Abstract
Observations of hereditary glomerular disease support the contention that podocyte intercellular junction proteins are essential for junction formation and maintenance. Genetic deletion of most of these podocyte intercellular junction proteins results in foot process effacement and proteinuria. This review focuses on the current understanding of molecular mechanisms by which podocyte intercellular junction proteins such as the Nephrin-Neph1-Podocin receptor complex coordinate cytoskeletal dynamics and thus intercellular junction formation, maintenance and injury-dependent remodeling.
Keywords: Podocyte, slit diaphragm, actin cytoskeleton
Introduction
The glomerular filter prevents the passage of macromolecules from the blood into the urinary space. Its tripartite structure consists of fenestrated endothelium, the basement membrane and glomerular visceral epithelial cells, also termed podocytes. Differentiated podocytes extend numerous actin-rich foot processes that interdigitate and cover the capillary walls of the glomerulus. At the site of interdigitation, a specialized intercellular junction is formed that is commonly called the “slit diaphragm”. Primary and secondary glomerular disease accounts for a considerable fraction of chronic kidney disease and therefore has a major impact on quality of life and life expectancy. The podocyte plays a central role in glomerular disease (1–3). Nearly invariably, the podocyte manifests dysfunction by undergoing changes in morphology resulting in a change in glomerular filter permselectivity and the clinical signs of proteinuria and the observation of “foot process effacement” in two dimensions by transmission electron microscopy. Therefore, it is of great interest to learn about the morphogenesis of podocytes, adaptation mechanisms of podocytes to physiological changes during their life time as well as reactions of this unique cell in pathology. The complex morphology of the podocyte is created and maintained by its cytoskeleton. While microtubules and intermediate filaments fashion podocyte major processes, the cytoskeleton of foot processes is built by a complex mesh of actin filament bundles (4–8). Therefore, well regulated actin dynamics are of great importance during podocyte foot process maturation, junction formation and maintenance of podocyte foot process structure during its life time. In podocyte disease the physiological course of cytoskeletal adaptation is dysregulated.
1. Podocyte development
The podocyte undergoes dramatic changes in cell morphology during development. At an early developmental stage, podocyte precursor cells show a cuboidal cell shape typical of other epithelial cells that are organized in a mono-layer on a basement membrane (Figure 1A) (1, 9). During this early stage, intercellular junctions are located apico-laterally. While the exact course of events and mechanisms governing the profound changes in podocyte morphology that take place thereafter remain to be elucidated, the following working model attempts to describe these remarkable developmental changes. The apical membrane domain of podocyte precursor cells appears to increase dramatically and the intercellular junctions shift from an apico-lateral to a basolateral position. During these events, podocyte precursor cells presumably begin to generate membrane protrusions which emerge at the basolateral aspect of the cell, move along the glomerular basement membrane and push into neighboring cells (9–10). These nascent processes presumably branch into secondary and tertiary processes which then form the typical interdigitation pattern observed in adult glomerular podocytes (Figure 1B) (1, 9). It is remarkable that at present the literature does not contain a complete set of images that documents podocyte morphogenesis from beginning to end. Thus, podocytes appear to undergo immense changes in their cellular polarity during development.
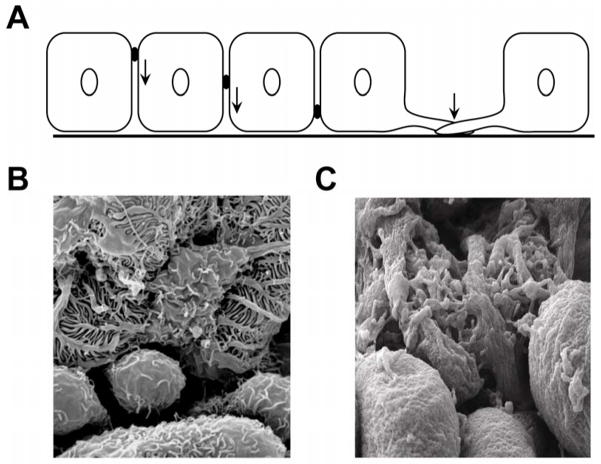
Figure 1.
Figure 1A. Podocyte precursor development from early (left) to late developmental stages (right). Note that the early podocyte precursor cell has a cuboidal cell shape with an apico-laterally located intercellular junction. In the course of development, the intercellular junction shifts to a baso-lateral position (see arrows). At this time, nascent podocyte processes presumably start to branch off.
Figure 1B. Scanning electron micrograph of wild type adult mouse podocytes. Podocytes show typical morphology with primary and secondary processes. Tertiary foot processes interdigitate regularly with neighboring podocytes, forming a thin sheet of cell processes and intercellular junctions around the glomerular capillaries
Figure 1C. Scanning electron micrograph of podocytes of Nephrin deficient mice. While primary and secondary podocyte processes develop, tertiary processes appear short without the typical interdigitating pattern. Instead, tertiary processes are oddly oriented and do not form regular junctions with their neighboring target cells.
1a. Podocyte polarity
Polarity is a fundamental characteristic of epithelial cells. It is characterized by asymmetric distribution of constituents in a single cell such as intercellular junction components, cytoskeletal organization and distribution of membrane or cytoplasmic proteins. Most polarized cells display polarity in apical-basal and planar dimensions. For a mono-layer of epithelial cells the axis of apical-basal polarity would be defined as perpendicular to the basement membrane, thus establishing an asymmetric distribution of cell constituents between membrane and a lumen. In contrast, cellular asymmetry relative to a pole in the plane defined by the basement membrane is termed planar cell polarity. In general, polarity is essential for forming physiological barriers and organizing cell orientation in tissue to achieve functionality (11). As described above, podocyte precursor cells appear to undergo changes in apical-basal polarity during development. This results in the orientation of the cell body to the urinary space while a network of cellular processes is extended along the basement membrane. Foot processes (tertiary processes) appear to take up a non-random orientation—or polarity—relative to secondary processes which we speculate forms a “pole” (Figure 1B). The molecular basis of this orientation in space has not been defined mechanistically, but it is interesting to speculate that cell membrane bound, GBM associated, or soluble gradients cues determine these relationships.
Many events that specify cellular polarity are mediated by protein complexes that are conserved in evolution. Among these well established polarity complexes is the Par3-Par6-aPKC complex which plays an essential role in epithelial polarity from the fly to mammals by promoting epithelial junction formation and segregating apical from basolateral membrane compartments (12). Recently, the Huber and Ohno groups approached podocyte polarity by investigating the functional roles of protein components of the Par3-Par6-aPKC complex in podocyte morphogenesis (13–15). Par3 is able to bind to Nephrin as well as mammalian Neph proteins at the mature podocyte intercellular junction (13). This raises the hypothesis that the Par polarity complex is necessary for junction formation and correct membrane partitioning in the podocyte. Although mice with podocyte-specific deletion of both aPKCλ/ι or aPKCλ alone showed regular foot process architecture and slit diaphragm formation at birth, in both models mice developed foot process effacement, proteinuria and progressive glomerulosclerosis subsequently (14–15). As previously discussed, both the Nephrin and Podocin promoters used in these studies to express Cre recombinase likely become active after early aPKC-mediated developmental events have been initiated. Thus, this technical detail could explain the surprising result that both studies did not show a developmental phenotype. While the fact that NPHS1-Cre-driven deletion of aPKCλ resulted in proteinuria at birth could point to a developmental role of this complex in podocyte foot process and junction development, further studies will be necessary to test whether these defects in junction formation/maintenance and cytoskeletal architecture are indeed caused by a polarity defect.
