Abstract
Gene therapy holds great promise for the treatment of diseases. The key problem of gene therapy is the choice of an effective vector. Ultrasound-mediated microbubble technique (UMMT) has already shown promising applications in numerous types of tumors apart from cervical carcinoma. In the present study, according to the results of an MTT assay, we initially chose an ultrasound intensity of 0.5 W/cm2, an ultrasound exposure time of 30 sec and a microbubble concentration of 10% as the optimum experimental condition for wtp53 plasmid transfection into HeLa cells. To further investigate the transfection efficiency of ultrasound combined with microbubbles, RT-PCR analysis was used to examine the mRNA level of p53. The transfection efficiency in the plasmid plus microbubbles and ultrasound group was significantly higher than that of the other groups. Following transfection of the wtp53 gene, flow cytometric analysis showed that the cell cycle of HeLa cells was arrested in the G1 phase. The results of the present study suggest that UMMT, a new gene delivery system, increases the transfection efficiency of the wtp53 gene. Moreover, the growth of HeLa cells was arrested by introducing wtp53. This study may afford a new trend for the gene therapy of cervical carcinoma.
Keywords: ultrasound microbubble, feasibility and effect, wild-type p53 gene, gene therapy, HeLa cells
Introduction
Cervical carcinoma, the most common malignant tumor of the female reproductive system, is a serious threat to the global physical and mental health of women (1). Surgical techniques, radiation equipment and technology are constantly improving, and chemotherapy drugs are continually being developed. However, the impact of surgery on patients particularly on the physical and mental status of young patients and the series of systemic side effect associated with radiation and chemotherapy reduce the quality of life of these patients (2). Therefore, the search for a safe, effective and targeted treatment that can ensure the fertility of young patients is an important research direction.
With the rapid development in molecular biology and in the understanding of the pathogenesis of tumors, recently, gene therapy has become a hotspot of research. Gene therapy is a new techno logy that was developed in the 1980s, aimed at the cause of disease. It is a new method of treatment for difficult diseases with a promising future. The p53 gene is a tumor-suppressor gene which is currently being extensively studied. Numerous experiments both in vitro and in vivo have confirmed that this new method can induce cell apoptosis and inhibit the growth of tumors by introducing the p53 gene (3). It has been reported that abnormal p53 proteins and elevation of cell proliferative activity are related to the incidence of cervical cancer and the pathological grade of tumors (4). Therefore, inhibition of the growth of cancer can be realized if p53 genes can be transferred into cancer tissues, so as to realize etiological therapy in the treatment of cervical carcinoma.
At present, the key problem involving gene therapy is the lack of safe, effective, tissue-specific and tissue-targeted gene-loaded systems (5). The use of viral vectors is considered to exhibit high transfection efficiency yet poor safety. They were found to pose potential insertional mutagenesis and interference response (6). Conversely, non-viral delivery systems are safe and easy to apply, but suffer from low transfection efficiency, biocompatibility and are biodegradable (7). In order to solve this contradiction, ultrasound-mediated microbubble technique (UMMT), a new type of atraumatic gene transfer technology, has achieved great breakthroughs, which ensures that gene transfection is both efficient and targeted. It not only realizes the location ‘blasting’ of microbubbles (8), but also increases the permeability of tissues and cells, which facilitates the transfer of plasmid DNA or drugs into the cell (9–11). The intake of genes or drugs into cells is increased significantly so that the effect of treatment is markedly improved (9,12). However, when gene transfection is mediated by ultrasound microbubbles, it may damage cells because of ultrasonic cavitation (13). Thus, we attempted to ascertain the optimized ultrasonic parameters in order to minimize the damage and maximize transfection efficiency. Although researchers in this field have made many achievements, studies involving the treatment of gynecological tumors are still lacking.
