SUMMARY
Although Aβ peptides are causative agents in Alzheimer's disease (AD), the underlying mechanisms are still elusive. We report that Aβ42 induces a translational block by activating AMPK, thereby inhibiting the mTOR pathway. This translational block leads to widespread ER stress, which activates JNK3. JNK3 in turn phosphorylates APP at T668, thereby facilitating its endocytosis and subsequent processing. In support, pharmacologically blocking translation results in a significant increase in Aβ42 in a JNK3-dependent manner. Thus, JNK3 activation, which is increased in human AD cases and a familial AD (FAD) mouse model, is integral to perpetuating Aβ42 production. Concomitantly, deletion of JNK3 from FAD mice results in a dramatic reduction in Aβ42 levels and overall plaque loads and increased neuronal number and improved cognition. This reveals AD as a metabolic disease that is under tight control by JNK3.
INTRODUCTION
Aβ peptide accumulation is a hallmark of Alzheimer's disease (AD), being released from neurons via extracellular and subsequent intramembrane cleavage reactions of the amyloid precursor protein (APP) (Tanzi and Bertram, 2005). Recent findings suggest that soluble oligomeric are the pathogenic forms, eliciting neurotoxic effects that culminate in synaptic dysfunction and neuronal loss (Haass and Selkoe, 2007). Discovery of Prp and EphB2 as receptors for oligomeric Aβ42 (Cisse et al., 2011; Lauren et al., 2009) provides support for the view that oligomeric Aβ peptides could function as neurotoxic ligands, initiating diverse cellular signaling events that range widely, including inflammation, mitochondrial dysfunction, oxidative stress, apoptosis/autophagy, intracellular calcium imbalance, and a block in LTP (Koo and Kopan, 2004), any of which could contribute to AD pathology. The mechanism by which oligomeric Aβ peptides elicit such diverse cellular outcomes, however, has remained elusive.
Here, we report that oligomeric Aβ42 exerts such diverse effects in part by inducing a translational block, which is accompanied by ER stress as indicated by increased phosphorylation of Eif2α in hippocampal neurons. Increased Eif2α phosphorylation was reported to inhibit the late phase of LTP and memory acquisition (Costa-Mattioli et al., 2007; Costa-Mattioli et al., 2009). Once induced, ER stress activates Unfolded Protein Response (UPR), inducing a widespread secondary reactions, some of which include changes in inflammatory responses as well as cell survival programs (Ron and Walter, 2007), the often reported phenotypes in AD.
As part of UPR, ER stress activates the JNK pathway (Urano et al., 2000). JNK proteins, especially JNK3, a brain-specific JNK isoform, have been reported to play roles under neurodegenerative conditions, such as Parkinson's disease: Deletion of JNK3 in combination with JNK2 prevented loss of dopaminergic neurons after MPTP administration (Hunot et al., 2004). Deleting JNK3 also resulted in a significant increase in neuronal and oliogodendrocyte survival after traumatic injuries in the CNS (Beffert et al., 2006; Li et al., 2007). Although JNK activation has been reported in human AD brains, its role in AD pathology development remained unclear. To address this question, we analyzed an AD mouse model with and without JNK3. Our results indicate that JNK3 activation is integral to AD pathology, where JNK3 deletion restores the translational block induced by oligomeric Aβ42 and the effect of UPR.
RESULTS
Oligomeric Aβ42 induces a translational block by activating AMPK, thereby inhibiting the mTOR pathway
Oligomeric Aβ42 inhibits LTP and impairs memory formation in vivo (Cleary et al., 2005; Walsh et al., 2002), suggesting that Aβ peptides are pathogenic species that disrupt normal synaptic function and cognition. Disrupting translational control by disabling eif2α phosphorylation or deleting its kinase, GCN2, also resulted in inhibition of LTP and memory acquisition (Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007). Considering these parallel findings, we decided to ask whether Aβ42 could induce a translational block. To address the question, we measured the amount of 35S-methionine incorporation in rat hippocampal neurons after treatment with 5 μM Aβ42 overnight. It should be noted that the actual concentration of oligomeric Aβ42 in 5 μM Aβ42 was estimated to be 250 nM (Figure 1A). As controls, parallel cultures were treated with Cycloheximide, a protein synthesis inhibitor, and Rapamycin and Thapsigargin, agents whose actions impinge on the translational machinery. Oligomeric Aβ42 treatment at 250nM inhibited 35S-methionine incorporation by 44% (n=3-5, p≤0.0001), while 10 nM Rapamycin and 0.5 μM Thapsigargin reduced 35S-methionine incorporation by 70-72% (n=3-5, p≤0.01 and 0.001, respectively, Figure 1B and C). The effect of 20 μM Cycloheximide was virtually complete, blocking translation by 99% (n=3-5, p≤0.00001). The reduction in 35S-methionine incorporation was not due to cell death induced by Aβ42, since there were a very few MAP2+ neurons that incorporated propidium iodide when alive (Figure 1D). We therefore conclude that Aβ42 induces a translational block in cultured neurons.
Figure 1. Oligomeric Aβ42 induced the translational block by activating AMPK.
(A) 6E10 Western blot of oligomeric Aβ42 and the control Aβ42R (reverse sequence) used in the study. Actual concentration of oligomeric species is approximately 250 nM in 5 μM total Aβ42.
(B) Oligomeric Aβ42 inhibits translation in rat hippocampal neurons. All 35S-methionine fluororadiograms were obtained from the same experiment.
(C) Quantification of 35S-methionine incorporation in (B). The rate of 35S-methionine incorporation in the control was regarded as 1.0. Data are represented as means ± SEM. P values were calculated student t-test: p≤0.001 for Aβ42, p≤0.005 for Rapamycin, p≤0.001 for Thapsigargin, and p≤0.00001 for Cycloheximide.
(D) Overnight treatment with 250 nM oligomeric Aβ42 in metabolic experiments did not induce cell death in hippocampal neurons. Note that the majority of live MAP2+ neurons failed to incorporate propidium iodide both in control and Aβ42-treated cultures. Instead, oligomeric Aβ42 induced neurite degeneration (arrow). After propidium iodide incorporation while they were alive, cells were fixed and immunostained for MAP2 to identify neurons.
(E) Oligomeric Aβ42 induces a translational block by activating AMPK, thereby inhibiting the mTOR pathway. Rat hippocampal neurons were treated with 250 nM oligomeric Aβ42, 0.5 μM Thapsigargin, and 10 nM Rapamycin for the indicated periods of time. Note that S792 phosphorylation in Raptor and S1387 phosphorylation in TSC2, both of which are phosphorylated by AMPK, were also increased. S6K phosphorylation declined gradually with oligomeric Aβ42, suggesting mTOR inhibition. Oligomeric Aβ42 also induces ER stress based on Eif2a phosphorylation.
(F) Monomeric and fibrillar forms of Aβ42 fail to activate AMPK significantly. Rat hippocampal neurons were treated with 250 nM monomeric and fibrillar Aβ42 for the indicated periods of time.
