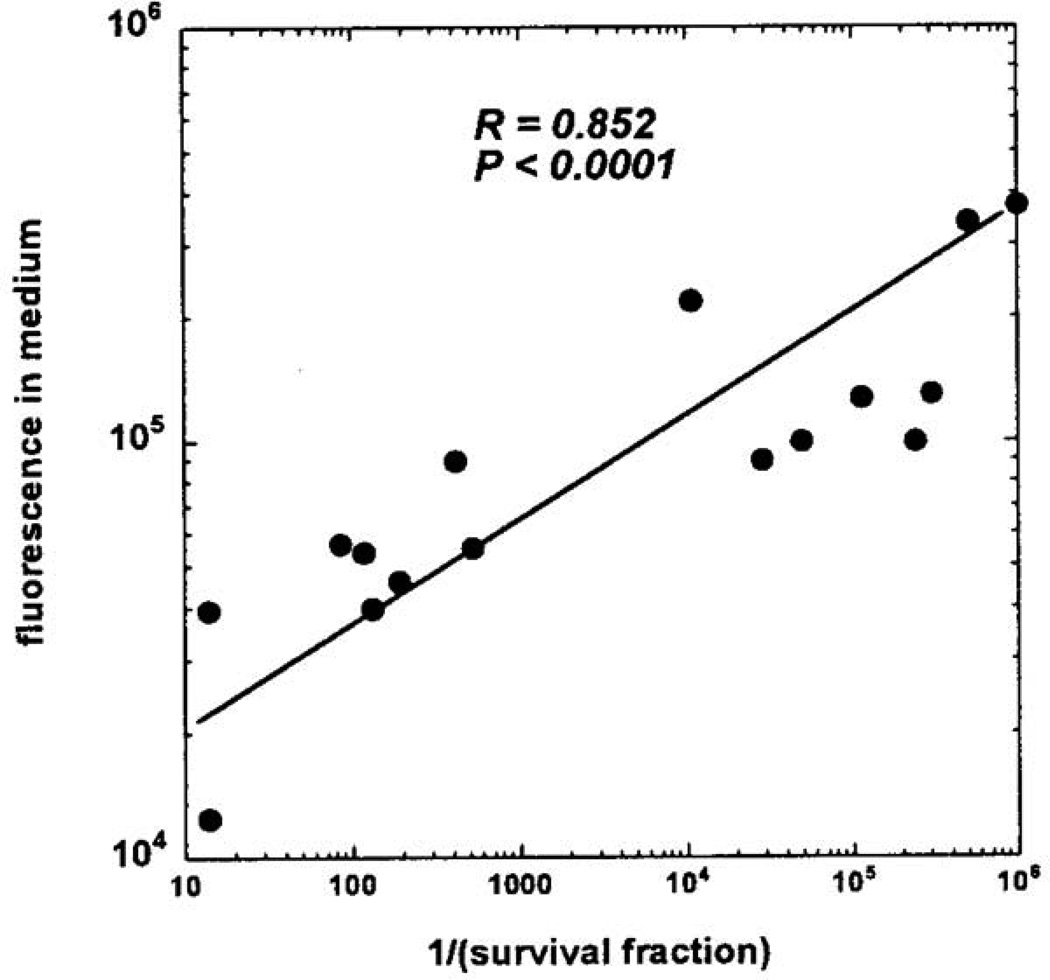
Hamblin et al (Hamblin et al., 2005) hypothesized that the inactivation of H. pylori by visible light with a peak in the action spectrum about 400 nm was due to the intracellular accumulation of a metal free porphyrin that produces ROS upon illumination. The hypothesis was tested by carrying out fluorescence emission spectroscopy on H. pylori cultures of five strains with different ages. After centrifuge, the culture supernatants from the H. pylori suspensions were excited at 405 nm and scanned the emission between 580 and 720 nm. It was observed that the supernatants contained an emission peak centered at 622 nm, almost midway between the emission peaks of coproporphyrin I dichloride (CP) (610 nm) and protoporphyrin IX (PPIX) (632 nm). The investigators then correlated the measures of the fluorescence intensity in the culture supernatants with the amount of inactivation observed in many experiments that gave variable degrees of light-mediated inactivation with different strains of H. pylori and particularly with cultures of different ages. The reciprocal of the survival fraction after 10 J/cm2 blue light was taken as the measure of cytotoxicity. The authors observed an excellent positive correlation over several orders of magnitude between the porphyrin fluorescence in the medium and the cytotoxicity (Figure 5). To further identify and confirm the existence of free porphyrins in the four-day old culture supernatant, the investigators carried out analysis by solvent extraction and capillary electrophoresis with-laser-induced fluorescence detection and comparison with porphyrin standards. The concentrations of both CP and PPIX measured in the supernatants from the five different H. pylori strains were roughly comparable, ranging from 10 to 20 nM for CP and from 12 to 42 nM for PPIX.
Fig. 5.
Correlation between height of porphyrin fluorescence emission from H. pylori culture supernatants and cytotoxicity expressed as reciprocal of surviving fraction after 10 J/cm2 of 405-nm light. Reprinted with permission from (Hamblin et al., 2005).
