Highlights
► Thermal and photo degradation of PVAc paint samples were studied by Py–GC/MS with double-shot and single-shot techniques. ► Changes of the PVAc paint samples before and after UV ageing were revealed. ► The effects of pigments and ageing status to the degradation of PVAc paint samples were illustrated.
Keywords: Polyvinyl acetate (PVAc), Py–GC/MS, Degradation, Paints, Artworks
Abstract
Photochemical degradation of commercial polyvinyl acetate (PVAc) homopolymer and PVAc paints mixed with burnt umber, cobalt blue, cadmium red dark, nickel azo yellow and titanium white commonly used for artworks were studied by pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) and Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR). Py–GC/MS with single-shot technique was used for the characterization of the thermal degradation of PVAc at different temperatures, while the double-shot technique of Py–GC/MS was used to reveal the differences in the specimens before and after UV ageing, including the changes of detectable amounts of deacetylation product – acetic acid and plasticizers such as diethyl phthalate (DEP). Furthermore, the relative concentration of the pyrolysis products of the paint samples could be measured and compared in the second step of the double-shot Py–GC/MS, which are highly dependent on the presence of pigments and the ageing status of PVAc paints.
1. Introduction
Polyvinyl acetate (PVAc) is prepared by polymerization of vinyl acetate monomer. It has been used as artists’ media for paintings and for conservation of art objects [1], [2]. The durability of the polymers and the prediction of the long-term stability of polymeric materials are of great interest for conservation and restoration of artworks. As known, deterioration of artist's media and varnishes is mainly a result of oxidation processes involving free radicals and hydrolytic processes of an ionic type. Auto-oxidation reactions are initiated by thermal or photochemical energy input. However, modern paint is a formulation where in addition to the polymers used as a binder, organic and/or inorganic pigments, fillers and other additives may also be used. Apart from thermal or photochemical sources, metal oxides and other salts present in pigments can have a notable effect on varnishes and media as they promote light-induced oxidation reactions [3]. Oxidants are usually chemical elements or substances with elements in high oxidation numbers or highly electronegative substances/elements that can gain one or two extra electrons by oxidizing an element or substance.
Concerning PVAc photodegradation under UV irradiation, it undergoes both chain scission and cross-linking accompanied by the release of volatile compounds, mainly are acetic acid, carbon monoxide, carbon dioxide and methane [4], [5]. The possible degradation pathways for PVAc proposed in the literature include the photo-oxidative mechanisms [6], [7], [8], which evolves through a series of reactive intermediates and radicals [9]. The intermediates can be inferred in an infrared spectrum of PVAc from the broadening of the carbonyl band by FTIR [10], [11]. The presence of metal oxides in formulations may have a stabilizing or sensitizing effect on the polymer [12], [13], [14]. As well known, titanium dioxide, especially in the anatase form, is photocatalytic [15], [16]. However, in recent study [17] about the effect of pigments to the photo degradation of PVAc, titanium dioxide and iron oxide displayed a protective effect on the paint shown by a decrease in the chain scission rate when compared to the pure PVAc. On the other hand, calcium carbonate and ultramarine blue promote an increase in the polymer scission rate [17]. From the above results (some are contradicting each other), it can be seen that the effect of pigments to the photodegradation of PVAc is a complex process, which is not clear yet. Pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) and thermally assisted hydrolysis and methylation with tetramethylammonium hydroxide (TMAH) have been introduced for the analysis of synthetic resins [18], [19]. More recently, Py–GC/MS with hexamethyldisilazane (HMDS) derivatization reagent has been used for the study of the changes of plasticizer in PVAc during ageing [20], [21]. The double-shot technique of Py–GC/MS allows a unique combination of thermal desorption (first step) for the analysis of volatile compounds and pyrolysis (second step) of the polymers themselves, which was applied for the study of the degradation of acrylic polymers, with promising results [22], [23].
The main objective of this work is to characterize the photochemical degradation properties of commercial polyvinyl acetate paints (with different pigments) mainly used in art works. For this purpose, a selection of commercial PVAc homopolymer and paints with different pigments from Kremer (Germany) were purchased. The intention of the selection of paints was to include inorganic and organic pigments in order to study the effects of the pigments on the paint ageing properties; thus Golden PVAc paints of burnt umber, cobalt blue, cadmium red dark, nickel azo yellow, titanium white were chosen. Specimens of polymer and paint films were prepared and subjected to accelerated aging in a Xenon arc solar simulator, which were analyzed by infrared spectroscopy (FTIR) and Py–GC/MS with single-shot/double-shot techniques. The thermal degradation of PVAc at different temperatures was characterized by single-shot Py–GC/MS. The effects of pigments on PVAc photo-stability were examined by double-shot Py–GC/MS. Especially in the thermal desorption step with double-shot Py–GC/MS, the differences of the amount of plasticizer and degradation products between the unaged and aged specimens could be revealed. Furthermore, the relative concentrations of the pyrolysis products of the PVAc paints (with different pigments unaged and aged specimen) could be evaluated and compared in the pyrolysis step with double-shot Py–GC/MS, which are highly dependent on the presence of pigments and the ageing status of the samples.
