Abstract
OBJECTIVES:
The objective of this study was to contribute long-term safety data for infliximab and other therapies in Crohn's disease (CD).
METHODS:
We prospectively evaluated CD patients enrolled in the large, observational Crohn's Therapy, Resource, Evaluation, and Assessment Tool registry, established to compare infliximab safety with conventional nonbiological medications in CD.
RESULTS:
A total of 6,273 patients were enrolled and evaluated on or before 23 February 2010; 3,420 received infliximab (17,712 patient-years; 89.9% received ≥2 infusions) and 2,853 received other-treatments-only (13,251 patient-years). Mean length of patient follow-up was 5.2 years. More infliximab- than other-treatments-only-treated patients had moderate-to-severe (30.6% vs. 10.7%) or severe-to-fulminant (2.5% vs. 0.6%) disease severity (P<0.001). In the year before enrollment, more infliximab- than other-treatments-only-treated patients required surgical intervention (17.4% vs. 13.6%), medical hospitalization (14.2% vs. 8.8%), prednisone (47.8% vs. 31.4%), immunomodulators (52.0% vs. 32.1%), and narcotic analgesics (17.3% vs. 9.1%). Patient mortality was similar for infliximab- and other-treatments-only-treated patients (0.58 vs. 0.59/100 patient-years). In multivariate logistic regression analyses, treatment with prednisone (hazard ratio (HR)=2.14, 95% confidence interval (CI)=1.55, 2.95; P<0.001) or narcotic analgesics (HR=1.79, 95% CI=1.29, 2.48; P<0.001) and age (HR=1.08, 95% CI=1.07, 1.09; P<0.001) were associated with increased mortality risk. Neither infliximab nor immunomodulator treatment was associated with increased mortality risk. Factors independently associated with serious infections included moderate-to-severe disease activity (HR=2.24, 95% CI=1.57, 3.19; P<0.001), narcotic analgesic treatment (HR=1.98, 95% CI=1.44, 2.73; P<0.001), prednisone therapy (HR=1.57, 95% CI=1.17, 2.10; P=0.002), and infliximab treatment (HR=1.43, 95% CI=1.11, 1.84; P=0.006).
CONCLUSIONS:
Mortality was similar between infliximab- and other-treatments-only-treated CD patients. An increased risk of serious infection with infliximab was observed, although CD severity and use of prednisone or narcotic analgesics carried higher risks.
INTRODUCTION
The pharmacological management of Crohn's disease (CD), an immune-mediated inflammatory disorder characterized by recurrent inflammation in the gastrointestinal tract, is determined by the location, extent, and severity of disease; disease-related complications (1); and patient preference related to the frequency and route of medication administration (2). In the past, therapeutic options were generally limited to aminosalicylates; antibiotics; immunosuppressive agents such as corticosteroids; and immunomodulators such as 6-mercaptopurine, azathioprine, and methotrexate. Over the past decade, tumor necrosis factor alpha (TNF-α) antagonists have become available as treatment options. Infliximab, adalimumab, and most recently certolizumab pegol are effective in inducing clinical remission and mucosal healing in patients with CD (3,4). When used in combination with immunomodulators, the early use of these biological therapies has been shown to induce remission more rapidly than the conventional “step-up” treatment, resulting in a more durable remission, decreased need for treatment with corticosteroids, and improved rates of mucosal healing (5).
All available therapies for CD have potential adverse side effects. For instance, corticosteroid therapy is associated with diabetes, hypertension, infection, and osteoporosis (6), and treatment with immunomodulators may be complicated by rare but serious side effects that can include bone marrow suppression, hepatotoxicity, opportunistic infection, an increased risk of lymphoma, and pancreatitis (6,7,8). Also, as both corticosteroids and immunomodulators have broad effects on the immune system, patients receiving such treatment at higher doses and/or for prolonged periods of time may be at increased risk for serious or opportunistic infections (9,10).
Potential side effects of TNF-α antagonists include serious opportunistic infections, such as tuberculosis and histoplasmosis, which occur at a higher incidence when concomitant immunosuppression (particularly corticosteroids) is employed (11,12). The absolute incidence of opportunistic infections, however, is generally low, as are the incidences of other potential side effects of anti-TNF-α therapy, including malignancies, lymphomas, demyelinating disorders, and lupus-like disorders (11,13,14). The rare occurrence of hepatosplenic T-cell lymphomas in inflammatory bowel disease patients is associated with thiopurine immunomodulatory therapy (azathioprine or 6-mercaptopurine), used either as monotherapy or in combination with TNF-α antagonists (7,11,12).
Based on the continued need to answer important safety questions related to anti-TNF-α therapy, the Crohn's Therapy, Resource, Evaluation, and Assessment Tool (TREAT™) registry was undertaken. The TREAT registry is a large, observational registry that was designed to examine the long-term clinical, economic, and humanistic outcomes of various treatment regimens, including infliximab, used in the management of CD in community-based and academic practice settings in North America. We previously reported the initial safety findings derived from the TREAT registry after an average of 1.9 years of follow-up (15). Results of that earlier analysis indicated that mortality rates were similar for infliximab- and other-treatments-only-treated patients (0.53 vs. 0.43 per 100 patient-years; relative risk (RR) ratio =1.24, 95% confidence interval (CI) =0.73, 2.10; P=0.43). While results of an unadjusted analysis showed an increased risk of serious infection with infliximab treatment, results of multivariate logistic regression analysis suggested that infliximab was not an independent predictor of serious infection (RR=0.99, 95% CI=0.64, 1.54; P=0.97). Factors independently associated with serious infection included moderate-to-severe disease activity, prednisone treatment, and narcotic analgesic treatment (15). Although the TREAT registry is ongoing, patient enrollment into the TREAT registry is complete, and all enrolled patients have been followed for at least 5 years. Results of analyses reflecting data collected as part of the TREAT registry through February 2010 are reported herein.
METHODS
Study design
The TREAT registry is an ongoing, prospective, observational, multicenter, long-term registry of North American patients with CD. The registry was initiated in 1999 to evaluate the clinical safety outcomes of various treatment regimens, including infliximab, in the management of CD. Approximately 350 gastroenterologists from both community-based and academic practice settings were each to enroll up to 150 patients, for a target enrollment of at least 5,000 patients. Physicians were generally identified from the membership list of the Crohn's and Colitis Foundation Association. Patients enrolled in the registry are treated at the discretion of their physicians (i.e., a treatment protocol is not predefined). At the start of the registry, it was stipulated that physician participation in the registry could be withdrawn if patient enrollment requirements were not met, complete data were not submitted, or a physician left the practice or elected to discontinue registry participation.
The design of the TREAT registry was approved by the institutional review boards at each participating site, and all patients provided written informed consent before participation in the registry. Physicians or their designees have been paid a small honorarium on a per patient basis as compensation for administering the registry. Janssen Biotech, Inc. (Spring House, PA), the manufacturer of infliximab, is the sponsor of the TREAT registry. The registry operates under the supervision and guidance of an Advisory Committee comprising several authors of this publication (GRL, BGF, RDC, BAS, and WJS) and the TREAT medical monitor (previously RHD and now XX).
Registry participants
Patients enrolled into the TREAT registry must have had a diagnosis of CD and could not be participating in any clinical trial. Upon TREAT's inception, both pediatric and adult patients were enrolled. However, the protocol was subsequently amended to limit enrollment to patients 18 years of age or older. The findings reported herein include limited data for 80 pediatric patients (defined as patients <18 years of age at the time of enrollment) who were subsequently discontinued early from the registry.
This report contains a summary of data collected for 6,273 patients, while a previous report cited 6,290 patients (15). Seventeen patients included in the previous report were found to be duplicates, i.e., sites either enrolled the same patient twice or treated a patient who was being seen at another TREAT registry site. These 17 patients are now excluded from the TREAT registry database.