In podocyte precursor cells, Nephrin is first expressed at the podocyte intercellular junction during capillary loop stage when the junction is already located basolaterally and at the point when nascent foot processes start to develop (16). Observations by Huber et al. show that Par3 and aPKCλ/ι are targeted to the podocyte intercellular junction earlier than Nephrin during the s-shape developmental stage when the podocyte junction is still located apico-laterally (14). Subsequently, the Par3-Par6-aPKC complex together with other junction proteins such as ZO-1 is displaced to the basolateral position where it co-localizes with Nephrin and Podocin. Thus, targeting of the Par3-Par6-aPKC complex to the junction seems to precede the targeting of the Nephrin-Neph1-Podocin receptor complex. Together with the data discussed above, the observation that Nephrin and Podocin expression is reduced and their distribution along the glomerular basement membrane appears impaired in adult aPKCλ/ι null mice argues that the Par3-Par6-aPKC complex might be instrumental in targeting the Nephrin-Neph1-Podocin receptor complex to the podocyte junction during development (14).
Once targeted to the podocyte basolateral intercellular junction the Nephrin receptor complex is well positioned to initiate foot process formation and extension. Indeed, live cell imaging in the fly eye suggests that appropriate interaction of the Neph1 homolog Roughest with mammalian Nephrin homolog Hibris is necessary for interommatidial cells (IOCs) to form directed extensions to reach and intensify contact with their target cells (also see schematic of fly eye development in Figure 2) (17–18). This appears to lead to a cellular phenotype where membranes are “scalloped” presumably to increase areas where Ig superfamily proteins can then interact (16). The observation that Nephrin and Neph1 family proteins appear to initiate cell process formation is further suggested by the fact that excess of cellular Roughest/Neph1 content leads to extreme extension of an IOC over more than its own niche in the fly eye (reviewed by Ross Cagan in (18)). While the Drosophila Par3-related protein bazooka and other members of this polarity complex are known to play important roles in polarization processes in diverse cells and tissues in the fly, it is not known whether the Par3-Par6-aPKC complex functions in the fly eye to specify cell sorting and junction formation (19).
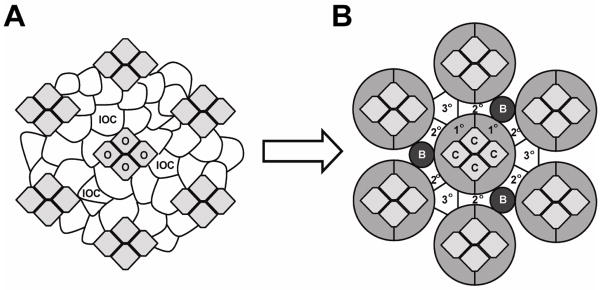
Figure 2.
Schematic of developing ommatidia in the fly eye. (A) During early development ommatidial clusters (cells marked in light grey, O) are surrounded by multiple layers of inter-ommatidial precursor cells (IOC; marked in white). (B) With differentiation, ommatidial clusters give rise to primary pigment cells (1°) and cone cells (C) (two shades of light grey). Preferential junction formation between Neph1 homolog Roughest expressing IOC and Nephrin homolog Hibris expressing 1° cells leads to sorting of IOC into a single column that spaces one ommatidium from the next. During this stage, IOC compete for interactions with 1° cells thus becoming secondary or tertiary (2°, 3°) pigment cells (marked in white) or undergoing apoptosis. Abbreviations: bristle group (B) (26).
It will be a challenging project to determine whether the Par3-Par6-aPKC complex determines podocyte polarity by recruiting the Nephrin-Neph1-Podocin complex and its associated intracellular protein complex or whether it also functions independently of this complex to drive foot process formation and junction maturation. Furthermore, other polarity protein complexes such as the Crumbs protein complex and environmental cues such as secreted proteins, the glomerular basement membrane and tension are likely to play a role in polarizing the podocyte.
1b. Junction formation
Junction formation between epithelial cells plays a major role in establishing polarity and is essential for tissue morphogenesis and homeostasis. This process requires the association of intercellular junctions with the actin cytoskeleton (20). Disruption of the actin cytoskeleton machinery impairs the formation of intercellular contacts and initiates disassembling of contacts that recently formed while more mature junctions are not affected (20–22). These observations suggest that the actin cytoskeleton provides the motive force for bringing together membranes from neighboring cells. Adhesion molecules and other receptors present in these membrane domains can then interact with their counterparts and junction formation is initiated.
During podocyte junction maturation several unique protein complexes are targeted to the specialized intercellular junction between foot processes of neighboring podocytes such as the Nephrin-Neph1-Podocin complex. Work in non-mammalian in vivo model systems indicates that Nephrin complex proteins govern junction formation and specification between specific cell types. In C. elegans the Nephrin homologue Syg-2 is transiently expressed on primary vulval epithelial cells where it acts as a central synaptic guidepost to guide motor neurons, which express Neph1 homologue Syg-1, to adjacent regions of vulval muscle. Syg-1 and -2 mutants exhibit fewer synapses with their natural targets and form aberrant synapses with incorrect target cells (23–25). The notion that Nephrin and Neph family proteins might indeed be instrumental in junction formation is further supported by work in Drosophila. The fly eye is a complex structure assembled by approximately 750 individual units, the ommatidia. Ommatidia are separated from each other by IOCs (Figure 2). During development IOCs are sorted from multiple rows into a single layer of cells. At this stage, local contacts between primary pigment cells and IOCs are essential in determining where the IOC will be located in the adult eye or whether it is dispensable and thus eliminated by apoptosis (17). This process is tightly regulated by interactions between Nephrin homologue Hibris which is expressed on primary pigment cells and Neph1 homologue Roughest on neighboring IOCs. In Hibris and Roughest mutant fly eyes IOCs fail to move into their proper niche and form aberrant junctions (26–27).
The fly eye model system was recently used to explore functional connections between cell adhesion molecules such as Neph1/Roughest and Drosophila E-cadherin with the cytoskeletal adaptor and Drosophila melanogaster member of the CD2ap family of proteins, Cindr, that determine cytoskeletal organization during fly development (28). Cell sorting during fly eye development requires precise movements of IOCs and thus extensive cytoskeletal dynamics. In this model system, loss of CD2ap/Cindr leads to severe disruption of ommatidial patterning. CD2ap/Cindr is required for targeting of E-cadherin and the Neph1 homologue Roughest to special membrane domains in specific cell populations in the Drosophila eye during junction formation and cell movement. CD2ap/Cindr functions together with regulators of the actin cytoskeleton such as the actin capping proteins alpha and beta (28). These observations emphasize the importance of the actin cytoskeleton in regulating Neph family protein-based junction formation. Interestingly, CD2ap deficiency in mice results in proteinuria within a few weeks after birth while foot processes appear to develop normally (29). Given the role of CD2ap/Cindr in targeting E-cadherin and Neph1 to intercellular junctions and its necessity for correct cell sorting in the fly eye it is astonishing that CD2ap null mice do not show developmental abnormalities of the mammalian kidney podocyte.