Toward this purpose, we initially screened the three main factors affecting transfection efficiency: ultrasound intensity, exposure time and microbubble concentration. Subsequently, different experimental groups were treated with the optimized parameters, respectively. Afterward, the transfection efficiency of the p53 gene was assessed. The transfection efficiency was higher in the ultrasound combined with microbubble group than that of the other groups. We aimed to explore the feasibility of gene transfer by UMMT and the effects after transfection. We hoped to discover a reliable vector for gene therapy that may provide a new concept for the comprehensive treatment of cervical carcinoma.
Materials and methods
Chemicals and reagents
RPMI-1640 medium and fetal bovine serum (FBS) were supplied by Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-thiazyl)-2,5-diphenyltetrazolium bromide (MTT) and dimethylsulfoxide (DMSO) were obtained from Sigma (St. Louis, MO, USA). TRIzol was purchased from Sangon Biological Engineering Technology and Services (Shanghai, China). Polymerase chain reaction (PCR) mixture was from Takara Biotechnology Co., Ltd., (Dalian, China). Lipofectamine™ 2000 reagent was obtained from Invitrogen (Carlsbad, CA, USA).
Preparation of microbubbles
Microbubbles used in the experiments were made by the Institute of Ultrasound Imaging, The Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). Before use, the microbubbles were washed with phosphate-buffered solution (PBS) three times and sterilized by 60Co γ-irradiation. The density of the microbubbles was ∼2.3×109/ml, with a diameter of 2–5 μm. The ultrasonic gene transfection instrument (UGT1025) was able to launch ultrasonic frequencies of 300 kHz at an acoustic intensity of 0.25–2.5 W/cm2 and was developed by the Institute of Ultrasound Imaging, The Second Affiliated Hospital of Chongqing Medical University (Chongqing, China).
Amplification and extraction of pEGFP-N1-p53 plasmid and preparation of the mixture for transfection
Recombinant pEGFP-N1-p53 plasmids were purchased from Promega Beijing Biotech Co., Ltd., (Beijing, China) and grown in Escherichia coli (obtained from the Key Laboratory of Infectious Diseases of Chongqing Medical University, Chongqing, China). After abundant amplification, the plasmid was purified using a plasmid extraction kit (Axygen Biosciences, Union City, CA, USA) and was suspended in 2.5 mM Tris-HCl (pH 8.5) at a concentration of 1.0 μg/μl. For preparation of the mixture of plasmid and microbubbles, 4.0 μg of plasmid pEGFP-N1-p53 (4.0 μl) was lightly blended with 200 μl of the microbubble suspension and the mixture was gently incubated for a few minutes at 4°C to help adhesion. Additionally, 4.0 μg pEGFPN1-p53 plasmid (4.0 μl) was lightly blended with 12 μl liposome (Lipofectamine™ 2000, Invitrogen) and then mixed at room temperature for 20 min. This was the mixture of plasmid and liposome used for subsequent experimentation.
Cell line and cell culture
Human cervical carcinoma HeLa cell line (KG-042; Biotech Co., Ltd., Nanjing, China) was grown as a monolayer in a 50-ml culture flask and passaged every 2–3 days. Cells were maintained in RPMI-1640 supplemented with 10% FBS and 100 U/ml penicillin, 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air. When cells reached subconfluence, they were counted with a hemocytometer (Burker Turk) and seeded in 6-well plates at a concentration of 4×105 cells/well.
Transfection and transfection rate assay
Cell transfections were performed under different ultrasonic conditions using 4 or 8 μg of pEGFP-N1-p53 plasmids according to the manufacturer’s instructions. Cells were incubated for 48 h after transfection and then the expression of enhanced green fluorescent protein (EGFP) was observed under a fluorescence microscope. Five horizons were selected randomly for each group. The intensity of EGFP reflected the transfection efficiency of the pEGFP-N1-p53 plasmids.
In the experiments, HeLa cells were divided into five groups as follow: i) control (no treatment); ii) plasmid only; iii) plasmid with ultrasound; iv) plasmid with microbubbles and ultrasound; and v) plasmid with liposome. The ultrasound treatment parameters for the HeLa cells were: continuous wave, 300 kHz, 0.5 W/cm2, 30 sec and a 10% concentration of microbubbles.