Registry evaluations
In this registry, patient data were collected at enrollment and then on a semi-annual basis (each January and July). Patients enrolled in the registry were to be followed for a minimum of 5 years. Patient demographic information and physicians' assessments of overall patient health and disease severity were documented upon registry enrollment. Physicians assessed disease severity according to the American College of Gastroenterology Guidelines (16) as remission, mild-to-moderate, moderate-to-severe, or severe-to-fulminant.
A wide array of patient data were collected at the semi-annual follow-up visits, including disease severity, medication use, adverse events, and the dates and outcomes of each infliximab infusion. Participating TREAT physicians were asked to provide information regarding an expansive list of specific adverse events, including infusion reactions, infections, and malignancies. The following infusion reaction adverse event terms were tracked: arthritis, cardiopulmonary symptoms, chills, headache, hypertension, hypotension, influenza-like illness, muscle spasm, nausea, pyrexia, and rash. All other infusion reactions were categorized as “other.” In addition, serious adverse events (SAEs), lymphoma, tuberculosis, congestive heart failure, hypersensitivity reaction, interstitial lung disease, amyotrophic lateral sclerosis, and changes in pregnancy status were to be documented as they occurred. Physicians were required to report SAEs within 24 h of occurrence. A detailed description of the SAE was also required, including dates of onset and resolution, designation of whether the SAE was expected or unexpected, the seriousness and intensity of the SAE, the relationship of the SAE to the underlying CD, concomitant CD medications or surgical procedures for CD related to the SAE, and SAE treatment and outcome.
Data analysis
Data from both active and discontinued (using data available through the last patient contact) patients are included in the data analyses. The cohort of 3,420 patients that received infliximab within 12 weeks before enrollment, who were scheduled to receive infliximab within 30 days of enrollment, or who received infliximab at some other point in the registry is termed “infliximab-treated patients.” As it is not possible to document infliximab administration before participation in the registry, only patients who met the criteria outlined above are considered “infliximab-treated” for data related to serious infections, infliximab dosing, infusion reactions, and baseline patient/disease characteristics. Patients who did not receive infliximab during the registry are grouped as having received “other-treatments-only” in these analyses. For analyses involving mortality, an additional 344 patients with infliximab exposure >3 but <12 months before registry enrollment were also included to yield a total of 3,764 patients “ever-exposed” to infliximab. Baseline demographic and disease characteristics are summarized using descriptive statistics. As such, means and s.d.'s are employed for continuous variables (age, body mass index, years between CD diagnosis and enrollment), and frequencies and percentages are employed for categorical outcomes (gender, race, involved intestinal segment(s), disease severity, immunomodulator treatment, prednisone treatment, narcotic analgesic therapy, and health resource utilization). Corticosteroid therapy represents systemic corticosteroids (i.e., prednisone or equivalent) exclusively, and immunomodulator therapy represents treatment with azathioprine, 6-mercaptopurine, methotrexate, and/or cyclosporine.
Serious infections were defined as any event in the Medical Dictionary for Regulatory Activities System-Organ Class “Infections and Infestations” that met the standard criteria for a SAE, including any infection reported as “serious” by the investigator and any infection that required hospitalization. Commonly observed serious infections were defined as those occurring at an overall incidence >0.01/100 patient-years of follow-up.
The rates of adverse events per 100 patient-years of follow-up were calculated for each medication category (infliximab-treated vs. other-treatments-only-treated) as the quotient of the total number of on-therapy events and patient-years of exposure to medication multiplied by 100. Comparisons of the resulting incidences were accomplished via generation of an RR ratio and its corresponding 95% CI; P-values were generated using Generalizing Estimation Equation methodology. For other treatment group comparisons, the Student t-test was used to test for equality of means across treatment groups, and the χ2 test was employed to evaluate the association between treatment group and categorical variables.
Before data analysis, the TREAT Registry Advisory Board prespecified that infections occurring within 3 months of an infliximab infusion would be considered potentially related to infliximab, while infections occurring outside of this timeframe would be considered not related to infliximab. Further, a Cox proportional hazards model with time-varying covariates was used to determine the relative contribution (in the form of hazard ratios [HR] and 95% CI) of different factors to the occurrence of death and serious infection. Factors assessed included age, sex, race, diseased segment(s), disease severity, years between diagnosis and enrollment in the TREAT registry, and medication use. For mortality analyses, CD medications designated as “ever used” were obtained from all available 6-month data collection periods that occurred between enrollment and the time of death. Medication use during the period in which the death occurred was included in this analysis. For analysis of serious infection, CD medication use was defined as any use in the 6-month data collection period before the onset of the serious infection. Medication use during the period in which the infection occurred was not included in these analyses because medication start and stop dates were not collected for any medications other than infliximab. Therefore, it was unknown whether the medication was given before or after the event. Note that for covariate analyses involving data collected at 6-month intervals (e.g., 1 January–30 June, 1 July–31 December), the most current collection period before the 23 February 2010 data cutoff would have pertained to data collected through 31 December 2009.
The effect of length of exposure on the infliximab safety profile was assessed by grouping each adverse event according to the number of infliximab infusions received by the patient before adverse event onset, i.e., 1–3, 4–9, 10–24, or ≥25 infusions. Adverse events in patients not exposed to infliximab or in infliximab-treated patients but before the first on-registry infliximab infusion were categorized within the other-treatments-only group. The 3,156 patients with at least two 6-month data collection periods as of 23 February 2010 were included in these analyses.
The registry data collection forms received through 23 February 2010 were analyzed using SAS software, Version 8.02 or higher of the SAS System for Windows (SAS Institute Inc., Cary, NC). Given the retrospective nature of several data analyses, no adjustments were made for multiple comparisons.
RESULTS
Patient disposition and baseline characteristics
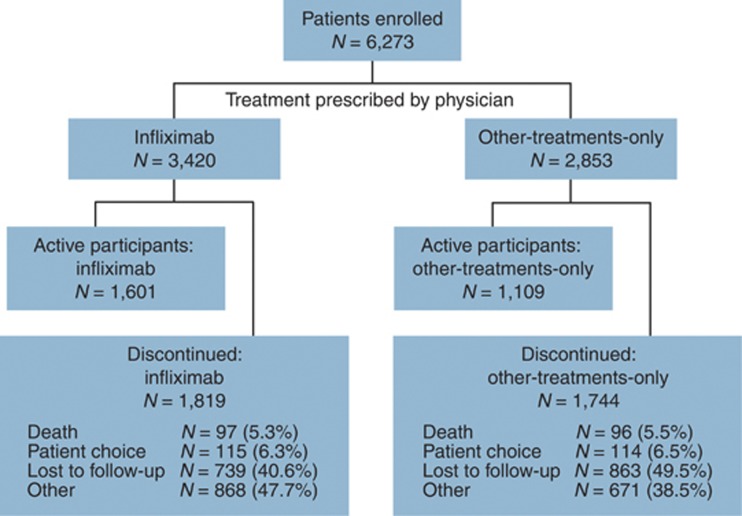
This report contains a summary of data collected for 6,273 patients, all of whom have been followed in the TREAT registry for at least 5 years. Data presented in this report reflect the time period from registry initiation (July 1999) through 23 February 2010. Patient enrollment was complete and closed as of 31 March 2004. The average duration of follow-up for all patients enrolled is currently 5.2 years. Infliximab-treated patients were followed for 17,712 patient-years, compared with 13,251 patient-years of follow-up for the other-treatments-only group. As of 23 February 2010, 43.2% (n=2,710) of enrolled patients were actively participating in the registry. Among these actively participating patients, the average duration of follow-up is currently 7.5 years. Both active and discontinued patients are included in this report. The most common reasons for patient discontinuation have been “patient lost to follow-up” (1,602/3,563, 45.0%) and reasons categorized as “other” (1,539/3,563, 43.2%), most of which were related to patient changes in care and changes in the physician's participation in the registry. Patient discontinuations are detailed in Figure 1.
Figure 1.
Flow of participation in the Therapy, Resource, Evaluation, and Assessment Tool registry. Infliximab-treated patients are those patients who received infliximab within 12 weeks before registration, who were scheduled to receive infliximab within 30 days of registration, or who received infliximab at some other point in the registry (i.e., exposed while participating in the registry). Note that one patient who died after 23 February 2010 is included.
