Abstract
A promoter-linked insertion/deletion polymorphism of the serotonin transporter gene (SLC6A4) has been implicated in autism spectrum disorders (ASDs) in numerous family-based association studies. However, the results of these investigations have been inconsistent in that both the long and short alleles have been shown to be over-transmitted to affected offspring. In order to further elucidate the relationship between the 5-HTTLPR variant and autism risk, we undertook a thorough study of parent-of-origin effects, maternal genotype effects, and offspring genotype effects in a sample of affected offspring from the Autism Genetic Resource Exchange (AGRE). Both the overall autism phenotype and measures of autism behaviors from the Autism Diagnostic Interview-Revised (Lord et al, 1994) were considered. We found evidence of over-transmission (risk allele short, p=0.012), maternal effects (risk allele long, p=0.035), and parent-of-origin effects (risk allele short from mother, p=0.018) of the 5-HTTLPR variant in the AGRE sample. Population-specific and gender-specific effects were also explored as associations may be heterogeneous across populations and sexes. Parent-of-origin effects of the variant were associated with maternally-inherited copies of the short allele that resulted in more impaired overall level of language (p=0.04). Our study was conducted to further investigate the 5-HTTLPR risk variants by identifying allelic associations that may be population-specific, phenotype-specific, or conferred by maternal or parent-of-origin effects. In light of conflicting observations from previous studies, these are just a few of the possible explanations that deserve attention.
Keywords: Autism, 5-HTTLPR, maternal effects, parent-of-origin effects, association
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is known to play a significant role in a number of psychiatric processes including mood regulation, cognition, and repetitive and stereotyped behaviors (Mendelsohn et al., 2009; Veenstra-VanderWeele et al., 2000). Converging evidence from genetic, imaging and drug treatment studies have implicated serotonin dysregulation in autism spectrum disorders (ASD) (Cook et al., 1996; Cook et al., 1997a; Mulder et al., 2004; Chandana et al., 2005; Coon et al., 2005; Hollander et al., 2005; Nakamura et al., 2010). Additionally, observations such as the presence of high levels of serotonin, or hyperserotonemia in patients and family members of individuals with autism sparked a number of studies investigating the role of serotonin in autism (Schain and Freedman, 1961; Abramson et al., 1989; Cook et al., 1990; Piven et al., 1991; Cook and Leventhal et al., 1996). Several genetic studies of autism have focused on the 5-HT transporter (5-HTT).
The 5HTTLPR was first described by Lesch and colleagues and is comprised of a 20–23 bp GC-rich sequence present in multiple lengths which can be primarily classified as long form and short forms although additional forms do occasionally occur in the population (Lesch et al., 1994, Nakamura et al., 2000). Several studies, with some exceptions (Willeit et al., 2001; Lim et al., 2008) have suggested that the long allele is associated with higher levels of 5HTT transcript and higher levels of serotonin uptake in lymphoblastoid cell lines when compared to the short allele (Lesch et al., 1996; Heils et al., 1996; Little et al., 1998; Mortenson et al., 1999; Greenberg et al., 1999). The effect of the 5HTTLPR on transporter expression in the heterozygote state is currently unclear with some groups finding a dominant effect of the short allele (Lesch et al., 1996) and others finding an additive effect of genotype (Hranilovic et al., 2004; Bradley et al., 2005).
The 5-HTTLPR has been the focus of candidate gene association studies in many different populations of children with autism (Longo et al., 2000; Brune et al., 2006; Cho et al., 2007; Guhathakurta et al., 2008;). The 5-HTTLPR variant is a functional common polymorphism with short and long alleles that have both been associated with autism and various endophenotypes (Cook et al., 1997a; Klauck et al., 1997; McCauley et al., 2004; Sutcliffe et al., 2005; Wassink et al., 2007). Although the largest sample and meta-analysis including the most studies replicated the initial finding of overtransmission of the short 5-HTTLPR variant, formal analysis revealed significant heterogeneity across studies (Devlin et al., 2005). This study attempts to identify the potential sources of heterogeneity previously found in studies of the 5-HTTLPR by using a recently developed method allowing discovery of potential maternal effects and/or parent-of-origin genotype effects (Kistner et al., 2006).
Maternal effects may be mediated through gestational exposure to maternal serotonin, whereas parent-of-origin effects may be mediated through maternal (or paternal) transmission of the risk alleles to offspring. Thus maternal effects depend only on the genotype of the mother, not the genotype of the offspring, while parent-of-origin effects depend on the genotype of the affected offspring. Parent-of-origin effects have already been shown for early-onset diseases, such as Angelman syndrome, Prader-Willi syndrome, and Autism Spectrum Disorder, particularly in regions of chromosome 15 (Buiting K et al., 1995; Schroer RJ et al., 1998). Maternal effects and parent-of-origin effects have not previously been explored, either independently or jointly, as explanations of the SLC6A4 findings in ASD.
