Abstract
This article summarizes the basic cellular and extracellular events in development of the glomerulus and assembly of the glomerular basement membrane (GBM), paying special attention to laminin and type IV collagen. Cellular receptors for GBM proteins, including the integrins, dystroglycan, and discoidin domain receptor 1 (DDR1) are also discussed. Evidence is reviewed showing that the laminin isoform present in the earliest GBM, LM-111, and final isoform found in the mature GBM, LM-521, are each derived from both endothelial cells and podocytes. Although the early collagen α1α2α1(IV) similarly derives from endothelial cells and podocytes, collagen α3α4α5(IV) found in fully mature GBM is a product solely of podocytes. Genetic diseases affecting laminin and type IV collagen synthesis are also presented, with an emphasis on mutations to LAMB2 (Pierson syndrome) and COL4A3, COL4A4, and COL4A5 (Alport syndrome) and their experimental mouse models. Stress is placed on the assembly of a compositionally correct GBM for the acquisition and maintenance of glomerular barrier properties.
Keywords: Laminin, Type IV collagen, Alport syndrome, fibrosis
The kidney glomerular basement membrane (GBM) is a specialized extracellular matrix that supports and informs its’ adherent cells, the glomerular endothelium and podocytes. This is particularly true during glomerulogenesis, when there is a burst of GBM synthesis, and during certain glomerular diseases. In both cases, the molecular composition and morphology of the GBM changes, which undoubtedly affects the developmental programs and pathobiological behaviors of the endothelium and podocytes. In this brief article, I review the cellular origins of the major GBM components, and discuss some of the genetic diseases resulting in GBM abnormalities.
GBM Building Blocks
Like all basement membranes, the GBM is composed of networks of laminin, networks of type IV collagen, nidogens, heparan sulfate proteoglycans (HSPGs),1 and probably other, as yet undefined extracellular matrix molecules. Unlike most basement membranes, however, the GBM laminin and type IV collagen isoforms change during glomerular development and maturation, and GBM laminin and type IV collagen composition are also altered in certain glomerular diseases.1
Laminin is a large (~800 kd) heterotrimer of α, β, and γ glycoprotein chains. Depending upon the αβγ chain composition, at least 15 different laminin heterotrimer isoforms exist. Perhaps the most common isoform with the widest distribution in basement membranes throughout the body is laminin α1β1γ1 (also designated LM-111).2 Laminin heterotrimers form intracellularly within the secretory pathway, and, once secreted, polymerize with one another through interactions between globular domains at the N-termini of each of the three polypeptide chains.3 The laminin polymerization process occurs preferentially on cell surfaces through interaction of the C-terminus of the laminin α chain with plasma membrane laminin receptors.4 Upon engaging laminin, the receptors mediate tyrosine phosphorylation events, reorganization of the internal cytoskeleton and rearrangement of the external laminin polymer itself.4 The net effect is the assembly of a laminin-rich matrix at the correct, cell surface microanatomical domain that corresponds with signal transduction and cell adherence.
Type IV collagen is secreted as a largely triple helical heterotrimer of different collagen IV α chains. Only 3 heterotrimers are possible: α1α2α1(IV), α3α4α5(IV), or α5α6α5(1V).5 Molecular recognition motifs on the C-terminal NC1 domains of collagen IV α chains dictate trimer formation.6 Binding interactions between N-terminal, 7S domains on neighboring heterotrimers, and binding of the three C-terminal, NC1 domains with NC1 domains on adjacent heterotrimers, results in a flexible, three dimensional meshwork of polymerized collagen IV.
Nidogen-1 and -2 were originally thought to be integral basement membrane proteins that connect the laminin network to the network of polymerized collagen IV. This may not be the case, however, as mice deficient for both proteins have what appear to be entirely normal basement membranes in many organs, including kidney, and embryonic development also appears to be unaffected (but these animals die shortly after birth with pulmonary and cardiac failure).7 The full function(s) of the nidogens therefore are yet to be determined.