More patients were female (58.6%) than male (41.4%), and most patients were Caucasian (90.8%). The average patient age at the time of enrollment was 42.5±14.7 years. Baseline demographic characteristics were similar between the infliximab-treated and other-treatments-only groups, with the exception of infliximab-treated patients being younger than patients in the other-treatments-only group (P<0.001) (Table 1). Note that 80 patients who were younger than 18 years were enrolled and then subsequently discontinued when the registry entrance criteria were amended to include only patients age 18 or older.
Table 1. Baseline patient demographics and disease characteristics.
| All patients | Infliximab-treateda | Other-treatments-only | P valueb | |
|---|---|---|---|---|
| N=6,273 | N=3,420 | N=2,853 | ||
| Total patients, N (%) | 6,273 (100.0) | 3,420 (54.5) | 2,853 (45.5) | |
| Age (years) at enrollment, N | 6,236 | 3,404 | 2,832 | <0.001 |
| Mean (s.d.) | 42.5 (14.7) | 40.5 (14.0) | 44.9 (15.3) | |
| Gender, N (%) | 6,163 | 3,372 | 2,791 | 0.46 |
| Male | 2,552 (41.4) | 1,382 (41.0) | 1,170 (41.9) | |
| Female | 3,611 (58.6) | 1,990 (59.0) | 1,621 (58.1) | |
| Baseline BMI, N | 5,822 | 3,180 | 2,642 | 0.84 |
| Mean (s.d.) | 25.8 (5.5) | 25.8 (5.6) | 25.8 (5.4) | |
| Race/ethnicity, N (%) | 6,153 | 3,368 | 2,785 | 0.59 |
| Caucasian | 5,586 (90.8) | 3,044 (90.4) | 2,542 (91.3) | |
| Black | 420 (6.8) | 244 (7.2%) | 176 (6.3) | |
| Asian | 24 (0.4) | 15 (0.4) | 9 (0.3) | |
| Hispanic | 81 (1.3) | 43 (1.3) | 38 (1.4) | |
| Other | 42 (0.7) | 22 (0.7) | 20 (0.7) | |
| Years between diagnosis and enrollment, N | 6,105 | 3,341 | 2,764 | 0.31 |
| Mean (s.d.) | 11.3 (10.2) | 11.2 (9.8) | 11.4 (10.7) | |
| Disease severity, N (%) | 5,999 | 3,280 | 2,719 | <0.001 |
| Remissionc | 1,528 (25.5) | 465 (14.2) | 1,063 (39.1) | |
| Mild–moderated | 3,076 (51.3) | 1,728 (52.7) | 1,348 (49.6) | |
| Moderate–severee | 1,296 (21.6) | 1,004 (30.6) | 292 (10.7) | |
| Severe–fulminantf | 99 (1.7) | 83 (2.5) | 16 (0.6) | |
| Involved intestinal area, N (%) | 6,034 | 3,308 | 2,726 | <0.001 |
| Ileum only | 1,801 (29.8) | 869 (26.3) | 932 (34.2) | |
| Colon only | 1,764 (29.2) | 971 (29.4) | 793 (29.1) | |
| Ileum and colon | 2,469 (40.9) | 1,468 (44.4) | 1,001 (36.7) | |
| Health resource utilization in year before enrollment, N (%) | ||||
| Any admission | 1,469 (23.4) | 930 (27.2) | 539 (18.9) | <0.001 |
| Surgical admission | 983 (15.7) | 596 (17.4) | 387 (13.6) | <0.001 |
| Medical admission | 736 (11.7) | 485 (14.2) | 251 (8.8) | <0.001 |
| Medication use at enrollment, N (%) | ||||
| Antibiotics | 1,763 (28.1) | 1,094 (32.0) | 669 (23.4) | <0.001 |
| Antidepressants | 698 (11.1) | 462 (13.5) | 236 (8.3) | <0.001 |
| Immunomodulators | 2,696 (43.0) | 1,780 (52.0) | 916 (32.1) | <0.001 |
| Narcotic analgesics | 853 (13.6) | 593 (17.3) | 260 (9.1) | <0.001 |
| Prednisone | 2,530 (40.3) | 1,635 (47.8) | 895 (31.4) | <0.001 |
BMI, body mass index.
Infliximab-treated patients are those who received infliximab within 12 weeks before enrollment, were scheduled to receive infliximab within 30 days of enrollment, or received infliximab at some other time during the registry.
P value from t-test (continuous variables) or χ2 test (categorical variables).
Remission refers to patients who are asymptomatic or without inflammatory sequelae and refers to patients who have responded to acute medical intervention or have undergone surgical resection without evidence of residual disease. Patients requiring corticosteroids to maintain well-being are considered to be “steroid-dependent” and are not in “in remission.”
Mild-moderate disease applies to ambulatory patients who are able to tolerate oral alimentation without manifestations of dehydration, toxicity (high fevers, rigors, and prostration), abdominal tenderness, painful mass, obstruction, or >10% weight loss.
Moderate-severe disease applies to patients who have failed to respond to treatment for mild-moderate disease or those with more prominent symptoms of fevers, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting (without obstructive findings), or significant anemia.
Severe-fulminant disease applies to patients with persistent symptoms despite the introduction of corticosteroids, or individuals presenting with high fever, persistent vomiting, evidence of intestinal obstruction, rebound tenderness, cachexia, or evidence of an abscess.
A somewhat higher proportion of patients had been treated with infliximab (n=3,420, 54.5%) than those who had received other-treatments-only (n=2,853, 45.5%). Approximately 90% (n=2,986) of infliximab-treated patients had received at least two infusions. Among the 53,003 infliximab infusions documented, most (81.5%) were at a dose of 5 mg/kg. Patients in the infliximab-treated group vs. the other-treatments-only group were more likely to have received prednisone (47.8% vs. 31.4%), immunomodulators (52.0% vs. 32.1%), antibiotics (32.0% vs. 23.4%), narcotic analgesics (17.3% vs. 9.1%), and antidepressants (13.5% vs. 8.3%) within 1 year of registry enrollment (P<0.001) (Table 1).
Patients in the infliximab-treated group also differed significantly from patients in the other-treatments-only group with regard to intestinal segment(s) affected by disease, disease severity, and health resource utilization in the year before enrollment (Table 1). Specifically, patients who received infliximab, compared with patients who received other-treatments-only, were more likely to have both their ileum and colon involved (44.4% vs. 36.7% P<0.001), and to have moderate-to-severe (30.6% vs. 10.7% P<0.001) or severe-to-fulminant (2.5% vs. 0.6% P<0.001) disease at enrollment. Hospitalization in the year before enrollment for either surgical (17.4% vs. 13.6% P<0.001) or medical (14.2% vs. 8.8% P<0.001) management was also more common among infliximab-treated patients compared with patients who received other-treatments-only.
Infusion reactions
Of the 3,420 registry patients in the infliximab-treated group, 3,322 had data available for assessment of infusion reactions. Three percent (1,571/53,003) of infliximab infusions were associated with an infusion reaction; 0.047% of reactions were serious. The most common infusion reactions were headache (0.5%) and arthritis (0.4%) (Table 2).
