Abstract
The isolation and characterization of lung stem and progenitor cells represent an important step towards the understanding of lung repair after injury, lung disease pathogenesis and the identification of the target cells of transformation in lung carcinogenesis. Different approaches using prospective isolation of progenitor cells by flow cytometry or lineage-tracing experiments in mouse models of lung injury have led to the identification of distinct progenitor subpopulations in different morphological regions of the adult lung. Genetically defined mouse models of lung cancer are offering new perspectives on the cells of origin of different subtypes of lung cancer. These mouse models pave the way to further investigate human lung progenitor cells at the origin of lung cancers, as well as to define the nature of the lung cancer stem cells. It will be critical to establish the link between oncogenic driver mutations recently discovered in lung cancers, target cells of transformation and subtypes of lung cancers to enable better stratification of patients for improved therapeutic strategies.
Keywords: lung stem cells, lung disease and repair, lung cancer, cell of origin of lung cancer, cancer stem cells
2. Introduction
Respiratory diseases are a major cause of mortality and morbidity worldwide, with over 10 million deaths attributed to lung disorders [1]. The lung is a complex organ with multiple functions that are critical for survival. Isolation and characterization of lung stem cells and understanding their capacity for repair, regeneration and tumourigenesis have an enormous potential impact on prevention and treatment of lung diseases. Lung stem cells may constitute a therapeutic option in poorly treated lung degenerative disorders, including cystic fibrosis, chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Comprehension of the epithelial hierarchical organization of the normal lung is also critical for the understanding of the initiation of lung carcinogenesis. Recent advances in the technologies available for cell tracking using engineered mouse models, as well as cell isolation by flow cytometry, have provided new tools to study lung stem/progenitor cells. In this review, we focus our interest on recent insights into the identification of lung stem and progenitor cells in the adult lung, and the evaluation of their role as possible cells of origin in lung cancer.
Lung cancer is the leading cause of cancer death worldwide. Five-year lung cancer survival is only 15 per cent [2], and lung cancer is responsible for more deaths than prostate, colon, pancreas and breast cancers combined. Major improvements in clinical outcome will depend on new insights into normal lung and tumour biology. Lung cancers are divided into distinct histopathological classes: small cell lung cancer (SCLC; 20% of all lung cancers), which has a neuroendocrine phenotype, and non-small cell lung cancer (NSCLC; 80% of all lung cancers), which can be further subdivided into adenocarcinomas, squamous cell, bronchioalveolar and large cell carcinomas [3–6]. Squamous cell carcinomas are thought to originate from the proximal airways, SCLC are predominantly located in the bronchioles while adenocarcinomas, the most common type of lung cancer, are more frequently detected in the distal part of the lung. It is speculated that these different subclasses arise from distinct cells of origin localized within a defined regional compartment [7,8]. Prospective isolation of stem/progenitor cells in the different compartments of the lung will enable further evaluation of their respective roles in tumour initiation.
A large number of cell types constitute the adult lung and are present at different frequencies according to the anatomical region of the respiratory system [9]. In the adult trachea and main bronchi (cartilaginous airways), the luminal epithelium contains two main columnar cell types: ciliated cells (expressing FoxJ1) and Clara-like cells (producing secretoglobins, the most abundant being Scgb1a1, or CC10). Ciliated cells are terminally differentiated cells that do not have self-renewal capacity [10,11]. A small number of neuroendocrine cells are also present. The cartilaginous airways contain a discontinuous population of basal cells that express p63, keratin 5, keratin 14 and nerve growth factor receptor (NGFR) [12]. In the mouse, basal cells are only detected in the trachea, whereas in humans basal cells are present in the bronchi and bronchioles [12]. In the more distal airways (small bronchi and bronchioles), the epithelium is columnar. Clara cells predominate over ciliated cells and there are more neuroendocrine cells than in the trachea. No basal cells are detected in the distal small airways [13,14]. The most distal region of the lung is organized into a complex system of alveoli composed of two types of epithelial cells: alveolar type I cells (AEC I), which provide the thin-walled gas exchange surface, and cuboidal alveolar type II cells (AEC II), containing secretory vesicles filled with surfactant, including surfactant protein C (SP-C). The transitional region between the terminal bronchiole and the alveoli is known as the bronchioalveolar duct junction [15]. These different regions of the lung appear to use different progenitor cells for maintenance and repair [15].