The two most prominent heparan sulfate proteoglycans within the GBM are agrin (with two heparan sulfate side chains), and perlecan (with three heparan sulfate side chains), although agrin appears to be the most abundant HSPG.8, 9 GBM HSPGs were originally thought to provide the basis for charge selectivity in the glomerular filtration barrier. This is a concept stemming from classic glomerular perfusion studies in which negatively charged macromolecules were shown to be less permeable than their neutral or cationic counterparts of the same size.10 More recently, this concept has been challenged. For example, mice with a genetic knockout of podocyte-derived agrin show no signs of permeability defects, despite the loss GBM anionic charge.11 In addition, in doubly mutant mice with a deletion of the perlecan heparan sulfate attachment domain, and podocyte-derived agrin, glomerular structure and function are normal, with no signs of proteinuria.12 Perhaps the cell surface glycocalyx on the glomerular endothelium and podocytes represents the most relevant charge selective sites in the glomerular permeability barrier.13
Morphogenesis of the GBM
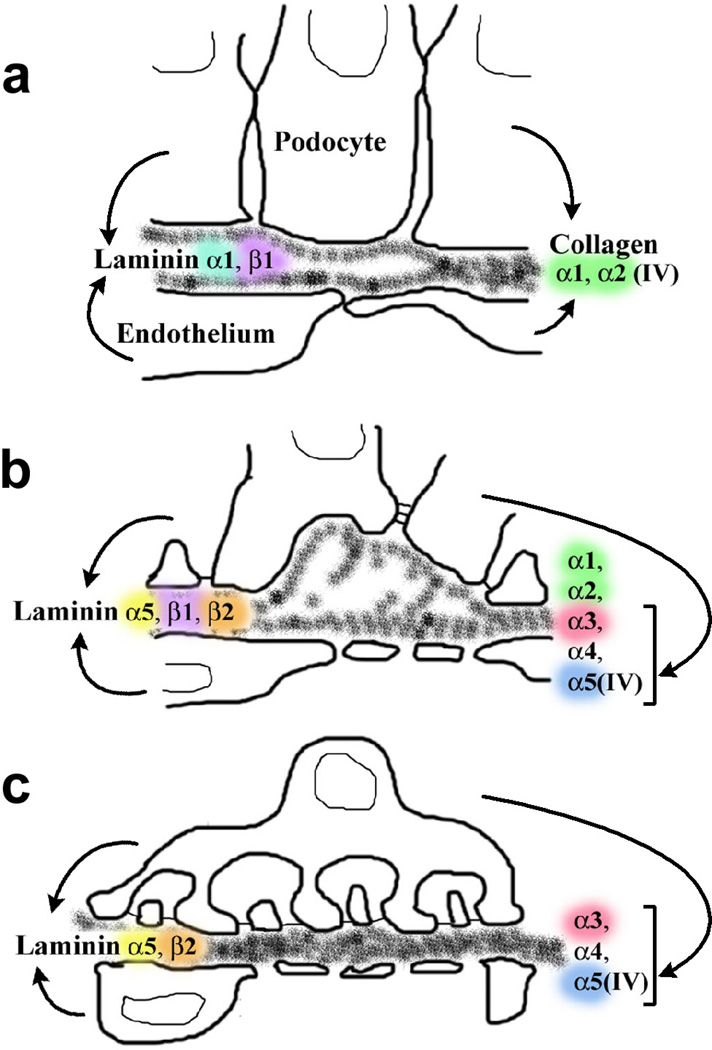
During the earliest stages of nephron formation, metanephric mesenchymal cells aggregating near tips of ureteric buds convert to a sphere of lumenized epithelial cells called a vesicle.14 Shortly thereafter, an invagination or vascular cleft forms in the vesicle and angioblasts (forerunners of endothelial cells) and probably mesangioblasts as well, migrate into this cleft. As the angioblasts make cell-cell contact and differentiate into endothelial cells, a layer of columnar epithelial cells below the cleft begin forming cytoplasmic interdigitations, and these cells subsequently differentiate into podocytes. At this time, there is a double layered basement membrane between the developing glomerular endothelial cells and podocytes. As glomerular capillary loops begin to form, the dual basement membrane fuses to produce a common GBM shared on its inner surface by endothelial cells and on its outer surface by podocytes. After basement membrane fusion, newly synthesized lengths of basement membrane material appear beneath developing podocyte foot processes that are somehow inserted or spliced into the fused GBM as capillaries inflate to their final dimensions.14 The cellular events taking place during formation of the glomerular capillary wall are shown schematically in Figure 1.
Figure 1.