Ashkenazi et al (Ashkenazi et al., 2003) confirmed that P. acnes is capable of producing endogenous porphyrins with no need for any trigger molecules such as 5-aminolevulinic acid. Extracts from growing cultures demonstrated emission peaks around 612 nm, which were characteristic for porphyrins. Endogenous porphyrins were determined and quantified after their extraction from the bacterial cells by fluorescence intensity and by elution retention time on high-performance liquid chromatography (HPLC). The porphyrins produced by P. acnes were mostly coproporphyrin, as shown by the HPLC elution patterns.
By using HPLC, Soukos et al (Soukos et al., 2005) identified and quantified the endogenous porphyrins in periodontal disease causing bacteria including P. intermedia, P. nigrescens, P. melaninogenica, and P. gingivalis. HPLC revealed that these bacteria expressed different porphyrin patterns including uroporphyrin, heptacarboxyl porphyrin, hexacarboxyl porphyrin, pentacarboxyl porphyrin, isocoproporphyrin, coproporphyrin, and protoporphyrin. The total amounts of porphyrin were 267, 47, 41, and 2.2 ng/mg-protein in P. intermedia, P. nigrescens, P. melaninogenica, and P. gingivalis, respectively. The authors suggested that blue light could achieve rapid and selective elimination of periodonto-pathogenic bacteria by exciting their endogenous porphyrins.
4.3. Summary
Studies have found that blue light inactivation of bacteria is oxygen dependent (Feuerstein et al., 2005; Maclean et al., 2008b), suggesting that the antimicrobial effect of blue light is associated with the photo-excitation of intracellular chromophores and the subsequent generation of cytotoxic ROS such as singlet oxygen. Intracellular photosensitizing porphyrins were found in P. acnes (Ashkenazi et al., 2003), H. pylori (Hamblin et al., 2005), and some oral bacteria (Soukos et al., 2005). Different porphyrin patterns were observed in different bacteria (Table 3). Although it is commonly considered that blue light inactivation of wound pathogenic bacteria (e.g., S. aureus, P. aeruginosa) is also due to the photo-excitation of intracellular porphyrins, this hypothesis has not yet been rigorously proved, and it is necessary to verify the existence of intracellular photosensitizing porphyrins or identify alternative non-porphyrin intracellular photosensitizing chromophores.
Table 3.
Intracellular porphyrin patterns observed in different bacteria
| Bacterial species | Intracellular porphyrins | Ref. |
|---|---|---|
| P. acnes | Coproporphyrin | (Ashkenazi et al., 2003) |
| H. pylori | Coproporphyrin, protoporphyrin | (Hamblin et al., 2005) |
| P. intermedia | Coproporphyrin, protoporphyrin | (Soukos et al., 2005) |
| P. nigrescens | Uroporphyrin, heptacarboxyl porphyrin, protoporphyrin | (Soukos et al., 2005) |
| P. melaninogenica | Coproporphyrin, uroporphyrin, protoporphyrin | (Soukos et al., 2005) |
| P. gingivalis | Coproporphyrin | (Soukos et al., 2005) |
5. Effects of blue light on mammalian cells
To employ blue light for the treatment of infections, it is clearly important to understand the effects of blue light on host cells and tissues so that unacceptable damage to host cells and tissues is not inflicted. However, very limited studies have been reported so far in this area.
5.1. In vitro studies
Shnitkind et al (Shnitkind et al., 2006) demonstrated that blue light has anti-inflammatory effects on keratinocytes. Two immortalized keratinocyte cell lines, HaCaT and h TERT1, were illuminated with narrow-band blue light (420 nm) at an exposure of 54 mJ/cm2 or 134 mJ/cm2. IL-1α and ICAM-1 were used as the markers for inflammation. The results showed that blue light treatment of HaCaT and hTERT cells resulted in inhibition of cytokine-induced production of IL-1α. The levels of IL-1α decreased by 82% in HaCaT and by 75% in hTERT cells.
Liebmann et al (Liebmann et al., 2010) evaluated the effects of blue light from LED arrays on endothelial cells and primary human keratinocytes. Panels of LED arrays with the following peak wavelengths were used: 412, 419, 426, and 453 nm, each with a bandwidth of 20–30 nm at 50% power. Cell cultures were placed at a distance of 50 mm under the LED array and were irradiated every 24 hours on 3 successive days with different radiant exposures. It was observed that irradiation with wavelengths of 412, 419, and 426 nm at high radiant exposures (66–100 J/cm2) and 453 nm wavelength at very high radiant exposures (4500 J/cm2) is cytotoxic for skin-derived endothelial cells as well as keratinocytes. Irradiation with nontoxic radiant exposures significantly and dose dependently reduces proliferation in both endothelial cells and keratinocytes. This reduction in proliferation was found to be due to an initiation of differentiation, as proven by the measured expressional increase of the respective markers in keratinocytes. Using bovine serum albumin (BSA) as a model protein, the authors showed that blue light up to a wavelength of 453 nm is capable of releasing nitric oxide (NO) from nitrosated proteins, but not from nitrite, and that NO can initiate differentiation in human keratinocytes.