2. Experimental
2.1. Materials
Polyvinyl acetate Mowilith® 50 and the Golden PVAc paints of Kremer (Kremer Pigmente Gmbh & Co. KG, Germany), including Golden PVAc of burnt umber, cobalt blue, cadmium red dark, nickel azo yellow and titanium white were chosen for this study (Table 1). To make specimen, Mowilith® 50 was dissolved in acetone and then casted on glass slides, while the Golden PVAc paints were directly casted on glass slides, allowing them to dry at room conditions (average thickness of the paint film is about 0.1 mm). Characterization of the pigments used by the manufacture is listed in Table 1; no extenders were mentioned in the data sheet of the products. The paint films were stored in a box for about one year as unaged samples. The UV aged samples were obtained by exposing the slides with the paint film in the UV ageing chamber for sixty days.
Table 1.
List of investigated samples (information from the data sheet: golden PVA paints are made from polyvinyl acetate resins, specifically a 1:1 blend of AYAA and AYAC type polyvinyl acetate resins dissolved in ethanol. Average molecular weight: AYAA is 83,000 amu, AYAC is 12,800 amu).
| Product no. | Commercial name | Description |
|---|---|---|
| 67040 | Mowilith® 50 (PVAc) | Polyvinyl acetate |
| GO00080 | Golden PVA cadmium red dark (CR) | PVAc + CdS, xCdSe |
| GO00225 | Golden PVA nickel azo yellow (NA) | PVAc + nickel complex azo |
| GO00030 | Golden PVA burnt umber (BU) | PVAc + Fe2O3 + MnO2 + Si + Al2O3 |
| GO00140 | Golden PVA cobalt blue (CB) | PVAc + CoAl2O4 |
| GO00380 | Golden PVA titanium white (TW) | PVAc + TiO2 |
2.2. Instruments and methods
2.2.1. Accelerated ageing
UV exposure of the samples was carried out in a UV chamber UVACUBE SOL 2/400F produced by Dr. Hönle GmbH UV-Technology, Germany. The radiation source of UV light was supplied by a 910-W/m2 Xenon arc solar simulator with an incorporated H2 filter, which provides radiation with wavelengths between 295 and 800 nm. The chamber temperature was 48.8 °C; the relative humidity (RH) varied between 30% and 35%, depending on the RH in the ambient atmosphere.
2.2.2. Pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS)
Py–GC/MS analyses were performed with a PY-2020 iD (Frontier Lab, Japan) combined with a GCMS-QP2010 Plus (Shimadzu, Japan) equipped with cryo trap. A capillary column Ultra alloy-5 MS (30 m × 0.25 mm × 0.25 μm, Frontier Lab, Japan) using bonded and highly cross-linked 5% diphenyl/95% methyl siloxane was used. The GC column temperature conditions were as follows: initial temperature 40 °C, hold for 5 min, and afterwards increased by 10 °C/min to 292 °C. The helium gas flow was set at 1 ml/min in splitless mode in the thermal desorption step, while in split mode (1:100) in the pyrolysis step. For the single-shot analyses, different pyrolysis temperatures were used respectively: 300 °C, 400 °C, 500 °C, 600 °C and 700 °C for 12 s.
The double-shot parameters were as follows: for the thermal desorption, several temperature gradients were tested: 50–120 °C, 50–200 °C, 100–250 °C, resulting the chromatogram obtained with the temperature range of 100–250 °C provides better reproducibility information, so it was chosen as thermal desorption temperature parameters. The temperature was set at 100 °C and increased by 20 °C/min to 250 °C, held for 2 min. The second step – pyrolysis temperature was set at 600 °C and held for 12 s. About 200 μg of the samples were scraped from the mock-ups with a scalpel and put in a sample cup (ECO-CUP Frontier Lab, Japan), which were introduced into the pyrolyzer by auto sampler. Mass spectra were recorded under electron impact ionization at 70 eV. NIST 05 and NIST 05s Library of Mass Spectra were used for identifying the compounds.
2.2.3. Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR)
FTIR-ATR analyses were performed with an Alpha FT-IR Platinum ATR instrument (Bruker Optics, Germany) equipped with a deuterated triglycine sulfate detector (DTGS) and with a diamond crystal. Spectra were acquired in a spectral range between 4000 and 370 cm−1 performing 64 scans at 4 cm−1 resolutions. The resulting spectra were collected and evaluated with the spectrum software OPUS® of Bruker Optics.
3. Results and discussion
3.1. Unaged and aged Mowilith® 50 (PVAc) samples
3.1.1. Py–GC/MS analysis with single-shot method
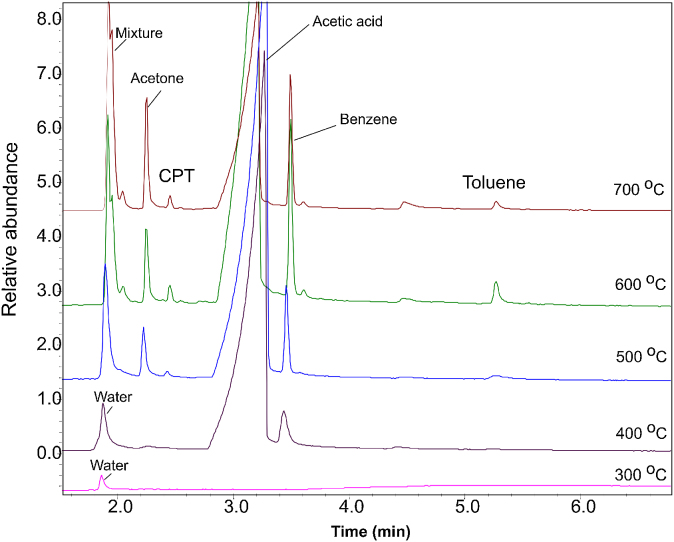
Thermal degradation of unaged PVAc was studied at different pyrolysis temperatures by Py–GC/MS. The pyrograms obtained are depicted in Fig. 1.