Diagram showing stages of glomerular capillary wall development and cellular origins of different laminin and collagen IV isoforms. A: During vascular cleft and early capillary loop stages, a double basement membrane is located between the endothelium and podocyte and this dual structure fuses. This immature GBM contains LM-111 and collagen α1α2α1(IV), both of which originate in endothelial cells and podocytes. B: During intermediate stages of glomerular development, newly synthesized segments of basement membrane are seen beneath developing podocyte foot processes. LM-521 can now be detected, and originates from both endothelial cells and podocytes. Collagen α3α4α5(IV) is also seen for the first time at this stage, but this isoform derives solely from podocytes. C: As glomeruli mature, the glomerular capillary loops achieve their final dimensions. The GBM contains only LM-521 (derived from endothelial cells and podocytes) and collagen α3α4α5(IV) (derived only from podocytes). Reprinted with permission.34
GBM Binding Proteins – Cementing the Cells in Place
The major cell surface laminin and collagen-binding proteins are the integrins, which are transmembrane proteins consisting of 18 different α and 8 β subunits that heterodimerize non-covalently. Their long ectodomains contain binding sites for extracellular matrix ligands such as laminin or collagen IV, and their comparatively short cytoplasmic tails bind cytoskeletal proteins and also mediate transmembrane signaling. The major laminin-binding integrin in the glomerulus is believed to be integrin α3β1, whereas integrin α1β1 and α2β1 are thought to be the chief collagen IV receptors.15
Gene targeting studies have emphasized the importance of integrins in establishment and maintenance of the glomerular capillary wall. For example, when floxed alleles of integrin β1 are selectively deleted in podocytes by podocin-Cre (podocin is a podocyte specific, slit diaphragm associated protein)16 mice are proteinuric at birth with splitting of the GBM, effacement of foot processes, loss of podocytes, and they die at 1–3 weeks of age with renal failure.17, 18 Mice with a global loss of integrin α1 are normal at birth with no overt phenotype.19 However, these animals are more susceptible to adriamycin-induced injury later in life than wildtype mice, and they produce higher levels of reactive oxygen species and more glomerular type IV collagen.20 Integrin α2 knockout mice are also normal at birth but develop mild proteinuria and irregular thickening and protrusions of the GBM by 5 months of age.21 Finally, mice with deletion of integrin α3 die shortly after birth with defects in kidney and lung branching morphogenesis.22 Glomeruli have abnormally large capillary loops, disorganized and split basement membranes and podocyte foot processes are absent.22
Dystroglycan (DG), originally described as part of the dystrophin-glycoprotein complex of skeletal muscle linking the extracellular matrix to the actin cytoskeleton,23 is comprised of an extacellular α subunit connected to a transmembrane β subunit. The DG α subunit binds to laminin-G (LG) modules present at the C-terminus of laminin α chains, and to LG-modules that are also found in agrin and perlecan.24 Dystroglycan has been immunolocalized to the apical and basolateral surfaces of podocytes and their foot processes, indicating that it may play a role not only in adherence to the GBM but also in maintenance of foot process registration.25 On the other hand, when DG is deleted specifically from podocytes, or from the entire kidney, there are no obvious glomerular structural or functional phenotypes and no increased susceptibility to injury.26 These findings indicate that the integrins are probably the primary laminin-binding proteins in glomeruli, or at least can fully substitute for the loss of DG .26
Discoidin domain receptor 1 (DDR1), a receptor tyrosine kinase, is also a receptor for type IV collagen.27 DDR1 knockout mice develop proteinuria, focal bulges in the GBM, and focal loss of slit diaphragms, but do not progress to renal failure.28
GBM Laminin and Collagen IV Isoform Substitutions
The earliest GBM within the vascular clefts contains LM-111 and LM-511. Shortly thereafter, these juvenile isoforms disappear entirely and are replaced by LM-521, which is the only laminin isoform found in the GBM of fully mature glomeruli.29 As discussed later however, the juvenile laminin isoforms can be re-expressed in certain glomerular diseases.
Post-fixation immunoelectron microscopy of early developing mouse glomeruli using chain-specific antibodies against LM-111 shows immunolabel within intracellular biosynthetic organelles of endothelial cells as well as in podocytes.30 The same approach also shows that both cell types synthesize LM-521.30 This later point was underscored in a recent study in which human laminin α5 is expressed in transgenic mice harboring a bacterial artificial chromosome (BAC) containing the human LAMA5 locus.31 As is the case for native mouse laminin α5, the human protein also originates from both endothelial cells and podocytes in BAC transgenics.31
Mechanisms for silencing expression of LM-111 and LM-511, and upregulating LM-521 during glomerular development are not understood. Similarly, how the early laminin isoforms are removed from the GBM, and the later isoform installed, are not known. Interestingly, the laminin isoform substitution process appears to rely on the presence of both endothelial cells and podocytes. For example, routine organ culture of embryonic day 12 mouse kidneys supports the development of avascular glomerular epithelial tufts. Cells within these tufts express the podocyte-differentiation markers, GLEPP-1 and nephrin, and assemble epithelial slit diaphragms at the correct microanatomical site between foot processes.32 These putative podocytes also assemble a basement membrane-like matrix that ultrastructurally resembles the GBM.32 The basement membranes laid down in organ culture contain laminin α1, α5, and β1 but not β2. When these organ cultured kidneys are grafted into the anterior eye chamber of adult mouse hosts, however, many of the glomerular epithelial tufts of the engrafted kidneys become vascularized by host-derived endothelial cells. In these cases, laminin β2 is now found within glomeruli.32 Signals derived specifically from endothelial cells, as well as circulating growth factors and chemokines, may be critically important for regulating the full laminin isoform substitution program, although hemodynamic forces associated with vascularization can not be wholly discounted.32
The immature GBMs found in the vascular cleft and early capillary loop stage glomeruli contain collagen α1α2α1(IV).33 As glomeruli mature, this network is replaced by collagen α3α4α5(IV), which is the only collagen IV network found in the fully mature GBM. Immunoelectron microscopic evidence from developing mouse kidneys shows that both glomerular endothelial cells and podocytes participate in synthesis of the collagen α1α2α1(IV) network.34 However, anti-collagen α3α4α5(IV) antibodies immunolabel the biosynthetic pathway only in podocytes; endothelial cells are negative (Figure 2).34 As is the case for laminins, the genetic, protein, and cellular mechanisms regulating collagen IV gene expression, removal of earlier isoforms, and deposition of the final isoform into the mature GBM, are not known. The composition of the GBM during different stages of glomerular development and the cellular origins of laminin and type IV collagen chains are diagrammed in Figure 1.