Wataha et al (Wataha et al., 2004b) assessed the effect of blue light from three common dental light sources, quartz–tungsten–halogen (QTH), plasma-arc (PAC), and laser, on the cellular function of 3T3 mouse fibroblasts in vitro. For each source, the majority of the light flux fell between 400 and 500 nm. Mouse fibroblasts were exposed to light from the dental light sources for the equivalent durations used in clinic, with total energy exposures ranging from 1.3 to 60 J/cm2. The light tip was placed 7.5 mm from the bottom of the culture well where cells were attached. To directly compare the three light sources, additional experiments were done using equivalent total light exposures from each source by adjusting the exposure durations for each light source. Cellular function was assessed by succinic dehydrogenase (SDH) activity of mitochondria. Results showed that exposures ranging from 5 J/cm2 (laser) to 15 J/cm2 (PAC, QTH) irreversibly suppressed SDH activity nearly 100% when compared to no-light controls up to 72 h post-exposure. For the PAC and QTH sources, exposures as low as 3.5 J/cm2 also irreversibly suppressed SDH activity. When equivalent radiant exposures were used from each light source, exposures of 1 J/cm2 did not suppress SDH activity for the QTH and laser sources, but significantly (50%) suppressed SDH for the PAC source, indicating a difference in the biological effects of the outputs of the different light units. Equivalent light exposure experiments also indicated a definite dependence of SDH activity on the total light exposure.
In another study, Godley et al (Godley et al., 2005) reported that blue light induced mitochondrial DNA damage and free radical production in human primary retinal epithelial (PRE) cells. Confluent cultures of human PRE cells were exposed to visible light (390–550 nm at 2.8 mW/cm2) for up to 6 h. A small loss of mitochondrial respiratory activity was observed at 6 h compared with dark-maintained cells, and this loss became greater with increasing time. Light exposure significantly damaged mitochondrial DNA at 3 h (0.7 lesion/10 kb DNA) compared with dark maintained controls. However, by 6 h of light exposure, the number of lesions was decreased in the surviving cells, indicating DNA repair. Isolated mitochondria exposed to blue light generated singlet oxygen, superoxide anion, and hydroxyl radical. Antioxidants confirmed the superoxide anion to be the primary species responsible for the mitochondrial DNA lesions. The authors conclude that visible light can cause cell dysfunction through the action of ROS on DNA and that this may contribute to cellular aging, age-related pathologies, and tumorigenesis.
Hockberger et al (Hockberger et al., 1999) showed that blue light stimulated H2O2 production in cultured mouse (3T3 fibroblasts), monkey (kidney epithelial cells), and human (foreskin keratinocytes) cells. The cultured cells were exposed to blue light in several wavelength ranges filtered from a xenon arc lamp: 400–410, 445–455, 450–490, or 485–495 nm. The production of H2O2 was detected by a classical histochemical staining. The investigators found that H2O2 originated in peroxisomes and mitochondria, and it was enhanced in cells over- expressing flavin-containing oxidases. These results support the hypothesis that photo-reduction of flavoproteins underlies light-induced production of H2O2 in cells. Because H2O2 and its metabolite, hydroxyl radicals, can cause cellular damage, these ROS may contribute to pathologies associated with exposure blue light.
5.2. In vivo studies
A small scale clinical trial was conducted by Kleinpenning et al (Kleinpenning et al., 2010) to investigate the effects of blue light, with a range of 390–460 nm and a peak emission of 420 nm, on normal skin of healthy volunteers. Eight (8) healthy volunteers with skin type I–III were irradiated with blue light on five consecutive days. Each day, 20 J/cm2 was given on the unprotected area of 15-cm×15-cm on the buttocks to a cumulative dose of 100 J/cm2. Skin biopsies were analyzed with respect to photodamage (p53, vacuolization, sunburn cells), skin ageing (elastosis, matrix metalloproteinase-1), and melanogenesis (Melan-A). No inflammatory cells and sunburn cells were visible before or after irradiation. A significant increase in the perinuclear vacuolization of keratinocytes was demonstrated during treatment (P=0.02) with a tendency towards significance after cessation of treatment (P=0.09). No significant change in p53 expression was observed. Signs of elastosis and changes in matrix metalloproteinase-1 (MMP-1) expression were absent. Minimal clinical hyperpigmentation of the irradiated skin was confirmed histologically with a significant increase in Melan-A-positive cells (P =0.03). The authors concluded that the (short-term) use of visible blue light in dermatological practice is safe.
5.3. Summary
Limited in vitro studies that have been reported so far (Godley et al., 2005; Hockberger et al., 1999; Liebmann et al., 2010; Wataha et al., 2004b) that showed that blue light, under certain wavelength range and light exposures, might be toxic to mammalian cells including keratinocytes, fibroblasts, retinal epithelial cells, skin-derived endothelial cells, etc. The mechanism of the cytotoxic effect of blue light on mammalian cells is similar to that on bacteria, which is associated with the photo-excitation of intracellular chromophores sensitive to blue light and the subsequent generation of cytotoxic ROS. It was suggested that the blue light damages mammalian cells by generating ROS from mitochondria or/and peroxisomes (Hockberger et al., 1999), which possess blue light sensitive chromophores: flavins and cytochromes. The light absorption of flavins peaks at 460 nm (Massey, 2000). These studies also indicated that blue light induced damages to mammalian cells in a wavelength dependent manner.