Table 2. Summary of infusion reactions among all infliximab-treated patients.
| Total infusions | |
|---|---|
| N=53,003 | |
| Total number of patients receiving an infliximab infusiona | 3,420 |
| Total number of patients with available data | 3,322 |
| Total number of infusions | 53,003 |
| Average infusion dose, N (%) | |
| 5 mg/kg | 43,195 (81.5) |
| 10 mg/kg | 9,094 (17.2) |
| Unknown | 714 (1.3) |
| Patients receiving ≥2 infusions | 2,986 (89.9) |
| Medications administered before infusion, N (%) | |
| Acetaminophen | 28,146 (53.2) |
| Antihistamines | 29,870 (56.5) |
| None | 18,930 (35.8) |
| Steroids | 7,453 (14.1) |
| Infusion reaction status, N (%) | |
| Delayed (>4 h to 14 days) | 594 (1.1) |
| Immediate (≤4 h) | 920 (1.7) |
| None | 51,432 (97.2) |
| Unknown | 57 (0.1) |
| Infusion reactions, N (%) | |
| Total number of infusion reactions/number of infusions | 1,571/53,003 (3.0) |
| Arthritis | 232 (0.4) |
| Cardiopulmonary symptomsb | 145 (0.3) |
| Chills | 76 (0.1) |
| Headache | 268 (0.5) |
| Hypertension | 35 (0.1) |
| Hypotension | 31 (0.1) |
| Influenza-like illness | 127 (0.2) |
| Muscle spasm | 130 (0.2) |
| Nausea | 177 (0.3) |
| Other | 815 (1.5) |
| Pyrexia | 44 (0.1) |
| Rash | 182 (0.3) |
Refers to treatment only during participation in the Crohn's Therapy, Resource, Evaluation, and Assessment Tool registry; this includes patients who received infliximab infusion(s) within 12 weeks before registration, were scheduled to receive infliximab within 30 days of registration, or received infliximab at some other point in the Registry.
Case report form (CRF) Verbatim Term—no equivalent Medical Dictionary for Regulatory Activities term available. This was one of the several terms provided to investigators to select from the CRF.
Mortality
One hundred and ninety-one (3%) of the 6,273 patients died over the course of registry participation; causes of death are detailed in Table 3. Of these 191 patients, 109 had received infliximab and 82 had received other-treatments-only, yielding similar time-adjusted mortality rates for patients who had received infliximab (0.58 per 100 patient-years; RR (95% CI) of 0.96 (0.72, 1.28; P=0.81) and those who had not (0.59 per 100 patient-years) (Table 4).
Table 3. Reported causes of death in the TREAT registry.
|
Infliximab-treated |
Other-treatments-only |
|||
|---|---|---|---|---|
| Cause of death | Incidents, N=109 | Average days since last infliximab infusion | Cause of death | Incidents, N=83a |
| Acute lymphocytic leukemia | 1 | 2334.0 | Accident | 1 |
| Anemia, aplastic | 1 | 556.0 | Anemia, severe | 1 |
| Aneurysm, thoracic | 1 | 88.0 | Aneurysm, cerebral | 1 |
| Anorexia | 1 | 104.0 | Aneurysm, ruptured | 1 |
| Aspiration into lungs | 1 | 2573.0 | Ascending aortic dissection | 1 |
| Aspiration pneumonia | 1 | 1517.0 | Cardiac arrest | 7 |
| Cardiac arrest | 2 | 513.5 | Cardiac arrhythmia | 1 |
| Cardiac arrhythmia | 1 | 417.0 | Cirrhosis of the liver | 1 |
| Cardiopulmonary event, acute | 1 | 398.0 | Colitis, ischemic | 1 |
| Cerebral amyloidosis | 1 | 788.0 | Decompensated cirrhosis | 1 |
| Cerebral vascular event | 1 | 1235.0 | Gastrointestinal bleeding | 1 |
| Drug overdose | 2 | 597.0 | Heart failure | 3 |
| Encephalopathy | 1 | 1224.0 | Heart failure, congestive | 2 |
| Gastroenteritis | 1 | 1754.0 | Human immunodeficiency virus | 1 |
| Gastrointestinal bleeding | 2 | 347.5 | Hypotension | 1 |
| Heart failure, congestive | 2 | 316.5 | Intracerebral hemorrhage | 1 |
| Ischemic ileocolitis | 1 | 420.0 | Leukemia | 1 |
| Liver failure | 2 | 284.5 | Lymphadenopathy | 1 |
| Liver transplant | 1 | 1237.0 | Lymphoma | 4 |
| Lymphoma, non-Hodgkin's | 2 | 757.5 | Malignancy, breast | 2 |
| Malignancy, brain | 1 | 134.0 | Malignancy, lung | 3 |
| Malignancy, colon | 4 | 391.3 | Malignancy, metastatic | 8 |
| Malignancy, lung | 4 | 459.0 | Malignancy, pancreatic | 1 |
| Malignancy, metastatic | 4 | 476.8 | Malignancy, renal | 2 |
| Malignancy, pancreatic | 2 | 1506.0 | Malignancy, squamous cell | 1 |
| Malignancy, peritoneal | 1 | 46.0 | Malignancy, throat | 1 |
| Malignancy, renal | 1 | 184.0 | Malignancy, tongue | 1 |
| Malignancy, small bowel | 1 | 405.0 | Malignancy, unspecified | 1 |
| Malnutrition | 1 | 211.0 | Malnutrition | 2 |
| Meningitis | 1 | 76.0 | Motor vehicle accident | 1 |
| Mesenteric venous thrombosis | 1 | 14.0 | Methicillin-resistant Staphylococcus aureus infection | 1 |
| Motor vehicle accident | 1 | 46.0 | Myeloma | 1 |
| Myocardial infarction | 5 | 563.6 | Myocardial infarction | 3 |
| Natural causes | 1 | 46.0 | Natural causes | 1 |
| Pneumonia | 5 | 1027.6 | Pneumonia | 1 |
| Pulmonary disease, chronic obstructive | 4 | 649.3 | Pulmonary complications, postsurgical | 1 |
| Pulmonary embolism | 1 | 55.0 | Renal failure | 3 |
| Renal failure | 4 | 1482.0 | Renal failure, chronic | 1 |
| Respiratory distress | 1 | 155.0 | Respiratory distress syndrome, acute | 1 |
| Respiratory failure | 7 | 771.1 | Respiratory failure | 2 |
| Sepsis | 9 | 355.7 | Sepsis | 6 |
| Stroke | 2 | 187.5 | Small bowel perforation | 1 |
| Sudden cardiac arrest | 1 | 3801.0 | Suicide | 2 |
| Sudden death | 1 | 20.0 | Unknown | 5 |
| Surgical complications—cholecystectomy | 1 | 476.0 | — | — |
| Surgical complications—unspecified | 2 | 443.0 | — | — |
| Unknown | 15 | 825.7 | — | — |
| Urinary sepsis | 1 | 316.0 | — | — |
| Urosepsis | 1 | 110.0 | — | — |
TREAT, Therapy, Resource, Evaluation, and Assessment Tool.
Includes an additional patient who died after 23 February 2010 and is not included in Table 4.
Table 4. Unadjusted incidences of neoplasia, mortality, and serious infection.
| Treatment groupa | Number of patientsb | Number of eventsc | Number of patient-years | Unadjusted rate per 100 patient-years | Unadjusted RR (95% CI) ratio | P valued |
|---|---|---|---|---|---|---|
| Neoplasia | ||||||
| Other-treatments-only | 4,010 | 113 | 13,251 | 0.85 | 1.00 (reference) | |
| Infliximab-treated | 3,764 | 139 | 17,712 | 0.78 | 0.90 (0.69, 1.18) | 0.46 |
| Mortality | ||||||
| Other-treatments-only | 4,113 | 82e | 13,979 | 0.59 | 1.00 (reference) | |
| Infliximab-treated | 3,764 | 109 | 18,825 | 0.58 | 0.96 (0.72, 1.28) | 0.81 |
| Serious infection | ||||||
| Other-treatments-only | 4,557 | 147 | 14,710 | 1.00 | 1.00 (reference) | |
| Infliximab-treated | 3,420 | 333 | 16,296 | 2.04 | 2.04 (1.45, 2.89) | <0.001 |
| Serious infection according to infliximab exposure within the previous 3 months | ||||||
| Other-treatments-onlyf | 5,597 | 317 | 22,344 | 1.42 | 1.00 (reference) | |
| Infliximab-treated | 2,942 | 163 | 7,923 | 2.06 | 1.45 (1.10, 1.91) | 0.008 |
CI, confidence interval; RR, relative risk.