Methods
Subjects
Subjects were selected based on the availability of their 5-HTTLPR genotype. A total of 378 males and 98 females subjects between 2 and 39 years old (mean = 7.8 years ± 4.6) were included in the initial analysis. The majority of the subjects (61%) came from the Autism Genome Research Exchange (AGRE); additional subjects came from research at Stanford (24%), Tufts (14%), and Vanderbilt (<1%) with ADI-R assessment and 5-HTTLPR genotyping previously described (Sutcliffe et al. 2005). The methods allow inclusion of subjects with missing genotype data from either the mother or the father, therefore only 85% of the 476 in the initial sample had complete 5-HTTLPR genotyping. If available, parental Affymetrix 10K GeneChip genotypes and Affymetrix Genome-wide Human SNP Array 5.0 genotypes were analyzed to estimate dimensions of population genetic variation, using Multidimensional scaling (MDS) in PLINK (Purcell S et al., 2007). Subjects with two parents of European ancestry were included in subpopulation analyses. In this cohort, there were 282 families identified as of European ancestry and 194 families identified as of non-European ancestry using MDS. Supplemental Figure S1 illustrates how the European ancestry subpopulation was defined. Because our analytic methods did not allow multiple affected siblings from each pedigree, one male and one female, if present, were chosen from each family to increase the number of subjects in the sex-stratified analysis. For the male only and female only subpopulation analyses, a total of 435 males and 153 females, with 98 pedigrees contributing both a male and female offspring to sex-stratified hypothesis testing. All subjects selected met criteria for classification of Autism on the Autism Diagnostic Interview-Revised. See Table I for a description of the initial sample.
Statistical Analyses
Exact tests of Hardy Weinberg Equilibrium were conducted for the initial sample and the sex-stratified European ancestry samples. Association analyses of the autism phenotype were conducted using a log-linear model framework described by Weinberg et al. (Weinberg CR, Wilcox AJ, Lie RT, 1998; Weinberg 1999). Using this approach, two degree-of-freedom likelihood ratio tests of association and maternal effects are reported. Tests of association allow quantifying the effects of inheriting 1 and 2 copies of the risk variant, while maternal effects tests allow for quantifying the gestational effects for mothers with 1 and 2 copies of the risk variant, in the presence of possible association. Both of these tests do not impose restrictions on the genetic model, as tests of additive, dominant, or recessive effects would. Instead, the tests allow detection of all of these models with a flexible two degree-of-freedom test, which allows discovery of either the heterozygous and/or homozygous carriers (of long/short alleles) being associated with the phenotype of interest. Reported parent-of-origin tests are one degree-of-freedom likelihood ratio tests to distinguish effects of a paternal and a maternal copy of an inherited variant, in the presence of possible maternal and offspring effects. Tests of genotype-phenotype association were conducted using a polytomous logistic regression model, incorporating tests of maternal effects and parent-of-origin effects on the phenotype as well (Kistner et al., 2006). Log-linear and polytomous logistic models of autism and related phenotypes incorporate offspring with missing maternal or paternal genotypes. Overall level of language was categorized as “0” (scores of 0 only, “presence of daily, functional fluent phrase speech”) or “1” (scores of 1 or 2, “no functional phrase speech or non-verbal”). This categorization was based on current language level at the time of administration of the ADI-R. For each population and subpopulation, specifically, the original sample, the European ancestry sample, the Non-European ancestry sample, the male sample, and the female sample, tests of offspring genotype, maternal genotype, and parent-of-origin were conducted for both the autism and overall language level phenotype. All tests were conducted with freeware developed using SAS V9.1 (Weinberg CR, Wilcox AJ, Lie RT, 1998; Weinberg 1999; Kistner et al., 2006).
Results
The 5-HTTLPR variant was in Hardy Weinberg Equilibrium in the overall sample (p=0.41). No Mendelian errors were detected. Using likelihood ratio tests and the log linear model from Weinberg (1999), we included 476 trios with at least the maternal or paternal genotype available in order to test offspring genotype effect, maternal genotype effect, and parent-of-origin genotype effect. A test of the offspring genotype effect was significant in this sample (p = 0.012) with the short allele as the risk conferring allele. Allowing for an offspring genotype effect, a maternal genotype effect was also significant (p=0.035) with the long maternal allele conferring risk. Lastly a parent-of-origin effect was tested after allowing for offspring and maternal genotype effects. The parent-of-origin test was also significant (p=0.018) with a maternal copy of the short allele conferring risk. See Table II.