In contrast to the in vitro studies discussed above, an in vivo study (Kleinpenning et al., 2010) reported minimal side effects of blue light on human skin. The discrepancies between different studies are attributable to many factors, for example, light exposure, the wavelength spectrum of the light source, etc. For in vivo situations, as the light decays when it penetrates into the tissue, the light energy received by the cells is significantly less than the light energy received on the skin surface. As a result, under the equivalent light exposures, cell damage observed in vivo will be significant lower than those in vitro. Further studies should be performed to examine the effect of blue light, in the therapeutic wavelength range used for infections and at the effective antimicrobial exposures (the exposures required to effectively treat infections), on host cells and tissues.
The identification of the blue light sensitive chromophores in mammalian host cells is of similar importance as it is in pathogenic bacteria. Ideally, the blue light wavelength used should selectively excite the chromophores in pathogenic bacteria while the photo-excitation of chromophores in mammalian cells should be avoided or minimal. Table 4 shows the summary of the studies on the blue light effects on mammalian cells.
Table 4.
Effects of blue light on mammalian cells
| In vitro studies | ||||
| Light source | Radiant exposure | Cell type | Treatment outcome | Ref. |
| Narrow-band blue light 420 nm. | 54 mJ/cm2 and 134 mJ/cm2. | Keratinocyte cell lines: HaCaT and hTERT. | Blue light has anti-inflammatory effects on keratinocytes by decreasing the cytokine-induced production of IL-1α and ICAM-1. | (Shnitkind et al., 2006) |
| LED 412, 419, 426, and 453 nm. | 66–100 J/cm2 for 412, 419, and 426 nm; 4500 J/cm2 for 435 nm. The exposures were measured at the LED apertures. | Human keratinocytes, skin-derived endothelial cells. | Irradiations with 412, 419, 426 nm at 66–100 J/cm2 and 453 nm at 4500 J/cm2 is cytotoxic for skin cells. | (Liebmann et al., 2010) |
| Dental light sources: quartz–tungsten–halogen (QTH), plasma-arc (PAC), and laser. The majority of the light flux fell between 400–500 nm. | 1.3 to 60 J/cm2. | 3T3 mouse fibroblasts. | Exposures ranging from 5 J/cm2 laser) to 15 J/cm2 (PAC, QTH) irreversibly suppressed SDH activity nearly 100% up to 72 h post-exposure. For the PAC and QTH sources, exposures as low as 3.5 J/cm2 also irreversibly suppressed SDH activity. | (Wataha et al., 2004b) |
| Visible light 390–550 nm. | Up to 6 h irradiation at 2.8 mW/cm2 (or up to 60.5 J/cm2). | Human primary retinal epithelial cells. | A small loss of mitochondrial respiratory activity was observed at 6 h. Light exposure significantly damaged mitochondrial DNA at 3 h. | (Godley et al., 2005) |
| Xenon arc lamp filtered to 400–410, 445–455, 450–490, or 485–495 nm. | 20–40 min illumination at 6.3 mW/cm2 (or 7.6 –15.2 J/cm2). | Mouse fibroblasts (3T3), African green monkey kidney epithelial cells, and human foreskin keratinocytes. | Blue light stimulated H2O2 production in cultured mammalian cells. | (Hockberger et al., 1999) |
| In vivo study | ||||
| Light source | Radiant exposure | Subject | Treatment outcome | Ref. |
| Visible light 380–480 nm with a peak emission at 420 nm. | Each day, 20 J/cm2 was given to a cumulative dose of 100 J/cm2. | Eight healthy volunteers with skin type I–III and an average age of 20.9 years (19–24 years). | No inflammatory cells and sunburn cells were visible after irradiation. A significant increase in the peri-nuclear vacuolization of keratinocytes was demonstrated. No significant change in p53 expression was seen. Signs of elastosis and changes in MMP-1 expression were absent. Minimal clinical hyperpigmentation of the irradiated skin was confirmed with a significant increase in Melan-A-positive cells. | (Kleinpenning et al., 2010) |
6. Effects of blue light on wound healing
Given the growing interest in blue light therapy for wound infections, it is important to investigate the utility that blue light may have in wound healing.
McDonald et al (McDonald et al., 2011) investigated whether blue light could be employed to maintain tissue sterility without damaging the wound-healing cells. The fibroblast (NIH/3T3)-populated collagen lattice (FPCL) was used as an in vitro model of wound healing, and the effect of blue light on contraction was examined. The FPCLs were exposed to blue light (405 ±10 nm) 24 h after seeding. Treatments were carried out using light intensities of 3.6–15 mW/cm2 for 1 h, giving a total dose of 13–54 J/cm2, respectively. The results showed that exposure of 3T3 fibroblast cells to 1 h of blue light with intensities ≤ 5 mW/cm2 (or light dose ≤ 18 J/cm2) did not have an inhibitory effect on fibroblast function. The authors concluded that exposing an open wound to these intensities for 1 h would not have a detrimental impact on the wound healing process.