For neoplasia and mortality, patients in the infliximab-treated group received infliximab during the registry before event onset, including the year before registration (i.e., patients “ever exposed” to infliximab). For serious infections, patients in the infliximab-treated group received infliximab during the registry participation and before event onset, including the 12 weeks before registration. Patients could contribute data to both treatment groups depending on the date of first infliximab use.
Over the course of the registry, a patient can contribute patient-years at risk to more than one medication or dosing category.
Data are available from the beginning of the registry (neoplasia and mortality) or beginning in 2002 (serious infections).
P value derived from Generalized Estimating Equations.
Note that one additional patient “ever exposed” (see footnote a of Table 3) to infliximab died after 23 February 2010 and is not included in this table but is shown in Table 3.
The other-treatments-only group included all patients without infliximab exposure within the previous 3 months.
Adjusted results of a multivariate regression analysis (Table 5) indicated that age was a significant predictor of death (HR=1.08, 95% CI=1.07, 1.09; P<0.001). Protective effects were observed among females (HR=0.71, 95% CI=0.53, 0.96; P=0.023), Caucasians (HR=0.64, 95% CI=0.39, 1.03, P=0.064), and patients with disease in only the ileum vs. those with disease in both the ileum and colon (HR=0.53, 95% CI=0.36, 0.77; P=0.001). In investigating medication use among patients who died relative to those who did not, neither infliximab (HR=0.83, 95% CI=0.60, 1.15; P=0.262) nor immunomodulator (HR=0.86, 95% CI=0.62, 1.18; P=0.338) treatment was a significant predictor of death. However, treatment with prednisone (HR=2.14, 95% CI=1.55, 2.95; P<0.001) or narcotic analgesics (HR=1.79, 95% CI=1.29, 2.48; P<0.001) significantly predicted death (Table 5).
Table 5. Predictors of mortality.
|
Unadjusted results |
Adjusted results |
|||||
|---|---|---|---|---|---|---|
| All patients (N=6,080) | Deaths (N=187) | Hazard ratio (95% CI) | P valuea | Hazard ratio (95% CI) | P valuea | |
| Age at enrollment | ||||||
| Mean (s.d.) | 42.5 (14.7) | 59.1 (15.5) | 1.074 (1.063, 1.084) | <0.001 | 1.078 (1.067, 1.090) | <0.001 |
| Sex | ||||||
| Male/unknown | 2,519 | 88/2,519 (3.49%) | — | — | ||
| Female | 3,561 | 99/3,561 (2.78%) | 0.806 (0.605, 1.074) | 0.14 | 0.714 (0.534, 0.955) | 0.023 |
| Race | ||||||
| Non-Caucasian | 573 | 19/573 (3.32%) | — | — | ||
| Caucasian | 5,507 | 168/5,507 (3.05%) | 0.779 (0.485, 1.253) | 0.30 | 0.635 (0.394, 1.026) | 0.06 |
| Involved intestinal area(s)b | ||||||
| Ileum and colon | 2,436 | 93/2,436 (3.82%) | — | — | ||
| Ileum only | 1,784 | 39/1,784 (2.19%) | 0.540 (0.371, 0.785) | 0.001 | 0.528 (0.362, 0.770) | 0.001 |
| Colon only | 1,742 | 54/1,742 (3.10%) | 0.767 (0.548, 1.073) | 0.12 | 0.741 (0.522, 1.052) | 0.09 |
| Unknown | 118 | 1/118 (0.85%) | 0.316 (0.044, 2.273) | 0.25 | 0.387 (0.054, 2.787) | 0.35 |
| Years between diagnosis and enrollment | ||||||
| Mean (s.d.) | 10.8 (10.2) | 16.7 (13.7) | 1.043 (1.031, 1.055) | <0.001 | 1.011 (1.000, 1.022) | 0.05 |
| Disease severityc | ||||||
| Remission | 569 | 19/569 (3.34%) | — | — | ||
| Mild | 3,127 | 85/3,127 (2.72%) | 1.072 (0.648, 1.774) | 0.79 | 1.078 (0.643, 1.807) | 0.78 |
| Moderate/severe/fulminant | 2,336 | 83/2,336 (3.55%) | 1.699 (1.019, 2.835) | 0.042 | 1.612 (0.910, 2.856) | 0.10 |
| Unknown | 48 | 0/48 (0.00%) | 0.000 (0.000, 0.000) | 0.98 | 0.000 (0.000, 0.000) | 0.98 |
| Infliximabd | ||||||
| No | 2,766 | 94/2,766 (3.40%) | — | — | ||
| Yes | 3,314 | 93/3,314 (2.81%) | 0.865 (0.648, 1.154) | 0.33 | 0.828 (0.595, 1.152) | 0.26 |
| Prednisoned | ||||||
| No | 3,249 | 77/3,249 (2.37%) | — | — | ||
| Yes | 2,831 | 110/2,831 (3.89%) | 1.973 (1.470, 2.648) | <0.001 | 2.140 (1.550, 2.954) | <0.001 |
| Immunomodoulatorsd,e | ||||||
| No | 2,097 | 82/2,097 (3.91%) | — | — | ||
| Yes | 3,983 | 105/3,983 (2.64%) | 0.713 (0.533, 0.955) | 0.023 | 0.856 (0.623, 1.176) | 0.34 |
| Narcotic analgesicsd | ||||||
| No | 4,586 | 124/4,586 (2.70%) | — | — | ||
| Yes | 1,494 | 63/1,494 (4.22%) | 1.855 (1.366, 2.520) | <0.001 | 1.789 (1.290, 2.479) | <0.001 |
CI, confidence interval.
Patients were eligible for this analysis if they had non-missing baseline covariates. Only data representing patient status between registration and 31 December 2009 are used within this analysis.
P value from Wald χ2 test.
This represents diseased area at baseline, as disease area was not collected longitudinally.
This represents time-varying maximum severity between enrollment and the 6-month data collection period of the event or censoring.
This represents time-varying medication use, and is defined as any use between enrollment and the 6-month data collection period before the event or censoring.
Immunomodulators include azathioprine, methotrexate, and 6-mercaptopurine.
Serious infection
Unadjusted rates of serious infection within 3 months of treatment were 2.06 and 1.42 per 100 patient-years, respectively, for the infliximab and other-treatments-only groups, yielding a RR (95% CI) of 1.45 (1.10, 1.91) (P=0.008). In a broader analysis including serious infections that occurred outside of the 3-month timeframe, unadjusted rates of serious infection were 2.04 and 1.00 per 100 patient-years, respectively, for the infliximab and other-treatments-only groups, yielding an RR (95% CI) of 2.04 (1.45, 2.89) (P<0.001) (Table 4). With the exception of six serious fungal infections (five in the infliximab group and one in the other-treatments-only group) and four serious mycobacterial infections (all in the infliximab group) (Table 6), all other serious infections were viral or bacterial in nature. The most commonly observed serious infections, defined as those occurring at an overall incidence >0.01/100 patient-years of follow-up, are displayed in Table 7. Without adjustment for other potential confounding factors (e.g., severity of CD and use of other immunosuppressive medications), the incidences of pneumonia, cellulitis, and perirectal abscess were higher among patients who had received infliximab (0.24, 0.15, 0.11/100 patient-years, respectfully) within the previous 3 months than among patients who had not (0.14, 0.03, 0.03/100 patient-years, respectively; Table 7).
Table 6. Summary of serious Mycobacterial and fungal infections.
|
Recenta
use of |
|||||
|---|---|---|---|---|---|
| Serious infection | Patient age at onset (years) | Treatment group | Immunomodulatorsb | Corticosteroids | Fatal? |
| Mycobacterial | |||||
| Mycobacterium avium complex infection | 47.3 | Infliximab | Yes | Yes | No |
| Mycobacterial TB | 71.9 | Infliximab | No | No | No |
| Mycobacterial pulmonary TB | 52.0 | Infliximab | Yes | No | No |
| Mycobacterial pulmonary TB | 33.3 | Infliximab | Yes | No | No |
| Mean | 51.1 | Yes/No: 3/1 | Yes/No: 1/3 | ||
| Fungal | |||||
| Pneumocystis jiroveci pneumonia | 69.5 | Infliximab | No | No | Yes |
| Candida glabrata fungaemia | 36.4 | Infliximab | No | Yes | No |
| Candida tropicalis candidiasisc | 58.8 | Infliximab | Yes | Yes | No |
| Systemic candidiasis | 40.1 | Other-treatments-only | Yes | No | No |
| Systemic fungaemiac | 59.3 | Inflixmab | Yes | Yes | No |
| Candida sepsis | 26.4 | Infliximab | No | No | No |
| Mean | 48.4 | Yes/No: 3/3 | Yes/No: 3/3 | ||
TB, tuberculosis.