Cell viability assay
Cell viability was evaluated by the MTT reduction assay. In brief, HeLa cells were seeded at a density of 5×103 cells/well in 96-well microtiter plates containing 150 μl of RPMI-1640 medium with 10% FBS. After growing to subconfluence, the cells were exposed to various concentrations of microbubbles (1, 5, 10 and 20%), different intensities of ultrasound (0.5, 0.75 and 1 W/cm2), different radiation times (10, 30 and 60 sec) and incubated for 24 h. Then incubation with MTT (5 mg/ml) was carried out in culture medium for 3 h at 37°C. After that, the medium was discarded and the formazan blue, which formed in the cells, was dissolved in 100 μl of DMSO. The absorbance was measured at 490 nm using a Sunrise Remote Microplate Reader (Grodlg, Austria), and then normalized according to the value of the control (untreated cells).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from HeLa cells using TRIzol. The quantity of RNA isolates was determined spectrophotometrically using a DNA/RNA Gene-Quant Calculator (Amersham Biosciences, USA). Reverse transcription was performed in 20 μl of reaction mixture containing 2 μg of total RNA, 5.0 units of AMV reverse transcriptase, 50 pmol of oligo(dT) primer, 40 nmol of dNTP mixture, 40 units of RNase inhibitor, 4 μl of 5X RT buffer (Bioer, Hangzhou, China) at 42°C for 1 h and 95°C for 5 min. The following primers for RT-PCR were used: p53 (568 bp), sense 5′-AGCATCTTATCCGAGTGGAAGGAA-3′; antisense 5′-TTATGGCGGGAGGTAGACTGACC-3′. β-actin (386 bp): sense 5′-GATGGTGGGAATGGGTCAGA-3′; antisense 5′-GGAGAGCATAGCCCTCGTAGAT-3′. RT-PCR analysis was performed in 20 μl of reaction mixture containing 1 μl of cDNA reaction mixture, 10 nmol of dNTP mixture, 10 pmol of sense and antisense primers and 2 units of BioReady rTaq polymerase (Bioer, Hangzhou, China). For PCR product analysis, 6 µl of each reaction mixture was electrophoresed on 1.5% agarose gel containing 1% Gold-View™. The band intensity was analyzed using the Geldoc 2000 system (Bio-Rad, USA) and presented as a percentage of β-actin expression.
Flow cytometry for cell cycle analysis
HeLa cells growing in 25-ml culture flasks were harvested, washed and fixed with ice cold alcohol (75%) for >24 h. After further being washed twice, cells were incubated with PBS (pH 7.4) containing RNase (5 units) and PI (50 μg/ml) for 15 min at 37°C. Flow cytometry was performed using a FACS Vantage SE flow cytometer.
Statistical analysis
All data are expressed as the means ± SD. Statistical analyses were performed using the SPSS 13.0 package (SPSS Inc., Chicago, IL, USA). Comparisons between groups were performed by the Student’s t-test and one-way analysis of variance (ANOVA). Statistical significance was accepted at a p-value <0.05.
Results
Effect of different ultrasonic conditions on the viability of HeLa cells
The viability of HeLa cells under different ultrasonic conditions, as assessed by MTT method, is shown in Table I and Fig. 1A. When ultrasound was conducted with 0.5, 0.7, 1.0 W/cm2 and 10 sec, 0.5, 0.75 W/cm2 and 30 sec, cell survival was approximately 80%. When ultrasound was 0.5 W/cm2 and 60 sec, viability was approximately 70%. As the intensity and time increased simultaneously, cell viability was decreased significantly.
Table I.
Effect of different ultrasonic conditions on the viability of HeLa cells.
| Ultrasound intensity (W/cm2)
|
|||
|---|---|---|---|
| Exposure time (sec) | 0.5 | 0.75 | 1 |
| 10 | 84.75±0.75 | 83.43±0.67 | 78.84±0.87 |
| 30 | 81.19±2.00 | 77.61±1.93 | 61.97±1.86 |
| 60 | 69.56±0.32 | 57.67±1.51 | 48.05±0.57 |
Figure 1.
