Abstract
The current study examined the Fear Avoidance (FA) model of chronic pain in pediatric chronic pain patients. Multiple structural equation models were tested in the current study with pairwise parameter comparisons made between younger children (8–12 years) and adolescents (13–17 years). Within a sample of 350 children and adolescents, we examined functional disability and depressive symptoms in separate models with the following predictor variables: pain, pain catastrophizing, fear of pain, and avoidance of activities, after controlling for duration of pain. For a subset of patients (n=151) we also tested a brief prospective outcome model with baseline predictor variables and functional disability at one-month follow-up. The FA models predicting functional disability concurrently and prospectively were an excellent fit to the data. The theorized FA model for depression was a poor fit. When the model was modified to include direct pathways from the cognitive processes of pain catastrophizing and fear of pain to depressive symptoms, the model fit was significantly improved. Examining developmental differences between younger children and adolescent patients, duration of pain contributed to the model for younger children while pain-related fears were more influential for adolescent patients.
Keywords: Fear avoidance model of pain, children and adolescents, chronic pain, psychological aspects of chronic pain
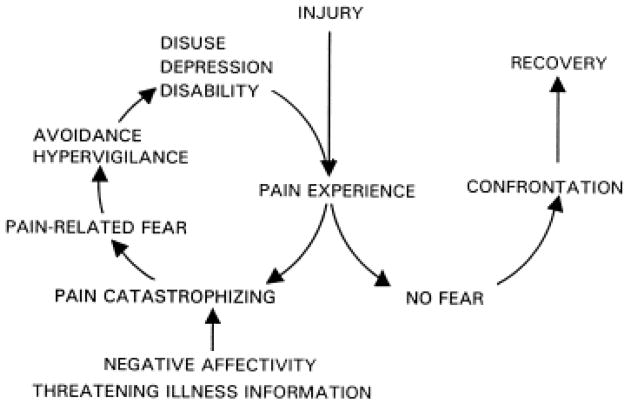
The fear avoidance (FA) model of chronic pain has garnered significant attention in adults. The model asserts that when an individual perceives pain as threatening, manifested as catastrophic thinking, (s)he responds with pain-related fear and avoidance behavior, which in turn results in declines in functioning 38; 39; 23 (see Figure 1). Evidence in adults supports the theoretical 14; 32 and clinical 8; 1 application of this model. Evaluations of the FA model have been pursued in cross-sectional adult chronic pain samples successfully predicting pain severity 15, disability and depression 5, and functional and social disability 14 and in whiplash injury to predict disability and depression 29; 20. Subsequently, investigators in adult chronic pain have examined prospective FA models predicting pain-related disability13; 20 and return-to-work45. Two of the primary predictive components of the FA model, pain catastrophizing and pain-related fear, have begun to be assessed and evaluated in children.
Figure 1.
Fear avoidance model of chronic pain (reproduced from Vlaeyen & Linton, 2000 with permission).
Pain catastrophizing, the initial response to pain theorized in the FA model, is consistently linked to poor outcomes in children. It has been associated with higher pain intensity ratings in children with idiopathic chronic pain 18 and juvenile idiopathic arthritis 33. Additionally, high levels of catastrophizing have been associated with poor performance across home, school, and social domains of functioning 7; 26; 36. In a prospective study among children, pain catastrophizing contributed to increased pain and disability six months later37. Pain-related fear, the next theorized step in the FA model, has also been shown to be a predictor of pain-related disability27; 31 and physical activity limitations 46 in pediatric chronic pain patients. Additionally, in acute post-surgical pain, pain related fear was associated with pain unpleasantness and functional disability in children and adolescents two weeks after surgery 12.
Although accumulating evidence supports the predictive value of pain catastrophizing and pain-related fear separately on pain-related outcomes, no studies to our knowledge have examined both variables from the fear avoidance model simultaneously in children and adolescents with chronic pain. The FA model has contributed to theoretical and clinical advances in our understanding and treatment of adults suffering with persistent pain problems, but we cannot simply assume that the FA model operates identically in children. Childhood is a time of rapid changes in cognitive development and behavior which may influence individual components of the FA model or the way in which components of the model interact. Thus it is important to examine the FA model across childhood for any potential development differences.
In this study, we conducted the first examination of the FA model in children with chronic pain using Structural Equation Modeling (SEM). With this approach we examined a model in which pain severity predicted pain catastrophizing, which predicted pain-related fear, which predicted pain-related avoidance, which predicted the outcomes, functional disability and depressive symptoms, consistent with the FA model. For each pathway we examined potential age-related differences between younger children (8–12 years) and adolescents (13–17).