(G) AMPK is necessary for oligomeric Aβ42 to inhibit the mTOR pathway. S6K phosphorylation was increased with Aβ42 when AMPK activation was inhibited by Compound C. Hippocampal neurons were treated with vehicle or 5 μM Compound C for 30 min prior to oligomeric Aβ42 addition for the indicated amount of time. As a control for Compound C, p-RaptorS792 blot is also shown along with total S6K and Raptor protein blots.
Rapamycin inhibits translation by blocking recruitment of mTOR to the translational initiation complex (Ma and Blenis, 2009), and Thapsigargin by inducing ER stress, which results in phosphorylation of Eif2α (Costa-Mattioli et al., 2009; Ron and Walter, 2007). In order to understand whether the mechanism by which oligomeric Aβ42 causes a translational block resembles that of Thapsigargin or Rapamycin, we examined the temporal changes in the phosphorylation status of various proteins that are known to be involved in the mTOR pathway and UPR in hippocampal neurons. Oligomeric Aβ42 induced a rapid increase in AMP-activated protein kinase α (AMPKα) phosphorylation (Figure 1E). Rapamycin and Thapsigargin also activated AMPK, but the kinetics of its activation differed from that by oligomeric Aβ42. Monomeric and fibrillar forms of Aβ42 did not activate AMPK in hippocampal neurons (Figure 1F). AMPK was shown to phosphorylate TSC2 and Raptor at S1387 and S792, respectively, thereby inhibiting the mTOR pathway (Gwinn et al., 2008; Inoki et al., 2003). Indeed, both TSC2 and Raptor were phosphorylated by oligomeric Aβ42 in kinetics similar to that of AMPK phosphorylation, and phosphorylation of S6 kinase decreased gradually over time (Figure 1D). We hypothesized that oligomeric Aβ42 inhibits the mTOR pathway in part by activating AMPK. Indeed, when AMPK activation was inhibited with 5 μM Compound C as indicated by a reduction in phospho-RaptorS792 levels, S6K phosphorylation was increased significantly with oligomeric Aβ42 at all the time points tested (Figure 1F). These results together suggest that oligomeric Aβ42 initiates a translational block by inhibiting the mTOR pathway via a mechanism that involves AMPK activation. It should also be noted that oligomeric Aβ42 increased phosphorylation of Eif2α, although the extent of its increase is much less than that by Thapsigargin (Figure 1E). We interpret this result as suggesting that oligomeric Aβ42-mediated translational block leads to UPR.
JNK3-dependent phosphorylation of APP increases APP endocytosis and processing
UPR was shown to activate the JNK pathway (Urano et al., 2000). We hypothesized that once UPR is induced, it activates JNK3, which in turn promotes further APP processing by phosphorylating it at T668P in neurons. Indeed, Thapsigargin and Rapamycin induced JNK3 activity in rat hippocampal neurons: JNK3 activity increased 5-30 min after treatments, which was sustained for up to 3-6 hrs (Figure 2A, B).
Figure 2. JNK-mediated phosphorylation of T668P regulates APP internalization and processing.
(A) Both Thapsigargin and Rapamycin activate JNK3 in rat hippocampal neurons. At the indicated times, the lysates were harvested and subjected to immunoprecipitation/kinase assays using GST-c-jun as a substrate. As controls, JNK3 Western results are also shown.
(B) Quantification of 32P-GST-c-jun in (A). The data are represented as means ± SEM (n=2-3).
(C) JNK3 phosphorylates APP at T668P in vitro. Purified GST-APPICD proteins were subjected to in vitro kinase assays in the presence of 32P-γ-ATP using active JNK3, p38, CDK5/p35, and GSK-3β according to manufacturer's instruction (Millipore). Only JNK3 and CDK5 phosphorylated APP, while p38 and GSK-3β did not. Note that JNK3 failed to phosphorylate APP, when T668 was mutated to A, but CDK5/p35-dependent phosphorylation still remained. These data suggest that JNK3 phosphorylates T668P, while CDK5/p35 phosphorylates sites other than T668P.
(D) JNK facilitates internalization of APP from the cell surface. To activate the endogenous JNK pathway, hippocampal neurons were treated with 50 ng/ml Anisomycin for the indicated amount of time. After Anisomycin treatment, cells were subjected to cell-surface biotinylation on ice, and the biotinylated APP on the cell-surface was detected following Neutravidin pulldown. Note that the amount of APP on the cell surface was increased initially, which was followed by a decrease with Anisomycin treatment over time. To test whether the changes in APP trafficking were due to JNK activation, a parallel set of cultures were treated with cell-permeable JNK inhibitor, TAT-TI-JIP peptides. Control cultures were treated with cell-permeable control peptides. When JNK activation was inhibited as indicated by the reduction in p-cjun and p-APPT668 levels, the amount of APP on the cell surface remained the same with Anisomycin treatment over time. These results suggest that JNK activation is necessary for inducing APP internalization.
(E) Quantification of cell-surface biotinylated APP after Anisomycin treatment. Data are represented as means ± SEM. P values were calculated by student t-test.
(F) JNK activation increased APP processing following internalization. The amount of CTF increased with Anisomycin treatment. Following cell-surface biotinylation on ice, neurons were incubated with Anisomycin for 2 hrs at 37°C. At the end of incubation, biotins left on the cell-surface were removed by treating cells with 50 mM DTT before protein extracts were prepared. The internalized APP and its products were subsequently detected by Neutravidin pulldown followed by APP Western blotting as well as 6E10 immunoprecipitation followed by Strepavidin-HRP blotting.
(G) T668P phosphorylation is necessary for APP internalization and processing. Anisomycin treatment reduced the full-length, biotinylated wild type APP from the cell surface, while it increased the amount of biotinylated CTF. On the other hand, Anisomycin failed to induce internalization of and CTF production from the A668P mutant. 293T cells were transfected with the wild type, full-length APP and A668P mutant, and subjected to the same cell surface biotinylation and internalization as with cortical neurons. Also shown are p-JNK and JNK blots in addition to a loading control, ERK blots.
(H) Quantification of the data in G. Data are represented as means ± SEM. P values were calculated by student t-test.
APP has a MAP kinase phosphorylation site at T668P, which becomes phosphorylated only in neurons (Iijima et al., 2000). We hypothesized that JNK3-mediated phosphorylation of APP results in internalization of APP into endosomal compartments, wherein it undergoes amyloidogenic processing. In support, Aβ42 has been detected in endosomes via immuno-EM in AD cases (Takahashi et al., 2002). We first asked whether JNK3 phosphorylated T668P in vitro. Active JNK3 indeed phosphorylated APP at T668P in vitro, as JNK3 failed to phosphorylate APP when T668 was mutated to A668 (Figure 2C). Active CDK5/p35 complex, on the other hand, still phosphorylated the mutant APPA668, suggesting that JNK3 phosphorylates T668P, while CDK5/p35 phosphorylates sites other than T668P.