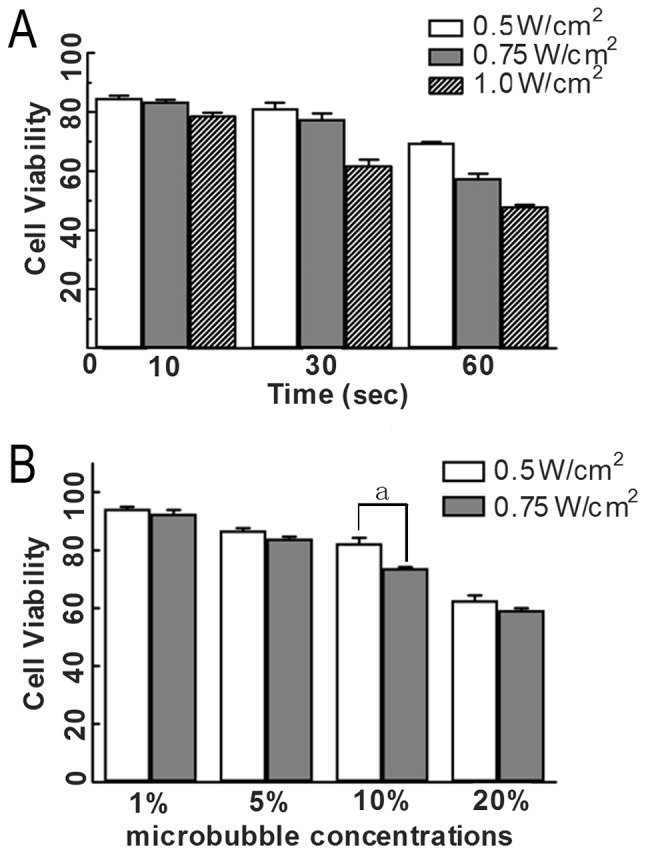
Effect of different ultrasonic conditions and different microbubble concentrations on HeLa cell viability measured by MTT assay. (A) Cells were treated with different ultrasound intensity (0.5, 0.75 and 1.0 W/cm2) and different exposure times (10, 30 and 60 sec) and cell viability was determined by MTT analysis (n=5) as described in Materials and methods. (B) Cells were treated with different microbubble concentrations (1, 5, 10 and 20%) and the ultrasound intensity was 0.5 and 0.75 W/cm2, and exposure time was 30 sec. Cell viability was determined by MTT assay. ap<0.05, two group comparison: the same microbubble concentrations, different ultrasound intensity.
Transfection rate of the wtp53 gene in HeLa cells under different ultrasonic conditions
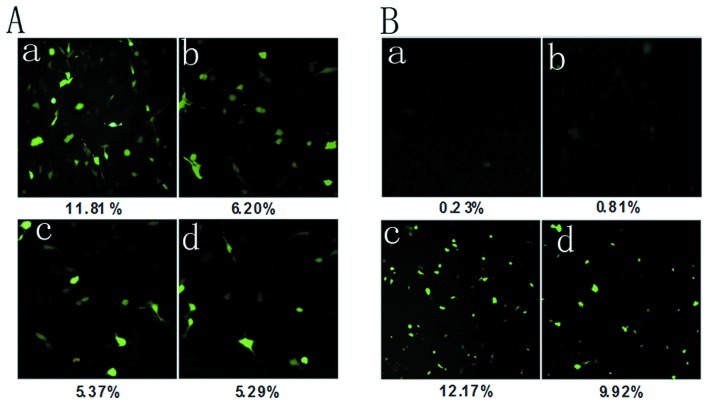
Based on the results of the MTT assay, we chose these ultrasound parameters (30 sec, 0.5 and 0.75 W/cm2; 60 sec, 0.5 W/cm2; 10 sec, 1 W/cm2) as the transfection conditions of the wtp53 gene, for which cell viability was >70%. Cells were incubated for 48 h after transfection and then the expression of EGFP was examined using fluorescence microscopy. As presented in Fig. 2A, when ultrasound was 0.5 W/cm2 and 30 sec, expression of EGFP in HeLa cells was highter than that in the other three groups. The result demonstrated that using this acoustic intensity and exposure time, the transfection efficiency of the wtp53 gene was highest.