We hypothesized that the FA model would be a good fit for pediatric patients across age groups due to the robust predictive power that has been observed for pain catastrophizing and pain-related fear in children. Specifically, we tested the FA model with three outcomes: 1) functional disability at evaluation, 2) depressive symptoms at evaluation, and 3) functional disability at one-month follow-up from a subset of the larger sample.
Method
Participants
Patients ages 8 to 17 years who participated in a multidisciplinary pain clinic evaluation at our hospital between September 2008 to August 2010 were invited to participate. Of the 401 patients approached, 350 were enrolled. Participants were primarily Caucasian (92.3%) and female (80.6%), consistent with the population of children seen in this tertiary care clinic setting. Mean age was 13.7 years (SD = 2.46). Primary pain diagnoses included neuropathic (34.3% [single limb 63%; diffuse 22%]), musculoskeletal (30.8% [single limb 71%; diffuse 22%]), back/neck (14.1%), chronic abdominal (8.9%), gynecological/genitourinary (5.8%), headache (2.0%) and other pain (e.g., chest, ear, bladder; 4.0%). Duration of pain varied greatly from 1 month to 206 months, with median duration of pain of 14 months. The majority of parents in this sample were married (80%). Family socioeconomic status (SES) based on the four-factor index of social status19 ranged from 22 (semi-skilled workers) to 66 (business owner; professional), with a mean of 45.5 (SD = 12.1). From March 2009 to May 2010 families were contacted one month after their evaluation to complete follow-up questionnaires as part of a larger Institutional Review Board (IRB) approved research protocol obtaining test-retest reliability data for validating the Fear of Pain Questionnaire31. Of the 208 families contacted, 151 participated in the follow-up assessment.
Procedure
Questionnaires in this study were collected at the child’s multidisciplinary pain evaluation. Some measures were completed as part of the standard clinic assessment battery (pain ratings, functional disability, depressive symptoms), while others were completed separately (pain-related fear and avoidance, pain catastrophizing) for a larger IRB approved research protocol. Clinic assessment measures were mailed to families prior to the pain evaluation. Parents and children were instructed to complete the measures independently and bring them to the appointment. During the clinic visit, families were approached to participate in the IRB-approved study. At that time, patients and parents completed study measures and provided assent/consent for use of clinic measures. For the subset of participants contacted for follow-up, questionnaires were mailed to families with follow-up phone calls from study staff to encourage completion.
Materials
Basic demographic (e.g, age, gender) and medical information (e.g., diagnosis) was collected from patient charts.
Pain intensity
Children were asked during the psychology interview to provide their average pain rating on a standard 11-point numeric rating scale 35 from 0 (no pain) to 10 (most pain possible).
Pain catastrophizing
The Pain Catastrophizing Scale (PCS-C,) 7; 16 assesses negative thinking associated with pain. It is comprised of 13 items rated on a 5-point scale. Items are summed to derive a total score from three subscales: rumination (“When I have pain, I want the pain to go away”), magnification (“When I have pain, I keep thinking of other painful events”), and helplessness (“When I have pain, I feel I can’t go on”). Higher scores indicated higher levels of catastrophic thinking. Internal consistency in the current sample was .91.
Pain-related fear and avoidance
The Fear of Pain Questionnaire (FOPQ-C) 31 assesses perceptions of child pain-related fears and avoidance behaviors. The FOPQ-C consists of 24 items and two subscales: Fear of Pain (“I worry when I am in pain”; 13 items) and Avoidance of Activities (“I avoid making plans because of my pain”; 11 items). Items are rated on a 5-point scale from 0= strongly disagree to 4= strongly agree. Higher scores indicate higher levels of pain-related fear and avoidance. In examining construct validity, Fear of Pain was more strongly associated with child somatization, anxiety, and catastrophizing while Avoidance of Activities was more strongly associated with functional disability and doctor visits in the previous three months31. Internal consistency in the current sample was .89 for Fear of Pain and .87 for Avoidance of Activities.
Functional disability
The Functional Disability Inventory (FDI) 42 is a scale that assesses difficulty in physical and psychosocial functioning due to physical health. The instrument consists of 15 items concerning perceptions of activity limitations during the past two weeks. Total scores are computed by summing the items with higher scores indicating greater disability. The FDI has excellent reliability and validity 4. This measure was completed at the evaluation and one month later by the child. Internal consistency at baseline and one month follow-up was .89 and .92, respectively.
Depressive symptoms
The Children’s Depression Inventory (CDI) is a valid and reliable 27-item child self-report measure of depressive symptoms. Higher scores indicate higher levels of depression 22. Alpha reliability of this measure was .88.