As a way to address whether JNK activation affects APP internalization, we asked whether JNK activation alters the amount of the full-length APP on the cell surface. In addition, since Anisomycin activates p38 besides JNK, cortical neurons were pre-incubated for 1 hr with 1 μM cell-permeable JNK-Interacting-Protein (JIP) peptides that inhibit JNK specifically (Barr et al., 2002; Farias et al., 2009), while control cultures were treated with cell-permeable control peptides. JIP peptides, TAT-TI-JIP, contain 22-amino acids, which were shown to be necessary for JIP to inhibit JNK activation (Dickens et al., 1997). The JIP peptides were also modified to make it permeable to mammalian cells by adding 10-amino acid sequences of HIV-TAT protein (Bonny et al., 2001; Schwarze et al., 1999). After treatments with 50 ng/ml Anisomycin at 37°C for 0, 5, 15, and 30 min., cells were subjected to cell surface biotinylation on ice for 30 min, and the amount of the cell surface-located, biotinylated, full-length APP was determined following Neutravidin pulldown and subsequent Western blotting for APP. In control cultures, Anisomycin treatment resulted in a rapid increase in biotinylated APP on the cell surface, which was followed by a gradual reduction in the amount of cell surface biotinylated APP, reaching 52% of the original APP level by 30 min (Figure 2D and E). In contrast, when the endogenous JNK was inhibited by the JIP peptides as evidenced by the reduction in phospho-cjun levels, cell surface biotinylated APP levels remained unchanged (Figure 2D and E). Anisomycin treatment also increased the extent of APP phosphorylation at T668 in control cultures, while it did not in cultures treated with JIP peptides. These results suggest that JNK activation induces rapid trafficking of APP to the cell surface and subsequent internalization in part by phosphorylating APP at T668.
In order to test whether a JNK-mediated increase in internalization results in greater APP processing, cortical neurons were subjected to cell surface biotinylation using a reversible biotin crosslinker, Sulfo-NHS-SS-Biotin, prior to treating them for 2 hrs with Anisomycin and also inducing internalization at 37°C. At the end of the incubation time, remaining biotins on cell surface proteins were removed by treating cells with 50 mM DTT on ice, thus allowing selective detection of the internalized, cell-surface biotinylated proteins via Neutravidin pulldown/APP blotting or Streptavidin-conjugated secondary antibody after immunoprecipitation with 6E10 (Figure 2F, (Yu et al., 2011)). Anisomycin treatment increased the amount of biotinylated CTF production significantly, which correlated with increased T668 phosphorylation on CTF (Figure 2F). These results together suggest that JNK activation rapidly induces APP internalization/endocytosis, thereby facilitates APP cleavage reactions.
We next determined whether T668P phosphorylation by JNK is required for the internalization and processing of APP. For this, the full-length APP and a point mutant, A668, were transfected into 293T cells, and subjected to cell surface biotinylation and internalization assays as described above. The amount of the full-length APP that was biotinylated on the cell surface decreased with 30 min Anisomycin treatment in the wild type, but not in the A668P mutant, suggesting that phosphorylation of T668P facilitates the internalization of the full-length receptor (Figure 2G, H). Upon internalization, a greater amount of biotinylated CTF was detected with the wild type APP after Anisomycin treatment, but not with the A668P mutant (Figure 2G). These results together suggest that T668P phosphorylation by JNK is necessary for APP to be internalized into endosomes and processed to generate Aβ peptides.
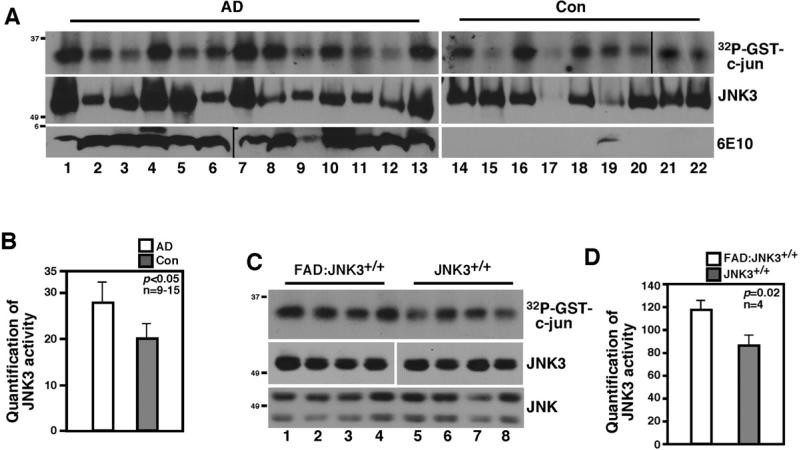
JNK3 activity is increased in human AD cases as well as in FAD mice
Since our in vitro data suggested that JNK-dependent phosphorylation of T668P in APP is necessary for APP internalization and processing, we wished to determine the role of JNK in AD pathology by deleting JNK3 from an AD mouse model. We focused on JNK3, since unlike JNK1 and 2, JNK3 is enriched in the nervous system and plays a role under pathological conditions but has little effect on normal development (Kuan et al., 1999; Yang et al., 1997). As our first step toward addressing the question, we asked whether JNK3 activity increased in human AD cases as well as in 5XFAD (henceforth called “FAD”) mice in comparison to normal and mice cases, respectively, by performing immunoprecipitation/kinase assays using JNK3-specific antibody. The specificity of JNK3 antibody has been demonstrated (Li et al., 2007). FAD mice express mutant human APP (Swe/Fl/Lon) and PS1 (M146L/L286V) genes, each under a neuronal Thy1 promoter, producing more Aβ42 than Aβ40 (Oakley et al., 2006). Indeed, JNK3 activity increased by 34% in human AD compared to normal cases (n=9-13, p≤0.05), and by 27% in FAD:JNK3+/+ mice compared to normal JNK3+/+ mice at 5-6 months (n=4, p≤0.05; Figure 3A-D). These results suggest that JNK3 activity correlates with AD pathology.
Figure 3. JNK3 activity is increased in human AD and FAD cases.
(A) JNK3 activity is increased in human AD cases compared to age-matched controls. The lysates from human frontal cortices were subjected to JNK3 immunoprecipitation/kinase assays using GST-c-jun as a substrate. As a control, JNK3 and 6E10 Western blots are also shown.
(B) Quantification of 32P-GST-c-jun in (A). The data are represented as means ± SEM.
(C) JNK3 activity is higher in FAD mice compared to normal mice at 5-6 months. Lanes 1 to 4 represent four different FAD:JNK3+/+ mice, and lanes 5 to 8, JNK3+/+ mice.
(D) Quantification of 32P-GST-c-jun in (C). The data are represented as means ± SEM.