Figure 2.
Expression of EGFP was examined by fluorescence microscopy. (A) The transfection rate of the wtp53 gene in HeLa cells under different ultrasonic conditions. Cells were incubated for 48 h after transfection and then observed under a fluorescence microscope. (a) 0.5 W/cm2 and 30 sec; (b) 0.75 W/cm2 and 30 sec; (c) 0.5 W/cm2 and 60 sec; (d) 1.0 W/cm2 and 10 sec.
Effect of different microbubble concentrations on HeLa cell viability
As shown in the result of the MTT assay (Table II and Fig. 1B), when the microbubble concentration was 20%, and ultrasound parameters were 0.5 W/cm2, 30 sec or 0.75 W/ cm2, 30 sec, cell survival was <70%. No significant difference was noted between the two groups (p>0.05). When micro-bubble concentration was 1 or 5% and ultrasound parameters were 0.5 W/cm2, 30 sec or 0.75 W/cm2, 30 sec, cell survival was >80%. That is to say, cell viability was not significantly affected. When the microbubble concentration was 10%, the difference between 0.5 W/cm2, 30 sec and 0.75 W/cm2, 30 sec was statistically significant (p<0.05). At the same time, we found that when the ultrasound parameters were 0.5 W/cm2, 30 sec or 0.75 W/cm2, 30 sec, the difference in cell viability was significant for the four concentrations of microbubbles.
Table II.
Effect of different microbubble concentrations on HeLa cell viability.
| Microbubble concentrations (%)
|
||||
|---|---|---|---|---|
| Ultrasound intensity (W/cm2) | 1 | 5 | 10 | 20 |
| 0.5 | 94.25±0.76b | 86.78±0.87b | 82.41±1.81a,b | 62.72±1.71b |
| 0.75 | 92.59±1.32c | 84.00±0.69c | 73.74±0.44a,c | 59.35±0.58c |
p<0.05 two-group comparison: the same microbubble concentrations, different ultrasound intensity.
p<0.05,
p<0.01 comparison of groups with the same ultrasound intensity, different microbubble concentrations.
Due to the slight effect on cell viability and high transfection efficiency, we chose 0.5 W/cm2, 30 sec, continuous wave and a microbubble concentration of 10% as the optimal transfection condition for transfection of the wtp53 gene in subsequent experiments.
wtp53 transfection rate in each experimental group
Fig. 2B indicates that the two groups, plasmid with microbubbles and ultrasound and plasmid with liposome, showed greater EGFP expression in HeLa cells than that in the other three groups. The transfection rate in the former was higher than that in the latter (p<0.05), while it was higher in the plasmid and liposome group than that in the plasmid only and plasmid and ultrasound groups (p<0.01).
Expression of wtp53 mRNA in each experimental group
The RT-PCR results (Fig. 3) showed that wtp53 expression was detected in the plasmid with microbubbles and ultrasound group and the plasmid with liposome group, while in the other three groups the expression was not apparent. The wtp53 mRNA level was also increased in the plasmid with microbubbles and ultrasound group compared to the plasmid with liposome group. This demonstrated that the carrier of ultrasound micro-bubbles increased the transfection rate of the wtp53 gene.
Figure 3.

mRNA levels of p53 in the different experiment groups. Cell were treated under five different conditions. After treatment, p53 mRNA levels were determined by RT-PCR analysis as described in Materials and methods. The amount of mRNA was normalized to that of β-actin. Data are expressed as means ± SD (n=3). #p<0.01, *p<0.05 compared to the control group.
