Abstract
Patients with chronic kidney disease (CKD) often exhibit associated endothelial dysfunction and inflammation. Systemic inflammation may contribute to the endothelial dysfunction and accelerated thrombosis observed in CKD patients. In this study, we assessed the relationships among endothelial dysfunction, a disintegrin-like and metalloprotease with thrombospondin type 1 repeats 13 (ADAMTS13) activity and levels of inflammatory cytokines in CKD patients. CKD patients were classified into three groups: The chronic glomerulonephritis group (CGN; n=31), the idiopathic nephritic syndrome group (NS; n=32) and the lupus nephritis group (LN; n=41). We measured the plasma levels of tumor necrosis factor-α (TNF-α), von Willebrand factor (VWF) antigen (VWF:Ag) and ADAMTS13 activity using an ELISA-based method in CKD patients (n=104) and normal controls (n=32). The ratio of the VWF:Ag levels to ADAMTS13 activity was calculated. The VWF:Ag levels were significantly higher and the ADAMTS13 activities were significantly lower in the disease groups compared to the controls (P<0.01). ADAMTS13 activity was lower in the NS group compared to the CGN and LN groups (P<0.05). The TNF-α levels were higher in the CKD group compared to the control group (P<0.01). TNF-α was positively correlated with the VWF:Ag levels (r=0.242, P=0.013) and negatively correlated with the glomerular filtration rate (GFR) (r=−0.193, P=0.049). ADAMTS13 activity was negatively correlated with the cholesterol levels in CKD patients (r=−0.2, P= 0.042). TNF-α levels in CKD were positively correlated with the VWF:Ag levels and negatively correlated with GFR, which indicates that inflammation may be a major cause of endothelial dysfunction and an index of renal function. The VWF:Ag levels increased and ADAMTS13 activity decreased in CKD patients, which indicates that CKD leads to a prothrombotic state.
Keywords: tumor necrosis factor-α, von Willebrand factor, ADAMTS13, chronic kidney disease
Introduction
Patients with chronic kidney disease (CKD) have increased risks of endothelial dysfunction and thrombosis (1,2). Systemic inflammation may contribute to endothelial dysfunction and accelerated thrombus formation in CKD (3).
The von Willebrand factor (VWF), a well-known index of endothelial damage, has been reported to increase in CKD and inflammatory states (4,5). A disintegrin-like and metalloprotease with thrombospondin type 1 repeats 13 (ADAMTS13) is a specific VWF-cleaving protease; severe deficiency of this protease leads to the excessive accumulation of ultralarge VWF multimers and platelet aggregation with microthrombus formation, and is the main cause of thrombotic thrombocytopenic purpura (TTP) (6). Previous studies have revealed that ADAMTS13 activity is often reduced in other diseases, such as cardiovascular disease, chronic liver disease, disseminated intravascular coagulation and CKD (7–11).
The pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α), plays a pivotal role in orchestrating innate inflammatory responses. It is a sufficient and necessary mediator of septic shock (12) and its levels are increased in patients with sepsis (13). TNF-α affects the release of VWF from endothelial cells into the circulation (14). Furthermore, TNF-α levels are increased in patients with CKD who have chronic low-grade inflammation; this indicates that this cytokine plays a critical role in the development of CKD by potentiating endothelial dysfunction.
In a cell-based study, TNF-α was found to be involved in the synthesis, release and cleavage of VWF and ADAMTS13 in cultured hepatic stellate and endothelial cells (15). However, the relationship among these factors in CKD has yet to be investigated. In addition, ADAMTS13 is a specific VWF-cleaving protease, and several studies have indicated that the ratio of VWF and ADAMTS13 may be more beneficial in the diagnosis and treatment evaluation of patients in the prothrombotic state (16,17); however, this ratio has yet to be studied in CKD. The present study was designed to investigate the plasma levels of the pro-inflammatory cytokine, TNF-α, VWF antigen (VWF:Ag) and ADAMTS13 activity in CKD patients. The relationships between the ratio of the VWF:Ag levels to ADAMTS13 activity and other parameters were also evaluated.