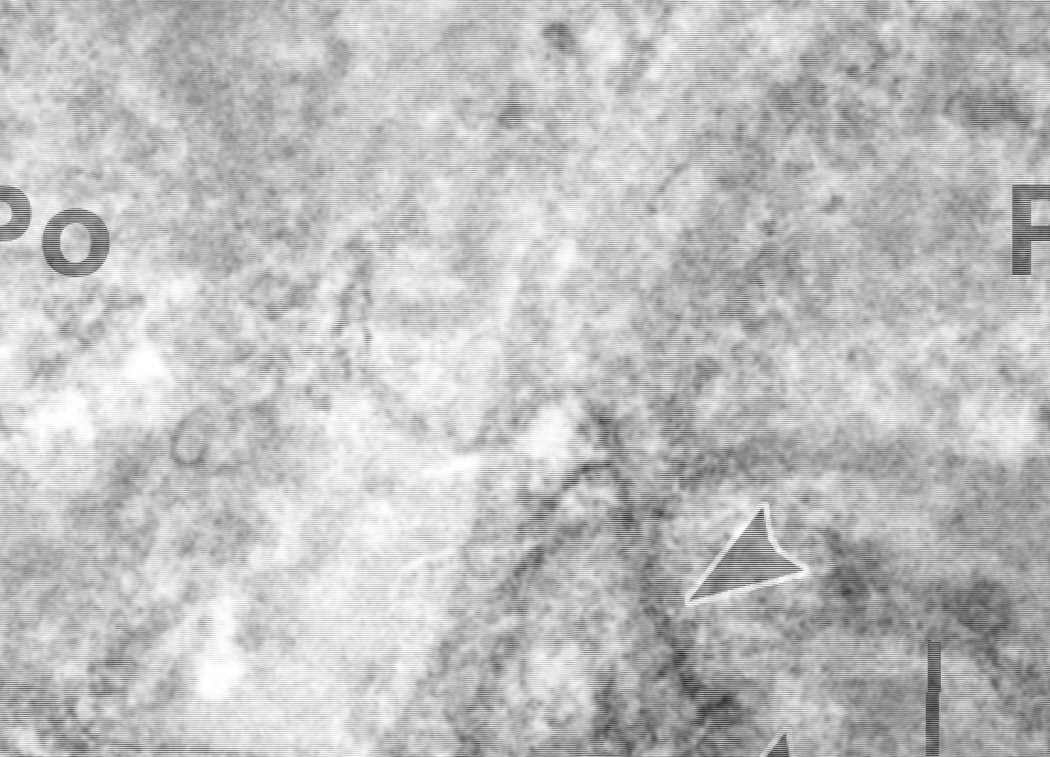
Figure 2.
Post-fixation immunoperoxidase electron microscopy of developing mouse glomerular capillary wall using anti-collagen α3α4α5(IV) IgG. Peroxidase reaction product appears within the GBM and intracellular biosynthetic organelles (arrowheads) of podocytes (Po). Labeling is absent in the glomerular endothelium (En). Reprinted with permission.34
Reasons for the removal of the juvenile isoforms, LM-111 and collagen α1α2α1(IV), and replacement with the adult LM-521 and collagen α3α4α5(IV) networks are also not completely understood. One possibility is that the juvenile laminin and collagen IV isoforms provide a more compliant or resilient GBM matrix. Conceivably, this may be important for the spreading of endothelial cells and podocytes on their respective GBM surfaces as the glomerular capillary loops inflate. Whether this cell mobility is facilitated by the expression of different densities or types of basement membrane binding proteins is not known. Nevertheless, there is genetic evidence from mice and humans that the laminin and collagen IV isoform substitution may be necessary for the attainment of the highly differentiated state assumed by glomerular endothelial cells and podocytes and for establishment and maintenance of the glomerular filtration barrier.