As in the fly eye, interaction of junction molecules and cytoskeletal-associated proteins also appears to be essential for process and junction formation in mammalian podocytes.
1c. Signaling from the podocyte junctional complex to the cytoskeleton
Observations of hereditary glomerular disease support the contention that the Nephrin-Neph1-Podocin receptor complex is involved in podocyte foot process development and junction formation. Loss of any of these three proteins in gene-targeted mice leads at birth to proteinuria and what is described as “foot process effacement” by transmission electron microscopy (30–32). Indeed, tertiary foot processes of Nephrin null mouse podocytes evaluated by scanning electron microscopy appear to be foreshortened, are oddly oriented, and fail to form regular contacts with neighboring podocytes (Figure 1B and C). Because in general cell junction formation and tissue morphogenesis are intimately connected, this phenotype is consistent with the conclusion that the Nephrin associated protein complex integrates both processes. Nephrin and Neph1 are structurally similar transmembrane Ig superfamily proteins. In the kidney podocyte Nephrin and Neph1 form hetero-oligomeric receptor complexes that associate via cis- and trans-interactions (33–35). The cytoplasmic domain of Nephrin interacts with Neph1 in cis. Its extracellular domain interacts with Nephrin itself as well as Neph1 presumably across the junction, while Neph1 does not form homophilic interactions (33–35). In distinction to the mammalian podocyte, Nephrin and Neph1 homologs in C. elegans are expressed in neighboring heterologous cell types and interact in trans across neighboring cells (23–25). This differential expression is essential for guiding motor neurons to form synapses with their appropriate target cells (21–23). As discussed above, lessons from C. elegans about the Nephrin/Neph1 homologs Syg-2/Syg-1are difficult to apply to the mammalian podocyte as interactions between Nephrin and Neph1 are formed between neighboring cells of the same type. It remains to be determined in which fashion Nephrin and Neph1 preferentially interact in situ. In other systems, transmembrane receptors appear to be sorted such that binding in trans is favored and binding in cis leads to reduced signal transduction and clearance of the signaling proteins from the membrane by endocytosis (36).
While only one Nephrin family gene exists in the human genome, there are three different gene loci encoding the Neph family proteins Neph1, Neph2 and Neph3. Like Neph1, Neph2 is located at the podocyte intercellular junction (37). Neph2 forms homodimers and its extracellular domain binds Nephrin. It seems that the extracellular domain of Neph2 is cleaved in healthy mice and can be detected in the urine (37). Genetic deletion of Neph1 in mice leads to foot process effacement, proteinuria and death early after birth comparable to the phenotype of Nephrin knockout in mice (31). Individual functional properties of mammalian Neph2 and Neph3 remain to be determined in detail. Recently, work using C. elegans showed that mammalian Neph proteins are able to partially rescue defects in synapse formation in worms lacking the Neph1 homologue Syg1 suggesting partial redundancy of mammalian Neph proteins (38).
The third member of this complex, the stomatin family member Podocin, interacts with both Nephrin and Neph1 (39–41). It is a membrane-associated protein with both N- and C-termini predicted to be located in the cytoplasm. Like other stomatin family members it recruits its complex partners to cholesterol-enriched membrane domains at the podocyte foot process junction thereby creating a cluster that can act as a unique signaling platform (42–43). Consistently, mutations in the human podocin gene NPHS2 were characterized which lead to foot process effacement, proteinuria and prevented the recruitment of Nephrin to lipid raft membrane domains (32, 42). The C. elegans stomatin family protein Mec-2 sheds light on possible functional properties of its mammalian homolog podocin. In the worm Mec-2 associates with two ion channel proteins Mec-4 and Mec-10 which are involved in transmitting touch sensation (44). Mec-2 appears to be required for regulating this cation channel and anchors it to a specialized underlying microtubular cytoskeleton (44). At the podocyte intercellular junction podocin is well positioned to perform similar functions. Other components necessary for assembling a mechano-sensing unit are indeed present at the slit diaphragm such as the calcium channel TrpC6. Indeed, podocin is involved in regulating TrpC6 channel activity (for review of calcium-dependent signaling at the slit diaphragm see respective chapter in this issue) (43, 45). It is not known whether Mec-2 is functionally involved in synapse formation in C. elegans.
Functionally, the Nephrin-Neph1-Podocin complex is now recognized as an outside-in signal transducing receptor complex. By being segregated in lipid raft micro-domains at the intercellular junction between neighboring podocyte foot processes, this complex assembles a specialized signaling cluster to relate outside-in information. While diverse post-translational modifications are likely to govern Nephrin-Neph1-Podocin receptor activation, activation by phosphorylation of residues within the cytoplasmic domain of Nephrin and Neph1 are the mechanisms best studied to date. Several protein kinases such as the Src family kinases Fyn and Yes, phosphatidyl-inositol-3 kinase, and Tec family kinases were identified to be associated with this receptor complex (16, 40, 46–51). Fyn binds directly to Nephrin via its SH3 domain (46). It phosphorylates Nephrin on multiple tyrosine residues within its cytoplasmic domain (16, 46). Deletion of Fyn in mice results in decreased Nephrin phosphorylation and foot process effacement while deletion of Yes does not change podocyte foot process morphology but dramatically increases Nephrin phosphorylation (46). Interestingly, mice deficient in Fyn and Yes show more severe alterations in foot process morphology than mice deficient in Fyn alone (46). While this illustrates the biological relevance of Fyn in mediating Nephrin phosphorylation, it remains elusive how Yes regulates Nephrin phosphorylation. It should be recognized that Src family kinases likely play a role in other aspects relevant to podocyte cytoskeletal dynamics besides Nephrin signaling. Very little is known so far about how the phosphorylation/activation of Nephrin is counterbalanced. Phosphorylation and dephosphorylation reactions are the most rapid and versatile regulations of signal propagation. Additional mechanisms nature employs include inhibition by other membrane-bound receptors or direct binding of negative-regulatory proteins to their targets (52–53). Recently, the c-maf inducing protein c-mip was identified to be increased in podocytes of patients with acquired idiopathic nephrotic syndrome. It has been suggested that c-mip competes with Nephrin for binding to Fyn thereby decreasing the level of Nephrin phosphorylation in a transgenic c-mip mouse model (54). Additional mechanisms that govern Nephrin complex phosphorylation or activation likely exist to provide refined regulation of Nephrin complex dependent function.