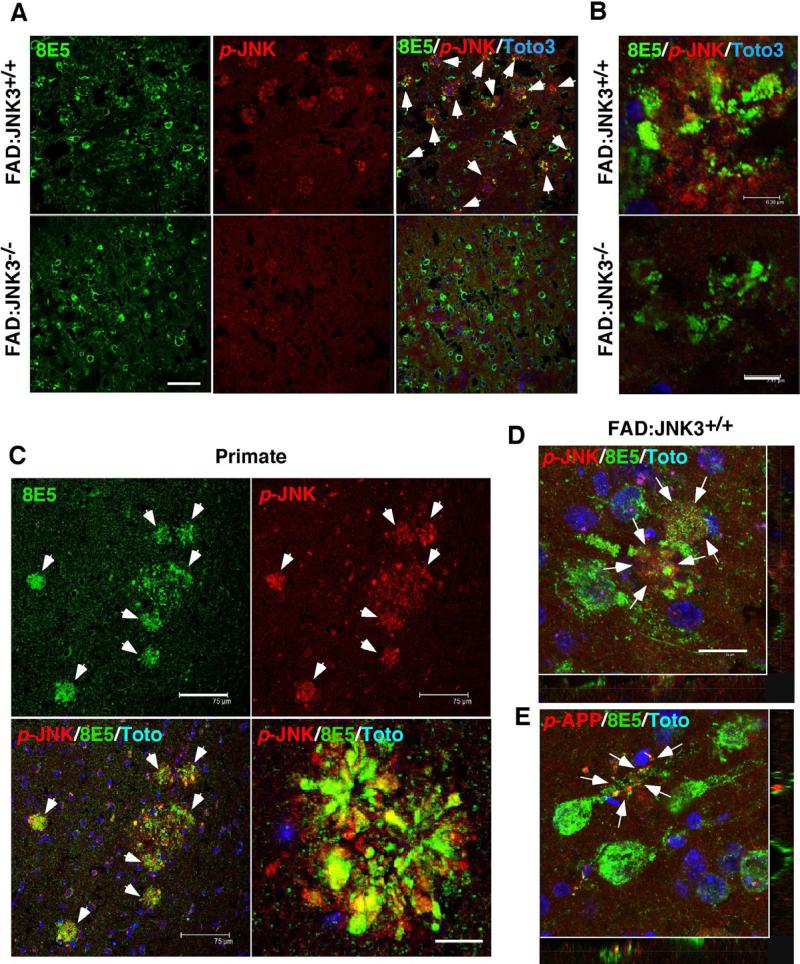
JNK3 is the principle JNK isoform activated in FAD mice
We next determined where active JNK is localized in FAD brains using active JNK or p-JNK antibody in immunohistochemistry. Beginning at 2-3 months, the time when plaques begin to appear in FAD:JNK3+/+ mice, p-JNK signals were predominantly detected near plaque structures, co-localizing with 8E5 immunoreactivity (Figure 4A, B, and D), a dystrophic neurite marker (Games et al., 1995). p-JNK signals were similarly reported to colocalize with 6E10 immunoreactivity in Tg2576/PS1M146L mice (Braithwaite et al., 2010). Also in aged monkey, p-JNK signals were detected near plaque structures (Figure 4C), suggesting that accumulation of active JNK near plaque structures is a common feature in primates as well. When FAD:JNK3-/- mouse brains were analyzed, on the other hand, p-JNK signals were reduced dramatically, to near background levels, and co-localization of p-JNK with 8E5 was not detected (Figure 4A and B). These results indicate that JNK3 is the principle JNK isoform that is activated in FAD mice.
Figure 4. JNK3 is the principle JNK isoform that is activated in FAD mice.
(A) JNK3 is the predominant JNK isoform that is activated near 8E5+ dystrophic neurites in FAD mice. Active JNK, detected with p-JNK antibody, colocalized with 8E5 in the FAD:JNK3+/+ brain (white arrows), which was mostly absent in the FAD:JNK3-/- brain. Scale bar; 75 μm.
(B) A high magnification view of the plaque structures that are positive for 8E5 and phospho-JNK in FAD:JNK3+/+ and FAD:JNK3-/- mice. Scale bar; 7 μm.
(C) p-JNK signals also associate with 8E5+ plaque structures in aged primates. Scale bar; 75 μm.
(D, E) A high-magnification view revealed that both the signals for p-JNK and p-T668-APP are found in degenerating neurtic processes that are positive for 8E5 (white arrows) in FAD:JNK3+/+ mice. The round 8E5 signals are endballs of damaged neuritic processes and not cell bodies, as evidenced by the lack of nuclear Toto staining. Scale bar; 15 μm.
A closer observation into FAD:JNK3+/+ mice revealed that p-JNK signals were detected predominantly at sites of neuritic damage assessed by 8E5 staining (Figure 4D): At 6 months, p-JNK signals are rarely detected in the soma. This result suggests that JNK3 becomes activated in damaged and degenerating neuritic processes, in agreement with previous reports (Abe et al., 2009; Cavalli et al., 2005; Muresan and Muresan, 2005). It should be noted that JIP and JNK3 have been reported to be transported along the axon under pathological conditions, presumably linking Kinesin-1 to receptor carrying vesicles, such as APP (Cavalli et al., 2005; Taru et al., 2002). APP itself has been known to be transported along the axon via fast axonal transport (Koo et al., 1990). We interpret these results as suggesting that JNK3 can potentially phosphorylate APP at T668P in the axon. Indeed, p-T668P signals were detected from damaged neurites in FAD:JNK3+/+ mice (Figure 4E), similarly to p-JNK signals. Unlike p-JNK signals, p-T668P signals were also prominent in cell bodies (data not shown). Together, these results suggest that JNK3 becomes activated in damaged and degenerating neuritic processes, where it can phosphorylate APP and regulate its processing. It should be noted that active JNK also co-localized with hyper-phosphorylated tau in FAD:JNK3+/+ mice (data not shown).
JNK3 deletion results in a dramatic reduction in Aβ42 levels and plaque loads in FAD mice
We next analyzed the effect of deleting JNK3 on overall plaque deposition in FAD mice. In FAD:JNK3-/- mice, insoluble Aβ42 levels were reduced dramatically, by 87% at 6 months (n=8, p=0.0004) and 70% at 12 months (n=8, p=0.005), compared to those in FAD:JNK3+/+ mice, based on Aβ40 and 42-specific sandwich Elisa analyses of the brain samples (Figure 5A). Soluble Aβ40 and 42 levels were also reduced with JNK3 deletion (data not shown), but levels of soluble Aβ peptides were negligible in FAD mice. Similar reductions were observed when the area occupied by plaques was quantified after 6E10 antibody labeling at 6 months (Figure 5B and C): 68% (n=4, p≤0.01), 71% (n=4, p≤0.01), and 65% (n=4, p≤0.05) reductions were found in the frontal cortex, the subiculum and the hippocampus, respectively. As evidenced by Thioflavin S staining (Figure 5C), the size and the number of plaques were also reduced in the frontal cortex and the hippocampus at 6 months by 58% (n=4, p≤0.01) and 47% (n=4, p≤0.01), respectively. Silver staining also indicated that JNK3 deletion resulted in a significant reduction in plaques throughout the brain at 6 months (Figure 5D). More importantly, the number of neurons in layers 5 and 6 of the frontal cortex was 17% higher in FAD:JNK3-/- compared to that in FAD:JNK3+/+ mice at 12-13 months, although it did not reach the levels found in non-FAD mice (n=5-6; Figure 5E). In line with these data, deletion of JNK3 from FAD mice resulted in a significant increase in long-term retention of fear memories at 12-13 months (n=12; Figure 5F). Similarly to NeuN data, the extent of improvement in cognitive function did not reach the normal levels found in non-FAD mice, although the difference was not statistically significant. Since modulation of associative plasticity in the amygdala where fear memories are encoded involves both the hippocampus and prefrontal cortex (Maren and Quirk, 2004), these results suggest that JNK3 activation affects cognitive function in FAD mice. Together, these results indicate that JNK3 plays a critical role in development of AD pathology by not only regulating Aβ peptide production but also impacting neuronal survival and associative learning capacity in FAD mice.