Hereditary Diseases of Laminin
When either the Lamb1 or Lamc1 gene, encoding the laminin β1 or γ1 chains, respectively, are deleted in mice, embryos do not survive beyond day 5.5 post coitum and completely lack recognizable basement membranes.35, 36 Lama1 knockouts survive until ~embryonic day 7, apparently because laminin α5 can partially substitute for the absence of laminin α1.35 Interestingly, the deletion in mice of only the nidogen binding domain on the laminin γ1 chain results in death immediately after birth, with renal agenesis and lung development deficiencies.37 This is surprising in view of the absence of a kidney phenotype in mice lacking both nidogen-1 and -2,7 suggesting that the nidogen-binding domain on laminin γ1 may have other functions.37 Probably because of the importance of basement membrane laminins in early embryogenesis, there are no known mutations to the human LAMA1, LAMB1 or LAMC1 genes.
Mice with a global deletion of the Lama5 gene, which encodes the laminin α5 protein, die before birth with neural tube closure defects and placental vascular abnormalities, among other deficits.38 (No LAMA5 mutations have thus far been identified in humans either, probably because they would also cause embryonic lethality). When embryonic kidneys from Lama5 knockout mice are examined, glomerular capillaries and GBMs are absent.39
Knowing that the laminin α5 chain normally originates in glomeruli from both endothelial cells and podocytes,30 we attempted to rescue the Lama5 null glomerular phenotype. Our approach was to transplant embryonic day-12 laminin α5 knockout kidneys under the renal capsule of newborn hosts carrying the cell-lineage marker, LacZ, but wildtype for Lama5. One week after transplantation, LacZ-positive, host-derived endothelial cells had migrated into engrafted kidney tissue, producing hybrid glomeruli containing wildtype endothelial cells and Lama5 null podocytes.40 Close examination by confocal immunofluorescence microscopy shows that GBMs within these hybrid glomeruli are stratified: laminin α5 occupies the inner, subendothelial layer of basement membrane, and laminin α1 is found in the outer layer beneath mutant podocytes.40 Electron microscopy of these hybrid glomeruli shows that the GBM is poorly condensed, and podocyte foot processes fail to develop.40 Quantification of the endothelial cell-derived laminin α5 by confocal image analysis shows that the hybrid GBMs contain ~50% of the normal complement of this laminin chain found in wildtype glomeruli.40 This signifies that, at least under these conditions, the endothelium can be a major source for GBM proteins. Further evidence that the laminin α1–α5 transition is important for podocyte development and maintenance comes from inactivation of Lama5 exclusively in podocytes. Conditional loss of Lama5 in podocytes alone results in proteinuria, progressive GBM thickening, and podocyte foot process effacement.41
Mice deficient for Lamb2, which encodes the laminin β2 chain, are born with an apparently intact GBM probably caused by the sustained, compensatory expression of laminin β1.42 A few weeks after birth, they become massively proteinuric and, subsequently, there is progressive fusion of podocyte foot processes.42 Albuminuria occurs well before podocyte foot process effacement, however, emphasizing the importance of a correctly assembled GBM on establishment of glomerular barrier properties.43 These mice also display extrarenal defects, including abnormal neuromuscular junctions where there is lack of active zones and dispersal of synaptic vesicles, intrusion of Schwann cell folds into the synaptic cleft, and marked reduction of junctional folds in the muscle fiber, and mice die ~3 weeks of age.42 Mutations to the human LAMB2 gene have been discovered and shown to cause Pierson syndrome,44 which results in a congenital, familial nephrosis, ocular anomalies, and life-threatening neonatal respiratory problems,45 which are generally similar to symptoms seen in Lamb2 knockout mice.
Hereditary Diseases of Type IV Collagen
Unlike the case for LM-111, deletion of much of the mouse Col4a1 and Col4a2 loci encoding the collagen α1α2α1(IV) network does not result in early lethality, and embryos survive as late as embryonic day-11.5.46 In earlier stage embryos, the absence of collagen α1α2α1(IV) also does not affect the expression of laminins or nidogen-1, and these proteins are assembled into extracellular matrices that ultrastructurally resemble basement membranes.46 The immunolabeling patterns in these basement membranes show discontinuities, however, and there are blood vessel dilations and hemorrhaging at embryonic day 10.5–11.5. Together, these results indicate that although collagen α1α2α1(IV) is not required for early embryogenesis and initial organogenesis, the network is necessary to stabilize basement membrane integrity at mid-gestation.46
Mutations in the human COL4A1 gene results in porencephaly and cerebral small vessel disease, predisposing affected individuals to intracerebral hemorrhage.47 A separate COL4A1 mutation(s) results in a novel spectrum of symptoms including hereditary angiopathy with nephropathy, aneurysms, and muscle cramps.48 With respect to renal defects, patients have hematuria, large cortical and medullary intrarenal cysts, and focal thickening and splitting of kidney tubular and peritubular capillary basement membranes.48 The GBM in these individuals appears to be unaffected, however, probably because of the replacement of the collagen α1α2α1(IV) network with collagen α3α4α5 (IV) during glomerular development.