Multiple direct or indirect interactions between the Nephrin-Neph1-Podocin receptor complex and proteins involved in actin cytoskeleton-associated processes were described such as α-actinin-4, CD2ap, ZO-1, synaptopodin, CASK, IQGAP1, β-arrestin, Nck, Crk, Grb2, MAGI-2 and others (16, 55–60). This as well as observations in other model systems discussed above suggested that the Nephrin-Neph1-Podocin receptor complex transduces a signal that governs actin cytoskeletal dynamics within the tertiary foot processes of podocytes. Three of the tyrosine residues phosphorylated by Fyn in the cytoplasmic domain of mouse Nephrin (Y1191, 1208, and 1232) lie within an SH2-domain binding motif where the SH2/SH3 domain-containing cytoskeletal adaptor proteins Nck1/2 was identified to bind in a tyrosine phosphorylation-dependent fashion via its SH2 domain (16). Nck family proteins play essential roles in assembling actin associated proteins such as N-WASP and components of the Arp2/3 complex which are necessary for initiation and regulation of actin polymerization (61). In cell culture, Nephrin phosphorylation leads to local actin polymerization in an Nck-dependent fashion (16, 62). Tyrosine residues within Nephrin necessary for Nck-binding appear to be phosphorylated during podocyte development while phosphorylation of these residues is attenuated in mature healthy mice (16). This transient phosphorylation profile argues that Nephrin-Nck signaling in the podocyte is especially active during periods where extensive changes in cytoskeletal dynamics result in altered morphology. This hypothesis is supported by the finding that mice with podocyte-specific deletion of Nck1 and Nck2 fail to develop “differentiated” foot processes with specialized intercellular junctions (62). (Whether foot processes fail to form entirely in this model or whether Nck1/2 null podocytes form dysmorphic foot processes similar to those observed in the Nephrin null mouse described above (Figure 1C) cannot be determined from published transmission electron micrographs (62)). Furthermore, deletion of Neph1 in mice results in a phenotype similar to Nephrin deletion in mice (31). This suggests that Nephrin and Neph1 functionally co-operate in vivo to transduce signals from the podocyte junction that lead to actin filament polymerization. In cell culture, activation of Neph1 in addition to Nephrin increases local actin polymerization efficiency (63). Upon Fyn-dependent phosphorylation of Neph1 the cytoskeletal adaptor protein Grb2 directly interacts with the cytoplasmic domain of Neph1 (63). Similar mechanisms are employed by viral or bacterial pathogens such as enteropathogenic E. coli (EPEC) or vaccinia virus (64–67). EPEC injects the transmembrane protein tir into the host cell membrane which subsequently acts as a receptor to a bacterial membrane protein. This is a crucial step in mediating EPEC virulence and initiates recruitment of host cell Nck to phosphorylated tir which results in local actin filament polymerization (64). Similarly, vaccinia virus membrane protein A36R is phosphorylated by host cell Fyn. Subsequently, the cytoskeletal adaptor proteins Nck as well as Grb2 associate with A36R and co-operate to initiate local actin tail formation at the plasma membrane (65–66).
Observations described above provide a mechanism for initiation of Nephrin-induced actin polymerization. Recently, the actin-binding protein Cofilin-1 which is involved in actin filament elongation and remodeling was shown to be associated with and regulated by Nephrin (50, 68). Cofilin activity is required to sever existing actin filaments generating barbed and pointed ends. Subsequently, rapid actin polymerization can take place at barbed ends while Cofilin-1 is able to remove actin monomers from pointed ends (69–71). Thus, Cofilin is crucial for sustaining actin remodeling after its initiation.
While mechanistic details describing how the Nephrin-Neph1-Podocin receptor junctional complex signals to the actin cytoskeleton recently emerged, little is known about additional cellular functions that this receptor might influence. For instance, loss of functional Nephrin in cultured podocytes was shown to lead to activation of NF-κB, a transcription factor that regulates inflammation, immune response, cell growth and survival in other model systems (72). Nephrin also associates with pI3 kinase which results in phosphorylation of AKT presumably activating AKT-dependent signaling processes in the podocyte (49, 51). AKT is a central regulator of cell survival. Moreover, it is likely that signaling from the Nephrin receptor complex is integrated with signals from other pathways.
Together, the evidence discussed above point to the importance of the interplay of podocyte junctional proteins with components of the actin cytoskeleton in regulating cytoskeletal dynamics thus establishing complex podocyte morphology and junction composition that is essential for a fully functional kidney filter.
2. Maintenance of podocyte junction composition and process morphology
The mature podocyte is located in a unique environmental niche. It must withstand fluctuations in hydrostatic pressure and the potentially injurious composition of primary filtrate. Thus, podocyte structure is unlikely to be static and it can be hypothesized that cytoskeletal and junction remodeling play a major role in adapting to environmental demands. Indeed, studies in cell culture and in vivo indicate that a key feature of an intercellular junction is its dynamic nature. While the regulation of adherens junction maintenance varies across experimental model systems, a close relationship between E-Cadherin-based junctional protein complexes, the actin cytoskeleton and Rho family GTPases such as Rho, Rac and Cdc42 exist (73). Signaling seems to occur in both directions. While junctional protein complexes control polarity, cell shape and cell movement via contact with the actin cytoskeleton and GTPases, Rho family GTPases regulate junction dynamics, position and turnover of proteins (73).
Observations of hereditary human glomerular disease with late onset and mouse models illustrate that polarity complex, intercellular junction as well as cytoskeletal adaptor proteins play a central role in maintaining podocyte function. In addition to mediating essential processes during development the Nephrin-Neph1-Podocin receptor complex likely has a major impact on maintaining podocyte morphology. While most mutations in the human NPHS1 gene encoding Nephrin lead to malformation of foot processes and intercellular junctions during development and thus do not provide information about the role of Nephrin in mature podocytes, recently several mutations were identified that show a delayed onset phenotype thus providing evidence that Nephrin indeed performs essential functions in the adult podocyte (74). Mutations in the human NPHS2 gene encoding Podocin typically result in FSGS with onset during early childhood or later in life (30, 32). Furthermore, podocin inactivation in adult mouse podocytes resulted in FSGS and proteinuria (75). Reminiscent of Congenital Nephrotic Syndrome of the Finnish-type in humans, Neph1 deficient mice develop proteinuria and foot process effacement shortly after birth (31). Although mutations of NPHS1 and NPHS2 produce a phenotype that hint at the importance of the Nephrin-Neph1-Podocin complex in junction maintenance, additional studies in this are required.
Inherited human mutations in several genes encoding actin-associated proteins were described that present with delayed appearance of proteinuria and foot process spreading: these include ACTN4, CD2ap, and INF2 encoding alpha-actinin 4, CD2ap, and inverted formin 2 (76–78). This reinforces the concept that cytoskeletal dynamics play an important role in maintenance of podocyte morphology and junction stability through life. This hypothesis is supported by observations from studies of knockout mice. Several of these cytoskeletal-associated proteins characterized in mouse models are involved in transducing signals from the Nephrin-Neph1-Podocin receptor complex to the cytoskeleton. Induction of dual Nck1 and Nck2 deficiency in podocytes of adult mice results in rapid development of proteinuria and foot process effacement (79). Furthermore, podocyte-specific knockout of the actin-binding protein Cofilin-1 which mediates actin filament elongation and remodeling leads to delayed appearance of foot process effacement and proteinuria (50). These observations underline the importance of signaling from the slit diaphragm to the actin cytoskeleton for maintaining podocyte integrity.