In an animal study using rats, Adamskaya et al (Adamskaya et al., 2011) examined the effects of blue (470 nm) light from LED arrays on the wound healing in excisional wounds. Male Sprague Dawley rats weighing 300–350 g were shaved and two circular full thickness excisional wounds were created under sterile conditions on the dorsum of each rat, including the panniculus carnosus. Animals were divided into two groups (n = 6): Group 1 treated with red LED and Group 2 was not illuminated. In the blue light treated group, the rats with excisional wounds on either the left or right side were illuminated post- wounding and on five consecutive days for 10 min at the irradiance of 50 mW/cm2. It was observed that blue light significantly decreased wound size on day 7, which correlated with enhanced epithelialization. Blue light also decreased keratin-1 mRNA on day 7 post-wounding, while keratin-10 mRNA level was elevated in both light treated group compared to control.
Another animal study was performed by Soyer et al (Soyer et al., 2011) to evaluate the effect of blue light on growth factor levels responsible for wound healing in neonatal rat skin. Eighteen (18) Wistar albino newborn rats less than 7 days old and weighing 7 ± 2 g with both sexes were included in the study. The animals were randomized into 3 groups: control, blue light, and sham (n = 6). Both the control group and blue light treated group had 1-cm median dorsal skin incision. In the control group, 1 × 1 cm of dorsal skin was sampled including the incised skin. Blue light exposure occurred shortly after the incision. The blue light treated group received blue light from 5 beams (Bilitron 3006, Fanem, Brasil) with an aperture-skin distance of 45 cm. Blue light therapy was started 24 h after birth with the mean light illumination time ranging from 15 ±1.5 min to 21±2 h. Sham group consisted of animals receiving one beam of white light with the same aperture-skin distance and a total illumination time ranging from 18 ± 9.1 min to 26 ± 3 h. The irradiance and the wavelength range of the blue light illumination were not reported in this study. After blue light exposure, 1 × 1 cm dorsal skin samples were obtained from both blue light treated group and sham group including the median incision. The effect of blue light treatment was evaluated for the expressions of vascular endothelial growth factor (VEGF), its receptor (VEGFR), and TGF-β in endothelial vessels and fibroblasts of neonatal skin samples. Results showed that there was no significant difference between groups in VEGF receptor and transforming growth factor β expressions. The VEGF levels in endothelial vessels were significantly decreased in blue light treated group and sham group when compared with the control group (P<0.05). The investigators concluded that VEGF is a mediator of angiogenesis and may decrease in neonatal rat skin after light exposure. It can be suggested that decreased levels of VEGF after blue light application may alter angiogenesis and also may adversely affect the healing features of neonatal skin.
In summary, discordant results have been reported by the limited studies regarding the effects of blue light on wound-healing. While two studies (Adamskaya et al., 2011; McDonald et al., 2011) have showed that blue light has no detrimental impact on wound-healing cells in vitro and can accelerate the healing of excisional wounds in adult rats, one study (Soyer et al., 2011) found that blue light decreased the levels of VEGF and altered angiogenesis in neonatal skin. The contradictory results might be attributable to different light sources (wavelength range), different light exposures (a biphasic dose response is frequently observed in low level light therapy (Huang et al., 2009; Huang et al., 2011)), and different models (in vitro or in vivo, ages of animals, etc) used.
7. Discussion
The alarmingly increasing emergence of antibiotic resistance in pathogenic bacteria has necessitated the search for alternative antimicrobial approaches. Blue light has attracted much attention due to its intrinsic antimicrobial properties without the involvement of added exogenous photosensitizers.
Blue light can be sensed by numerous microorganisms and can induce physiological responses elicited by blue light receptors. As a result of this, blue light can regulate bacterial motility, suppress biofilm formation, and potentiate light inactivation of bacteria. On the other hand, the presence of blue light may also up-regulate bacterial virulence factors.
Blue light therapy is now an accepted protocol for acne vulgaris, and additional clinical trials have been carried out using blue light for H. pylori gastritis showing promising outcomes. Blue light may also be effective in oral infections by killing dental anaerobic bacterial species.
It has been reported in several studies that blue light is effective in inactivating wound pathogens (both S. aureus (including MRSA) and P. aeruginosa). There are surprisingly no published preclinical and clinical reports on using blue light for wound infections, which is one of most common problems encountered in clinical practice. Future studies are of great interest to test the efficacies of blue light for wound infections in animal models (these are in progress in our laboratory) and even in clinical trials.