Transfection of wtp53 results in cell cycle arrest
To test the role of wtp53 in the cell cycle progression of HeLa cells, the cell cycle distribution was assessed by flow cytometry. As indicated in Table III and Fig. 4, in the plasmid with microbubbles and ultrasound group, 83.33±3.43% of the total number of cells was in the G1 phase of the cell cycle. Compared to the other groups, the number of G1 phase cells was increased significant, while the number of cells in the G2 phase was decreased. This indicated that the wtp53 gene arrested the cell cycle of HeLa cells in the G1 phase.
Table III.
Flow cytometry for cell cycle analysis.
| Groups | G1 | S | G2 |
|---|---|---|---|
| Control | 66.17±3.50 | 25.35±3.70 | 8.48±4.32 |
| Plasmid | 68.42±4.12 | 28.81±4.28 | 2.77±3.91 |
| Plasmid with ultrasound | 69.92±3.71 | 27.69±4.53 | 2.39±4.11 |
| Plasmid with microbubbles and ultrasound | 83.33±3.43a | 15.34±4.15 | 1.33±3.50 |
| Plasmid with liposome | 76.51±4.23b | 21.91±3.53 | 1.58±3.64 |
p<0.01,
p<0.05, compared to the control group.
Figure 4.
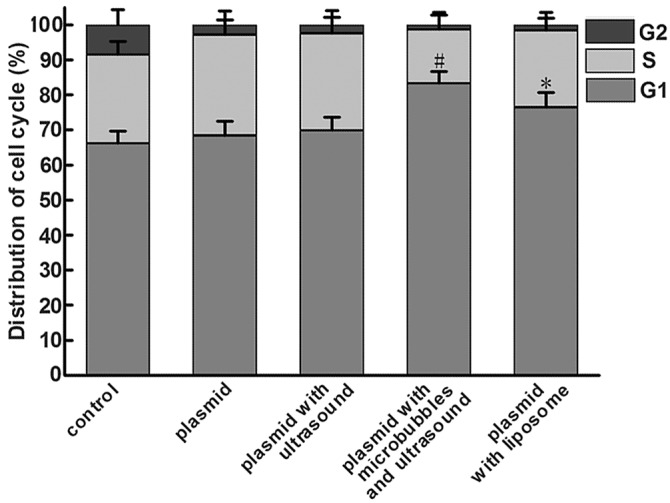
Flow cytometry for cell cycle analysis of HeLa cells treated under different conditions. Cell were treated under five different conditions, harvested and labeled with PI, and then analyzed by measuring the fluorescence intensity of PI. Values are means ± SD (n=3). *p<0.05, #p<0.01 compared to the vehicle-treated control group.
Discussion
Cervical cancer is a common gynecological malignancy. Its therapy faces the task of reducing recurrence by the killing of most tumor cells and the removal of the tumor tissue, but which simultaneously reduces the fertility of the patient. Therefore, researchers need to explore better methods with which to optimize the treatment of cervical cancer.
One of the most common tumor-suppressor genes involved in human malignancies is p53 (14). When there is a mutation in the p53 gene, its monitoring capacity is lost and it becomes an oncogene. Mutated or inactivated p53 can lead to selective growth advantage and tumor formation (4). In view of the high mutation rate of the p53 gene in human malignant neoplasms, the use of the wtp53 gene in cancer therapy has become a main focus of research. The wtp53 gene inhibits tumor cell proliferation by blocking the cell cycle and accelerating cell apoptosis (15,16). It has been reported that wtp53 also increases tumor sensitivity to radiotherapy and chemotherapy (17) and reduces the side effects of radiotherapy and chemotherapy (18).
Gene therapy is a promising new method. The main difficulty involves the choice of gene vector. As a novel gene transfer vector, the microbubble contrast agent can be broken down by ultrasound exposure in designated areas in order to release the gene it carries (19,20). At present, researchers working on gene transfection mediated by UMMT have achieved some important breakthroughs. It has been demonstrated that genes could be highly transfected into various types of tissues using this technique (21). UMMT plays an ever increasing role in enhancing the delivery of therapeutic agents into various tissues, such as the myocardium, blood vessels, skeletal muscle, tumor and even fetal tissues. These deliverable agents currently include genetic material, proteins and chemotherapeutic drugs (22–28).