Fig. 1.
Chromatograms of Mowilith® 50 obtained by Py–GC/MS analysis: (a) 300 °C; (b) 400 °C; (c) 500 °C; (d) 600 °C; (e) 700 °C; mixture: CO, CO2 and H2O; CPT, 1-3-cyclopendadiene.
From the comparison between the pyrograms obtained at different temperatures by Py–GC/MS, it can be seen that at 300 °C, only water at retention time (RT) 2.0 min was detected, which was probably absorbed by the sample from the environment. No acetic acid was detected, which means that deacetylation reaction did not occur at this temperature. In contrast to that result, in the pyrogram obtained at 400 °C the most dominant peak is that of acetic acid at RT of 3.2 min (m/z = 60), with a smaller amount of water at RT 2.0 min (m/z = 18) and benzene at RT 3.5 min (m/z = 78), indicating deacetylation reaction occurs at 400 °C. At temperatures above 500 °C, the main pyrolysis products are carbon dioxide, carbon monoxide and water at RT 2.0 min based on the ions (m/z = 18, 28, 42, 44), acetone at RT 2.2 min (m/z = 15, 43, 58), 1-3-cyclopendadiene at RT 2.4 min (m/z = 39, 66), acetic acid, benzene and toluene at RT 5.3 min (m/z = 91). It can be seen the pyrolyses at temperature above 500 °C allowed the formation of more products such as 1-3-cyclopentadiene, benzene and toluene, showing the decomposition of PVAc during deacetylation reaction into a highly regular unsaturated material or polyene. The results are in agreement with the thermal degradation mechanism of PVAc by non-isothermal and dynamic thermalgravimetry including the formation of acetone [24], and solid-state NMR, thermogravimetry coupled with mass spectrometry and differential thermal analysis. In the literature, it was reported the first and most intense degradation step-deacetylation occurs between 300 °C and 400 °C with temperature rate at 20 °C/min [25]. The chain scission reaction occurs at the end of the polymer main chain during deacetylation.
3.1.2. Py–GC/MS analysis with double-shot method
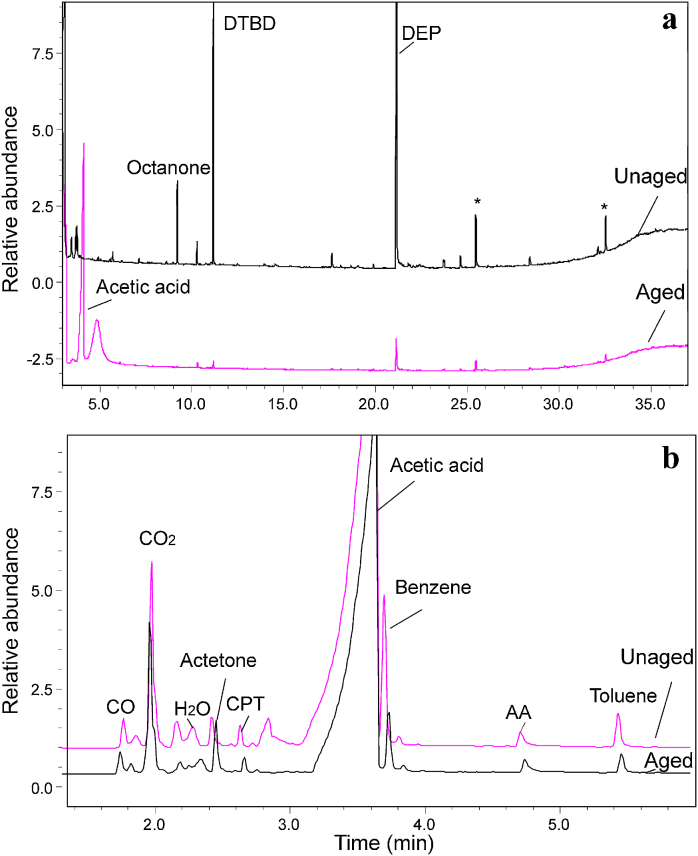
The double-shot technique of Py–GC/MS was applied for the characterization of unaged and aged PVAc samples. In order to achieve in the pyrograms a good resolution of the molecules with low molecular weight, a cryo trap was used. The pyrograms of unaged and aged PVAc samples obtained by Py–GC/MS in the thermal desorption are depicted in Fig. 2a. Acetic acid is the degradation product of PVAc and diethyl phthalate (DEP) is the plasticizer usually used in PVAc [21]. Di-tert-butyl dicarbonate (DTBD) is a reagent widely used in organic synthesis. However, its detection in the sample is probably due to the impurity of the PVAc product, as it is also the case for octanone. By comparison of those two chromatograms, it can be seen that a significant amount of acetic acid was detected in the UV aged sample, which is in agreement with a Norrish Type II mechanism based on the formation of an excited carbonyl group following absorption of light [10], [26]. In addition, the decrease of the amount of the plasticizer DEP was observed after ageing. It was reported that the change in the mechanical properties of PVAc products has been related to the slow migration and evaporation of such plasticizers, resulting in severe embrittlement and potential damage of the paint film [20], [21].