Statistical Analyses
Preliminary analyses were conducted in SPSS version 19. Data were screened for normality (i.e., skewness <3.0; kurtosis<10.0)21. Descriptive statistics and correlations were conducted to determine whether the pattern of relationships among the variables was consistent with the hypothesized model. Correlations and one-way ANOVAs were also conducted to examine associations among demographic (SES, gender, ethnicity), pain (pain duration, pain diagnosis), and study variables (pain, catastrophizing, fear of pain, avoidance of activities, functional disability, depressive symptoms); demographic and pain variables that were associated with study variables were included as control variables in the SEM model. We conducted one-way ANOVAs between younger children (8–12 years) and adolescents (13–17 years) across study variables in anticipation of examining developmental differences across the two age groups. As the one-month post-evaluation is a relatively brief window of time, we ran a paired sample t-test to examine whether there was a significant change in functional disability from the evaluation to follow-up. If there was no significant change, we would not proceed with examining functional disability at follow-up.
Path analysis within a structural equation modeling (SEM) framework with AMOS version 20.0 for SPSS was employed to evaluate the study hypotheses. SEM was considered superior to other analytic techniques, such as multiple regression, because it is possible to simultaneously evaluate the overall fit of complex models as well as the significance of individual model pathways, to reduce measurement error, to compare alternative models, and to include cases with missing data in the model 28. Full information maximum likelihood estimation (FIML) was employed to account for missing data. Based on recommendations by Bentler and Bonett 2 and Ullman 34, the following statistics were used to evaluate model fit: χ2, χ2/df (<2 acceptable); Comparative Fit Index (CFI; >.90 acceptable, >.95 excellent); and Root Mean Square Error of Approximation (RMSEA; <.08 acceptable, <.05 excellent). A sample size of 100–200 subjects is generally considered adequate for testing complex models in SEM 21, thus there was a sufficient sample size for the cross-sectional (n=350) and prospective (n=151) models.
Using the FA model framework we tested separate models for each outcome of interest, functional disability and depression. We then evaluated the fit of the models and the strength and direction of individual model parameters. To examine potential developmental differences in the FA model we conducted pairwise parameter comparisons between younger children (8–12 years) and adolescent (13–17 years) patients. Separate models were specified simultaneously within the same overall model for both groups, younger children (n=108 concurrent sample; n=46 prospective sample) and adolescents (n=242 concurrent sample; n=105 prospective sample). We examined the critical ratios for differences between parameters between groups, with a z-score≥ 1.96 considered significantly different. For each model, we describe the overall model fit and note specific parameter estimate differences for younger children and adolescent subgroups.
Results
Preliminary and Descriptive Analyses
We examined the relations between demographic and pain characteristics (pain diagnosis, duration of pain) and all variables of interest in this study. Duration of pain was significantly correlated with pain and pain catastrophizing; thus we controlled for this variable in all models (Table 2). No one-way ANOVAs were significant (e.g., gender); thus no additional control variables were included in the SEM models. In conducting one-way ANOVAs examining differences between younger children and adolescents across study variables, we found that adolescents had significantly higher avoidance of activity scores and higher functional disability scores at follow-up compared to younger children (Table 1). Using paired-sample t-tests, we found that functional disability decreased significantly from evaluation (M=24.3, SD=12.6) to one-month follow-up (M=20.5, SD=12.6), t(149) = 4.46, p < .00; thus, we examined functional disability at follow-up as a prospective outcome in this study. We also explored any potential sampling bias between the 151 participants in the follow-up assessment and the 57 who were contacted but did not participate. There was no significant difference between responders and non-responders on age, pain duration, pain level, and functional disability at evaluation, thus no evidence of sampling bias on those parameters.
Table 2.
Intercorrelations for all model variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | N |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child age (years) | -- | .15** | .01 | .03 | .02 | .13* | −.03 | .08 | .16* | 350 |
| 2. Duration of pain (months) | -- | −14** | −.11* | .01 | −.01 | −.09 | −.02 | −.06 | 349 | |
| 3. Pain severity | -- | .30** | .23** | .21** | .32** | .23** | .14 | 347 | ||
| 4. Pain catastrophizing | -- | .74** | .53** | .29** | .56** | .29** | 346 | |||
| 5. Fear of pain | -- | .67** | .39** | .60** | .43** | 348 | ||||
| 6. Avoidance of activities | -- | .48** | .44** | .50** | 348 | |||||
| 7. Functional disability | -- | .36** | .65** | 345 | ||||||
| 8. Depressive symptoms | -- | .33** | 340 | |||||||
| Follow-up | ||||||||||
| 9. Functional disability | -- | 151 | ||||||||
Note.
p < .05,
p < .01; Correlations are two-tailed.
Table 1.