In order to further investigate these findings, we identified trios of European ancestry (EA) using MDS as we hypothesized that population admixture may be contributing to results showing putative effects of both the long and short alleles. In this EA cohort, the parent-of-origin test was statistically significant, with the risk allele being the maternally derived copy of the short allele (p = 0.046). In the families of non-European ancestry, the offspring genotype effect was significant, with the short allele conferring risk (p=0.033).
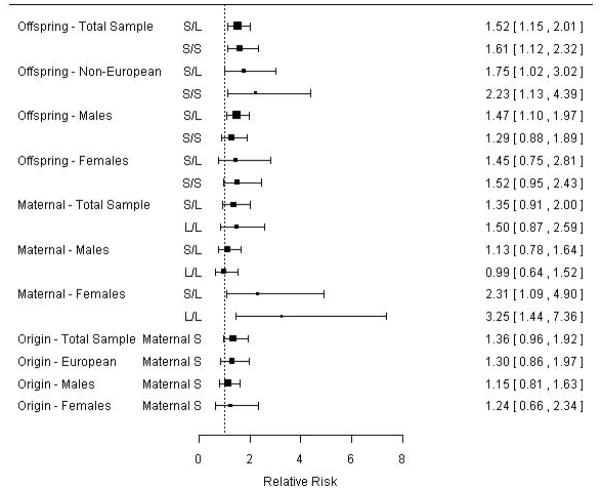
Affected siblings were included in sex-specific analyses. In the sample of affected males of both EA and non-EA, a test of the offspring genotype effect was not statistically significant (p = 0.190). In the same sample allowing for an offspring genotype effect, a maternal genotype effect was also not statistically significant (p=0.368). Lastly a parent-of-origin effect was tested after allowing for offspring and maternal genotype effects. The parent-of-origin test was also not significant (p=0.292). Similarly in the sample of affected females of both EA and non-EA, a test of the offspring genotype effect was not statistically significant (p = 0.654). Allowing for an offspring genotype effect in this sample, a maternal genotype effect was statistically significant in this sample (p=0.029) with the long (“528”) maternal allele conferring risk. After allowing for offspring and maternal genotype effects, the parent-of-origin test was not statistically significant in the affected females (p=0.489). Genotype relative risks and 95% confidence intervals are estimated using families without missing parental genotypes, as the estimates of standard error are biased in the presence of missing data. See Table II and Figure 1 for estimated effects from each analysis.
Figure 1.
Forest plot of relative risks and 95% confidence intervals associated with offspring, maternal, and parent-of-origin genotypes, corresponding to estimates shown in Table II. Relative risks and confidence intervals are constructed from families without missing parental genotypes. Offspring and maternal genotype relative risks are relative to the genotype not shown. Parent-of-origin relative risks represent the risk for a child with a maternally-derived copy of the short allele relative to a paternally-derived copy of the short allele.
Lastly, we conducted tests of offspring genotype, maternal effects, and parent-of-origin effects on phenotype separately in the male and female European ancestry samples. All genotype-phenotype analyses were non-significant, except boys showed a parent-of-origin effect for overall language level, such that maternally-inherited copies of the short allele were associated with more severe language impairment (p = 0.040). Although non-significant, girls who inherited maternal copies of the short allele also showed more severe language impairment. For boys, 47% of those inheriting the maternal short allele demonstrated language impairment, while only 33% of those inheriting the paternal short allele demonstrated impairment. Similarly for girls, 50% of those inheriting the maternal short allele demonstrated language impairment, while only 40% of those inheriting the paternal short allele demonstrated impairment.
Discussion
In an initial sample of subjects, tests of association, maternal effects, and parent-of-origin effects were conducted in an attempt to discover possible explanations for the previously reported contradictory effects of the 5-HTTLPR variant. Maternal effects, if present, may be misconstrued as parent-of-origin effects such that offspring who have inherited the maternal allele appear to be at increased risk when in fact any offspring of a mother with the maternal risk variant will be at increased risk. For example, maternal effects of SLC6A4 may manifest through effects of maternally expressed serotonin transporter in the placenta, such that formally testing a maternal genetic effect of the 5-HTTLPR variant is of interest.