Figure 5. JNK3 deletion in FAD results in a dramatic reduction in Aβ-peptides and plaques.
(A) JNK3 deletion from FAD results in an 87% and 70% reduction in insoluble Aβ-42 levels at 6 and 12 months, respectively. Quantification of Aβ-40 and Aβ-42 using Elisa assays at 6 and 12 months. Data are represented as means ± SEM; n=8. P values were calculated by student t-test.
(B) Quantification of areas that were occupied by plaques in the frontal cortex at 6 months. Data are represented as means ± SEM. P values were calculated by student t-test.
(C) Representative images of Aβ staining with 6E10 in the cortex at 6 months. Scale bar; 150 μm. Also shown are the images of Thioflavin S staining from the frontal cortex at 6 months. Scale bar; 75 μm.
(D) JNK3 deletion from FAD results in a significant reduction in plaque loads. Silver stained sections of the brain at 6 month; plaques appear black. Scale bar; 1.5 mm (upper), 150 μm (lower).
(E) JNK3 deletion in FAD results in an increase in neuronal survival at 12-13 months. NeuN+ cells were quantified from cortical layers 5 and 6. Data are represented as means ± SEM. P values were calculated by student t-test.
(F) JNK3 deletion in FAD mice results in an improvement in cognitive function at 12-13 months. Mice were subjected to fear conditioning tests (n=12). Data are represented as means of 8 trials per mouse ± SEM. P values were calculated by student t-test.
(G) T668 phosphorylation is reduced in FAD:JNK3-/- compared to FAD:JNK3+/+ mice. Protein levels of the full-length APP and CTF in membrane fractions are reduced in FAD:JNK3-/- compared to FAD:JNK3+/+ mice. In contrast, their levels were similar between the genotypes when whole cell extracts were prepared. Also reduced in membrane fractions are p-T668P levels of the full-length APP and CTF.
(H) Neither BACE1 and PS1 levels nor the extent of tau phosphorylation were very different between FAD:JNK3+/+ and FAD:JNK3-/- mice. Tau phosphorylation was, however, increased in FAD compared to non-FAD controls.
We next asked whether JNK3 phosphorylates APP in the brain, thereby regulating β and/or γ cleavage processes by preparing whole cell lysates as well as membrane fractions from 6 month-old mice. JNK3 indeed phosphorylates APP at T668P in vitro and in FAD brains, without affecting the total APP protein levels (Figure 5G): While the human APP levels in whole cell lysates were not very different between FAD:JNK3+/+ and FAD:JNK3-/- mice, p-T668P signals as well as human APP protein levels were significantly reduced when membrane fractions were analyzed (Figure 5G). It should be noted that sw192 antibody is specific to Swedish mutation in human APP (Haass et al., 1995), thus it was used as a marker for FAD mice. In particular, p-T668P levels in membrane fractions were reduced to a much greater extent in α and β CTF than in the full-length APP with JNK3 deletion. This finding closely parallels the observation in human AD brains, wherein increased T668P phosphorylation mainly associated with α and β CTF and not the full-length APP (Lee et al., 2003). In addition, total protein levels of α and β CTF were also reduced to a much greater extent than those in the full-length APP in the membrane fraction (Figure 5G). These results correlate faithfully with our Aβ42 Elisa results at 6 months. We therefore interpret these results as suggesting that JNK3 phosphorylates APP preferentially in membranous compartments, such as vesicles/endosomes, thereby promoting APP processing. It should be noted that although BACE1 and PS1 levels were increased in FAD mice compared to those in normal mice as reported (O'Connor et al., 2008), JNK3 deletion did not affect their levels greatly (Figure 5H). Similarly, neither the levels nor extent of tau phosphorylation was altered by JNK3 deletion in FAD mice (data not shown).
A widespread translational block is a prominent feature in FAD mice
In a preliminary RNAseq-based transcriptome analysis of 3 month-old FAD mice with and without JNK3 and the control cortices from JNK3+/+ and JNK3-/- mice, we obtained the results that suggest that there is a general translational block in FAD:JNK3+/+mice; genes involved in translation, such as ribosomes and translation-initiation factors were dramatically reduced in FAD:JNK3+/+ compared to JNK3+/+ mice and JNK3 deletion restored the effect on these genes to nearly normal levels (data not shown). We therefore tested whether there is indeed a global translational block in FAD mice by Western blotting cortical lysates with an antibody against phospho-S6235/236 ribosomal protein, a marker for active translation. Indeed, the p-S6 signal was reduced by 48% in FAD: JNK3+/+ mice, compared to that in the normal mice and FAD:JNK3-/- mice (Figure 6A, B). Immunohistochemistry with p-S6 antibody also revealed similar findings: both the number of cells that are positive for p-S6 signals and the intensity of its signals decreased significantly in the cortex of FAD:JNK3+/+ mice, compared to those in other genotypes (Figure 6C). It should also be pointed out that p-RaptorS792 levels were increased by 4-fold in FAD:JNK3+/+ compared to those in FAD:JNK3-/- (Figure 6A, B). Since S792 in Raptor is phosphorylated by AMPK (Gwinn et al., 2008), these results suggest that AMPK activity is also reduced in FAD:JNK3-/- compared to FAD:JNK3+/+ mice. These results agree also with our culture data in Figure 1.
Figure 6. JNK3 activation is integral to FAD pathology, regulating the translational block induced by inhibition of the mTOR pathway.
(A) Phosphorylation of S6 ribosomal protein, an indicator of active translation is reduced, while phosphorylation in Raptor at S792, an AMPK phosphorylation site, was increased in FAD:JNK3+/+ compared to that in other genotypes. Also shown are control Western blots for the JNK3 genotype and FAD genotype: 192SW is specific to the Swedish mutation in human APP.
(B) Quantification of p-S6 and p-Raptor protein levels in (A). Y-axis on the left is for p-S6 and the one on the right is for p-Raptor. Data are represented as means ± SEM. P values were calculated by student t-test.
(C) Both the number of cells that express p-S6 and the level of p-S6 immunoreactivity decreased in FAD:JNK3+/+ mice compared to other genotypes in the cingulate cortex at 3-4 months. Scale bars, 75 μM.