One of the most extensively studied genetic diseases of the GBM is Alport syndrome, which in humans is caused by mutations to the COL4A3, COL4A4, and/or COL4A5 genes.49 Alport is a familial nephropathy with hearing impairment and ocular defects and, in most families, is caused by mutations to COL4A5. In these cases, it is transmitted as an X-linked dominant trait causing greater disease severity in males than females. More than 300 mutations have been identified in COL4A5, ranging from deletions of various sizes, duplications and rearrangements, insertions and single base changes.49 Many of these occur in codons that affect the Gly-X-Y repeats in the type IV collagenous domains, making it impossible to form a stable collagen α3α4α5(IV) heterotrimer. In kidney there is variable thickening, thinning, and splitting of the GBM and widespread effacement of podocyte foot processes, especially in areas overlying irregular projections or outpockets of GBM. This results in progressive hematuria and proteinuria and, in most individuals eventually, renal failure.50, 51
Unlike what occurs during normal glomerular development, and because of the absence of the collagen α3α4α5(IV) heterotrimer, there is retention of the collagen α1α2α1(IV) network in the Alport GBM. This network is more susceptible to proteolysis than collagen α3α4α5(IV), and helps explain why the Alport GBM eventually deterioriates.52 In many affected individuals, however, there is no evidence of kidney malfunction for up to several decades, indicating that the presence of the collagen α3α4α5(IV) network per se within the GBM is not required for the initial development and maintenance of the glomerulus and its barrier properties.
Alport syndrome can also be transmitted less frequently in humans as an autosomal recessive disorder involving homozygous or compound heterozygous mutations to COL4A3 and COL4A4.49 Mutations to these genes do not always result in full-blown Alport, however. Many cases of thin basement membrane nephropathy, also known as benign familial hematuria, have also been linked to COL4A3 and COL4A4, but these individuals do not progress to Alport and the expression of collagen IV chains in the GBM is normal.49 These results indicate that this disorder represents a heterozygous condition of autosomal recessive Alport, and/or that it is genetically heterogeneous.49
There have been several studies on the structure and composition of the Alport GBM from human patients and in canine and mouse genetic models of the disease.53 We began studying the Col4a3 knockout mouse model of Alport some time ago, and reported that LM-111 underwent normal downregulation during initial glomerular development. However, this isoform was ectopically re-expressed by both endothelial cells and podocytes in maturing Alport mouse glomeruli.54 Immunoelectron microscopy showed that the LM-111 was deposited specifically in many of the irregular subepithelial GBM projections typically seen in Alport disease. We speculated that the presence of this juvenile laminin isoform, which is more characteristic of immature glomeruli, may have contributed to the foot process effacement seen in the overlying podocytes.54 Subsequently, we quantified laminin α1 and laminin α5 chains in Alport mouse GBMs and found both to be significantly increased over wildtype.55
We also carried out ferritin permeability studies and electron microscopy showed that Alport mouse GBMs are more permeable than wildtype. These abnormal permeabilities occurred even in areas of Alport glomerular capillary walls with ultrastructurally normal GBMs, normal foot process morphologies and slit diaphragms.55 Further, permeabilities are even greater in those regions of the GBM previously shown to contain ectopic deposits of LM-111.55 Taken together, we believe that the Alport GBM is defective not only due to an absence of collagen α3α4α5(IV) and prolonged presence of collagen α1α2α1(IV), but due also to the dysregulation of the laminins, which may represent a compensatory response. The abnormal GBM that results likely conveys inappropriate signals to the adherent endothelial cells and podocytes, which, in turn, causes progressive disease. Interestingly, loss of the collagen IV receptor, DDR1, results in a milder form of the disease in Alport mice, and downregulation of the pro-fibrotic cytokines TGFβ and CTGF.56 These results further implicate a key role for basement membrane receptor proteins in monitoring changes in GBM composition and transmitting this information to adherent cells.