Patients and methods
Patient selection
We studied 104 patients who visited our hospital and were diagnosed with CKD according to the standards set by the American National Kidney Foundation (18). We excluded patients with infection, acute or chronic hepatic diseases, malignant cancer or diabetic nephropathy, and those under immunosuppressive therapy. Patients who underwent hemodialysis or peritoneal dialysis were also excluded. In all, 31 patients with primary chronic glomerulonephritis (CGN) and 32 patients with idiopathic nephrotic syndrome (NS) were investigated. Renal biopsy was performed for 42% (13/31) of the CGN patients and 81% (26/32) of the NS patients. Additionally, 41 patients with lupus nephritis (LN) were also enrolled in this study. The diagnosis was made according to the diagnostic criteria for systemic lupus erythematosus (SLE), as defined by the American Rheumatism Association in 1997 (19).
The control group contained 32 healthy controls. The local ethics committee approved the study, and all patients provided written informed consent for participation in the study.
Sample collection
For ADAMTS13 activity and VWF:Ag, venous blood was collected in 5-ml tubes (3.8% sodium citrate) and centrifuged at 3,000 rpm for 10 min. The plasma samples were then stored at −80°C until they were assayed.
For TNF-α, venous blood was collected in 5-ml tubes (EDTA-containing) and centrifuged at 3,000 rpm for 10 min. The plasma samples were stored at −80°C until they were assayed.
Measurement of serum lipid and creatinine concentrations
Fasting serum levels of cholesterol (CHOL), triglycerides, albumin (ALB), creatinine and blood urea nitrogen (BUN) were measured in an automatic biochemical analyzer (AU5400; Olympus Optical Ltd., Japan).
Renal function was estimated in terms of the glomerular filtration rate (GFR; ml/min/1.73 m2) using the Cockcroft-Gault formula, which is as follows: GFR (ml/min/1.73 m2) = [140 -age (years)] x weight (kg)/[72 x serum creatinine (mg/ dl)] x 0.85 (if female).
The SLE disease activity index (SLEDAI) was determined using the 24-parameter scoring system defined by Bombardier et al (20).
VWF:Ag and TNF-α assay
VWF:Ag in plasma was assayed by enzyme-linked immunosorbent assay (ELISA) using the kit prepared by the Department of Thrombosis and Haemostasis of Jiangsu Institute of Haematology, China. Plasma levels of TNF-α were measured using ELISA kits (R&D Systems, Minneapolis, MN, USA).
ADAMTS13 activity analysis
The VWF-cleaving activity of ADAMTS13 in plasma was assayed using the FRETS-VWF73 ELISA kit (Peptide Institute Company, Japan) (21). A dilution series of normal pooled plasma was used to calibrate the assay. Assay buffer was used as the negative control. The relative proteolytic activity in the samples was derived directly from the slope and compared to that of normal human plasma. The proteolytic activity in normal human plasma was arbitrarily defined as 100%.
Statistical analysis
The results of statistical analysis are presented as the means ± standard deviation. Comparisons among the studied groups were performed using the one-way ANOVA or the Kruskal-Wallis test. The correlations were calculated using Pearson's or Spearman's correlation co-efficient test. All analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). A probability level of <0.05 was considered statistically significant.
Results
Subject characteristics
Table I shows the baseline characteristics of the subjects. The three groups did not differ in terms of age, blood pressure and GFR. The serum ALB levels in the NS group were significantly lower than those in the CGN and LN groups, and the urine protein and CHOL levels in the NS group were clearly higher than those in the CGN and LN groups (Table I).
Table I.
Demographic features and clinical information.
| LN | CGN | NS | Controls | |
|---|---|---|---|---|
| Gender (M/F) | 2:39 | 20:11 | 21:11 | 18/14 |
| Age (years) | 34.80±14.43 | 37.13±17.54 | 39.81±15.67 | 40.44±10.80 |
| SBP (mmHg) | 126.17±24.87 | 123.70±20.57 | 133.53±22.92 | 125.37±13.40 |
| DBP (mmHg) | 77.73±12.85 | 77.10±11.16 | 81.78±14.25 | 77.72±11.02 |
| GFR (ml/min/1.73 m2) | 89.14±37.67 | 88.59±50.58 | 92.87±40.62 | 96.87±31.11 |
| Albumin (g/l) | 33.42±9.05b,d | 34.39±8.17b,d | 23.77±9.45d | 46.62±4.05 |
| Urine protein (g/24 h) | 2.15±2.59b | 1.41±1.24b | 4.38±2.99 | - |
| CHOL (mmol/l) | 5.51±1.86b,c | 5.63±2.14b,c | 8.63±2.38d | 4.67±0.66 |
| TRIG (mmol/l) | 2.60±2.47d | 1.88±1.45a | 3.22±2.66d | 1.45±0.91 |
vs. NS:
P<0.05,
P<0.01; vs. control:
P<0.05,
P<0.01. LN, lupus nephritis; CGN, primary chronic glomerulonephritis; NS, nephrotic syndrome; GFR, glomerular filtration rate; CHOL, cholesterol; TRIG, triglycerides.