However, a high concentration of microbubbles may have a negative impact on cell activity, and the use of ultrasound at a certain range of sound intensity and period of time when used to break the microbubbles, can harm cells and even cause cell death (29). Thus, in the present study, different ultra-sound intensities, ultrasound exposure times and different microbubble concentrations were used in preliminary experiments to identify the optimal experimental conditions. MTT assay showed that ultrasound microbubbles had a certain inhibitory effect on the proliferation of the HeLa cell line. From the result of our preliminary experiments, we chose an ultrasound intensity of 0.5 W/cm2, an ultrasound exposure time of 30 sec and a microbubble concentration of 10% as the optimum experimental condition for wtp53 plasmid transfection into HeLa cells.
To further investigate the transfection efficiency of ultra-sound combined with microbubbles, RT-PCR analysis was used to examine the mRNA level of p53. Under different transfection conditions, the transfection efficiency of wtp53 varied. It was clear that the transfection efficiency in the plasmid plus microbubbles and ultrasound group was significantly higher than that in the plasmid with liposome group. No expression was detected, however, in the control group or plasmid with ultrasound group. This indicates that microbubbles as a novel transfection medium may meet the need for efficient gene transfer. Flow cytometric evaluation of the cell cycle showed that as a novel gene transfer vector, ultrasound microbubble contrast agents can increase the intracellular levels of the wtp53 gene, affect the cell cycle, and thereby inhibit tumor cell growth.
In fact, there are many factors relevant to ultrasound microbubble-mediated gene transfer. Relevant conditions can be further optimized to improve transfection efficiency and reduce damage to normal cells. Our study was limited to experiments in vitro. Experiments in vivo will be the focus of further research. This technology in gynecological disease is still in its infancy. Based on the existing technology, more in-depth study and investigation are warranted.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (project no. 30770566).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, Williams C, Collingwood M. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005:CD002225. doi: 10.1002/14651858.CD002225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, Ji L. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo. Clin Cancer Res. 2001;7:2887–2897. [PubMed] [Google Scholar]
- 4.Oki E, Tokunaga E, Nakamura T, et al. Genetic mutual relationship between PTEN and p53 in gastric cancer. Cancer Lett. 2005;227:33–38. doi: 10.1016/j.canlet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Ding JH, Bekeredjian R, et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci USA. 2006;103:8469–8474. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberle V, de Jong G, Drayer JI, Hoekstra D. Efficient transfer of chromosome-based DNA constructs into mammalian cells. Biochim Biophys Acta. 2004;1676:223–230. doi: 10.1016/j.bbaexp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 9.Xing W, Gang WZ, Yong Z, Yi ZY, Shan XC, Tao RH. Treatment of xenografted ovarian carcinoma using paclitaxel-loaded ultrasound microbubbles. Acad Radiol. 2008;15:1574–1579. doi: 10.1016/j.acra.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Xie M, Wang X, Lv Q, Ding S. Efficient gene delivery to myocardium with ultrasound targeted microbubble destruction and polyethylenimine. J Huazhong Univ Sci Technolog Med Sci. 2008;28:613–617. doi: 10.1007/s11596-008-0528-4. [DOI] [PubMed] [Google Scholar]