Recent use is defined as use within the 6-month data collection period before serious adverse event (SAE) onset and/or use within the same data collection period as SAE onset.
Includes azathioprine, 6-mercaptopurine, and/or methotrexate.
Two fungal infections occurred in the same patient.
Table 7. Most common (>0.01/100 patient-years among all patients) serious infections.
| All patients | Infliximab-treateda | Other-treatments-onlyb | |
|---|---|---|---|
| N=6,273 | N=2,942 | N=5,597 | |
| Total patient-years of follow-up | 30,268 | 7,923 | 22,344 |
|
Common adverse events |
Rate/100 patient-years (no. of events) |
||
| Pneumonia | 0.17 (50) | 0.24 (19) | 0.14 (31) |
| Abdominal abscess | 0.09 (28) | 0.11 (9) | 0.09 (19) |
| Catheter sepsis | 0.09 (27) | 0.06 (5) | 0.10 (22) |
| Sepsis | 0.07 (21) | 0.03 (2) | 0.09 (19) |
| Cellulitis | 0.06 (18) | 0.15 (12) | 0.03 (6) |
| Central line infection | 0.06 (17) | 0.04 (3) | 0.06 (14) |
| Gastroenteritis | 0.05 (16) | 0.05 (4) | 0.05 (12) |
| Perirectal abscess | 0.05 (16) | 0.11 (9) | 0.03 (7) |
| Diverticulitis | 0.05 (15) | 0.04 (3) | 0.05 (12) |
| Pelvic abscess | 0.05 (14) | 0.04 (3) | 0.05 (11) |
| Intestinal abscess | 0.04 (13) | 0.01 (1) | 0.05 (12) |
| Viral gastroenteritis | 0.04 (11) | 0.04 (3) | 0.04 (8) |
| Appendicitis | 0.03 (10) | 0.06 (5) | 0.02 (5) |
| Wound infection | 0.03 (10) | 0.05 (4) | 0.03 (6) |
| Abscess | 0.03 (9) | 0.03 (2) | 0.03 (7) |
| Urinary tract infection | 0.03 (9) | 0.06 (5) | 0.02 (4) |
| Catheter bacteremia | 0.02 (7) | 0.03 (2) | 0.02 (5) |
| Staphylococcal infection | 0.02 (7) | 0.03 (2) | 0.02 (5) |
| Clostridium difficile colitis | 0.02 (6) | 0.01 (1) | 0.02 (5) |
| Postoperative abscess | 0.02 (6) | 0.03 (2) | 0.02 (4) |
| Anal abscess | 0.02 (5) | 0.04 (3) | 0.01 (2) |
| Infection (not otherwise specified) | 0.02 (5) | 0.04 (3) | 0.01 (2) |
| Rectal abscess | 0.02 (5) | 0.01 (1) | 0.02 (4) |
| Urosepsis | 0.02 (5) | 0.03 (2) | 0.01 (3) |
| Viral infection | 0.02 (5) | 0.01 (1) | 0.02 (4) |
Infliximab-treated patients are those patients who received infliximab within 12 weeks before the serious infection.
Includes all patients who did not receive infliximab within the previous 3 months.
Adjusted results of multivariate regression analyses (Table 8) indicated that moderate-to-severe disease severity was the strongest predictor of serious infection (HR=2.24, 95% CI=1.57, 3.19; P<0.001), while colon only involvement (vs. both ileum and colon involvement) was protective against the development of serious infections (HR=0.73, 95% CI=0.54, 1.00; P=0.046). When investigating medications used by patients with a serious infection relative to patients who did not have a serious infection, infliximab treatment conferred a significant HR of 1.43 (95% CI=1.11, 1.84; P=0.006). Treatment with immunomodulators (HR=1.23, 95% CI=0.96, 1.57; P=0.10) was not a significant predictor of serious infection, while narcotic analgesic (HR=1.98, 95% CI=1.44, 2.73; P<0.001) and prednisone (HR=1.57, 95% CI=1.17, 2.10; P=0.002) use were both significant predictors of serious infection (Table 8).
Table 8. Predictors of serious infections.
|
Unadjusted results |
Adjusted results |
|||||
|---|---|---|---|---|---|---|
| All patients (N=5,394) | Serious infections (N=297) | Hazard ratio (95% CI) | P valuea | Hazard ratio (95% CI) | P valuea | |
| Age at enrollment | ||||||
| Mean (s.d.) | 43.0 (14.6) | 45.3 (15.5) | 1.010 (1.002, 1.017) | 0.017 | 1.013 (1.004, 1.023) | 0.007 |
| Sex | ||||||
| Male/unknown | 2,222 | 121/2,222 (5.45%) | — | |||
| Female | 3,172 | 176/3,172 (5.55%) | 1.044 (0.828, 1.316) | 0.72 | 1.006 (0.789, 1.283) | 0.96 |
| Race | ||||||
| Non-Caucasian | 501 | 30/501 (5.99%) | — | |||
| Caucasian | 4,893 | 267/4,893 (5.46%) | 0.776 (0.532, 1.132) | 0.19 | 0.764 (0.511, 1.142) | 0.19 |
| Involved intestinal area(s)b | ||||||
| Ileum and colon | 2,121 | 141/2,121 (6.65%) | — | |||
| Ileum only | 1,631 | 85/1,631 (5.21%) | 0.762 (0.582, 0.997) | 0.048 | 0.858 (0.646, 1.139) | 0.29 |
| Colon only | 1,579 | 67/1,579 (4.24%) | 0.613 (0.459, 0.821) | 0.001 | 0.729 (0.535, 0.995) | 0.046 |
| Unknown | 63 | 4/63 (6.35%) | 0.821 (0.302, 2.229) | 0.70 | 1.412 (0.513, 3.891) | 0.50 |
| Years between diagnosis and enrollment | ||||||
| Mean (s.d.) | 10.9 (10.2) | 14.0 (11.9) | 1.025 (1.015, 1.035) | <0.001 | 1.018 (1.007, 1.030) | 0.002 |
| Severityc | ||||||
| Remission | 2,468 | 99/2,468 (4.01%) | — | |||
| Mild | 2,113 | 122/2,113 (5.77%) | 1.197 (0.917, 1.562) | 0.19 | 0.976 (0.731, 1.302) | 0.87 |
| Moderate/severe/fulminant | 459 | 71/459 (15.47%) | 3.473 (2.553, 4.723) | <0.001 | 2.239 (1.569, 3.194) | <0.001 |
| Unknown | 62 | 0/62 (0.00%) | 0.000 (0.000, 0.000) | 0.96 | 0.000 (0.000, 0.000) | 0.97 |
| Infliximabd | ||||||
| No | 2,283 | 146/2,283 (6.40%) | — | |||
| Yes | 827 | 129/827 (15.60%) | 1.749 (1.380, 2.219) | <0.001 | 1.431 (1.110, 1.844) | 0.006 |
| Prednisoned | ||||||
| No | 4,496 | 217/4,496 (4.83%) | — | |||
| Yes | 599 | 75/599 (12.52%) | 2.317 (1.778, 3.019) | <0.001 | 1.571 (1.173, 2.103) | 0.002 |
| Immunomodoulatorsd,e | ||||||
| No | 3,255 | 148/3,255 (4.55%) | — | |||
| Yes | 1,849 | 146/1,849 (7.90%) | 1.296 (1.030, 1.629) | 0.027 | 1.227 (0.961, 1.566) | 0.10 |
| Narcotic analgesicsd | ||||||
| No | 4,721 | 237/4,721 (5.02%) | — | |||
| Yes | 374 | 55/374 (14.71%) | 2.906 (2.166, 3.899) | <0.001 | 1.980 (1.436, 2.729) | <0.001 |
CI, confidence interval.