Similarly, parent-of-origin effects may be detected as a weak association between the risk variant and the phenotype, if not directly tested. Previous work has implicated parent-of-origin effects as contributing to Autism Spectrum Disorders. A region on chromosome 15q has been identified in which maternal duplications increase risk of ASD, while variants in the same region have been associated with the development of Angelman syndrome, for which ASD is often a co-morbidity, when the maternally-inherited genes are deleted (Cook et al., 1997b; Knoll et al., 1989; Buiting et al., 1995).
Given the previously reported effects of the 5-HTTLPR variant, we undertook an extensive study of association, maternal effects, and parent-of-origin genetic effects for the 5-HTTLPR variant. In a sample of 476 offspring, we detected evidence of association between ASD and the short allele, maternal effects of the long allele, and parent-of-origin effects of the short maternal allele. None of these effects are necessarily contradictory, in that the mechanism of the maternal effect is not expected to be functionally similar to identified parent-of-origin effects or linkage and association effects with the offspring genotype. We also further explored these effects in sex-stratified analyses and subpopulations of European and non-European descent. Tests of offspring genotype were significant in the non-European ancestry cohort, while parent-of-origin tests were significant in the European ancestry cohort. Replications of these results may implicate ancestry specific genetic mechanisms in autism risk.
Although parent-of-origin effects have been explored as a genetic mechanism of disease in autism and other early onset disorders, little attention has been given to maternal genetic effects on prenatal environment that may influence susceptibility after birth. Evidence from the tph1 knockout mouse suggests that reduced maternal serotonin has a critical impact on embryonic development. A recent study found that heterozygous tph1 embryos generated by crosses between null mothers and wild type fathers showed striking cortical abnormalities not seen in wild type, heterozygous or homozygous offspring from tph1 heterozygous mothers (Cote et al., 2009). This finding indicates that at early developmental stages, the murine maternal tph1 genotype is more influential than the genotype of the concepti and that maternal serotonin is necessary for normal CNS development.
Here we considered both offspring and maternal genetic effects of the SLC6A4 promoter-linked insertion/deletion polymorphism in ASD. Evidence of offspring genetic effects had been previously shown, however little attention had been paid to the maternal genotype. Tests of these effects, after controlling for the offspring genotype, allow exploration of the intrauterine environment on autism risk, in addition to the transmitted risk conferring variants. Evidence of both maternal genetic effects and parent-of-origin effects was observed in this study, however without either a biologic model or a larger cohort available for testing, results remain inconclusive.
Supplementary Material
Figure S1. European ancestry clusters as defined using Multidimensional scaling. (A) Parents typed using Affymetrix 5.0 genotyping array were defined as being of European ancestry if the corresponding first dimension scale from MDS was less than 1.0 as shown above. All other parents were defined as of unknown ancestry. A total of 495 parents were genotyped using the Affymetrix 5.0 array. (B) Parents typed using Affymetrix 10K GeneChip genotyping array were defined as being of European ancestry if the corresponding first dimension scale from MDS was less than 0.01 as shown above. All other parents were defined as of unknown ancestry. A total of 666 parents were genotyped using the 10K GeneChip, 399 of which were also typed using the Affymetrix 5.0 array above.
Acknowledgments
The analysis described was supported by Award Number P50HD055751 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. This work was also supported by National Institutes of Health Grants MH061009 and NS049261 to JSS. Biomaterials and phenotypic data from Stanford and Tufts were provided through the NIMH Autism Genetics Initiative. We acknowledge support from the Autism Genetic Resource Exchange (AGRE) and Autism Speaks. We gratefully acknowledge the resources provided by the AGRE consortium and the participating AGRE families. The Autism Genetic Resource Exchange (AGRE) is a program of Autism Speaks and is supported, in part, by grant 1U24MH081810 from the National Institute of Mental Health to Clara M. Lajonchere (PI).