Plasma TNF-α levels in CKD patients
The results showed that plasma TNF-α levels (pg/ml) were significantly higher in patients with CKD than in the healthy controls (CGN, 3.19±1.01; NS, 3.26±1.18; LN, 3.24±1.24; control, 2.27±1.23; P<0.01). Patient groups classified according to the disease did not exhibit any statistically significant difference in terms of plasma TNF-α levels. The TNF-α levels were positively correlated with the VWF:Ag levels (r=0.242, P=0.013) and negatively correlated with the GFR (r=-0.193, P=0.049) (Fig. 1). The TNF-α levels were not correlated with ADAMTS13 (r=-0.043, P=0.668).
Figure 1.
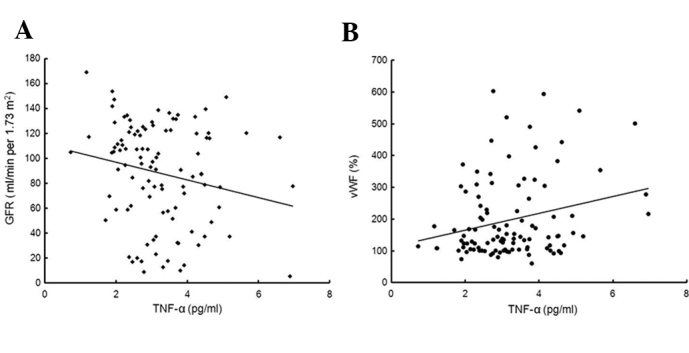
(A) Correlation between the TNF-α levels and GFR (n=104; r=−0.193, P= 0.049). (B) Correlation between the TNF-α levels and VWF (n=104; r=0.242, P=0.013). TNF-α tumor necrosis factor-α; VWF, von Willebrand factor.
Plasma VWF:Ag levels, ADAMTS13 activity and their ratio in CKD patients
Plasma VWF:Ag levels and ADAMTS13 activity were measured in healthy volunteers (n=32) and CKD patients (CGN, n=31; NS, n=32; LN, n=41), and the VWF/ ADAMTS13 ratio was calculated (Table II). Compared to the healthy volunteers (controls), CKD patients exhibited higher levels of VWF, significantly higher VWF/ADAMTS13 ratio and markedly lower levels of ADAMTS13 activity. Furthermore, the VWF:Ag levels and ADAMTS13 activity in the NS group were lower than those in the CGN and LN groups (P<0.05) (Fig. 2). However, the VWF/ADAMTS13 ratios in the three CKD groups exhibited no clear difference. The VWF:Ag levels were not correlated with the ADAMTS13 activity in CKD patients. ADAMTS13 activity in CKD patients was negatively correlated with the CHOL levels (n=104; r=−0.2, P=0.042) (Fig. 3).
Table II.
Plasma VWF:Ag levels, ADAMTS13 activity and the VWF/ADAMTS13 ratio in the CGN, NS, LN and control groups.
| No. | VWF (%) | ADAMTS13 (%) | VWF/ADAMTS13 | |
|---|---|---|---|---|
| CGN | 31 | 198.25±140.20a | 61.93±22.47a,c | 3.83±3.29a |
| NS | 32 | 149.94±74.50a | 48.87±18.63a | 3.42±1.59a |
| LN | 41 | 234.75±134.91a,b | 67.81±22.30a,b | 3.79±2.52a |
| Control | 32 | 99.55±21.37 | 94.37±23.66 | 1.12±0.37 |
vs. control
P<0.01; vs. NS
P<0.01,
P<0.05. VWF, von Willebrand factor; LN, lupus nephritis; CGN, primary chronic glomerulonephritis; NS, nephrotic syndrome.
Figure 2.