Rho family small GTPases are known regulators of cytoskeletal dynamics and play an important role in adherens junction maintenance (73). They cycle between two conformational forms, the GTP-bound active and the GDP-bound inactive form. Their activity status is carefully governed by GDP/GTP exchange proteins (GEP), GTPase activating proteins (GAP), and GDP dissociation inhibitors (GDI) (80–81). Recently, the GTPase activating protein Arhgap24 was found to be associated with familial focal segmental glomerulosclerosis (Akilesh et al, JCI, in press). Arhgap24 inactivates Rac1and thus seems to disrupt the equilibrium between RhoA and Rac1 signaling (Akilesh et al, JCI, in press). Several studies indicate that decreased RhoA and increased Rac1 activity is harmful to the podocyte and is associated with proteinuria and foot process effacement (82–83). Mice lacking the GDP dissociation inhibitor Rho GDIα show normal podocyte development but present with a delayed FSGS phenotype (82). This was attributed to increased Rac1 and mineralocorticoid receptor signaling in the kidney without changes in systemic aldosterone status (83). Treatment of Rho GDIα mice with either a mineralocorticoid receptor inhibitor or a Rac1 inhibitor reduces proteinuria and histological changes in this model (83). These observations indicate that proper balance between RhoA and Rac1 signaling is important for maintaining podocyte junction stability. Interestingly, glomerular Arhgap24 is enriched in podocytes where it co-localizes with the slit diaphragm marker synaptopodin (Akilesh et al, JCI, in press). This suggests that Arhgap24 might be involved in signal transduction from the podocyte intercellular junction to the actin cytoskeleton. It will be interesting to discover upstream cues that regulate Arhgap24 activity at the slit diaphragm.
During the life of a podocyte its polarity needs to be maintained. Therefore, it is not surprising that the polarity complex protein aPKC was shown to be essential for conserving podocyte functionality. Gene delivery of a dominant-negative aPKC construct into adult mice lead to rapid-onset proteinuria within one day (13). Similarly, when glomeruli were treated with an aPKC inhibitor podocytes promptly developed foot process effacement (13). As detailed in the previous section of this review, podocyte-specific knockout of aPKC isoforms results in delayed loss of junction integrity and foot process architecture in adult mice (14–15). Taken together, these observations build a strong case to support the contention that the Par3-Par6-aPKC polarity complex is essential for maintaining podocyte structure.
Furthermore, extensive work in Drosophila as well as mammalian cell culture shows that polarity proteins of the Par complex and small GTPases of the Rho family act together to regulate junction protein turnover by endocytosis (73, 84–86). In the fly, loss of Cdc42 activity in the ventral ectoderm disrupts adherens junctions and increases the endocytic uptake of apical polarity complex proteins such as Par proteins, Crumbs and Patj (85). Interestingly, loss-of-function mutations in the Baz/Par3, Par6 or aPKC genes strongly enhance the Cdc42 phenotype (85). In mammalian epithelial cell culture, both small GTPases Rac1 and Cdc42 are required to regulate actin cytoskeletal dynamics to mediate E-cadherin endocytosis thus showing a redundancy of mechanisms governing junction turnover that is conserved in evolution (87–88). Indeed, in podocyte culture Nephrin and Podocin appear to be internalized into endosomes following Nephrin phosphorylation (89). This appears to require CIN85/RukL, a cytoskeletal adaptor protein of the same family as CD2ap, which seems to compete with CD2ap for binding to both Nephrin and Podocin and enhance their ubiquitination and endocytosis of this receptor complex (90). This mechanism sheds interesting light on the phenotype of CD2ap-deficient mice. Given the function of CD2ap as a cytoskeletal adaptor that connect Nephrin to the actin cytoskeleton it is surprising that CD2ap deficiency of the podocyte does not result in disruption of foot process and junction formation as observed in Nephrin-deficient mice. While CD2ap does not appear to be necessary for podocyte development, mice develop proteinuria and foot process effacement after birth, arguing that CD2ap is essential for maintenance of podocyte structure (77). Interestingly, this breakdown of the filtration barrier in CD2ap null mice is accompanied by increased cellular CIN85/RukL and ubiquitination implying that dysregulated endocytosis of junction components contributes to filter failure (90). Other regulators of Nephrin endocytosis during podocyte maintenance and injury were identified such as β-arrestin-2 and PKCα (60, 91). As CD2ap is associated with both actin filaments as well as endosomes it is tempting to speculate that local actin cytoskeletal dynamics mediate slit diaphragm protein recycling thus regulating signaling events that take place at the podocyte junction.
Remodeling of junction composition and podocyte morphology/cytoskeleton after injury
While mutations in human genes encoding actin-associated proteins such as ACTN4 or CD2ap lead to podocyte disease that develops chronically, it is known from observations of human podocyte disease and experimental models that stimuli exist that result in acute and dramatic changes of podocyte morphology. A good example of an acute human disease of the podocyte is steroid-sensitive minimal change disease (92). It may present with proteinuria and foot process effacement that is reversible within days of initiating glucocorticoid therapy. Podocyte effacement as a major feature of acute podocyte injury directly correlates with the clinical sign of proteinuria. By electron microscopy, podocyte foot process effacement is characterized by spreading and retraction of foot processes.
While animal models might be considered artificial relative to human disease, they provide a valuable and convenient means to explore disease mechanisms. For instance, protamine sulfate (PS) perfusion of rodent kidneys induces dramatic foot process spreading within a few minutes which can be reversed by subsequent perfusion of heparin sulfate (93). Similar to initial effacement, this recovery takes place within minutes. While it was proposed that repulsive negative charges resident on apical surfaces of podocytes would be disintegrated by protamine sulfate and thus lead to breakdown of foot process spacing (93), the rapid changes in podocyte process morphology following PS perfusion argue that extensive cytoskeletal remodeling is initiated.
Little is known so far about signaling events that trigger actin dynamics during foot process remodeling in human podocyte disease such as minimal change disease or acute experimental models. There is evidence that the Nephrin-Neph1-Podocin receptor complex is phosphorylated following PS-induced injury (16). Thus, it is intriguing to speculate that Nephrin signaling is evoked following PS-induced acute podocyte injury. Interestingly, the actin-severing protein Cofilin-1 which is regulated by signaling via the Nephrin-Neph1-Podocin receptor complex is necessary for reconstituting regular foot process architecture in this model (50). Cofilin-1 activity severs existing actin filaments to generate filament fragments necessary for rapid actin polymerization (69). Thus, observations using the PS model indicate that Cofilin-1 is necessary for actin remodeling that presumably accompanies regeneration following acute foot process spreading.