One-way ANOVAs between younger children and adolescents for all study variables
| Variable | Children Mean (SD) |
Adolescents Mean (SD) |
ANOVA F |
|---|---|---|---|
| Duration of Pain | 20.4 (29.3) | 25.9 (29.6) | 2.43 |
| Pain severity | 6.0 (2.1) | 5.9 (2.7) | .06 |
| Pain catastrophizing | 27.6 (11.6) | 26.6 (11.1) | .58 |
| Fear of pain | 24.2 (11.1) | 23.2 (10.5) | .64 |
| Avoidance of activities | 17.0 (8.37) | 19.3 (9.55) | 4.28* |
| Functional disability | 23.8 (12.2) | 22.5 (11.9) | .83 |
| Depressive symptoms | 9.66 (7.48) | 10.0 (7.05) | .18 |
| Follow-up | |||
| Functional disability | 17.5 (12.7) | 21.9 (12.3) | 3.90* |
Note.
p < .05,
p < .01.
Correlations were conducted between pain, catastrophizing, fear of pain, avoidance of activities, functional disability, and depressive symptoms (Table 2). All relations between variables of interest were significant, except pain severity and functional disability at follow-up (r = .14, p = .09).
Model Testing
All variables met the SEM requirements for normality. Missing data was considered missing completely at random for SEM analyses due to the small amount of missing data across variables (see Table 2). Model parameters reported represent standardized values.
Fear of Pain Model with Concurrent Outcomes
Functional disability
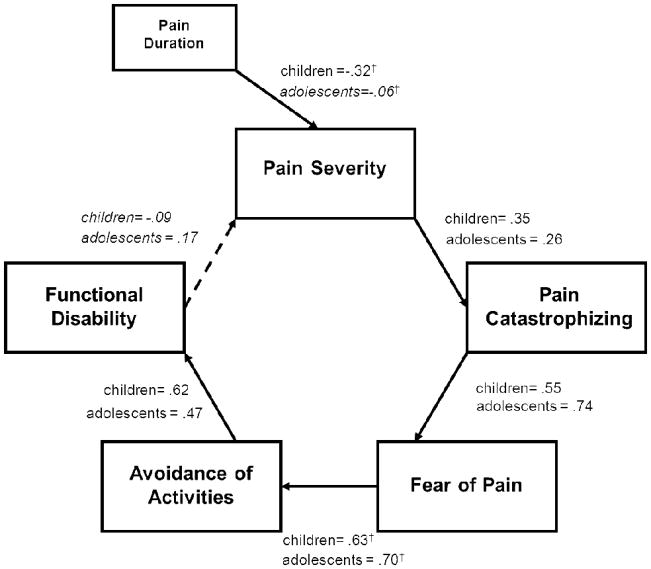
The model predicting functional disability provided a good fit to the data [χ2(18) = 36.5, p = .006; χ2/df = 2.025; CFI = .97, RMSEA = 0.054 (90% CI = 0.028–0.080), CAIC = 247.34]. The FA model sequence from pain to disability was supported, as all model pathways were statistically significant and in the expected direction (Figure 2). The pathway from disability back to pain was not significant contrary to the feedback loop theorized within the FA model.
Figure 2.
Model with Functional Disability as Concurrent Outcome. Error variances were not included in the figure for simplicity and interpretability. Italicized regression coefficients are non-signficant pathways. †denotes values that significantly different between children and adolescents within a specific pathway.
To further support the hypothesized FA model, we tested an alternative regression model in which each predictor variable independently contributed to functional disability simultaneously. The model fit was extremely poor, χ2(20) = 550.92, p = .00, χ2/df = 27.55; CFI = .084, RMSEA = 0.276 (90% CI = 0.257–0.296), CAIC = 750.09] providing additional validation of the FA path model in this pediatric sample.
In examining pairwise parameter comparisons, two age-related differences emerged. The pathway between duration of pain and pain ratings was significant for younger children (β=.−32), whereas this pathway was non-significant among adolescents (β=−.06), z=2.31, p=.02, with shorter pain duration associated with higher pain ratings among younger children. In addition, the pathway between fear of pain and avoidance of activities was significantly different between younger children (β=.63) and adolescents (β=.70), z=2.26, p=.02, with fear of pain having a stronger indirect relationship between catastrophizing and avoidance behaviors for adolescents.
Depressive symptoms
The regression model predicting depression provided a poor fit to the data [χ2(18) = 110.3, p < .00; χ2/df = 6.12; CFI = .87, RMSEA = 0.12 (90% CI = 0.100–0.144), CAIC = 321.10]; thus suggesting that the theorized FA model sequence from pain to depressive symptoms does not adequately account for depressive outcomes in the current pediatric sample (Figure 3). We chose to modify the model to include direct pathways from pain catastrophizing and fear of pain to depressive symptoms based on a review of the correlation matrix and given that depressive symptoms are predominantly cognitive and often involve catastrophic or fearful thoughts. These modifications improved the model fit substantially [χ2(14) = 24.4, p = .04; χ2/df = 1.74; CFI = .99, RMSEA = 0.046 (90% CI = 0.009–0.076), CAIC = 258.73]. Inclusion of these two pathways resulted in the direct pathway from avoidance of activities to depressive symptoms to be greatly reduced (from β = .22 to β = −.15 for younger children and from β=.51 to β=.15 for adolescents) (Figure 3). This suggests, at least for this sample, that catastrophic and fearful cognitions drive depressive symptoms, rather than avoidant behaviors. Similar to the model with functional disability, the pathway from depressive symptoms back to pain was not significant, contrary to the feedback loop theorized within the FA model.