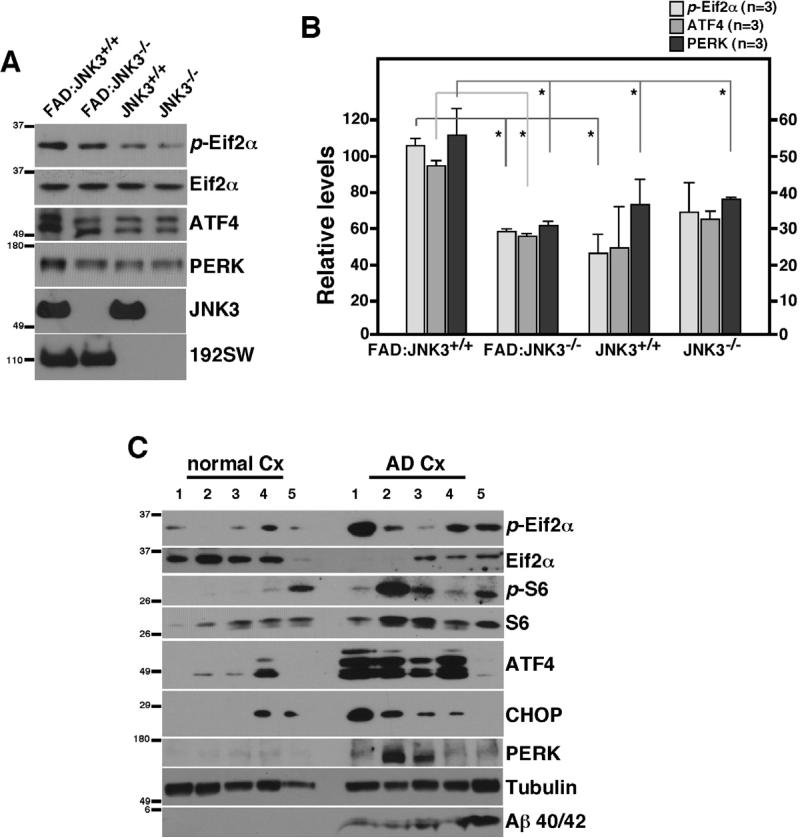
UPR is a prominent feature in FAD mice as well as in human AD cases
Oligomeric Aβ42 treatment in hippocampal neurons also resulted in phosphorylation of Eif2α, albeit at a lower extent than that by Thapsigargin, suggesting that Aβ42 induces ER stress (Figure 1E). A reduction in the global rate of translation is one of the earliest events in UPR, which leads to induction of a widespread secondary response that includes transcriptional activation of the target genes for UPR, namely apoptotic as well as survival promoting genes (Ron and Walter, 2007). Western blotting of a selected set of ER stress transducers, such as p-Eif2α ATF4, and PERK, supported the finding that expression of these proteins was increased by approximately 2-fold in 3 month-old FAD:JNK3+/+ compared to those in normal and FAD:JNK3-/- mice (p<0.05; Figure 7A, B). More importantly, cortical samples from five AD patients demonstrated a significant increase in ER stress markers as well as translational block, including p-S6, p-Eif2α, ATF4, CHOP, and PERK compared to five age-matched control cases (Figure 7C). Together, these suggest that the ER stress response occurs in human AD, and JNK3 activation may contribute to it.
Figure 7. The ER stress response is a predominant feature in human AD cases as well as in FAD.
(A) An increase in ER markers is detected in FAD:JNK3+/+ mice, which becomes reduced as a result of deleting JNK3.
(B) Quantification of ER markers in (A) (n=3-4). Data are represented as means ± SEM. P values were calculated by student t-test. * denotes p<0.05.
(C) An increase in the ER stress response in human AD cases, compared to age-matched control cases.
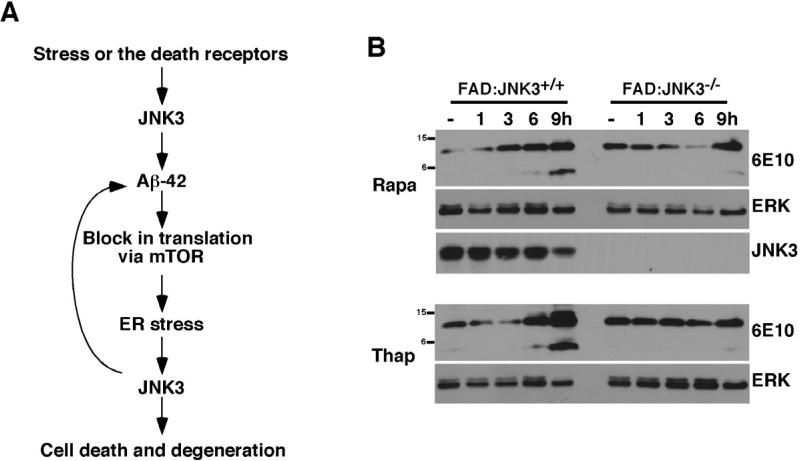
JNK3 activation maintains a positive feedback loop of cyclic Aβ42 production
We hypothesized that once UPR is induced, it activates JNK3, which in turn promotes further APP processing by phosphorylating it at T668P. T668P phosphorylation facilitates increased internalization of the receptor into endosomal vesicles wherein APP undergoes processing to generate more Aβ42. As the cycle repeats itself, more Aβ42 accumulates, exacerbating the pathology (Figure 8A). To test the hypothesis that translational block and/or ER stress increase APP processing in a JNK3-dependent manner, organotypic slices were prepared from 8-10 week-old FAD:JNK3+/+ and FAD:JNK3-/- mice and treated with the vehicle, 0.5 μM Thapsigargin or 10 nM Rapamycin for the indicated amounts of time. Both CTF and Aβ peptide levels increased gradually over the 9 hr period in FAD:JNK3+/+ slices with Thapsigargin as well as Rapamycin treatments (Figure 8B). These results suggest that ER stress or inhibiting the mTOR pathway is sufficient to induce Aβ peptide production. Importantly, this increase in APP processing was dependent on JNK3, since the overall CTF and Aβ peptide levels were significantly reduced in FAD:JNK3-/- slices (Figure 8B). These results together indicate that JNK3 activation is necessary to perpetuate the cycle of translational block via mTOR, ER stress, JNK3 activation, and further production of Aβ42.
Figure 8. Translational block exacerbates APP processing in JNK3-dependent manner.
(A) A model of how JNK3 activation exacerbates the development of AD pathology. We hypothesize that JNK3 is initially activated by death receptors and/or under certain stress conditions. Once activated, JNK3 phosphorylates APP at T668P, facilitating APP processing by enhancing its internalization, which results in greater Aβ42 production. Aβ42 then blocks translation, leading to UPR, which activates JNK3. Activated JNK3 will target APP again in a positive feedback loop, thereby perpetuating a cycle that exacerbates Aβ42 production.
(B) Both Thapsigargin and Rapamycin increase Aβ42 production in a JNK3-dependent manner. Organotypic brain slices from FAD:JNK3+/+ and FAD:JNK3-/- were treated with the vehicle, 0.5 μM Thapsigargin, and 10 nM Rapamycin for the indicated amount of time and the resulting whole cell lysates were analyzed for 6E10 Western. In order to facilitate detection of CTF and Aβ peptides, the slices were also treated with 10 μM MG132 at the same time. Note that both CTF and Aβ42 levels were increased in FAD:JNK3+/+ cultures upon treatments compared to those in the vehicle control. This increase was significantly attenuated in FAD:JNK-/- cultures.
DISCUSSION
Here, we report that JNK3 activation is critical for maintaining a positive feedback loop that culminates in continued production of Aβ42. This conclusion is supported by a dramatic reduction in overall Aβ42 levels upon deleting JNK3 from FAD mice. Such a dramatic reduction is not only due to the ability of JNK3 to phosphorylate APP directly, but also due to the cyclic activation of JNK3 by a translational block and subsequent UPR that had been induced by oligomeric Aβ42 in the first place.