In comparison to PDT, blue light inactivates bacteria without the involvement of exogenous photosensitizer. As a result, the use of blue light inactivation is easier to accomplish. Delivery of photosensitizers to the target microbes has been challenging when PDT is used for infectious diseases. In addition, as the intracellular porphyrins (or other blue light sensitive chromophores) of bacteria are located inside the bacterial cells, blue light at the appropriate parameters could selectively inactivate bacteria while preserving host cells. The ideal wavelength range of blue light should be the one that is selectively absorbed by the chromophores in pathogenic bacteria but not those in host cells.
One question that will have to be addressed is “Can microbial cells develop resistance to blue light inactivation?” To our knowledge this question has not yet been experimentally addressed. The possible development of microbial resistance to photodynamic inactivation has been studied. After repeated cycles of partial inactivation followed by regrowth, different bacterial species failed to develop resistance to the photodynamic process after 10 (Tavares, 2010) or even 20 cycles (Giuliani et al., 2010). However development of microbial resistance to UVC inactivation may be possible (Dai et al., 2012b). At the very least it will be necessary to repeatedly deliver sub-eradication doses of blue light to susceptible cultures with regrowth between cycles to investigate whether resistant clones can be selected, or mutants with increased blue light damage repairing enzymes can be produced.
The biological effects of blue light are often viewed as innocuous and very limited studies have been carried out in this area. It is reported by limited in vitro cell culture studies that blue light at certain wavelength and exposures may induce dysfunction of mammalian cells. The effect is considered to be attributable to the photo-excitation of chromophores in mitochondria or peroxisomes (Hockberger et al., 1999; Wataha et al., 2004a). In comparison to UVC irradiation therapy, there is much less concern about blue-light-induced mutagenesis effects in mammalian cells. Blue light (>400 nm) absorption by DNA is weak (Sutherland and Griffin, 1981). A clinical study demonstrated that blue light with a cumulative dose of 100 J/cm2 did not cause significant changes in p53 expression (Kleinpenning et al., 2010).
A bottleneck in light-based therapy is the limitation of light penetration in tissue. While the penetration depth of blue light in tissue is lower than that of red light (Ankri et al., 2010), which is commonly employed in PDT, blue light can penetrate deeper in tissue than UVC irradiation. In addition, an increasing number of creative approaches are being investigated to increase the depth of light penetration in tissue, including optical clearing, interstitial light delivery, etc.
In general, the use of blue light for the treatment of infectious diseases is a new area that warrants further investigations. The mechanisms of the bactericidal effect of blue light as well as the effects of blue light on mammalian cells are still not fully understood. Further studies should be performed to ensure an effective and safe blue light therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42:917–921. doi: 10.1016/j.injury.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Ammad S, Gonzales M, Edwards C, Finlay AY, Mills C. An assessment of the efficacy of blue light phototherapy in the treatment of acne vulgaris. J Cosmet Dermatol. 2008;7:180–188. doi: 10.1111/j.1473-2165.2008.00386.x. [DOI] [PubMed] [Google Scholar]
- Ankri R, Lubart R, Taitelbaum H. Estimation of the optimal wavelengths for laser-induced wound healing. Lasers Surg Med. 2010;42:760–764. doi: 10.1002/lsm.20955. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Bache SE, Maclean M, Macgregor SJ, Anderson JG, Gettinby G, Coia JE, Taggart I. Clinical studies of the High-Intensity Narrow-Spectrum light Environmental Decontamination System (HINS-light EDS), for continuous disinfection in the burn unit inpatient and outpatient settings. Burns. 2011 doi: 10.1016/j.burns.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob Agents Chemother. 2011a;55:5710–5717. doi: 10.1128/AAC.05404-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Garcia B, Murray CK, Vrahas MS, Hamblin MR. UVC Light Prophylaxis for Cutaneous Wound Infections in Mice. Antimicrob Agents Chemother. 2012a;56:3841–3848. doi: 10.1128/AAC.00161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009a;6:170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d'Enfert C, Hamblin MR. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 2011b;87:342–349. doi: 10.1111/j.1751-1097.2011.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009b;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillinresistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012b;10:185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady EA, Gloor M, Leyden JJ. Propionibacterium acnes resistance: a worldwide problem. Dermatology. 2003;206:54–56. doi: 10.1159/000067822. [DOI] [PubMed] [Google Scholar]
- Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillinresistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 2009;27:221–226. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photodestroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2008;40:734–737. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- Fan X, Xing YZ, Liu LH, Liu C, Wang DD, Yang RY, Lapidoth M. Effects of 420-nm intense pulsed light in an acne animal model. J Eur Acad Dermatol Venereol. 2012 doi: 10.1111/j.1468-3083.2012.04487.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 2005;81:1186–1189. doi: 10.1562/2005-04-06-RA-477. [DOI] [PubMed] [Google Scholar]
- Feuerstein O, Persman N, Weiss EI. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochem Photobiol. 2004;80:412–415. doi: 10.1562/0031-8655(2004)080<0412:PEOVLO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Filice GA, Nyman JA, Lexau C, Lees CH, Bockstedt LA, Como-Sabetti K, Lesher LJ, Lynfield R. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31:365–373. doi: 10.1086/651094. [DOI] [PubMed] [Google Scholar]
- Fukui M, Yoshioka M, Satomura K, Nakanishi H, Nagayama M. Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis. J Periodontal Res. 2008;43:174–178. doi: 10.1111/j.1600-0765.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002;16:1047–1057. doi: 10.1046/j.1365-2036.2002.01276.x. [DOI] [PubMed] [Google Scholar]
- Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- Gold MH, Andriessen A, Biron J, Andriessen H. Clinical Efficacy of Self-applied Blue Light Therapy for Mild-to-Moderate Facial Acne. J Clin Aesthet Dermatol. 2009;2:44–50. [PMC free article] [PubMed] [Google Scholar]
- Gomelsky M, Hoff WD. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 2010;19:441–448. doi: 10.1016/j.tim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 2006;24:684–688. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger PE, Skimina TA, Centonze VE, Lavin C, Chu S, Dadras S, Reddy JK, White JG. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc Natl Acad Sci U S A. 1999;96:6255–6260. doi: 10.1073/pnas.96.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Ren S, Maekawa M, Hasegawa K, Arisaka F, Hyodo M, Hayakawa Y, Ohta H, Masuda S. Biochemical and physiological characterization of a BLUF protein-EAL protein complex involved in blue light-dependent degradation of cyclic diguanylate in the purple bacterium Rhodopseudomonas palustris. Biochemistry. 2010;49:10647–10655. doi: 10.1021/bi101448t. [DOI] [PubMed] [Google Scholar]
- Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. J Dermatol Sci. 2002;30:129–135. doi: 10.1016/s0923-1811(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Kim RH, Armstrong AW. Current state of acne treatment: highlighting lasers, photodynamic therapy, and chemical peels. Dermatol Online J. 2011;17:2. [PubMed] [Google Scholar]
- Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC, 3rd, Lindmark WR, McGrath JR, Hamblin MR. Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: a pilot clinical trial. Lasers Surg Med. 2009;41:337–344. doi: 10.1002/lsm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130:259–269. doi: 10.1038/jid.2009.194. [DOI] [PubMed] [Google Scholar]
- Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg Med. 2010;42:467–472. doi: 10.1002/lsm.20948. [DOI] [PubMed] [Google Scholar]
- Losi A, Gärtner w. Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochem Photobiol. 2011;87:491–510. doi: 10.1111/j.1751-1097.2011.00913.x. [DOI] [PubMed] [Google Scholar]
- Maclean M, MacGregor SJ, Anderson JG, Woolsey G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol Lett. 2008a;285:227–232. doi: 10.1111/j.1574-6968.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol. 2009;75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008b;92:180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Maclean M, Macgregor SJ, Anderson JG, Woolsey GA, Coia JE, Hamilton K, Taggart I, Watson SB, Thakker B, Gettinby G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J Hosp Infect. 2010;76:247–251. doi: 10.1016/j.jhin.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Massey V. The chemical and biological versatility of riboflavin. Biochem Soc Trans. 2000;28:283–296. [PubMed] [Google Scholar]
- Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R, Macgregor SJ, Anderson JG, Maclean M, Grant MH. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: an in vitro model of wound healing. J Biomed Opt. 2011;16:048003. doi: 10.1117/1.3561903. [DOI] [PubMed] [Google Scholar]
- Megraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1333–1343. doi: 10.1046/j.1365-2036.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- Mochon AB, Garner OB, Hindler JA, Krogstad P, Ward KW, Lewinski MA, Rasheed JK, Anderson KF, Limbago BM, Humphries RM. New Delhi metallo-beta-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J Clin Microbiol. 2011;49:1667–1670. doi: 10.1128/JCM.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton CA, Scholefield RD, Whitehurst C, Birch J. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. J Dermatolog Treat. 2005;16:219–223. doi: 10.1080/09546630500283664. [DOI] [PubMed] [Google Scholar]
- Mussi M, Gaddy JA, Cabruja M, Arivett BA, Viale AM, Rasia R, Actis LA. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol. 2010;192:6336–6345. doi: 10.1128/JB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noborio R, Nishida E, Kurokawa M, Morita A. A new targeted blue light phototherapy for the treatment of acne. Photodermatol Photoimmunol Photomed. 2007;23:32–34. doi: 10.1111/j.1600-0781.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- Nordmann P, Poirel L, Toleman MA, Walsh TR. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J Antimicrob Chemother. 2011;66:689–692. doi: 10.1093/jac/dkq520. [DOI] [PubMed] [Google Scholar]