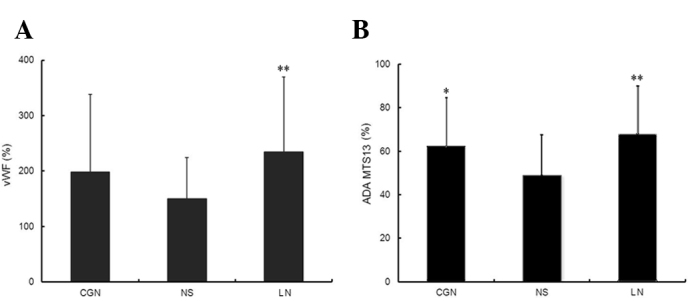
(A) VWF:Ag in different CKD groups. (B) ADAMTS13 activity in different CKD groups vs. the NS group. *P<0.05; **P<0.01. VWF:Ag, von Willebrand factor antigen; CKD, chronic kidney disease; NS, idiopathic nephrotic syndrome; CGN, chronic glomerulonephritis; LN, lupus nephritis.
Figure 3.
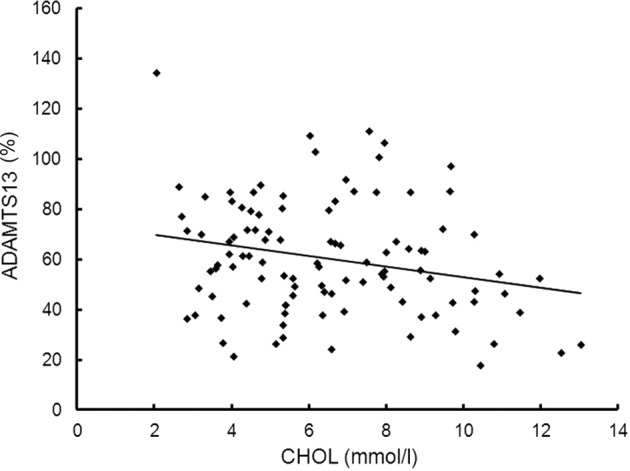
Correlation between ADAMTS13 activity and CHOL levels (n=104; r=−0.2, P=0.042). CHOL, cholesterol.
The plasma VWF:Ag levels, ADAMTS13 activity and their ratio did not show any clear difference between the different pathological types of CKD (data not shown).
Correlation between renal function and ADAMTS13 activity, VWF:Ag levels and the VWF/ADAMTS13 ratio
Patients with CGN and NS were divided into two groups: The GFR reference group, comprising patients with a GFR of ≥60 ml/ min/1.73 m2, and the low-GFR group, comprising patients with a GFR of 0–59 ml/min/1.73 m2. The difference between renal function and ADAMTS13 activity, VWF:Ag levels and VWF/ADAMTS13 ratio was examined in these groups. No significant inter-group differences were noted in these factors (data not shown).
Although no significant correlation was observed between the VWF/ADAMTS13 ratio and GFR (r=−0.127, P=0.2), a significant negative correlation was observed between the VWF:Ag levels and GFR (r=−0.194, P=0.048).
Relationship between SLEDAI and TNF-α, ADAMTS13 activity, VWF:Ag levels and VWF/ADAMTS13 ratio in LN patients
In the linear regression analysis, SLEDAI in LN patients was positively correlated with the levels of TNF-α (r= 0.354, P= 0.023) and VWF (r=0.472, P=0.002) (Fig. 4), but did not correlate with ADAMTS13 activity and the VWF/ ADAMTS13 ratio.
Figure 4.
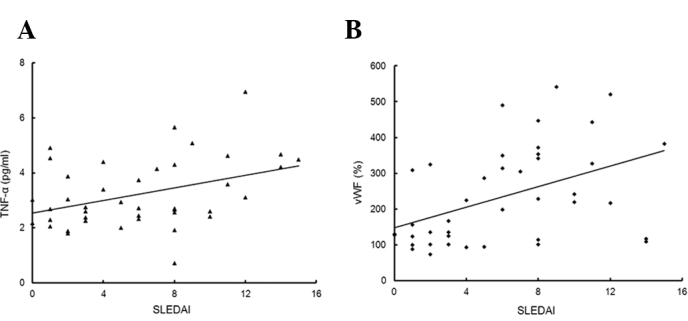
(A) Correlation between SLEDAI and TNF-α levels (n=41; r=0.354, P= 0.023). (B) Correlation between SLEDAI and VWF:Ag levels (n=41; r=0.472, P=0.002). SLEDAI, systemic lupus erythematosus disease activity index; TNF-α tumor necrosis factor-α; VWF, von Willebrand factor; VWF:Ag, VWF antigen.