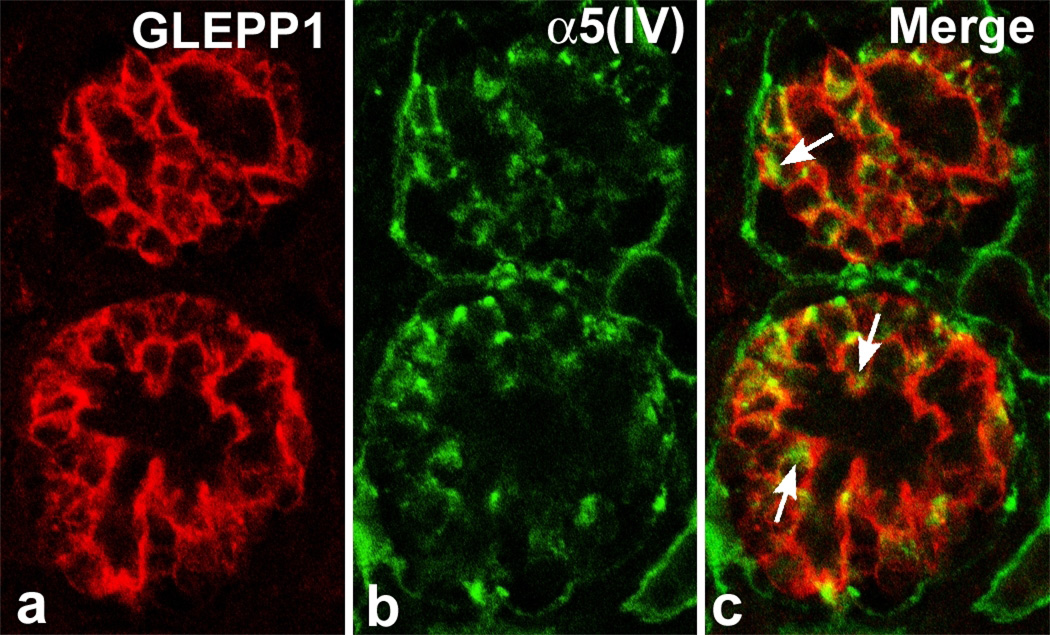
As we had done before with the partial rescue of the laminin α5-null glomerular phenotype,40 we grafted mouse embryonic day-12 Col4a3 knockout kidneys into wildtype hosts carrying a transgenic lineage marker.34 Hybrid glomeruli containing wildtype endothelial cells and mutant (Alport) podocytes develop within grafts. Unlike the outcome with the laminin α5 grafts, however, there is no expression of collagen α3α4α5(IV) within graft glomeruli containing wildtype endothelial cells.34 In addition, confocal microscopy of developing Alport glomeruli shows that collagen α5(IV) immunolocalizes specifically intracellularly within developing podocytes but, in the absence of one of its usual GBM binding partners, collagen α3(IV), it is not deposited in the GBM (Figure 3).34 These results provide additional evidence that collagen α3α4α5(IV) originates solely from podocytes, and that glomerular Alport disease is caused by a genetic defect manifest specifically within this cell.34
Figure 3.
Section of immature Alport mouse glomerulus double labeled with (A): podocyte-specific anti-GLEPP1 and (B): anti-collagen α5(IV). Merged image (C) shows intracellular immunolabeling for collagen α5(IV) (arrows) exclusively within podocytes. Reprinted with permission.34
Construction completed; home repairs
Once the normal GBM has been fully assembled and has assumed its mature status, the endothelial cells and podocytes engaged in secretion of the various GBM constituents downregulate their GBM biosynthetic programs. Indeed, the normal GBM appears to be a remarkably stable structure. For example, classic metabolic labeling studies published 35 years ago show that the in vivo loss of basement membrane protein radioactivity from adult rat GBM collagen is as slow as that for tail tendon collagen (with a half life of more than 100 days).57 As is the case for regulation of the different laminin and collagen IV isoforms during glomerular development, however, details regarding the suppression of basement membrane protein gene transcription and/or message translation after glomerular maturation are almost completely lacking. Nevertheless, in almost every example of primary glomerular disease (including postinfection glomerulonephritis, immunoglobulin A nephropathy, anti-GBM disease, minimal change nephrotic syndrome, focal segmental glomerulosclerosis, mesangial proliferative disease, hereditary nephritis) and metabolic or hematologic diseases affecting the kidney (including hypertension, diabetes mellitus, lupus, HIV), the normal architecture of the glomerular capillary wall is affected. In many cases, these changes may either be caused by, or result in, alterations to the GBM, including renewed biosynthesis of GBM proteins that can then progress to glomerular fibrosis and eventually, end stage renal disease. To slow or prevent fibrosis, we urgently need to understand controls that silence GBM protein gene expression and prevent matrix deposition. Similarly, we need to learn how to selectively repair laminin and type IV collagen genes to restore normal protein expression patterns in hereditary diseases affecting the GBM.