Furthermore, the actin-associated protein Synaptopodin that is enriched in kidney podocytes appears to be involved in actin dynamics during PS injury (59). While Synaptopodin deficient mice have normal podocytes, Synaptopodin is necessary for heparin-induced recovery following foot process effacement in the PS model (59, 94). Synaptopodin interacts with the actin-associated protein α-actinin and elongates α-actinin-induced actin filaments (59). In vitro, Synaptopodin is essential for cell migration and de-regulates the balance of small GTPases of the Rho family such as RhoA and Cdc42 (95). Thus, podocyte intercellular junction protein complexes such as the Nephrin-Neph1-Podocin receptor complex or the Synaptopodin complex and their interaction with actin-associated proteins appear to be essential for regenerative actin remodeling that presumably takes place following acute podocyte injury.
Conclusion
Hereditary mutations and genetic deletion of genes encoding podocyte intercellular junction proteins such as the Nephrin-Neph1-Podocin receptor complex lead to malformation or breakdown of the kidney filtration barrier. It is now recognized that the Nephrin-Neph1-Podocin receptor complex as well as other intercellular junction proteins interact with cytoskeletal-associated proteins thereby signaling to regulate foot process cytoskeletal dynamics and morphology. Lessons from non-mammalian model systems imply that this complex is essential for specifying podocyte polarity, process and junction formation during development. Furthermore, observations of human disease and rodent models suggest that the connections of junctional proteins with the cytoskeleton are essential for podocyte intercellular junction maintenance and remodeling during podocyte injury. Understanding these molecular details will facilitate tailoring podocyte disease-specific therapeutics.
Table 1.
lists podocyte proteins that are part of the slit diaphragm junctional protein complex and/or regulate foot process/junction development, maintenance or response to injury. Protein localization in the glomerular podocyte was classified by evaluating published data from immune-gold electron microscopy, tissue immunofluorescence analysis or interaction studies with known slit diaphragm proteins. Abbreviations: FP (foot process), SD (slit diaphragm).
| Protein | Localization | Null Phenotype | References |
|---|---|---|---|
| Nephrin | slit diaphragm | Proteinuria, FP effacement | (30, 74, 96) |
| Podocin | slit diaphragm | Proteinuria, FP effacement | (32, 37, 97) |
| Neph1 | slit diaphragm | Proteinuria, FP effacement | (31, 37) |
| Neph2 | slit diaphragm | Not known | (37) |
| Neph3 | slit diaphragm | Not known | (98) |
| FAT1 | slit diaphragm | Proteinuria, FP effacement | (99–100) |
| JAM4 | podocyte and slit diaphragm | Not known | (101) |
| Neurexin-1 | slit diaphragm | Not known | (102) |
| P-Cadherin | slit diaphragm | Not known | (103) |
| TRPC6 | slit diaphragm | Proteinuria, FP effacement | (104) |
| kAE1 | podocyte and near slit diaphragm | Proteinuria, FP effacement | (105) |
| Fyn | podocyte and associated with SD complex | FP effacement, variable proteinuria | (46) |
| Yes | podocyte and associated with SD complex | No proteinuria | (46) |
| pI3k C2α | podocyte and associated with SD complex | Proteinuria, FP effacement | (49–50, 106) |
| PLCE1 | podocyte | Proteinuria, FP effacement | (107) |
| Synaptopodin | associated with actin | Impaired recovery from injury | (59) |
| ZO-1 | podocyte and associated with SD complex | Not known | (108) |
| CD2AP | podocyte and associated with SD complex | Proteinuria, FP effacement | (77, 109) |
| CIN85/RukL | podocyte and associated with SD complex | Not known | (90) |
| α-actinin-4 | podocyte and associated with SD complex | Proteinuria, FP effacement | (78) |
| Nck1/2 | podocyte and associated with SD complex | Proteinuria, FP effacement | (16, 62, 79) |
| Cofilin-1 | podocyte and associated with SD complex | Delayed onset proteinuria | (50, 68) |
| Grb2 | podocyte and associated with SD complex | Not known | (63, 110) |
| β-Arrestin | podocyte and associated with SD complex | Not known | |
| Inverted Formin | podocyte and associated with SD complex | Proteinuria, FP effacement | (76) |
| IQGAP1 | podocyte and associated with SD complex | Not known | (58) |
| Rho GDIα | podocyte | FP effacement, proteinuria | (79–80) |
| Arhgap24 | podocyte and near slit diaphragm | FP effacement, proteinuria | Akilesh et al. |
| CASK | podocyte and associated with SD complex | Not known | (56) |
| MAGI-2 | podocyte and associated with SD complex | Not known | (58, 101) |
| aPKCι/λ | podocyte and slit diaphragm | Proteinuria, FP effacement | (14–15) |
| Par3 | podocyte and slit diaphragm | Not known | (13) |
| MyH9 | podocyte | Variable proteinuria | (111) |
| MYO1E | podocyte | Proteinuria, FP effacement | (112) |
| Myo1c | podocyte and associated with SD complex | Not known | (113) |
| CRIM1 | near slit diaphragm | Proteinuria, FP effacement | (114–116) |
| Galectin-1 | podocyte and near slit diaphragm | Not known | (117) |
| Densin | near slit diaphragm | Not known | (118) |
| EphrinB1 | slit diaphragm | Not known | (119) |
| JAM-A | slit diaphragm | Not known | (120) |
| Occludin | slit diaphragm | Not known | (120) |
| Cingulin | slit diaphragm | Not known | (120) |
| Dendrin | Near slit diaphragm | Not known | (121) |
| WTIP | podocyte and associated with SD complex | Not known | (122–124) |
| β-Catenin | podocyte | Protects against podocyte injury | (125) |
| Semaphorin3a | Podocyte, collecting tubule | FP effacement, proteinuria | (126) |
Acknowledgments
Supported in part by grants from the NIDDK (DK080751) and the Department of Veterans Affairs to L.B.H., and a grant from the Deutsche Forschungsgemeinschaft (GE 2158/1-1) to B.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 2.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5(8):463–8. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 3.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57(4):189–95. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 4.Andrews PM. Investigations of cytoplasmic contractile and cytoskeletal elements in the kidney glomerulus. Kidney Int. 1981;20(5):549–62. doi: 10.1038/ki.1981.176. [DOI] [PubMed] [Google Scholar]
- 5.Cortes P, Mendez M, Riser BL, Guerin CJ, Rodriguez-Barbero A, Hassett C, et al. F-actin fiber distribution in glomerular cells: structural and functional implications. Kidney Int. 2000;58(6):2452–61. doi: 10.1046/j.1523-1755.2000.00428.x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Mundel P. A role of microtubules during the formation of cell processes in neuronal and non-neuronal cells. Cell Tissue Res. 1998;291(2):163–74. doi: 10.1007/s004410050988. [DOI] [PubMed] [Google Scholar]
- 7.Vasmant D, Maurice M, Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec. 1984;210(1):17–24. doi: 10.1002/ar.1092100104. [DOI] [PubMed] [Google Scholar]
- 8.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem. 2003;51(12):1589–600. doi: 10.1177/002215540305101203. [DOI] [PubMed] [Google Scholar]
- 9.Kriz W. Ontogenetic development of the filtration barrier. Nephron Exp Nephrol. 2007;106(2):e44–50. doi: 10.1159/000101792. [DOI] [PubMed] [Google Scholar]
- 10.Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56(4):1481–91. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20(2):227–34. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119(Pt 6):979–87. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 13.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, et al. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem. 2008;283(34):23033–8. doi: 10.1074/jbc.M803143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, et al. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol. 2009;20(4):798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, et al. An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PLoS One. 2009;4(1):e4194. doi: 10.1371/journal.pone.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116(5):1346–59. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson DE, Liberman Z, Cagan RL. Cellular behavior in the developing Drosophila pupal retina. Mech Dev. 2008;125(3–4):223–32. doi: 10.1016/j.mod.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cagan RL. The Drosophila nephrocyte. Curr Opin Nephrol Hypertens. 2011;20(4):409–15. doi: 10.1097/MNH.0b013e328347ae02. [DOI] [PubMed] [Google Scholar]