Discussion
The results of this study show that in CKD patients, the plasma TNF-α levels were associated with the VWF:Ag levels, but did not correlate with the ADAMTS13 activity levels. The TNF-α levels were also negatively correlated with the GFR. Compared to the control group, the CGN, NS and LN groups showed significantly higher VWF:Ag levels and VWF/ADAMTS13 ratio (P<0.01) and markedly low ADAMTS13 activity (P<0.01). Furthermore, the VWF:Ag levels and ADAMTS13 activity in the NS group were lower than those in the CGN and LN groups (P<0.05 and P<0.01). However, no clear difference was noted between the three CKD groups in terms of the VWF/ ADAMTS13 ratio. The VWF:Ag levels in CKD patients were negatively correlated with the GFR. ADAMTS13 activity was negatively correlated with the CHOL levels.
Our study revealed that the TNF-α levels were positively correlated with the VWF:Ag levels in CKD patients, which is consistent with the findings of a previous study by Bolton et al (2) on the relationship between inflammatory cytokines and endothelial dysfunction in chronic renal failure patients, as well as the results of a study on Henoch-Schönlein purpura patients (22). We also found that the plasma TNF-α levels were not correlated with ADAMTS13 activity in CKD patients, although certain studies have indicated that TNF-α inhibits ADAMTS13 synthesis without affecting VWF secretion in hepatic stellate and endothelial cells (15). On the one hand, in the above-mentioned study, the incubation of TNF-α with purified ADAMTS13 did not inhibit the proteolytic cleavage of VWF in vitro. On the other hand, the plasma TNF-α concentrations in sepsis patients were higher than those in CKD patients (23). In the Cao et al study, the incubation concentrations of TNF-α in hepatic stellate and endothelial cells were considerably higher than the plasma concentration (15). This may explain why our results for the plasma TNF-α concentration differ from those at a cellular level.
In our study, we found that the VWF:Ag levels in CKD patients were significantly higher than those in the control group (P<0.01); in addition, the ADAMTS13 activities in CKD patients were markedly lower than those in the control group (P<0.01). We hypothesized that low ADAMTS13 activity causes an increase in ultralarge VWF multimers, resulting in renal microvascular platelet thrombosis, and is therefore relevant to the development and progression of CKD. Thus, the balance between the VWF:Ag levels and ADAMTS13 activity may be more important in estimating thrombus formation. The VWF/ADAMTS13 ratio has also been reported to be more useful than the ADAMTS13 assay alone in the differential diagnosis of TTP (23). In our study, we found that the VWF:Ag levels and ADAMTS13 activity in the NS group were lower than those in the CGN and LN groups (P<0.05 and P<0.01). However, the three groups did not clearly differ with respect to the VWF/ ADAMTS13 ratio. Thus, our results were not consistent with those obtained for TTP.
CKD patients generally have damaged glomerular filtration membrane, which leads to considerable protein loss via the urine. A previous study revealed that VWF in urine increased in patients with large amounts of urine protein in insulin-dependent diabetes mellitus (24). Since NS involves the excretion of large amounts of urine protein, the VWF:Ag decrease in the NS group can be mainly attributed to the urinary loss of VWF:Ag. Therefore, the ratio of VWF:Ag levels and ADAMTS13 activity may be not a suitable index for the evaluation of CKD. Somewhat unexpectedly, we found that in CKD patients, the serum CHOL levels were associated with ADAMTS13 activity. Furthermore, the ADAMTS13 activity was the lowest in the NS group, which had the highest CHOL levels. Perhaps the abnormality in lipid metabolism in NS influences the ADAMTS13 activity. However, this is only a speculation and requires investigation in future studies.
Ono et al (9) found that the ADAMTS13 activity was associated with acute renal failure and serum creatinine levels. However, other reports do not indicate a clear association between the changes in ADAMTS13 or VWF and disease severity, organ failure or outcome (25). We classified the CGN and NS patients according to the GFR: One group with a GFR of ≥60 ml/ min/ 1.73 m2 and another with a GFR of 0–59 ml/ min/1.73 m2; however, the two groups did not differ with respect to the VWF:Ag levels, ADAMTS13 activity and their VWF/ADAMTS13 ratio. This can be explained by the fact that all the patients in our study had chronic diseases, and the pathological changes in chronic diseases were different from those in acute conditions.
In the LN group, we studied the correlation between SLEDAI and TNF-α levels, VWF:Ag levels, ADAMTS13 activity and the VWF/ADAMTS13 ratio. In LN patients, SLEDAI was positively correlated with the levels of TNF-α and VWF:Ag, but did not correlate with ADAMTS13 activity and the VWF/ ADAMTS13 ratio. Increased serum VWF concentration has been shown to be a marker of endothelial dysfunction in human diseases (26). Our data indicate that TNF-α is not only associated with endothelial dysfunction, but also with LN activity.