Patients were eligible for this analysis if they had non-missing baseline covariates. Only data representing patient status between registration and 31 December 2009 are used within this analysis.
P value from Wald χ2 test.
This represents diseased area at baseline, as disease area was not collected longitudinally.
This represents time-varying maximum severity between enrollment and the 6-month data collection period of the event or censoring.
This represents time-varying medication use and is defined as any use in the 6-month data collection period before the event or censoring.
Immunomodulators include azathioprine, methotrexate, and 6-mercaptopurine.
There was no evidence of an increase in the occurrence of serious infections with receipt of more infliximab infusions. Specifically, the incidences of serious infections were 2.58, 2.16, 1.65, and 1.92 per 100 patient-years in association with receipt of 1–3, 4–9, 10–24, and ≥25 infliximab infusions, respectively. Escalation of the infliximab dose from 5 to 10 mg/kg also had no important effect on the occurrence of serious infections (data not shown).
Pregnancy
Maternal and paternal (i.e., treated male partner) pregnancies were monitored for outcome and clinical condition of live births. For both female and male registry participants, the vast majority of pregnancies resulted in lives births, i.e., 83.1% (118/142) and 95.3% (41/43), respectively, for infliximab-treated patients and 90.7% (68/75) and 91.7% (11/12), respectively, for patients receiving only other treatments. Among the maternal and paternal live births documented, the vast majority of babies were healthy with no defect or other adverse event, i.e., 92.4% (109/118) and 90.2% (37/41), respectively, for infliximab-treated patients and 85.3% (58/68) and 90.9% (10/11), respectively, for patients receiving only other treatments. A more detailed analysis of pregnancy in the TREAT registry will be the subject of a separate publication.
Neoplasia
The incidences of neoplasia (including benign, malignant, and unspecified growths) were similar between infliximab-treated patients (0.78 per 100 patient-years) and patients who received other-treatments-only (0.85 per 100 patient-years), yielding an RR (95% CI) of 0.90 (0.69, 1.18) (P=0.46) (Table 4). While a more detailed analysis of malignancy in the TREAT registry will be the subject of a separate publication, as a top-level summary, the overall incidences of solid tumors (0.42 vs. 0.45 events per 100 patient-years), non-melanoma skin cancer (0.16 vs. 0.18 events per 100 patient-years), and lymphoma (0.05 vs. 0.06 events per 100 patient-years) were similar in infliximab-treated patients and those who received other-treatments-only, respectively.
DISCUSSION
We are reporting on the updated safety information acquired throughout the TREAT registry, which is a large, prospective, observational research program designed to address the long-term safety of medications, including infliximab, used to treat CD. This report, which describes the analysis of the prespecified follow-up period of at least 5 years per patient, is thus the principal registry report and updates previously reported findings through ∼2 years of registry participation. Consistent with the initial registry report (15), with an average of 5.2 years of patient follow-up in this registry study, the occurrence of death has been similar between patients treated with infliximab and those who have received other-treatments-only (0.58 vs. 0.59 per 100 patient-years, respectively; RR=0.96, 95% CI=0.72, 1.28, P=0.81). For both treatment groups, death rates remain below annual death rates for CD patients documented in epidemiological studies (17,18,19,20). Results of multivariate logistic regression analyses indicated that age, prednisone use, and narcotic analgesic use were significant predictors of death, while neither infliximab treatment nor immunomodulator therapy predicted death in patients with CD. These findings are particularly notable given that patients treated with infliximab had a worse prognosis at baseline. Although the multivariate model adjusted the HR estimate for known predictors of mortality, such as clinical disease activity, it could not account for unknown confounders associated with disease severity that likely would have conveyed a greater risk of death in the infliximab group. However, improved control of disease activity in patients treated with infliximab might have offset mortality risks related to therapy from other causes such as serious infection.
A higher incidence of infections in general was observed in the infliximab-treated patients relative to patients receiving other-treatments-only. However, infliximab-treated patients in the TREAT registry had more severe CD at registry entry and also were more likely to be using other immunosuppressive agents or prednisone. Multivariate regression analyses indicated that moderate-to-severe disease activity was the strongest significant predictor of serious infection (HR=2.24, P<0.001), followed by treatment with narcotic analgesics (HR=1.98, P<0.001), treatment with prednisone (HR=1.57, P=0.002), and then infliximab treatment (HR=1.43, P=0.006). The statistical significance of infliximab in this regard is a new finding relative to the previous registry report (15). It is of note that the increased risk of serious infection with infliximab did not lead to a higher mortality risk. As mentioned, it is likely that infliximab lessened the disease burden and thus counterbalanced any mortality risk that might have been associated with other causes such as serious infection. It should be noted that while this type of analysis allows the relative comparison of the risk associated with one immunosuppressive drug with that of another, it does not allow for the assessment of risk associated with various combinations of therapies. In addition, the analysis does not take into consideration contributing risk factors such as duration of use, dose, or cumulative exposure of a particular immunosuppressive agent or combination regimen. However, in an analysis that grouped serious infections according to the number of infliximab infusions the patient had received before the event, there was no evidence of an increase in the occurrence of serious infections with increasing number of infliximab infusions.
The significant effect of narcotic analgesic use on the risk of serious infection and mortality could result from such therapy serving as a proxy for patients with the most severe cases of CD, although we would anticipate that differences in disease severity would have been corrected by the multivariate regression analyses conducted. In addition, it could be that narcotic analgesic use, which is known to slow intestinal motility, yields longer gut processing times and thus longer periods of patient exposure to infection-causing organisms. Finally, it may also be possible that narcotic analgesic use can mask the signs and symptoms of infections until they become serious.
When assessed on a “per infusion” basis, the incidences of infusion reactions were similar across the TREAT registry (1,571/53,003 or 3%) and previously conducted clinical trials of infliximab in CD patients, i.e., 106/2,026 or 5% in ACCENT I (21) and 70/1,728 or 4% in ACCENT II (22).
With an approximate average of 5.2 years of patient follow-up, based on the TREAT registry data, infliximab does not appear to be associated with an increased risk of development of neoplasia in general or with an increased risk for the development of malignant solid tumors, non-melanoma skin cancer, or lymphoma. There have been no signals indicating an increased risk of fetal malformation associated with infliximab therapy analyses to date. Note that results of more detailed analyses of TREAT registry malignancy and pregnancy data will be reported in separate publications.
While registries generally allow a large number of patients to be followed prospectively in a “real-world” setting, they are not as rigorously monitored as randomized controlled studies. As such, it is possible that some SAEs occurred and were not captured in the registry and, thus, such events could be under-reported in the TREAT registry. However, most analyses performed involved comparisons between different CD treatment regimens, and there is no evidence indicating that SAE reporting would be inconsistent between treatment groups, outside of the fact that maintenance infliximab therapy requires regular office visits and thus a stronger likelihood for adverse event reporting. Registries can also be limited by comprising a population that is not as “sick” as the general CD population, possibly leading to an under-representation of certain adverse events. To address this point, the rate of hospitalizations and surgeries in TREAT was compared with an age-matched (18–65 years of age) general CD population (from the MedStat MarketScan 2003 insurance claims database). In this analysis, the proportions of TREAT registry participants who had at least one CD-related hospitalization was similar to (5.36% vs. 5.15%, respectively) and the proportion who had at least one CD-related surgery was higher than (3.95% vs. 1.90%, respectively) those observed in the MarketScan database (data not shown). These data suggest that patients in the TREAT registry were representative of the general CD population.