Acknowledgments
Supported in part by NIH grant P01 DK065123
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miner JH. Organogenesis of the kidney glomerulus. Focus on the glomerular basement membrane. Organogenesis. 2011;7:75–82. doi: 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Cheng YS, Champilaud RE, Burgeson MP, Yurchenco PD. Self-assemby of laminin isoforms. J Biol Chem. 1997;272:31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 4.Colognato H, Winkelman DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeletal network. J Cell Biol. 1999;143:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 6.Koshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 7.Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groffen AJ, Ruegg MA, Dijkman H, van de Veldon TJ, Buskins CA, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem. 1998;46:19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 9.Groffen AJA, Veekamp JH, Monnens LAH, van den Heuvel LPWJ. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant. 1999;14:2119–2129. doi: 10.1093/ndt/14.9.2119. [DOI] [PubMed] [Google Scholar]
- 10.Bohrer MP, Baylis C, Humes HD, Glassock RJ, Robertson CR, et al. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest. 1978;61:72–78. doi: 10.1172/JCI108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permeability. Am J Pathol. 2007;171:139–152. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24:2044–2051. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamson DR. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis. 2009;5:275–287. doi: 10.4161/org.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew S, Chen X, Pozzi A, Zent R. Integrins in renal development. Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-1890-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, et al. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, et al. β1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, et al. Integrin β1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin α1 by homologous recombination permits normal renal development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, et al. Lack of integrin α1β1 lead to severe glomerulosclerosis after glomerular injury. Am J Pathol. 2004;165:617–630. doi: 10.1016/s0002-9440(10)63326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, et al. Integrin α2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissure Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 23.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 24.Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–317. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 25.Vogtlander NPJ, Dijkman H, Bakker MAH, Campbell KP, van der Vlag J, Berden JHM. Localization of α dystroglycan on the podocyte: from top to toe. J Histochem Cytochem. 2005;53:1345–1353. doi: 10.1369/jhc.4A6596.2005. [DOI] [PubMed] [Google Scholar]
- 26.Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development of function, in health or injury. Am J Physiol Renal Physiol. 2011;300:F811–F820. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 28.Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, et al. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, et al. The laminin alpha chains: Expression, developmental transitions, and chromosomal locations of alpha 1–5, identification of laminins 8–11, and cloning of a novel alpha 3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- 31.Steenhard BM, Zelenchuk A, Stroganova L, Isom K, St John PL, et al. Transgenic expression of human LAMA5 suppresses murine Lama5 mRNA and laminin α5 protein deposition. PLoS ONE. 2011;6:e23926. doi: 10.1371/journal.pone.0023926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St John PL, Wang R, Yin Y, Miner JH, Robert B, et al. Glomerular laminin isoform transitions: Errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol. 2001;280:F695–F705. doi: 10.1152/ajprenal.2001.280.4.F695. [DOI] [PubMed] [Google Scholar]
- 33.Miner JH. Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens. 1998;7:13–19. doi: 10.1097/00041552-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Abrahamson DR, Hudson BG, Stroganova, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 36.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, et al. specific ablation of the nidogen-binding site in the laminin γ1 chain interferes with kidney and lung development. Development. 2002;129:2711–2722. doi: 10.1242/dev.129.11.2711. [DOI] [PubMed] [Google Scholar]
- 38.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the α5 chain. J Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miner JH, Li C. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–298. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamson DR, St John PL, Isom K, Robert B, Miner JH. Partial rescue of glomerular laminin alpha5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 2007;18:2285–2293. doi: 10.1681/ASN.2007020207. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin α5. J Am Soc Nephrol. 2010;21:579–586. doi: 10.1681/ASN.2009091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin β2: Nephrosis despite compensation by laminin β1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 43.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenker M, Aigner T, Wendler O, Tralau T, Munterfering H, Fenski R, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 45.Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, et al. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: An autosomal recessive syndrome. Am J Med Genet A. 2004;130:138–145. doi: 10.1002/ajmg.a.30310. [DOI] [PubMed] [Google Scholar]
- 46.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 47.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 48.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 49.Gubler MC. Inherited diseases of the glomerular basement membrane. Nature Clin Prac Nephrol. 2008;4:24–37. doi: 10.1038/ncpneph0671. [DOI] [PubMed] [Google Scholar]
- 50.Hudson BG, Tryggvason K, Sundarmoorthy M, Neilson EG. Alport syndrome, Goodpasture syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 51.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: Beacons for discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 52.Kalluri R, Shield CF, Todd P, Hudson BG, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashtan CE, Kim Y, Lees G, et al. Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: Aberrant laminin α2 deposition is species independent. J Am Soc Nephrol. 2001;12:252–260. doi: 10.1681/ASN.V122252. [DOI] [PubMed] [Google Scholar]
- 54.Abrahamson DR, Prettyman AC, Robert B, St John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–834. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- 55.Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, et al. Laminin compensation in collagen α3(IV) knockout (Alport) glomeruli contributes to permeability defects. J Am Soc Nephrol. 2007;18:2465–2472. doi: 10.1681/ASN.2007030328. [DOI] [PubMed] [Google Scholar]
- 56.Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, et al. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Price RG, Spiro RG. Studies on the metabolism of the renal glomerular basement membrane. J Biol Chem. 1977;252:8597–8602. [PubMed] [Google Scholar]