- 11.Bekeredjian R, Kroll RD, Fein E, et al. Ultrasound targeted microbubble destruction increases capillary permeability in hepatomas. Ultrasound Med Biol. 2007;33:1592–1598. doi: 10.1016/j.ultrasmedbio.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Anwer K, Kao G, Proctor B, et al. Ultrasound enhancement of cationic lipid mediated gene transfer to primary tumors following systemic administration. Gene Ther. 2000;7:1833–1839. doi: 10.1038/sj.gt.3301302. [DOI] [PubMed] [Google Scholar]
- 13.Nie F, Xu HX, Tang Q, Lu MD. Microbubble-enhanced ultra-sound exposure improves gene transfer in vascular endothelial cells. World J Gastroenterol. 2006;12:7508–7513. doi: 10.3748/wjg.v12.i46.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomkova K, Tomka M, Zajac V. Contribution of p53, p63, and p73 to the developmental diseases and cancer. Neoplasma. 2008;55:177–181. [PubMed] [Google Scholar]
- 15.Nork TM, Poulsen GL, Millecchia LL, Jantz RG, Nickells RW. p53 regulates apoptosis in human retinoblastoma. Arch Ophthalmol. 1997;115:213–219. doi: 10.1001/archopht.1997.01100150215011. [DOI] [PubMed] [Google Scholar]
- 16.Ghule P, Kadam PA, Jambhekar N, et al. p53 gene gets altered by various mechanisms: studies in childhood sarcomas and retinoblastoma. Med Sci Monit. 2006;12:BR385–BR396. [PubMed] [Google Scholar]
- 17.Xu L, Pirollo KF, Tang WH, Rait A, Chang EH. Transferrin liposome mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10:2941–2952. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 18.Haupt S, Haupt Y. Importance of p53 for cancer onset and therapy. Anticancer Drugs. 2006;17:725–732. doi: 10.1097/01.cad.0000217422.52208.fa. [DOI] [PubMed] [Google Scholar]
- 19.Tiukinhoy SD, Mahowald ME, Shively VP, et al. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol. 2000;35:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Tachibana K, Kuroki M, Kuroki M. Gene transfer with echo-enhanced contrast agents: comparison between Albunex, Optison, and Levovist in mice-initial results. Radiology. 2003;229:423–428. doi: 10.1148/radiol.2292020500. [DOI] [PubMed] [Google Scholar]
- 21.Shimamura M, Sato N, Taniyama Y, et al. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther. 2004;11:1532–1539. doi: 10.1038/sj.gt.3302323. [DOI] [PubMed] [Google Scholar]
- 22.Zhigang W, Zhiyu L, Haitao R, et al. Ultrasound-mediated microbubble destruction enhances VEGF gene delivery to the infarcted myocardium in rats. Clin Imaging. 2004;28:395–398. doi: 10.1016/j.clinimag.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Wang Z, Ran H, et al. Enhanced gene delivery into skeletal muscles with ultrasound and microbubble techniques. Acad Radiol. 2006;13:363–367. doi: 10.1016/j.acra.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Mitragotri S, Blankschtein D, Langer R. Transdermal drug delivery using low-frequency sonophoresis. Pharm Res. 1996;13:411–420. doi: 10.1023/a:1016096626810. [DOI] [PubMed] [Google Scholar]
- 25.Mitragotri S, Kost J. Low-frequency sonophoresis: a noninvasive method of drug delivery and diagnostics. Biotechnol Prog. 2000;16:488–492. doi: 10.1021/bp000024+. [DOI] [PubMed] [Google Scholar]
- 26.Tachibana K, Uchida T, Tamura K, Eguchi H, Yamashita N, Ogawa K. Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett. 2000;149:189–194. doi: 10.1016/s0304-3835(99)00358-4. [DOI] [PubMed] [Google Scholar]
- 27.Munshi N, Rapoport N, Pitt WG. Ultrasonic activated drug delivery from Pluronic P-105 micelles. Cancer Lett. 1997;118:13–19. doi: 10.1016/s0304-3835(97)00218-8. [DOI] [PubMed] [Google Scholar]
- 28.Yu T, Hu K, Bai J, Wang ZB. Reversal of adriamycin resistance in ovarian carcinoma cell line by combination of verapamil and low-level ultrasound. Ultrason Sonochem. 2003;10:37–40. doi: 10.1016/s1350-4177(02)00106-2. [DOI] [PubMed] [Google Scholar]
- 29.Dalecki D, Raemen CH, Child SZ, Cox C, Francis CW, Meltzer RS, Carstensen EL. Hemolysis in vivo from exposure to pulsed ultrasound. Ultrasound Med Biol. 1997;23:307–313. doi: 10.1016/s0301-5629(96)00203-7. [DOI] [PubMed] [Google Scholar]