Fig. 2.
Pyrograms of Mowilith® 50 obtained by Py–GC/MS with double shot technique: (a) in the first step – thermal desorption; (b) in the second step – pyrolysis. DTBD, di-ter-butyl dicarbonate; DEP, diethyl phthalate; AA, acetic anhydride; CPT, 1-3-cyclopentadiene; *not identified compounds.
After the thermal desorption analysis, the remains of the sample was dropped again into the pyrolyzer for the second step-pyrolysis. The chromatograms of unaged and aged samples obtained are shown in Fig. 2b. The pyrolysis products, retention time and the peak areas are listed in Table 2. It can be seen that the separation of all pyrolysis products is good with the cryo trap on, which enables the identification of the individual compounds. Pyrolysis products including carbon monoxide, carbon dioxide, water, acetone, 1-3-cyclopentadiene, acetic acid, benzene, acetic anhydride and toluene could be well separated and identified in both the unaged and aged specimens. However, the relative concentration of the pyroylsis products are found different in the unaged and aged samples, which is discussed in detail in Section 3.2.1 in comparison with the paint samples.
Table 2.
The compounds identified in the sample of PVAc obtained by Py–GC/MS with double-shot technique in the pyrolysis step.
| Peak code | RT | Area% | Products characterization |
|---|---|---|---|
| CO | 1.74 | 0.9 | Oxidization product |
| CO2 | 1.95 | 6.2 | Oxidization product |
| H2O | 2.25 | 1.3 | Oxidization product |
| Acetone | 2.39 | 1.1 | Oxidization product |
| CPT | 2.61 | 0.6 | 1-3-Cyclopendadiene, chain scission product |
| Acetic acid | 3.60 | 83.4 | Deacetylation product |
| Benzene | 3.67 | 4.6 | Chain scission product |
| AA | 4.68 | 0.7 | Acetic anhydride, deacetylation product |
| Toluene | 5.40 | 1.2 | Chain scission product |
3.1.3. FTIR-ATR analysis
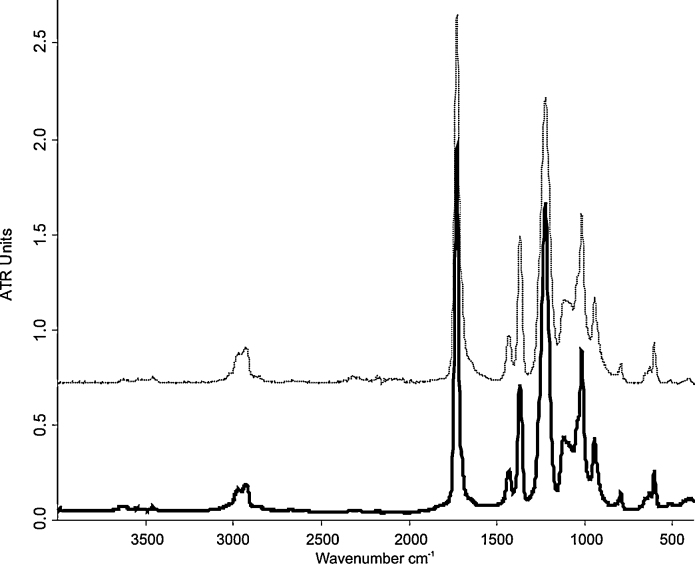
The FTIR-ATR results of the polyvinyl acetate (PVAc) Mowilith® 50 binder are shown in the Table 3. The infrared spectrum of the PVAc (Fig. 3) has a characteristic finger print characterized by several sharp and intense peaks. The most dominant band is related to the —C O stretching vibration associated to acetate groups with a molecular vibration at 1729 cm−1 and complemented by two less intense peaks at 1433 and 1370 cm−1 due to the —CH3 asymmetric and symmetric bending vibration, respectively. The second most intense peak was detected at 1,225 cm−1 according to the asymmetric stretching mode of —C—(C O)—C— of ester groups, followed at lower wavelengths by a double peak with the maximum at 1018 cm−1 and the less intense one at 945 cm−1. Additionally, different less intense peaks such as the doublet of the —CH3 and —CH2 antisymmetric stretching vibration at 2973 and 2926 cm−1 and the C—H rocking vibration at 795, 632 and 604 cm−1 were detected. The spectra of the unaged PVAc binder were compared with the spectra of the aged sample in order to see any effect of the UV light on their chemical stability. By comparing the IR spectra of the unaged and aged polyvinyl acetate binding medium (Fig. 3) no significant changes in the absorption were recorded. The only signal of the photo-oxidative reaction on the synthetic binder is given by the weak broadening of the carbonyl peak in the wavenumber range between 1700 and 1600 cm−1, which suggests the increasing of free acetic acid [27] after UV ageing according to the Norrish Type II mechanism [17] as well as with the results obtained by the Py–GC/MS analysis with the double-shot mode. Although a loss of ester groups with a consequent formation of the free acetic acid took place during the UV ageing, the absorption of the carbonyl peak at 1729 cm−1 remained unchanged. This is probably due to the compensation of the loss of ester groups with some new oxidation products such as aldehyde groups absorbing in the same region and formed by reaction of secondary macroradical with oxygen [28].