Figure 3.
Final Model with Depressive Symptoms as Concurrent Outcome. Error variances were not included in the figure for simplicity and interpretability. Italicized regression coefficients are non-signficant pathways. †denotes values that significantly different between children and adolescents within a specific pathway.
We also tested an alternative regression model where each predictor variable independently contributed to depression simultaneously. The model fit was extremely poor, χ2(20) = 548.75, p = .00, χ2/df = 27.44; CFI = .24, RMSEA = 0.276 (90% CI = 0.256–0.296), CAIC = 747.92]. further supporting the FA path model in this sample.
In examining pairwise parameter comparisons, three age-related differences emerged. The pathway between duration of pain and pain ratings was significant for younger children (β=.−31), whereas this pathway was non-significant among adolescents (β= −.06), z=2.27, p=.02, with shorter pain duration associated with higher pain ratings among younger children. The pathway between fear of pain and avoidance of activities was significantly different between younger children (β=.62) and adolescents (β=.70), z=2.44, p=.01, with fear of pain having a stronger indirect role between catastrophizing and avoidance behaviors for adolescents. Lastly, the pathway between avoidance of activities and depressive symptoms was significantly different between younger children (β= −.15) and adolescents (β=.15), z=2.33, p=.02, with avoidance of activities a marginally significant predictor of depressive symptoms for adolescents and non-significant for younger children.
Fear of Pain Model with Prospective Outcome
Functional disability at follow-up
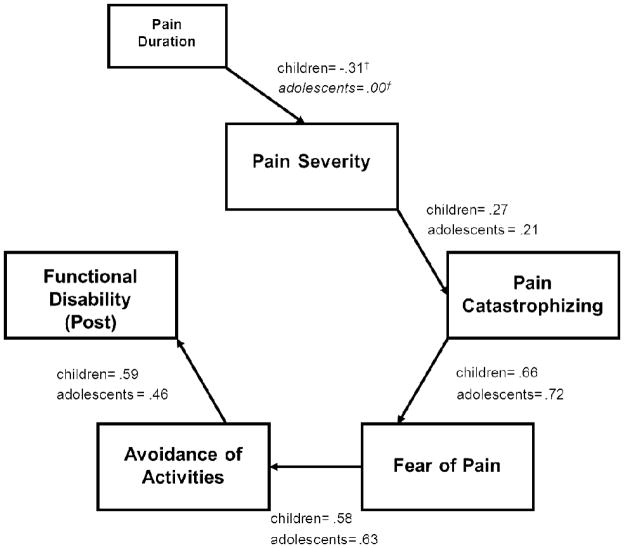
Using baseline FA model variables to predict functional disability at one-month follow-up, we examined a prospective model. This provided a good fit to the data [χ2(20) = 34.23, p = .03; χ2/df = 1.71; CFI = .94, RMSEA = 0.069 (90% CI = 0.025–0.108), CAIC = 204.82] with the FA model variables predicting functional disability at follow-up (Figure 4).
Figure 4.
Model with Functional Disability as Prospective Outcome. Error variances were not included in the figure for simplicity and interpretability. Italicized regression coefficients are non-signficant pathways. †denotes values that significantly different between children and adolescents within a specific pathway.
We also tested an alternative regression model where each predictor variable independently contributed to functional disability at follow-up simultaneously. The model fit was extremely poor, χ2(20) = 199.22, p = .00, χ2/df = 9.96; CFI = .19, RMSEA = 0.245 (90% CI = 0.215–0.277), CAIC = 369.81]. further supporting the FA path model in this sample.
In examining pairwise parameter comparisons, one developmental difference emerged. The pathway between duration of pain and pain ratings was relatively stronger in younger children (β=. −31) than adolescents (β=.00) although the difference was not statistically significant, likely due to the smaller sample size (z=1.89, p=.06). Shorter pain duration was associated with higher pain ratings among younger children but not adolescents.