In conclusion, we found that in CKD patients, plasma TNF-α levels were correlated with the VWF:Ag levels, but not associated with ADAMTS13 activity. Our study indicates that plasma TNF-α increased in CKD patients and did not directly influence the ADAMTS13 activity, although previous studies have shown that TNF-α inhibits ADAMTS13 synthesis in hepatic stellate and endothelial cells. The circulating ADAMTS13 activity was found to correlate with the levels of CHOL.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 30670904, to C.R.) and was a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Ocak G, Verduijn M, Vossen CY, et al. Chronic kidney disease stages 1–3 increase the risk of venous thrombosis. J Thromb Haemost. 2010;8:2428–2435. doi: 10.1111/j.1538-7836.2010.04048.x. [DOI] [PubMed] [Google Scholar]
- 2.Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI, Pinkney JH. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16:1189–1197. doi: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- 3.Guebre-Egziabher F, Fouque D. Metabolic consequences of inflammation in kidney failure. Nephrologie. 2003;24:383–386. [PubMed] [Google Scholar]
- 4.Patel TV, Mittal BV, Keithi-Reddy SR, Duffield JS, Singh AK. Endothelial activation markers in anemic non-dialysis chronic kidney disease patients. Nephron Clin Pract. 2008;110:c244–c250. doi: 10.1159/000167872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Aguirre Y, Paramo JA. Endothelial cell and hemostatic activation in relation to cytokines in patients with sepsis. Thromb Res. 1999;94:95–101. doi: 10.1016/s0049-3848(98)00200-x. [DOI] [PubMed] [Google Scholar]
- 6.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TC, Liu A, Liu L, et al. Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica. 2007;92:121–124. doi: 10.3324/haematol.10262. [DOI] [PubMed] [Google Scholar]
- 8.Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49–55. [PubMed] [Google Scholar]
- 9.Ono T, Mimuro J, Madoiwa S, et al. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107:528–534. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 10.Lu GY, Shen L, Wang ZY, Guo XF, Bai X, Su J, Ruan CG. Significance of plasma von Willebrand factor level and von Willebrand factor-cleaving protease activity in patients with chronic renal diseases. Chin Med J. 2008;121:133–136. [PubMed] [Google Scholar]
- 11.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation. 2008;117:1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 12.Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 14.Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 15.Cao WJ, Niiya M, Zheng XW, Shang DZ, Zheng XL. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J Thromb Haemost. 2008;6:1233–1235. doi: 10.1111/j.1538-7836.2008.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsukawa M, Kaikita K, Soejima K, et al. Serial changes in von Willebrand factor-cleaving protease (ADAMTS13) and prognosis after acute myocardial infarction. Am J Cardiol. 2007;100:758–763. doi: 10.1016/j.amjcard.2007.03.095. [DOI] [PubMed] [Google Scholar]
- 17.Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009;101:239–247. [PubMed] [Google Scholar]
- 18.National Kindney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19.Berden JH. Lupus nephritis. Kidney Int. 1997;52:538–558. doi: 10.1038/ki.1997.365. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 21.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 22.Del Vecchio GC, Penza R, Altomare M, et al. Cytokine pattern and endothelium damage markers in Henoch-Schonlein purpura. Immunopharmacol Immunotoxicol. 2008;30:623–629. doi: 10.1080/08923970801973646. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Wada H, Nishioka N, et al. ADAMTS13 related markers and von Willebrand factor in plasma from patients with thrombotic microangiopathy (TMA) Thromb Res. 2008;121:849–854. doi: 10.1016/j.thromres.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Silveira AM, Sjoberg S, Blomback M, Ostman J. von Willebrand factor antigen in plasma and urine in patients with type I (insulin-dependent) diabetes mellitus with and without nephropathy. J Diabetes Complications. 1992;6:89–95. doi: 10.1016/1056-8727(92)90017-f. [DOI] [PubMed] [Google Scholar]
- 25.Kremer Hovinga JA, Zeerleder S, Kessler P, et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5:2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 26.Nadar SK, Al Yemeni E, Blann AD, Lip GY. Thrombomodulin, von Willebrand factor and E-selectin as plasma markers of endothelial damage/dysfunction and activation in pregnancy induced hypertension. Thromb Res. 2004;113:123–128. doi: 10.1016/j.thromres.2004.02.010. [DOI] [PubMed] [Google Scholar]