Table 3.
ATR infrared absorption of the investigated polyvinyl acetate samples.
| Bond type | Mowilith® 50 and golden PVAc paints (cm−1) |
|---|---|
| C—H stretching | 2973, 2926 |
| C O stretching | 1729 |
| C—H bending | 1433 |
| 1370 | |
| C—O and C—C stretching | 1225 |
| 1120 | |
| 1018 | |
| 945 | |
| C—H rock | 795 |
| 632 | |
| 604 | |
Fig. 3.
FTIR-ATR spectra of the polyvinyl acetate (PVAc) Mowilith® 50 before (solid line) and after UV ageing (dashed line).
3.2. Unaged and aged golden PVAc paints
3.2.1. Py–GC/MS analysis with double-shot method
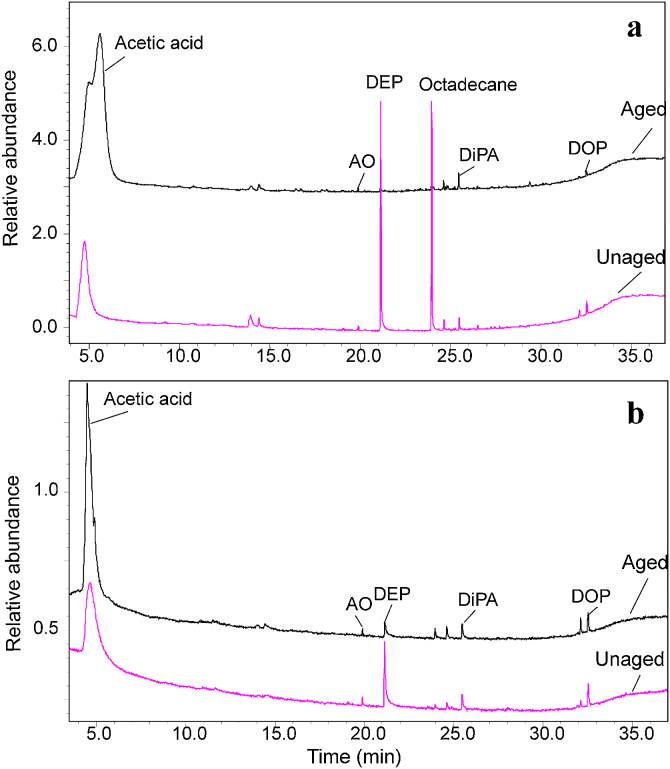
In order to characterize PVAc paints of different pigments and to see their degradation properties after UV ageing, the golden paints (Table 1) unaged and aged specimens were analyzed by Py–GC/MS with double-shot technique. Due to the similarity of the results, only the pyrograms of golden PVAc cobalt blue and titanium white unaged and aged samples obtained by Py–GC/MS in the thermal desorption step are depicted in Fig. 4a and b. It can be seen that the deacetylation product – acetic acid was detected in both unaged and aged samples. However, in the aged samples the amount of acetic acid is higher than in the unaged ones indicating the degradation of the film after drying and accelerated by the UV ageing procedure. Diethyl phthalate (DEP) at RT of 21.1 min and di-n-octyl phthalate (DOP) at RT of 32.5 min were detected in the samples, which are the plasticizers used in the products. The antioxidant KB (butylated hydroxytoluene) at RT of 19.9 min and N,N-diethyl-N′-phenylethylenediamine at RT of 32.5 in the samples were used as additives for the paint products. The reason for the presence of octadecane in the Golden paint PVAc with cobalt blue samples is not clear and probably due to the impurity of the product. In the aged samples the increase amount of acetic acid and the decrease of the plasticizer could be observed.
Fig. 4.
Pyrograms obtained by Py–GC/MS with double shot technique in the first step-thermal desorption of unaged/aged samples of (a) golden paint PVAc cobalt blue, (b) golden PVAc paint titanium white; AO, antioxidant KB (butylated hydroxytoluene); DEP, diethyl phthalate; DiPA, N,N-diethyl-N′-phenylethylenediamine, DOP, di-n-octyl phthalate.