Discussion
The aim of the current study was to conduct the first examination of the FA model of chronic pain in a pediatric population. For disability, we found support for this model concurrently and at one-month follow-up using path analysis in SEM. For depression, the results were less straightforward. The theorized FA model did not accurately represent associations among pain, pain catastrophizing, pain-related fear and avoidance, and depression in this pediatric sample. Modifying the model to include direct pathways from pain catastrophizing and fear of pain to depression resulted in improved model fit, with the modified model potentially more accurately characterizing the inter-relations among these variables.
Several interesting developmental differences emerged across models. Consistently, a shorter duration of pain was associated with higher pain ratings among younger children whereas this variable was not a significant predictor of pain severity among adolescent patients. Although this finding needs further replication, it may be that duration of pain is only associated with pain severity ratings in younger children presenting with pain complaints. This may be due to referral patterns, in that briefer and more severe pain in younger children may result in a referral to a tertiary care chronic pain clinic more frequently than in adolescents. Specific to the FA model, fear of pain appears to play a stronger indirect role between catastrophizing and avoidant behaviors among adolescents as compared to younger children with chronic pain. This suggests that targeting anxiety-related pain cognitions (“I walk around in constant fear of hurting”) when working with adolescents will likely yield greater gains in returning to previously avoided activities, than in younger pain patients. Lastly, in examining one-way ANOVA analyses between younger children and adolescent patients, adolescents reported higher levels of avoidant behaviors, perhaps due to greater agency in choosing to avoid social or academic activities whereas younger children may have less choice in doing so (e.g., “I cancel plans when I am in pain”). Adolescents also reported higher levels of functional disability at the one month follow-up compared to younger children. This could be attributed to several factors. It could be younger children and their parents may respond more enthusiastically to the multidisciplinary clinic experience and treatment recommendations, whereas the increased agency adolescents have may hinder their enthusiasm and willingness to re-engage in activities.
The results in this study provide a nuanced understanding of the FA model of chronic pain for children and adolescents. For functional disability as predicted by the FA model, the process builds from the child perceiving pain as threatening (pain catastrophizing), to responding with fear, and in turn engaging in avoidance behaviors. Thus, functional disability, where patients report perceived activity limitations42; 4 is the logical consequence of behavioral avoidance. Divergent from the theorized adult model, functional disability did not contribute back to pain severity. In reviewing models previously tested in the adult literature, this feedback loop is often not incorporated and it may be due to the lack of variance remaining to be explained. Further support for the FA model was observed when examining functional disability at follow-up as the outcome. These results suggest that despite improvements in functional disability from baseline to follow-up, FA model variables (pain, catastrophizing, fear, avoidance) assessed at evaluation may serve as potential risk factors significantly contributing to levels of disability reported one month later. Although this is not a true prospective examination of the FA model of chronic pain in children and adolescents because most variables were concurrent, the findings suggest further inquiry with longitudinal data to extend and confirm these preliminary results. A true longitudinal study with each variable assessed prospectively would be required to more fully validate the FA model.
For depressive symptoms, the FA model of chronic pain in children and adolescents may altogether operate somewhat differently. The original theorized model, with indirect pathways from catastrophizing and fear to depression through activity avoidance, was insufficient. When the model was modified by adding direct pathways from pain catastrophizing and fear of pain to depressive symptoms, the fit improved substantially and avoidance of activities was no longer a significant predictor of depression for younger children and a marginal predictor for adolescents. This likely reflects the important influence of the cognitive aspects of the FA model for depression and suggests that targeting catastrophic interpretations and fearful cognitions could improve depressive symptoms, at least in the context of pediatric chronic pain. Although these results seem to be to some degree conflicting with a large body of evidence that supports behavioral activation for depression 9, it may not be “either/or” but rather that in this context, pain-specific negative cognitions are more relevant in understanding depressive symptoms. Furthermore, behavioral activation may provide a way to indirectly challenge catastrophic and fearful cognitions by providing a positive experience that contrasts with the feared or anticipated outcome (i.e., increased pain), thus targeting negative cognitions indirectly via behavioral strategies. Similar to the model with functional disability as an outcome, there was a lack of a feedback loop from depressive symptoms to perceived pain ratings. This may be because the child’s cognitive interpretation of and reaction to the pain is more important in the development of depressive symptoms than the severity of the pain. It may also be that the other variables in the model accounted for such a large proportion of the variance in depressive symptoms that there was no longer adequate unexplained variance to allow for an association with pain.