- 19.Doe CQ. Cell polarity: the PARty expands. Nat Cell Biol. 2001;3(1):E7–9. doi: 10.1038/35050684. [DOI] [PubMed] [Google Scholar]
- 20.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 21.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13(1):76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 22.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142(4):1105–19. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116(6):869–81. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 24.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112(5):619–30. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 25.Chao DL, Shen K. Functional dissection of SYG-1 and SYG-2, cell adhesion molecules required for selective synaptogenesis in C. elegans. Mol Cell Neurosci. 2008;39(2):248–57. doi: 10.1016/j.mcn.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8(6):925–35. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Bao S, Fischbach KF, Corbin V, Cagan RL. Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev Biol. 2010;344(2):948–56. doi: 10.1016/j.ydbio.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RI, Seppa MJ, Cagan RL. The Drosophila CD2AP/CIN85 orthologue Cindr regulates junctions and cytoskeleton dynamics during tissue patterning. J Cell Biol. 2008;180(6):1191–204. doi: 10.1083/jcb.200706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286(5438):312–5. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 30.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–82. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 31.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21(14):4829–36. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24(4):349–54. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 33.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, et al. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol. 2003;163(6):2337–46. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14(4):918–26. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- 35.Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278(21):19266–71. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- 36.Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465(7294):86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerke P, Sellin L, Kretz O, Petraschka D, Zentgraf H, Benzing T, et al. NEPH2 is located at the glomerular slit diaphragm, interacts with nephrin and is cleaved from podocytes by metalloproteinases. J Am Soc Nephrol. 2005;16(6):1693–702. doi: 10.1681/ASN.2004060439. [DOI] [PubMed] [Google Scholar]
- 38.Neumann-Haefelin E, Kramer-Zucker A, Slanchev K, Hartleben B, Noutsou F, Martin K, et al. A model organism approach: defining the role of Neph proteins as regulators of neuron and kidney morphogenesis. Hum Mol Genet. 2010;19(12):2347–59. doi: 10.1093/hmg/ddq108. [DOI] [PubMed] [Google Scholar]
- 39.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276(45):41543–6. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- 40.Sellin L, Huber TB, Gerke P, Quack I, Pavenstadt H, Walz G. NEPH1 defines a novel family of podocin interacting proteins. FASEB J. 2003;17(1):115–7. doi: 10.1096/fj.02-0242fje. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108(11):1621–9. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, et al. Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet. 2003;12(24):3397–405. doi: 10.1093/hmg/ddg360. [DOI] [PubMed] [Google Scholar]
- 43.Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103(46):17079–86. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr Biol. 2004;14(21):1888–96. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Huber TB, Schermer B, Benzing T. Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp Nephrol. 2007;106(2):e27–31. doi: 10.1159/000101789. [DOI] [PubMed] [Google Scholar]
- 46.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278(23):20716–23. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol. 2004;15(12):3006–15. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 48.Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, et al. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. 2003;64(2):404–13. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556–66. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 50.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, et al. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285(29):22676–88. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23(14):4917–28. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartman NC, Groves JT. Signaling clusters in the cell membrane. Curr Opin Cell Biol. 2011;23(4):370–6. doi: 10.1016/j.ceb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choudhuri K, Dustin ML. Signaling microdomains in T cells. FEBS Lett. 2010;584(24):4823–31. doi: 10.1016/j.febslet.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang SY, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, et al. c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal. 2010;3(122):ra39. doi: 10.1126/scisignal.2000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112(2):209–21. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, et al. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem. 2003;278(15):13417–21. doi: 10.1074/jbc.C200678200. [DOI] [PubMed] [Google Scholar]
- 57.Huber TB, Kwoh C, Wu H, Asanuma K, Godel M, Hartleben B, et al. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest. 2006;116(5):1337–45. doi: 10.1172/JCI27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A. 2005;102(28):9814–9. doi: 10.1073/pnas.0504166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115(5):1188–98. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, et al. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A. 2006;103(38):14110–5. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14(9):723–31. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 62.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440(7085):818–23. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 63.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27(24):8698–712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gruenheid S, DeVinney R, Bladt F, Goosney D, Gelkop S, Gish GD, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat Cell Biol. 2001;3(9):856–9. doi: 10.1038/ncb0901-856. [DOI] [PubMed] [Google Scholar]
- 65.Newsome TP, Scaplehorn N, Way M. SRC mediates a switch from microtubule- to actin-based motility of vaccinia virus. Science. 2004;306(5693):124–9. doi: 10.1126/science.1101509. [DOI] [PubMed] [Google Scholar]
- 66.Scaplehorn N, Holmstrom A, Moreau V, Frischknecht F, Reckmann I, Way M. Grb2 and Nck act cooperatively to promote actin-based motility of vaccinia virus. Curr Biol. 2002;12(9):740–5. doi: 10.1016/s0960-9822(02)00812-6. [DOI] [PubMed] [Google Scholar]
- 67.Carlier MF, Nioche P, Broutin-L’Hermite I, Boujemaa R, Le Clainche C, Egile C, et al. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J Biol Chem. 2000;275(29):21946–52. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- 68.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, et al. Cofilin-1 inactivation leads to proteinuria--studies in zebrafish, mice and humans. PLoS One. 2010;5(9):e12626. doi: 10.1371/journal.pone.0012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ichetovkin I, Grant W, Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol. 2002;12(1):79–84. doi: 10.1016/s0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- 70.Yahara I, Aizawa H, Moriyama K, Iida K, Yonezawa N, Nishida E, et al. A role of cofilin/destrin in reorganization of actin cytoskeleton in response to stresses and cell stimuli. Cell Struct Funct. 1996;21(5):421–4. doi: 10.1247/csf.21.421. [DOI] [PubMed] [Google Scholar]
- 71.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 72.Hussain S, Romio L, Saleem M, Mathieson P, Serrano M, Moscat J, et al. Nephrin deficiency activates NF-kappaB and promotes glomerular injury. J Am Soc Nephrol. 2009;20(8):1733–43. doi: 10.1681/ASN.2008111219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192(6):907–17. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santin S, Garcia-Maset R, Ruiz P, Gimenez I, Zamora I, Pena A, et al. Nephrin mutations cause childhood- and adult-onset focal segmental glomerulosclerosis. Kidney Int. 2009;76(12):1268–76. doi: 10.1038/ki.2009.381. [DOI] [PubMed] [Google Scholar]