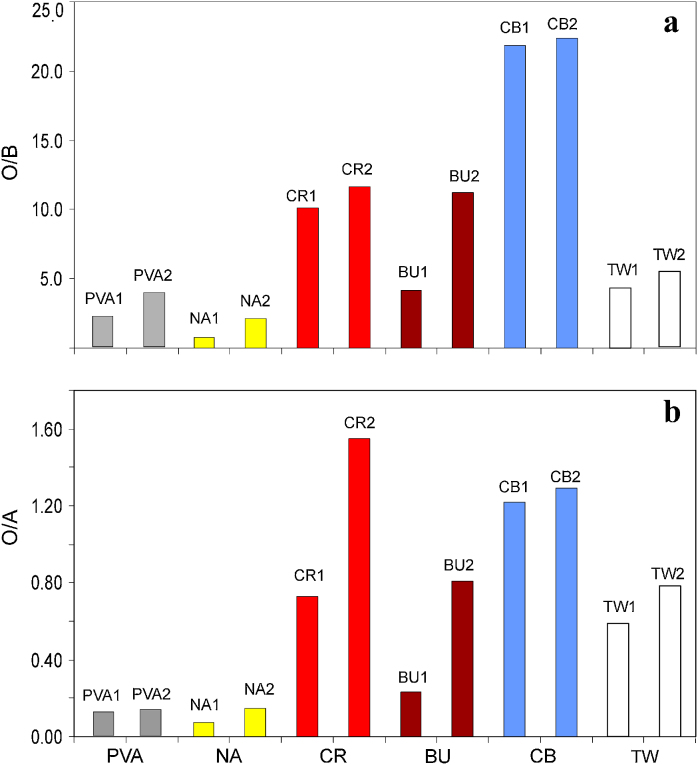
In the second step-pyrolysis of the paint samples, the main pyrolysis products are the same as for PVAc (Fig. 2b), which are carbon monoxide, carbon dioxide, water, acetone, 1-3-cyclopentadiene, acetic acid, benzene, acetic anhydride and toluene (chromatograms are not present here due to the similarity to Fig. 2b). However, the relative concentration of the pyrolysis products is different in the studied paint samples, respectively. The ratios of oxidization products of CO, CO2, H2O (O) to the deacetylation product – acetic acid (A) – labelled as O/A and the oxidization products (O) to the main chain scission product benzene (B) – labelled as O/B were calculated using the peak areas in the pyrograms, which are illustrated in Fig. 5. It can be seen that the values of O/A and O/B for all the aged samples are higher than their unaged counterparts. Furthermore, by comparing these results with those for PVAc itself, the golden PVAc paints with pigments of burnt umber, cobalt blue, cadmium red dark, titanium white have significant higher O/A and O/B values, while golden PVAc paint with nickel azo yellow has lower O/A and O/B values.
Fig. 5.
Comparison of unaged and aged golden PVAc paints: (a) values of O/B; (b) values of O/A; PVA, Mowilith® 50; NA, nickel azo yellow; CR, cadmium red dark; BU, burnt umber; CB, cobalt blue; TW, titanium white; 1, unaged, 2, aged.
It is obvious that the increase of O/A value in the aged samples in comparison to the unaged counterparts is either due to the decrease of acetic acid (A) or increase of the oxidization products (O). It is not difficult to explain the decrease of the amount of acetic acid in the UV aged samples obtained by double-shot Py–GC/MS in the pyrolysis step, since significant amount of acetic acid was formed during UV ageing, which already released and detected in the thermal desorption step, so less amount of acetic acid remained in the samples. Therefore the O/A values are higher in the UV aged PVAc samples than that in the unaged ones. The O/A value could be used as an indicator for the status of the degradation of the PVAc. The higher the value of O/A is, the more degradation of PVAc probably occurred, and vice versa. Since the O/B value has the same trend as O/A value, both of those two values can be used as a clue of the degradation status. From Fig. 4, it can be seen that the Golden PVAc paints with pigments of cadmium red dark, burnt umber, cobalt blue and titanium white have higher values of O/A and O/B than PVAc itself, this may indicate that those pigments accelerate the degradation of PVAc. Contrary to that, the PVAc paint with nickel azo yellow has lower values of O/A and O/B, representing the nickel azo yellow probably behavior as stabilizer for PVAc.
3.2.2. FTIR-ATR analysis
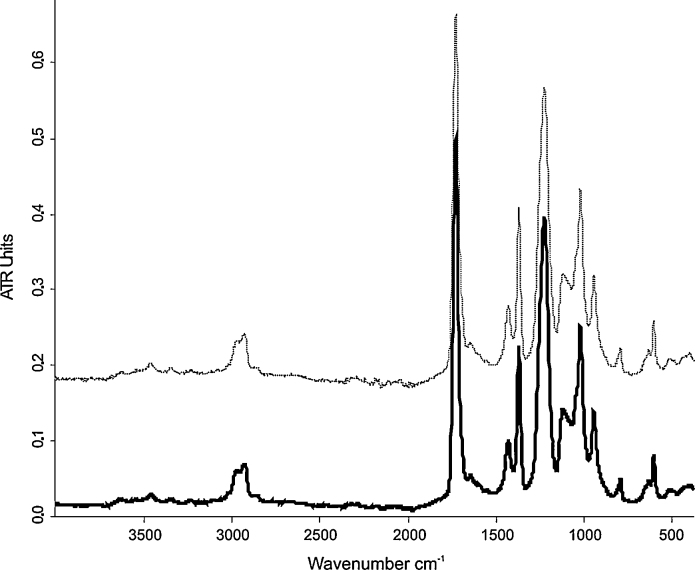
Polyvinyl acetate (PVAc) paints have a more complex chemical formulation in comparison to the pure synthetic binding medium. This is mainly due to the addition in the paint of other components such as organic and/or inorganic pigments, extenders as well as plasticizers, which might interfere with the polyvinyl acetate binder on the infrared absorption in the IR spectrum. The plasticizers are commonly included in these commercial products as external type in an amount up to 50% for improving the chemical and physical properties of PVAc. Additionally, some studies on the PVAc paints, which were carried out also by FTIR, reported the presence of a plasticizer as well as its loss during the irradiation [29]. Except for the nickel azo yellow sample (Fig. 6) with three weak and small peaks in the region between 1665 and 1500 cm−1 and for the cobalt blue sample in the range between 720 and 480 cm−1, the aged polyvinyl acetate paints did not show additional bands that could mask in some infrared regions the detection of the binding medium with their absorption.