References
- Abramson RK, Wright HH, Carpenter R, Brennan W, Lumpuy O, Cole E, Young SR. Elevated blood serotonin in autistic probands and their first-degree relatives. J Autism Dev Disord. 1989;19:397–407. doi: 10.1007/BF02212938. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am J Med Genet B Neuropsychiatr Genet. 2005;5:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism. Am J Psychiatry. 2006;163:2148–56. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Buiting K, Saitoh S, Gross S, Dittrich B, Schwartz S, Nicholls RD, Horsthemke B. Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nature Genetics. 1995;9:395–400. doi: 10.1038/ng0495-395. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhasz C, Muzik O, Rothermel RD, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–82. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cho IH, Yoo HJ, Park M, Lee YS, Kim SA. Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios. Brain Res. 2007;1139:34–41. doi: 10.1016/j.brainres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: relationships between serotonin and norepinephrine levels and intelligence. J Neuropsychiatry Clin Neurosci. 1990;2:268–74. doi: 10.1176/jnp.2.3.268. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997a;2:247–50. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. Am J Hum Genet. 1997b;60:928–34. [PMC free article] [PubMed] [Google Scholar]
- Coon H, Dunn D, Lainhart J, Miller J, Hamil C, Battaglia A, Tancredi R, Leppert MF, Weiss R, McMahon W. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) Am J Med Genet B Neuropsychiatr Genet. 2005;135B:42–6. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA. 2007;104:329–34. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD CPEA Genetics Network. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–6. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–87. [PubMed] [Google Scholar]
- Guhathakurta S, Sinha S, Ghosh S, Chatterjee A, Ahmed S, Gangopadhyay PK, Usha R. Population-based association study and contrasting linkage disequilibrium pattern reveal genetic association of SLC6A4 with autism in the Indian population from West Bengal. Brain Res. 2008;1240:12–21. doi: 10.1016/j.brainres.2008.08.063. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;11:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30:582–9. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- Kistner EO, Infante-Rivard C, Weinberg CR. A method for using incomplete triads to test maternally mediated genetic effects and parent-of-origin effects in relation to a quantitative trait. Am J Epidemiol. 2006;163:255–61. doi: 10.1093/aje/kwj030. [DOI] [PubMed] [Google Scholar]
- Klauck SM, Poustka F, Benner A, Lesch KP, Poustka A. Serotonin transporter (5- HTT) gene variants associated with autism? Hum Mol Genet. 1997;6:2233–8. doi: 10.1093/hmg/6.13.2233. [DOI] [PubMed] [Google Scholar]
- Knoll JK, Nicholls RD, Lalande M. On the parental origin of the deletion in Angelman syndrome. Hum Genet. 1989;83:205–7. doi: 10.1007/BF00286723. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lim JE, Papp A, Pinsonneault J, Sadee W, Saffen D. Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTLPR. Mol Psychiatry. 2006;11:649–662. doi: 10.1038/sj.mp.4001797. [DOI] [PubMed] [Google Scholar]
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- Longo D, Schuler-Faccini L, Brandalize AP, dos Santos Riesgo R, Bau CH. Influence of the 5-HTTLPR polymorphism and environmental risk factors in a Brazilian sample of patients with autism spectrum disorders. Brain Res. 2009;1267:9–17. doi: 10.1016/j.brainres.2009.02.072. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Coueur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:104–12. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33:926–52. doi: 10.1016/j.neubiorev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Thomassen M, Larsen MB, Whittemore SR, Wiborg O. Functional analysis of a novel human serotonin transporter gene promoter in immortalized raphe cells. Brain Res Mol Brain Res. 1999;68:141–148. doi: 10.1016/s0169-328x(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–9. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Piven J, Tsai GC, Nehme E, Coyle JT, Chase GA, Folstein SE. Platelet serotonin, a possible marker for familial autism. J Autism Dev Disord. 1991;21:51–9. doi: 10.1007/BF02206997. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–20. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Schroeder RJ, Phelan MC, Michaelis RC, Crawford EC, Skinner SA, Cuccaro M, Simensen RJ, Bishop J, Skinner C, Fender D, Stevenson RE. Autism and maternally derived aberrations on chromosome 15q. Am J Med Genet. 1998;76:327–36. doi: 10.1002/(sici)1096-8628(19980401)76:4<327::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-complusive behaviors. Am J Hum Genet. 2005;77:265–79. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Dawson G, Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–17. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Weinberg CR. Allowing for missing parents in genetic studies of case-parent triads. Am J Hum Genet. 1999;64:1186–93. doi: 10.1086/302337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Lie RT. A log-linear approach to case-parent triad data: assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parental imprinting. Am J Hum Genet. 1998;62:969–78. doi: 10.1086/301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. European ancestry clusters as defined using Multidimensional scaling. (A) Parents typed using Affymetrix 5.0 genotyping array were defined as being of European ancestry if the corresponding first dimension scale from MDS was less than 1.0 as shown above. All other parents were defined as of unknown ancestry. A total of 495 parents were genotyped using the Affymetrix 5.0 array. (B) Parents typed using Affymetrix 10K GeneChip genotyping array were defined as being of European ancestry if the corresponding first dimension scale from MDS was less than 0.01 as shown above. All other parents were defined as of unknown ancestry. A total of 666 parents were genotyped using the 10K GeneChip, 399 of which were also typed using the Affymetrix 5.0 array above.