This study must be considered in the context of several limitations. Most importantly, most of the variables in this study were collected cross-sectionally rather than longitudinally; thus, we cannot establish causality or a clear direction of effect among the variables. Although we were able to examine functional disability at follow-up and the results further supported the FA model, we did not collect depressive symptoms at follow-up and thus were unable to test this model in a brief prospective fashion. As the FA model was designed to explain the transition from acute to chronic pain, prospective longitudinal analyses of this multivariate model are essential. In relation to pain diagnoses, the FA model was originally developed for adult chronic low back pain patients 24 and although several studies in the adult literature have now successfully examined this model among diverse pain conditions5, it may be beneficial to examine if the model performs differently among children and adolescents specifically diagnosed with chronic low back pain or within other specific pain groups (e.g., headache). Additionally, all measures were completed by the child, and shared method variance may contribute to the strength of the relations among the variables. 6; 3; 25,17 High rates of female and Caucasian patients limit the generalizability of findings to males and diverse ethnic groups; however, our sample is commensurate with other pediatric pain clinics10.
Although we examined developmental differences within this pediatric sample, the current model may not be complete as it currently does not encompass parent influences of emotional distress and protective behavioral responses 6; 3; 25 that likely impact this model at multiple points (Goubert & Simons, 2012). This study represents a first step in applying the FOP model in pediatric populations, and expansion of the model to include the interpersonal context of the parent-child relationship would likely not only enrich our understanding, but also increase the predictive ability of this already robust model to understand pain-related disability in childhood.
With regards to treatment implications, the primacy of negative cognitions and behavioral avoidance in maintaining chronic pain among children and adolescents assessed in this study provides support for the use of cognitive behavioral interventions, found to be effective with this population11; 30. Graded in-vivo exposure, developed by Vlaeyen and colleagues40; 41, has also been shown to be effective at reducing pain and associated disability by directly targeting fear of pain. Additionally, one study with adolescents in an exposure and acceptance based intervention43 identified pain reactivity, which primarily involves worries about pain, as a significant mediator for improvements in pain-related functioning and depressive symptom outcomes44. Results of these treatment studies suggest that targeting individual or multiple aspects of the FA model of chronic pain will likely result in improvement, given the relations among the various components of the model.
Future research focusing on longitudinal evaluations of the FA model to support the direction of effect is essential. Laboratory studies which allow for greater control and artificial manipulation of the cognitive and behavioral components of the model would also be beneficial for establishing the causal pathways in the FA model of chronic pain. It will also be important to conduct additional treatment studies with pediatric chronic pain samples to determine whether relations among pain, catastrophizing, fear, and avoidance change following an intervention designed to address these issues. In summary, the FA model of chronic pain appears to be appropriate for pediatric chronic pain patients, with nuanced differences for younger children and adolescents.
Supplementary Material
Perspective.
The FA model of chronic pain appears to be applicable for pediatric patients with some modification to account for developmental differences across childhood. We discuss the developmental, theoretical, and clinical implications of these results.
Footnotes
Disclosures: This investigation was supported by NIH grant K23HD067202 (LS) and the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment, and the Department of Anesthesiology, Perioperative and Pain Medicine at Children’s Hospital Boston. There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey KM, Carleton RN, Vlaeyen JW, Asmundson GJ. Treatments addressing pain-related fear and anxiety in patients with chronic musculoskeletal pain: a preliminary review. Cognitive behaviour therapy. 2010;39:46–63. doi: 10.1080/16506070902980711. [DOI] [PubMed] [Google Scholar]
- 2.Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–600. [Google Scholar]
- 3.Caes L, Vervoort T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child’s pain and its relationship with activity restriction: the mediating role of parental distress. Pain. 2011;152:212–222. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook AJ, Brawer PA, Vowles KE. The fear-avoidance model of chronic pain: validation and age analysis using structural equation modeling. Pain. 2006;121:195–206. doi: 10.1016/j.pain.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Craig KD, Versloot J, Goubert L, Vervoort T, Crombez G. Perceiving pain in others: automatic and controlled mechanisms. The journal of pain: official journal of the American Pain Society. 2010;11:101–108. doi: 10.1016/j.jpain.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 8.de Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clinical Journal of Pain. 2005;21:9–17. doi: 10.1097/00002508-200501000-00002. discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 9.Dimidjian S, Barrera M, Jr, Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- 10.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–229. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009:CD003968. doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Gabrielle Page M, Campbell F, Isaac L, Stinson J, Martin-Pichora AL, Katz J. Reliability and validity of the Child Pain Anxiety Symptoms Scale (CPASS) in a clinical sample of children and adolescents with acute postsurgical pain. Pain. 2011 doi: 10.1016/j.pain.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Gheldof EL, Crombez G, Van den Bussche E, Vinck J, Van Nieuwenhuyse A, Moens G, Mairiaux P, Vlaeyen JW. Pain-related fear predicts disability, but not pain severity: a path analytic approach of the fear-avoidance model. Eur J Pain. 2010;14:870, e871–879. doi: 10.1016/j.ejpain.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Gheldofz EL, Vinck J, Van den Bussche E, Vlaeyen JW, Hidding A, Crombez G. Pain and pain-related fear are associated with functional and social disability in an occupational setting: evidence of mediation by pain-related fear. Eur J Pain. 2006;10:513–525. doi: 10.1016/j.ejpain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: a structural equations approach. Pain. 2004;107:234–241. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006;123:254–263. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Goubert L, Simons L. Cognitive styles and processes in paediatric pain. In: McGrath P, Stevens B, Walker S, Zempsky W, editors. Oxford Textbook of Pediatric Pain. Oxford: Oxford University Press; 2012. [Google Scholar]
- 18.Hermann C, Hohmeister J, Zohsel K, Ebinger F, Flor H. The assessment of pain coping and pain-related cognitions in children and adolescents: current methods and further development. The journal of pain: official journal of the American Pain Society. 2007;8:802–813. doi: 10.1016/j.jpain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Hollingshead AB. Four Factor Index of Social Status. 1975. [Google Scholar]
- 20.Kamper SJ, Maher CG, Menezes Costa LD, McAuley JH, Hush JM, Sterling M. Does fear of movement mediate the relationship between pain intensity and disability in patients following whiplash injury? A prospective longitudinal study. Pain. 2011 doi: 10.1016/j.pain.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Kline RB. Principles and Practice of Structural Equation Modeling. New York: Guilford Press; 2010. [Google Scholar]
- 22.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- 23.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 24.Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behav Res Ther. 1983;21:401–408. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 25.Logan DE, Simons LE, Carpino EA. Too sick for school? Parent influences on school functioning among children with chronic pain. Pain. 2012;153:437–443. doi: 10.1016/j.pain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. J Pain. 2006;7:244–251. doi: 10.1016/j.jpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Martin AL, McGrath PA, Brown SC, Katz J. Anxiety sensitivity, fear of pain and pain-related disability in children and adolescents with chronic pain. Pain Res Manag. 2007;12:267–272. doi: 10.1155/2007/897395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthen LK, Muthen BO. User’s guide. Los Angeles: Muthen & Muthen; 2004. Mplus. The comprehensive modeling program for applied researchers. [Google Scholar]
- 29.Nieto R, Miro J, Huguet A. The fear-avoidance model in whiplash injuries. European journal of pain. 2009;13:518–523. doi: 10.1016/j.ejpain.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148:387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12:677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Swinkels-Meewisse IE, Roelofs J, Schouten EG, Verbeek AL, Oostendorp RA, Vlaeyen JW. Fear of movement/(re)injury predicting chronic disabling low back pain: a prospective inception cohort study. Spine. 2006;31:658–664. doi: 10.1097/01.brs.0000203709.65384.9d. [DOI] [PubMed] [Google Scholar]
- 33.Thastum M, Herlin T, Zachariae R. Relationship of pain-coping strategies and pain-specific beliefs to pain experience in children with juvenile idiopathic arthritis. Arthritis and rheumatism. 2005;53:178–184. doi: 10.1002/art.21081. [DOI] [PubMed] [Google Scholar]
- 34.Ullman JB. Structural equation modelling. Boston: Allyn and Bacon; 2001. [Google Scholar]
- 35.Varni JW, Thompson KL, Hanson V. The Varni/Thompson Pediatric Pain Questionnaire: I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28:27–38. doi: 10.1016/0304-3959(87)91056-6. [DOI] [PubMed] [Google Scholar]
- 36.Vervoort T, Goubert L, Eccleston C, Bijttebier P, Crombez G. Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain. Journal of Pediatric Psychology. 2006;31:674–683. doi: 10.1093/jpepsy/jsj059. [DOI] [PubMed] [Google Scholar]
- 37.Vervoort T, Eccleston C, Goubert L, Buysse A, Crombez G. Children’s catastrophic thinking about their pain predicts pain and disability 6 months later. Eur J Pain. 2010;14:90–96. doi: 10.1016/j.ejpain.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 39.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 40.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clinical Journal of Pain. 2002;18:251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Vlaeyen JW, De Jong JR, Onghena P, Kerckhoffs-Hanssen M, Kole-Snijders AM. Can pain-related fear be reduced? The application of cognitive-behavioural exposure in vivo. Pain Res Manag. 2002;7:144–153. doi: 10.1155/2002/493463. [DOI] [PubMed] [Google Scholar]
- 42.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 43.Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Wicksell RK, Olsson GL, Hayes SC. Mediators of change in Acceptance and Commitment Therapy for pediatric chronic pain. Pain. 2011;152:2792–2801. doi: 10.1016/j.pain.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Wideman TH, Adams H, Sullivan MJ. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145:45–51. doi: 10.1016/j.pain.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 46.Wilson AC, Lewandowski AS, Palermo TM. Fear-avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Res Manag. 2011;16:178–182. doi: 10.1155/2011/296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.