Fig. 6.
FTIR-ATR spectra of the golden PVAc nickel azo yellow before (solid line) and after UV ageing (dashed line).
Similar to the ATR results obtained on the aged Mowilith® 50, no noticeable differences could be determined by comparing the spectra of the unaged and aged golden PVAc paints (Fig. 6). The shape of the peaks and their intensity in absorption remained almost unchanged, without formation of new shoulders and/or bands in the infrared spectra of the aged samples. Although by the Py–GC/MS with double shot method it was evinced an increasing of the acetic acid, no broadening of the carbonyl peak in the spectra of the aged sample that could be attributed to the appearance of other carbonyl function was noticed. Additionally, the formation of the acetic acid according to the Norrish type II mechanism is complemented by the C C double bond in the main chain, which is possibly due to its low amount [28], not detectable in the infrared spectrum. Moreover, several possible intermediates such as lactones, ketones, peracids as well as other volatile compounds could be formed during the photo degradation by chain scission reactions. The relative concentration of these products would still be in very low and therefore not noticed in the infrared spectrum.
4. Conclusion
The thermal and photo-oxidative degradation of PVAc was characterized by Py–GC/MS and FTIR. Thermal degradation products of Mowilith® 50 (PVAc) at different temperatures were examined with single-shot technique of Py–GC/MS. The double-shot technique of Py–GC/MS was applied to monitor the PVAc degradation products. In the thermal desorption step of double-shot Py–GC/MS analysis, significant differences were observed in the unaged and aged PVAc paint samples, such as the detectable amount of acetic acid increased, while the content of plasticizers highly decreased in the aged PVAc paint samples. In the second step of double-shot Py–GC/MS analysis, the relative concentration of the pyrolysis products (O/A and O/B) of the paint samples could be measured and compared, with very interesting results. The O/A and O/B values of commercial golden PVAc paints with pigments of burnt umber, cadmium red dark, cobalt blue and titanium white are higher, while those values are lower in Golden PVAc paint with nickel azo yellow in comparison with PVAc itself, representing the two groups of pigments may play different roles to the degradation of the PVAc. In addition, the O/A and O/B values in the UV aged PVAc paint samples are higher than the unaged ones, so they could be used as indicators for the degradation status of the PVAc.
Acknowledgments
This work was funded by the Austrian Science Fund (FWF) project no. L699-N17 and Regione Sardegna (Italy), “Programma Master and Back anno 2009” Alta Formazione.
References
- 1.Crook J., Learner T. Tate Gallery Publishing Ltd; London: 2000. The Impact of Modern Paints. [Google Scholar]
- 2.Down J., MacDonald M., Tétreault J., Williams R. Adhesive testing at the Canadian Conservation Institute—an evaluation of selected poly(vinyl acetate) and acrylic adhesives. Studies in Conservation. 1996;41:19–44. [Google Scholar]
- 3.Rabek J. Chapman & Hall; London: 1995. Polymer Photodegradation: Mechanisms and Experimental Methods. [Google Scholar]
- 4.Geuskens G., Borsu M., David C. Photolysis and radiolysis of polyvinylacetate. III. Effect of temperature on the photolysis. European Polymer Journal. 1972;8:1347–1353. [Google Scholar]
- 5.Buchanan K.J., McGill W.J. Photodegradation of poly(vinyl esters). I. Formation and quantitative measurement of volatile products. European Polymer Journal. 1980;16:308–312. [Google Scholar]
- 6.De la Rie, René E. vol. 105. Royal Society of Chemistry (Special publication); Great Britain: 1992. Stability and function of coatings used in conservation. (Polymers in Conservation: Proceedings of an International Conference Organized by Manchester Polytechnic and Manchester Museum, Manchester, 17–19 July 1991). [Google Scholar]
- 7.McNeill I. In: Polymers in Conservation. Allen N., Edge M., Horie C., editors. The Royal Society of Chemistry; Cambridge: 1992. Fundamental aspects of polymer degradation; pp. 14–23. [Google Scholar]
- 8.Horie C.V. Butterworths; London: 1987. Materials for Conservation, Organic Consolidants, Adhesives and Coatings. pp. 93–101. [Google Scholar]
- 9.Ferreira J.L., Melo M.J., Ramos A.M., Avila M.J. Eternity is in love with the productions of time: Joaquim Rodrigo's classical palette in a vinyl synthetic medium. In: Learner T.J.S., Smithen P., Krueger J.W., Schilling M.R., editors. Proceedings from the Modern Paints Uncovered Symposium; 16–19 May 2006, Tate Modern, London; Getty Conservation Institute; 2006. [Google Scholar]