The potential for patient attrition can also limit interpretation of registry data (23). In an evaluation of 6,346 patients with rheumatoid arthritis who were followed semi-annually across 11 long-running databases, results of multivariable analyses indicated that younger age, lower levels of education, and non-Caucasian race predicted attrition (23). In two other registries involving a total of 6,185 patients with rheumatoid arthritis who initiated etanercept treatment, patients with follow-up data available through year 5 ranged from 42 to 57% across the registries (24). We observed similar proportions of patients with available data through an average of 5.2 years of follow-up in the TREAT registry (∼50% of patients overall).
Another potential limitation of these data is that only medication use during the registry or during the period before enrollment was considered. It is possible that some patients categorized as other-treatments-only had received infliximab in the more distant past. However, with regard to the risk for serious infection, the assessment of whether or not serious infections occurred within 3 months of an infliximab infusion addresses this potential concern. In addition, the prior remote use of infliximab is unlikely in most patients as the registry was initiated soon after infliximab was approved and became available.
In conclusion, the results presented herein, derived from CD patients enrolled in the TREAT registry with an overall average of more than 5 years of follow-up, indicate that infliximab does not confer an increased risk of mortality. Moderate-to-severe CD was the strongest predictor of serious infection, followed by treatment with narcotic analgesics, prednisone therapy, and infliximab treatment. There has been no indication that infliximab is associated with an increased risk of neoplasia or an increased risk of fetal malformation in the TREAT registry.
STUDY HIGHLIGHTS
Acknowledgments
We thank Mark Molenda (Janssen Biotech, Inc.) for analytical support, and Michelle Perate, MS, and Mary Whitman, PhD (Janssen Services, LLC) for writing and editorial support.
Guarantor of the article: Gary R. Lichtenstein, MD.
Specific author contributions: Gary R. Lichtenstein, Brian G. Feagan, Russell D. Cohen, Bruce A. Salzberg, Robert H. Diamond, and William J. Sandborn were involved in the registry design and conduct; Robert H. Diamond, Samiyeh Price, Wayne Langholff, and Anil Londhe were involved in data collection and analysis; and all authors provided critical content review and final approval of this manuscript.
Financial support: Funding for the TREAT registry is provided by Janssen Biotech Inc., a Johnson and Johnson (J&J) pharmaceutical company.
Potential competing interests: Dr. Lichtenstein has received research grants and/or has served as a consultant for Abbott, Alaven, Bristol-Myers Squibb, Elan, Ferring, Janssen, Meda Pharmaceuticals, Millenium Pharmaceuticals, Pfizer Pharmaceuticals, Proctor and Gamble, Prometheus Laboratories, Salix Pharmaceuticals, Santarus, Merck/Schering-Plough, Shire Pharmaceuticals, UCB, Warner Chilcotte, and Wyeth. Dr. Feagan has received research grants and/or has served as a consultant for Abbott, Actogenix, Alba Therapeutics, Albireo Pharma, Astra Zeneca, Athersys, Axcan, Berlex, Boehringer Engelheim, Bristol-Myers Squibb, Celgene, Cerimon Pharma, CombinatoRx, Elan/Biogen, Funxional Therapeutics, GeneLogic, Genentech, Given Imaging, Gilead, Glaxo Smithkline, ISIS, Janssen, Merck/Schering-Plough, Millennium, Napo Pharma, Nektar, Novartis, Novonordisk, Ore Pharm. (previously GeneLogic), Osiris, Otsuka, Pfizer, Proctor and Gamble, Prometheus Therapeutics and Diagnostics, Protein Design Labs, Salix Pharma, Santarus, Schering Canada, Serono, Shire, Synta, Teva Pharma, Tillotts Pharma AG, Tioga Pharma, UCB Pharma, Unity Pharma, Wyeth, and Zealand Pharma. Dr Cohen has received research grants and/or has served as a consultant for Janssen. Dr Salzberg has received consulting and/or speaker fees for Abbott Laboratories, Janssen, and Shire. Dr Sandborn has received consulting fees and research support from Abbott Laboratories, Janssen, and UCB Pharma, as well as consulting fees from Merck/Schering-Plough. R.H. Diamond and S. Price are employed by Janssen, a J&J company, and own stock in J&J. W. Langholff and A. Londhe are employees of J&J Pharmaceutical Research Division and own stock in J&J.
References
- Lichtenstein GR. Emerging prognostic markers to determine Crohn's disease natural history and improve management strategies: a review of recent literature. Gastroenterol Hepatol (NY) 2010;6:99–107. [PMC free article] [PubMed] [Google Scholar]
- Allen PB, Lindsay H, Tham TC. How do patients with inflammatory bowel disease want their biological therapy administered. BMC Gastroenterol. 2010;10:1. doi: 10.1186/1471-230X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Fedorak RN. Tumor necrosis factor-α monoclonal antibodies in the treatment of inflammatory bowel disease: clinical practice pharmacology. Gastroenterol Clin North Am. 2010;39:543–557. doi: 10.1016/j.gtc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Oussalah A, Danese S, Peyrin-Biroulet L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: a systematic review. Curr Drug Targets. 2010;11:156–175. doi: 10.2174/138945010790309939. [DOI] [PubMed] [Google Scholar]
- Lin MV, Blonski W, Lichtenstein GR. What is the optimal therapy for Crohn's disease: step-up or top-down. Expert Rev Gastroenterol Hepatol. 2010;4:167–180. doi: 10.1586/egh.10.4. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41. doi: 10.1016/j.cgh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Botoman VA, Bonner GF, Botoman DA.Management of inflammatory bowel disease Am Fam Physician 19985757–68.71–2. [PubMed] [Google Scholar]
- Bijlsma JW, Boers M, Saag KG, et al. Glucocorticoids in the treatment of early and late RA. Ann Rheum Dis. 2003;62:1033–1037. doi: 10.1136/ard.62.11.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NC, Go CH, Cunha BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001;15:423–432. doi: 10.1016/s0891-5520(05)70154-9. [DOI] [PubMed] [Google Scholar]
- Hoentjen F, van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J Gastroenterol. 2009;15:2067–2073. doi: 10.3748/wjg.15.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Sturm A. Concomitant use of immunomodulators with anti-TNF in Crohn's disease: yes or no. Curr Drug Targets. 2010;11:176–178. doi: 10.2174/138945010790309948. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflamm Bowel Dis. 2009;15:1308–1319. doi: 10.1002/ibd.20956. [DOI] [PubMed] [Google Scholar]
- Lees CW, Ali AI, Thompson AI, et al. The safety profile of anti-tumour necrosis factor therapy in inflammatory bowel disease in clinical practice: analysis of 620 patient-years follow-up. Aliment Pharmacol Ther. 2009;29:286–297. doi: 10.1111/j.1365-2036.2008.03882.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry Clin Gastroenterol Hepatol 20064621–630.Erratum in Clin Gastroenterol Hepatol. 2006;4:931. Comment in: Inflamm Bowel Dis 2007;13:933–4. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, Sandborn W, Practice Parameters Committee of the American College of Gastroenterology Management of Crohn's disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- Jess T, Winther KV, Munkholm P, et al. Mortality and causes of death in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Gastroenterology. 2002;122:1808–1814. doi: 10.1053/gast.2002.33632. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Jr, Silverstein MD, Sandborn WJ, et al. Crohn's disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn's disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818–1825. doi: 10.1016/0016-5085(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Helmick CG, Zack M, et al. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology. 1992;103:954–960. doi: 10.1016/0016-5085(92)90029-x. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- Krishnan E, Murtagh K, Bruce B, et al. Attrition bias in rheumatoid arthritis databanks: a case study of 6346 patients in 11 databanks and 65,649 administrations of the Health Assessment Questionnaire J Rheumatol 2004311320–1326.Comment in: J Rheumatol 2004;31:1244–5. [PubMed] [Google Scholar]
- Gibofsky A, Palmer WR, Keystone EC, et al. Rheumatoid arthritis disease-modifying antirheumatic drug intervention and utilization study: safety and etanercept utilization analyses from the RADIUS 1 and RADIUS 2 registries. J Rheumatol. 2011;38:21–28. doi: 10.3899/jrheum.100347. [DOI] [PubMed] [Google Scholar]