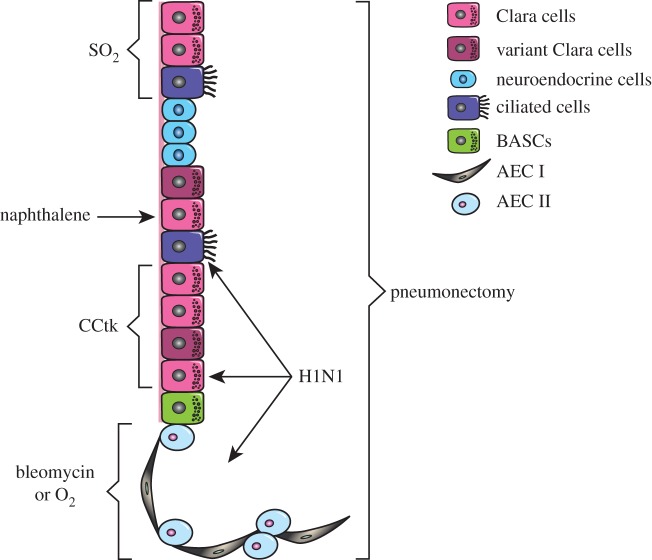
3. The importance of models of lung injury to study lung stem cells
Different models have been proposed for the maintenance and regeneration of adult solid tissues. In breast and gut, a small number of undifferentiated stem cells can self-renew and produce differentiating progeny for normal tissue function [16–18]. In the skin, Clayton et al. [19] proposed a committed progenitor model in which the epidermis is maintained by a population of progenitor cells that can undergo unlimited cell divisions and terminal differentiation [20,21]. Other organs (such as the pancreas and the liver) seem to regenerate by simple proliferation of existing mature cells such as β-cells or hepatocytes, but can also use ‘facultative’ stem cells to regenerate the tissue [22–26]. The model followed by the lung epithelium at steady state and after injury is still a matter of debate. Compared with the intestine or the skin, the adult lung has a slow turnover time. It is constantly exposed to potential toxic agents and pathogens present in the environment, however, and must therefore be able to respond quickly and effectively to cellular damage, suggesting the existence of lung stem/progenitor cells. Myelo-ablation and competitive repopulation assay have been used for many years in the haematopoietic field to study haematopoietic stem cell activity [27]. Similarly, in the lung, several experimental protocols (described below, and summarized in table 1 and figure 1) have been developed in mice to challenge the lung and stimulate activation of stem/progenitor cells [15,40]. Each model is unique in the injury caused, the degree of immune cell infiltration and fibrosis, the cell types affected, and resulting regeneration. In-depth description of lung injury models have been reviewed elsewhere [15,40]. Here, we describe mouse models most recently used in the search for adult lung stem cells (table 1 and figure 1).
Table 1.
Models of lung injury to study lung stem cells.