- Omi T, Bjerring P, Sato S, Kawana S, Hankins RW, Honda M. 420 nm intense continuous light therapy for acne. J Cosmet Laser Ther. 2004;6:156–162. doi: 10.1080/14764170410023785. [DOI] [PubMed] [Google Scholar]
- Ondrusch N, Kreft J. Blue and red light modulates SigB-dependent gene transcription, swimming motility and invasiveness in Listeria monocytogenes. PLoS One. 2011;6:e16151. doi: 10.1371/journal.pone.0016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A. Antibiotics. NDM-1 how dangerous is the mutation? Time. 2010;176:20. [PubMed] [Google Scholar]
- Pisciotta J, Zou Y, Baskakov IV. Light-dependent electrogenic activity of cyanobacteria. PLoS One. 2010;5:e10821. doi: 10.1371/journal.pone.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci U S A. 2007;104:18241–18246. doi: 10.1073/pnas.0705887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell N, Martin SS, Feoktistova K, Lagarias JC. Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. Proc Natl Acad Sci U S A. 2011;108:11854–11859. doi: 10.1073/pnas.1107844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R. Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol. 2010;64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- Savard P, Gopinath R, Zhu W, Kitchel B, Rasheed JK, Tekle T, Roberts A, Ross T, Razeq J, Landrum BM, Wilson LE, Limbago B, Perl TM, Carroll KC. First NDM-positive Salmonella sp. strain identified in the United States. Antimicrob Agents Chemother. 2011;55:5957–5958. doi: 10.1128/AAC.05719-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitkind E, Yaping E, Geen S, Shalita AR, Lee WL. Anti-inflammatory properties of narrow-band blue light. J Drugs Dermatol. 2006;5:605–610. [PubMed] [Google Scholar]
- Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49:1391–1396. doi: 10.1128/AAC.49.4.1391-1396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyer T, Ayva S, Aliefendioglu D, Aktuna Z, Aslan MK, Senyucel MF, Cakmak M. Effect of phototherapy on growth factor levels in neonatal rat skin. J Pediatr Surg. 2011;46:2128–2131. doi: 10.1016/j.jpedsurg.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Sutherland JC, Griffin KP. Absorption spectrum of DNA for wavelengths greater than 300 nm. Radiat Res. 1981;86:399–409. [PubMed] [Google Scholar]
- Suzuki H, Nishizawa T, Hibi T. Helicobacter pylori eradication therapy. Future Microbiol. 2010;5:639–648. doi: 10.2217/fmb.10.25. [DOI] [PubMed] [Google Scholar]
- Swartz TE, Tseng TS, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim JG, Mudgett MB, Splitter GA, Ugalde RA, Goldbaum FA, Briggs WR, Bogomolni RA. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- Tan AW, Tan HH. Acne vulgaris: a review of antibiotic therapy. Expert Opin Pharmacother. 2005;6:409–418. doi: 10.1517/14656566.6.3.409. [DOI] [PubMed] [Google Scholar]
- Tang Y, Cao Z, Livoti E, Krauss U, Jaeger KE, Gärtner W, Losi A. Interdomain signalling in the blue-light sensing and GTP-binding protein YtvA: a mutagenesis study uncovering the importance of specific protein sites. Photochem Photobiol Sci. 2010;9:47–56. doi: 10.1039/b9pp00075e. [DOI] [PubMed] [Google Scholar]
- Tavares A, Carvalho CM, Faustino MA, Neves MG, Tomé JP, Tomé AC, Cavaleiro JA, Cunha A, Gomes NC, Alves E, Almeida A. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Leeming JP, Bannister GC. Effect of antiseptics, ultraviolet light and lavage on airborne bacteria in a model wound. J Bone Joint Surg Br. 1993;75:724–730. doi: 10.1302/0301-620X.75B5.8376427. [DOI] [PubMed] [Google Scholar]
- Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manage. 2005;51:32–45. [PubMed] [Google Scholar]
- Tschowri N, Busse S, Hengge R. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev. 2009;23:522–534. doi: 10.1101/gad.499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst M, Stalcup TP, Kaledhonkar S, Kumauchi M, Hara M, Xie A, Hellingwerf KJ, Hoff WD. Locked chromophore analogs reveal that photoactive yellow protein regulates biofilm formation in the deep sea bacterium Idiomarina oihiensis. J Am Chem Soc. 2009;131:17443–17451. doi: 10.1021/ja9057103. [DOI] [PubMed] [Google Scholar]
- van der Horst MA, Stalcup TP, Kaledhonkar S, Kumauchi M, Hara M, Xie A, Hellingwerf KJ, Hoff WD. Locked chromophore analogs reveal that photoactive yellow protein regulates biofilm formation in the deep sea bacterium Idiomarina loihiensis. J Am Chem Soc. 2009;131:17443–17451. doi: 10.1021/ja9057103. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) The Journal of antimicrobial chemotherapy. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- Wataha JC, Lewis JB, Lockwood PE, Hsu S, Messer RL, Rueggeberg FA, Bouillaguet S. Blue light differentially modulates cell survival and growth. J Dent Res. 2004a;83:104–108. doi: 10.1177/154405910408300204. [DOI] [PubMed] [Google Scholar]
- Wataha JC, Lockwood PE, Lewis JB, Rueggeberg FA, Messer RL. Biological effects of blue light from dental curing units. Dent Mater. 2004b;20:150–157. doi: 10.1016/s0109-5641(03)00086-1. [DOI] [PubMed] [Google Scholar]
- Wheeland RG, Dhawan S. Evaluation of self-treatment of mild-to-moderate facial acne with a blue light treatment system. J Drugs Dermatol. 2011;10:596–602. [PubMed] [Google Scholar]