- 10.Lemaire J., Gardette J., Lacoste J., Delprat P., Vaillant D. In: Polymer Durability: Degradation, Stabilization and Lifetime Predictions. Clough R.L., Billingham N.C., Gillen K.T., editors. Boston American Chemical Society; 1996. Mechanisms of photo oxidation of polyolefins: prediction of lifetime in weathering condition; pp. 577–598. [Google Scholar]
- 11.Melo M.J., Ferreira J.L., Ramos A.M., Avila M.J. In: Contributions of the Seventh Biennial Gathering of the Infrared and Raman User's Group, New York MoMA. McGlinchey C., editor. Museum of Modern Art; New York: 2006. Vinyl paints in Potuguese modern art (1960–90): A FTIR study; pp. 57–60. [Google Scholar]
- 12.Allen N.S., Edge M., Ortega A., Liauw C.M., Stratton J., McIntyre R. Behaviour of nanoparticle (ultrafine) titanium dioxide pigments and stabilisers on the photooxidative stability of water based acrylic and isocyanate based acrylic coatings. Polymer Degradation and Stability. 2002;78:467–478. [Google Scholar]
- 13.Allen N.S., Katami H. Comparison of various thermal and photoageing conditions on the oxidation of titanium dioxide pigmented linear low density polyethylene films. Polymer Degradation and Stability. 1996;52:311–320. [Google Scholar]
- 14.Vijayalakshmi S.P., Madras G. Photocatalytic degradation of poly (ethylene oxide) and polyacrylamide. Journal of Applied Polymer Science. 2006;100(5):3997–4003. [Google Scholar]
- 15.Silva C.G., Wang W., Faria J.L. Photocatalytic and photochemical degradation of mono-, di- and tri-azo dyes in aqueous solution under UV irradiation. Journal of Photochemistry and Photobiology A. 2006;181(2–3):314–324. [Google Scholar]
- 16.Wu T., Li Y., Chu M. In: Nalwa H.S., editor. vol. 1. American Scientific Publishers; Los Angeles: 2003. p. 250. (Handbook of Photochemistry and Photobiology. Inorganic Photochemistry). [Google Scholar]
- 17.Ferreira J.L., Melo M.J., Ramos A.M. Poly(vinyl acetate) paints in works of art: a photochemical approach. Part 1. Polymer Degradation and Stability. 2010;95:453–461. [Google Scholar]
- 18.Capitelli F., Learner T., Cummings A.S. Proceedings of the 13th Triennial Meeting of the ICOM Committee for Conservation; vol. 1; London: James & James; 2002. [Google Scholar]
- 19.Osete-Cortina L., Doménech-Carbó M.T. Characterization of acrylic resins used for restoration of artworks by pyrolysis–silylation–gas chromatography/mass spectrometry with hexamethyldisilazane. Journal of Chromatography A. 2006;1027:228–236. doi: 10.1016/j.chroma.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 20.Doménech-Carbó M.T., Bitossi G., Osete-Cortina L., de la Cruz-Cañizares J., Yusá-Marco D.J. Characterization of polyvinyl resins used as binding media in paintings by pyrolysis–silylation–gas chromatography–mass spectrometry. Analytical and Bioanalytical Chemistry. 2008;391:1371–1379. doi: 10.1007/s00216-007-1783-0. [DOI] [PubMed] [Google Scholar]
- 21.Silva M.F., Doménech-Carbó M.T., Fuster-Lopéz L., Martín-Rey S., Mecklenburg M.F. Determination of the plasticizer content in poly(vinyl acetate) paint medium by pyrolysis–silylation–gas chromatography–mass spectrometry. Journal of Analytical and Applied Pyrolysis. 2009;85:487–491. [Google Scholar]
- 22.Pintus V., Schreiner M. Characterization and identification of acrylic binding media: influence of UV light on the ageing process. Analytical and Bioanalytical Chemistry. 2011;399(9):2961–2976. doi: 10.1007/s00216-010-4357-5. [DOI] [PubMed] [Google Scholar]
- 23.Pintus V., Wei S., Schreiner M. UV ageing studies: evaluation of lightfastness declarations of commercial acrylic paints. Analytical and Bioanalytical Chemistry. 2012;402(4):1567–1584. doi: 10.1007/s00216-011-5369-5. [DOI] [PubMed] [Google Scholar]
- 24.Blazevska-Gilev J., Spaseska D. Thernal degradation of PVAc. Journal of the University of Chemical Technology and Metallurgy. 2005;40(4):287–290. [Google Scholar]
- 25.Rimez B., Rahier H., Van Assche G., Artoos T., Biesemans M., Van Mele B. The thermal degradation of poly(vinyl acetate) and poly(ethylene-co-vinyl acetate). Part I. Experimental study of the degradation mechanism. Polymer Degradation and Stability. 2008;93:800–810. [Google Scholar]
- 26.Turro N.J. University Science Books; Mill Valley: 1991. Modern Molecular Photochemistry. [Google Scholar]
- 27.Doménech-Carbó M.T., Silva M.F., Aura-Castro E., Fuster-López L., Kröner S., Martínez-Bazán M.L., Más-Barberá X., Mecklenburg M.F., Osete-Cortina L., Doménech A., Gimeno-Adelantado J.V., Yusá-Marco D.J. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Analytical and Bioanalytical Chemistry. 2011;399(9):2921–2937. doi: 10.1007/s00216-010-4294-3. [DOI] [PubMed] [Google Scholar]
- 28.Chiantore O., Trossarelli L., Lazzari M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer. 2000;41(5):1657–1668. [Google Scholar]
- 29.Learner T. The Getty Conservation Institute; Los Angeles: 2004. Analysis of Modern Paints. [Google Scholar]