JNK3 phosphorylates APP at the T668P site in its cytoplasmic domain both in vitro and in vivo. The fact that JNK or JNK3 phosphorylates APP is also supported by a study, where double deletion of putative upstream JNK kinases, MKK4 and MKK7, from another FAD mouse line resulted in a reduction in T668P phosphorylation (Mazzitelli et al., 2011). T668P phosphorylation was increased in AD brains, wherein the β-CTF rather than the full-length APP exhibited increased T668P phosphorylation compared to the control (Lee et al., 2003). Supporting the notion that T668 phosphorylation contributes to APP processing, T668P to A668P mutation reduced Aβ peptide generation in vitro (Lee et al., 2003). Our data also support this view: When JNK phosphorylated APP at T668P, the amount of CTF increased. This was in part due to the fact that JNK phosphorylation of T668P in APP facilitated rapid internalization of the receptor as indicated by a reduction in the amount of the full-length APP on the cell surface. Since JNK3 is not the only kinase that phosphorylates APP at T668P in vivo as indicated by our data, and JNK3 can also be activated in the axon under pathological conditions (Falzone et al., 2009; Morfini et al., 2009), we hypothesize that JNK3 is the predominant kinase that phosphorylates APP within particular endosomal compartments in the axon where APP encounters BACE1 (Abe et al., 2009; Cavalli et al., 2005).
Structurally, phosphorylation at T668P induces propyl isomerization, converting the p-T668P peptide from trans to cis configuration (Ramelot and Nicholson, 2001). Pin1, a phosphorylation-dependent propyl isomerase, indeed binds p-T668P in vitro, thereby facilitating cis to trans conversion (Pastorino et al., 2006). Since Pin1 deletion from an AD mouse line resulted in a 46% increase in Aβ peptide production, cis configuration induced by T668 phosphorylation is believed to render APP vulnerable to amyloidogenic processing (Pastorino et al., 2006). Our data and those of Lee et al. (Lee et al., 2003) also support that T668P phosphorylation is critical for Aβ peptide generation in vitro. Whether T668P phosphorylation causes greater Aβ peptide generation in vivo is, however, still unresolved. In normal aged mice, A668P knock-in mutation did not affect β CTF generation (Sano et al., 2006), leading the authors to conclude that T668P phosphorylation plays no role in APP processing. Such a conclusion is premature especially with gain-of-function mutations such as phosphorylation, until the role of T668P phosphorylation is assessed in AD mouse models. A case in point is that although hyper-phosphorylation of tau is clearly indicated as pathologic, deleting tau alone showed a relatively minor defect in axon degeneration (Dawson et al., 2001; Gomez de Barreda et al., 2010; Harada et al., 1994). It was only when tau was deleted under an AD background that improvement in cognitive behavior was observed (Roberson et al., 2007). Although critical experiments are still needed to address whether T668P phosphorylation causes APP processing in vivo, our study provides additional support to the idea that T668P phosphorylation significantly contributes to APP processing in vivo.
We provide compelling evidence that a translational block is a prominent feature in FAD mice and to some extent in human AD cases. Since oligomeric Aβ42 induced a translational block in hippocampal neurons in culture, it is highly likely that oligomeric Aβ42 has a similar effect in vivo. Oligomeric Aβ42 is widely believed to be the central pathologic species that is responsible for inhibiting LTP and memory formation in vivo (Cleary et al., 2005; Walsh et al., 2002). Since inhibiting normal translational processes by disabling eif2α phosphorylation or deleting its kinase, GCN2, resulted in inhibition of LTP (Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007), it is tempting to speculate that such synaptotoxicity observed with oligomeric Aβ42 is likely be due to its inhibitory effect on translation.
Our data indicate that oligomeric Aβ42 inhibits translation in part by blocking the mTOR pathway. Dysregulation of the mTOR pathway or loss of energy balance has been identified as causative in normal aging as well as type 2-diabetes and obesity (Cohen et al., 2009; Demontis and Perrimon, 2010; Koo et al., 2005; Mair et al., 2011; Song et al., 2010). Our findings that widespread disruption of normal energy balance is prominent in FAD mice and to some extent in human AD cases suggest that in progressive diseases whose symptoms develop over a long period time, chronic metabolic imbalance becomes a pervasive phenotype. Our data clearly illustrate that oligomeric Aβ42 perturbs energy homeostasis, as indicated by activation of AMPK, a kinase that responds to energy imbalance in the cell (Steinberg and Kemp, 2009). AMPK was shown to play a critical role in aging in yeast and C. elegans, although the loss of snf1p, the yeast homolog of AMPK, increased the life span (Lin et al., 2001), while mutation in aak-2, the worm homolog of AMPK, decreased the life extension induced by stress (Apfeld et al., 2004). Besides this apparent species-related difference in homolog roles, the role of AMPK itself in aging appears clear. It is of special interest in this regard that oligomeric Aβ42 activates AMPK, thereby inhibiting the mTOR pathway. Aβ peptides are normally produced and cleared rapidly in human brains (Bateman et al., 2006). It is plausible that normal production of Aβ peptides contributes to the aging process in part by activating AMPK.
AMPK activation was rapid but transient by oligomeric Aβ42, detectable at 10 min, but greatly reduced by 3 hr after Aβ42 addition. Although transiently activated, AMPK substrates, Raptor and TSC2 remain phosphorylated up to 16 hrs, providing an explanation for a prolonged translational inhibition. What will be the mechanism by which oligomeric Aβ42 activates AMPK? AMPK was shown to be activated by LKB1 (Hawley et al., 2003; Shaw et al., 2004), CaMKK (Anderson et al., 2008; Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005), and TAK1 (Momcilovic et al., 2006). In the nervous system, however, LKB1 does not seem to serve as a kinase for AMPK, since AMPKα phosphorylation at T172 was not changed in LKB1 null mice (Barnes et al., 2007). A most likely candidate is CaMKK, since it is highly expressed in the brain and it associates with AMPK α and β subunits (Anderson et al., 2008). If that is the case, it is possible that oligomeric Aβ42 itself modulates intracellular calcium levels, thereby activating CaMKK. It will be of interest to test whether intracellular calcium levels change with oligomeric Aβ42 addition in neurons.
Can mTOR activity modulation be developed into a therapy for AD? This is an attractive idea, since there are already many FDA approved drugs that were designed to target the mTOR pathways for treating other progressive metabolic diseases. Although attractive, the idea appears too premature at the present time mainly because the role of the mTOR pathway in AD is not fully understood. For instance, some reported improvement in cognitive function and neuronal toxicity with Rapamycin administration (Berger et al., 2006; Bove et al., 2011; Caccamo et al., 2010; Khurana et al., 2006; Spilman et al., 2010), while others reported the opposite (Lafay-Chebassier et al., 2005). Similarly, the reports vary as to whether there is an inhibition or activation of the mTOR pathway in AD mouse models and/or human cases (Caccamo et al., 2011; Ma et al., 2010). Our data indicate that there is a significant translational block early in FAD mice. This notion was also supported by a global transcriptome analysis via RNAseq, which demonstrated a dramatic reduction in transcripts for ribosomes and elongation factors in FAD compared to the wild type mice (data not shown). It is possible that the use of different animal models at different ages in each study contributed to the opposite outcomes. It seems safe to surmise that before one takes further steps to alter the mTOR pathway or AMPK activity in pursuit of a treatment for AD, more systematic and consistent analyses are necessary.