- 75.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, et al. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol. 2009;20(10):2181–9. doi: 10.1681/ASN.2009040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42(1):72–6. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300(5623):1298–300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24(3):251–6. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 79.Jones N, New LA, Fortino MA, Eremina V, Ruston J, Blasutig IM, et al. Nck proteins maintain the adult glomerular filtration barrier. J Am Soc Nephrol. 2009;20(7):1533–43. doi: 10.1681/ASN.2009010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci. 2010;123(Pt 11):1841–50. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 81.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9(9):630–44. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18(39):5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 83.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 84.Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol. 2009;185(6):1111–25. doi: 10.1083/jcb.200901029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183(6):1129–43. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lecuit T. Junctions and vesicular trafficking during Drosophila cellularization. J Cell Sci. 2004;117(Pt 16):3427–33. doi: 10.1242/jcs.01312. [DOI] [PubMed] [Google Scholar]
- 87.Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12(4):847–62. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166(2):237–48. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol. 2009;20(12):2534–45. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, et al. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem. 2010;285(33):25285–95. doi: 10.1074/jbc.M109.087239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quack I, Woznowski M, Potthoff SA, Palmer R, Konigshausen E, Sivritas S, et al. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem. 2011;286(15):12959–70. doi: 10.1074/jbc.M110.204024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 1978;13(2):159–65. doi: 10.1038/ki.1978.23. [DOI] [PubMed] [Google Scholar]
- 93.Kerjaschki D. Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli: the effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest. 1978;39(5):430–40. [PubMed] [Google Scholar]
- 94.Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, et al. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100(18):10494–9. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171(2):415–27. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96(14):7962–7. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24(2):550–60. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ihalmo P, Schmid H, Rastaldi MP, Mattinzoli D, Langham RG, Luimula P, et al. Expression of filtrin in human glomerular diseases. Nephrol Dial Transplant. 2007;22(7):1903–9. doi: 10.1093/ndt/gfm135. [DOI] [PubMed] [Google Scholar]
- 99.Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001;59(3):1003–12. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- 100.Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23(10):3575–82. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirabayashi S, Mori H, Kansaku A, Kurihara H, Sakai T, Shimizu F, et al. MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest. 2005;85(12):1528–43. doi: 10.1038/labinvest.3700347. [DOI] [PubMed] [Google Scholar]
- 102.Saito A, Miyauchi N, Hashimoto T, Karasawa T, Han GD, Kayaba M, et al. Neurexin-1, a presynaptic adhesion molecule, localizes at the slit diaphragm of the glomerular podocytes in kidneys. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R340–8. doi: 10.1152/ajpregu.00640.2009. [DOI] [PubMed] [Google Scholar]
- 103.Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11(1):1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 104.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37(7):739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu F, Saleem MA, Kampik NB, Satchwell TJ, Williamson RC, Blattner SM, et al. Anion exchanger 1 interacts with nephrin in podocytes. J Am Soc Nephrol. 2010;21(9):1456–67. doi: 10.1681/ASN.2009090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harris DP, Vogel P, Wims M, Moberg K, Humphries J, Jhaver KG, et al. Requirement for class II phosphoinositide 3-kinase C2alpha in maintenance of glomerular structure and function. Mol Cell Biol. 2011;31(1):63–80. doi: 10.1128/MCB.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38(12):1397–405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 108.Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111(3):1255–63. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159(6):2303–8. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, et al. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem. 2008;283(14):9177–86. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnstone DB, Zhang J, George B, Leon C, Gachet C, Wong H, et al. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol. 2011;31(10):2162–70. doi: 10.1128/MCB.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365(4):295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arif E, Wagner MC, Johnstone DB, Wong HN, George B, Pruthi PA, et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol. 2011;31(10):2134–50. doi: 10.1128/MCB.05051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nystrom J, Hultenby K, Ek S, Sjolund J, Axelson H, Jirstrom K, et al. CRIM1 is localized to the podocyte filtration slit diaphragm of the adult human kidney. Nephrol Dial Transplant. 2009;24(7):2038–44. doi: 10.1093/ndt/gfn743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pennisi DJ, Wilkinson L, Kolle G, Sohaskey ML, Gillinder K, Piper MJ, et al. Crim1KST264/KST264 mice display a disruption of the Crim1 gene resulting in perinatal lethality with defects in multiple organ systems. Dev Dyn. 2007;236(2):502–11. doi: 10.1002/dvdy.21015. [DOI] [PubMed] [Google Scholar]
- 116.Wilkinson L, Gilbert T, Kinna G, Ruta LA, Pennisi D, Kett M, et al. Crim1KST264/KST264 mice implicate Crim1 in the regulation of vascular endothelial growth factor-A activity during glomerular vascular development. J Am Soc Nephrol. 2007;18(6):1697–708. doi: 10.1681/ASN.2006091012. [DOI] [PubMed] [Google Scholar]
- 117.Shimizu M, Khoshnoodi J, Akimoto Y, Kawakami H, Hirano H, Higashihara E, et al. Expression of galectin-1, a new component of slit diaphragm, is altered in minimal change nephrotic syndrome. Lab Invest. 2009;89(2):178–95. doi: 10.1038/labinvest.2008.125. [DOI] [PubMed] [Google Scholar]
- 118.Ahola H, Heikkila E, Astrom E, Inagaki M, Izawa I, Pavenstadt H, et al. A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol. 2003;14(7):1731–7. doi: 10.1097/01.asn.0000075553.33781.9f. [DOI] [PubMed] [Google Scholar]
- 119.Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, et al. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 2007;72(8):954–64. doi: 10.1038/sj.ki.5002454. [DOI] [PubMed] [Google Scholar]
- 120.Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG. Slit diaphragms contain tight junction proteins. J Am Soc Nephrol. 2009;20(7):1491–503. doi: 10.1681/ASN.2008101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patrakka J, Xiao Z, Nukui M, Takemoto M, He L, Oddsson A, et al. Expression and subcellular distribution of novel glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and 2310066E14Rik. J Am Soc Nephrol. 2007;18(3):689–97. doi: 10.1681/ASN.2006060675. [DOI] [PubMed] [Google Scholar]
- 122.Srichai MB, Konieczkowski M, Padiyar A, Konieczkowski DJ, Mukherjee A, Hayden PS, et al. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem. 2004;279(14):14398–408. doi: 10.1074/jbc.M314155200. [DOI] [PubMed] [Google Scholar]
- 123.Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, et al. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol. 2005;289(2):F431–41. doi: 10.1152/ajprenal.00389.2004. [DOI] [PubMed] [Google Scholar]
- 124.Kim JH, Konieczkowski M, Mukherjee A, Schechtman S, Khan S, Schelling JR, et al. Podocyte injury induces nuclear translocation of WTIP via microtubule-dependent transport. J Biol Chem. 2010;285(13):9995–10004. doi: 10.1074/jbc.M109.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20(9):1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, et al. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136(23):3979–89. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]