In conclusion, our findings suggest that JNK3 activation is central to the development of AD pathology by exacerbating metabolic stress that is induced by Aβ42 accumulation. This study thus identifies JNK3 as a promising new target of therapeutic intervention for Alzheimer's disease.
EXPERIMENTAL PROCEDURES
Human samples
Tissues from the frontal cortex were obtained through UCSD Experimental Neuropath Laboratory.
Mouse breeding
FAD mice in B6/SJL F1 hybrid background were initially crossed with JNK3 in knockout mice in B6 background to obtain FAD:JNK3+/- and control non-transgenic:JNK3+/-.
Rat hippocampal neuron cultures
The hippocampal neurons were isolated from E18 rat embryos, and cultured for 7 days in Neurobasal medium supplemented with B27 and 2 mM glutamine.
Organotypic slice preparations
500 μm-thick organotypic slices were prepared according to (del Rio and Soriano, 2010) from 12-14 week-old FAD:JNK+/+ and FAD:JNK3-/- mice.
JNK3 immunoprecipitation/kinase assays
The lysates from hippocampal neurons were subjected to immunoprecipitation with JNK3 antibody, and the immune complexes were used in kinase reactions using GST-c-jun as a substrate as described (Li et al., 2007).
Tissue preparation for Aβ40 and 42 Elisa
Brain tissues that contain the cortex, the hippocampus, the septum and the striatum were used to extract proteins using 70% formic acid.
Preparations of crude membrane fractions
Brain tissues were processed to obtain membrane and soluble fractions for biochemical analyses as described (Pastorino et al., 2006).
Quantification of plaque areas and the numbers of neurons
For the quantification of the areas occupied by plaques, two 60 μm, floating sections from the bregma positions from +0.26 to +0.5 for the frontal cortex were processed for staining with 6E10 (n=4).
Silver staining
Coronal sections of the brains (60 μm) were processed for silver staining using a FD NeuroSilver kit from FD Neurotechnologies as directed by the manufacturer.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Elan pharmaceuticals for the gift of 8E5 and 192sw antibodies, and Dr. Li Huei Tsai for APP-wild type and T668A mutant constructs. We also thank Drs. Gary Landreth, Bruce Carter, and Joachim Herz for valuable comments on the manuscript. This work was funded by a grant from The Alzheimer's Association (IIRG-08-90129) and NINDS (RO1NS050585) to S.O.Y. and The Ohio State Neuroscience Center Core from NINDS (P30NS045758), P30 CA016058-30 National Cancer Institute. RNA sequencing was performed at the OSUCCC Nucleic Acid Shared Resource-Illumina Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barr RK, Kendrick TS, Bogoyevitch MA. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002;277:10987–10997. doi: 10.1074/jbc.M107565200. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol. 2006;16:2446–2452. doi: 10.1016/j.cub.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Schmid RS, He DN, Sung ML, Cho S, Resnick L, Monaghan MM, Hirst WD, Essrich C, Reinhart PH, et al. Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer's disease. Neurobiol Dis. 2010;39:311–317. doi: 10.1016/j.nbd.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A, Oddo S. Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem. 2011;286:8924–8932. doi: 10.1074/jbc.M110.180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- del Rio JA, Soriano E. Regenerating cortical connections in a dish: the entorhino-hippocampal organotypic slice co-culture as tool for pharmacological screening of molecules promoting axon regeneration. Nat Protoc. 2010;5:217–226. doi: 10.1038/nprot.2009.202. [DOI] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Rogers RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Falzone TL, Stokin GB, Lillo C, Rodrigues EM, Westerman EL, Williams DS, Goldstein LS. Axonal stress kinase activation and tau misbehavior induced by kinesin-1 transport defects. J Neurosci. 2009;29:5758–5767. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gomez de Barreda E, Perez M, Gomez Ramos P, de Cristobal J, Martin-Maestro P, Moran A, Dawson HN, Vitek MP, Lucas JJ, Hernandez F, et al. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol Dis. 2010;37:622–629. doi: 10.1016/j.nbd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 Is Necessary for c-jun N-Terminal Kinase-Mediated Apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Iijima K, Ando K, Takeda S, Satoh Y, Seki T, Itohara S, Greengard P, Kirino Y, Nairn AC, Suzuki T. Neuron-specific phosphorylation of Alzheimer's beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem. 2000;75:1085–1091. doi: 10.1046/j.1471-4159.2000.0751085.x. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Koo EH, Kopan R. Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat Med. 2004;10(Suppl):S26–33. doi: 10.1038/nm1065. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer's disease. J Neurochem. 2005;94:215–225. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QM, Tep C, Yune TY, Zhou XZ, Uchida T, Lu KP, Yoon SO. Opposite regulation of oligodendrocyte apoptosis by JNK3 and Pin1 after spinal cord injury. J Neurosci. 2007;27:8395–8404. doi: 10.1523/JNEUROSCI.2478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mazzitelli S, Xu P, Ferrer I, Davis RJ, Tournier C. The loss of c-Jun N-terminal protein kinase activity prevents the amyloidogenic cleavage of amyloid precursor protein and the formation of amyloid plaques in vivo. J Neurosci. 2011;31:16969–16976. doi: 10.1523/JNEUROSCI.4491-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan Z, Muresan V. c-Jun NH2-terminal kinase-interacting protein-3 facilitates phosphorylation and controls localization of amyloid-beta precursor protein. J Neurosci. 2005;25:3741–3751. doi: 10.1523/JNEUROSCI.0152-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, et al. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- Ramelot TA, Nicholson LK. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J Mol Biol. 2001;307:871–884. doi: 10.1006/jmbi.2001.4535. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Sano Y, Nakaya T, Pedrini S, Takeda S, Iijima-Ando K, Iijima K, Mathews PM, Itohara S, Gandy S, Suzuki T. Physiological mouse brain Abeta levels are not related to the phosphorylation state of threonine-668 of Alzheimer's APP. PLoS One. 2006;1:e51. doi: 10.1371/journal.pone.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, et al. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468:933–939. doi: 10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotthibundhu A, Sykes AM, Fox B, Underwood CK, Thangnipon W, Coulson EJ. Beta-amyloid(1-42) induces neuronal death through the p75 neurotrophin receptor. J Neurosci. 2008;28:3941–3946. doi: 10.1523/JNEUROSCI.0350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Taru H, Kirino Y, Suzuki T. Differential roles of JIP scaffold proteins in the modulation of amyloid precursor protein metabolism. J Biol Chem. 2002;277:27567–27574. doi: 10.1074/jbc.M203713200. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yu T, Calvo L, Anta B, Lopez-Benito S, Southon E, Chao MV, Tessarollo L, Arevalo JC. Regulation of trafficking of activated TrkA is critical for NGF-mediated functions. Traffic. 2011;12:521–534. doi: 10.1111/j.1600-0854.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.