| model | dose and route of administration | target cell(s) | maximal injury | repair | references |
|---|---|---|---|---|---|
| bleomycin | 2.3 units kg–1 intratracheal instillation or, 120 mg kg–1 i.v. | EC, AEC I, AEC II | 6–10 days | 21 days | [28–30] |
| naphthalene | 250 mg kg–1, i.p. | Cyp2f2-containing Clara cells | 3 days | 10 days | [31,32] |
| ganciclovir (CCtk mice) | 4.5 mg d–1 GCV (375 mg ml–1 in saline) via miniosmotic pump for 6 or 12 days | CC10+ Clara cells, ciliated cells susceptible to delayed ablation, AEC II | 7–12 days | [33–35] | |
| pneumonectomy | n.a.; entire left lung removed | all lung epithelia, vasculature and support cells | at time of surgery | 15 days | [36] |
| H1N1 (PR8) influenza virus | 250 PFU, intratracheal inhalation | Clara cells, ciliated cells, AEC II | 11 days | 21–60 days | [13] |
| O2 | 70–100% O2, chamber for 56 h or if longer, alternating to room air every 24 h | alveolar cells (distal) | 3 days | 14 days | [37,38] |
| SO2 | 500 ppm SO2 in room air, chamber for 3 h | luminal cells of tracheo-bronchial epithelium | 36 h | 7 days | [11,14,39] |
Figure 1.
Models of lung injury to study lung stem cells. Schematic diagram of the selective effect of different injuries in proximal and distal lung.
3.1. Naphthalene
Naphthalene is an aromatic hydrocarbon found in tobacco smoke and in mothballs. Administered i.p. naphthalene becomes cytotoxic when metabolized by Cyp2f2, a specific P450 mitochondrial cytochrome contained in a subset of Clara cells located in the bronchioles [31,32]. Approximately 3 days after naphthalene administration, the majority of Clara cells lining the bronchioles are destroyed. This effect is abolished in mice lacking Cyp2f2 [31]. A small subset of Clara cells, termed variant Clara cells, are resistant to naphthalene and are proposed to be responsible for repletion of the bronchiolar epithelium after injury [31,32,41].
3.2. Ganciclovir (CCtk mice)
To target all Clara cells independent of Cyp2f2 expression, Reynolds et al. [33] generated a transgenic mouse strain, termed CCtk, which possess the herpes simplex virus thymidine kinase (HSVtk) under the control of the CC10 promoter. Temporal and site-specific ablation is achieved by the addition of ganciclovir, which results in production of toxic HSVtk metabolites in cells expressing HSVtk, in this case Clara cells [33]. Whereas variant Clara cells are resistant to naphthalene, the CCtk mouse model results in complete depletion of CC10+ cells, making it a useful model to identify early Clara cell, progenitors. Secondary loss of AEC II was observed in these mice and was characteristic of an end-stage disease [34].
3.3. Bleomycin
Bleomycin is an antibiotic produced by Streptomycin vercillus that has been used extensively as anti-cancer agent owing to its ability to cause DNA strand breaks. A major side effect of the drug is pulmonary fibrosis, specifically bronchioalveolar damage. In mice, reduction in the number of AEC I and AEC II was observed after intranasal or intratracheal instillation [28,42,43]. Intratracheal administration, the most frequently used method, results in maximum AEC I and AEC II loss 6–10 days following treatment [29,30,44,45].
3.4. Pneumonectomy
Partial pneumonectomy (PNX), whereby one lobe is removed by surgical resection, results in compensatory expansion of the remaining lung lobes, which increase in volume to fill the void and maintain ventilation [46–48]. Recently, Ding et al. [36] provided new insight into this long-standing yet unexplored model of lung regeneration. Use of PNX demonstrates the interplay between endothelial and epithelial compartments, long known to be essential for developmental alveologenesis, but only recently appreciated as critical for regenerative alveologenesis [36].
3.5. H1N1
Sublethal infection of mice with murine adapted (PR8) H1N1 influenza virus results in widespread bronchiolar and alveolar damage, with loss of Clara cells, ciliated cells and AEC II. Remarkably, post-H1N1 lung regeneration appears to occur in the absence of fibrosis [13]. The infiltration of macrophages appears essential for the post-H1N1 regenerative process to occur without fibrosis [49].
3.6. O2 and SO2
The diffusible gases O2 and SO2 are administered via ventilation supply of the gases to mice housed in airtight chambers, and result in lung injury with immune cell infiltration and fibrosis. Hyperoxia has long been known to cause lung injury and changes in normal alveolar development in premature infants on artificial ventilation. In adult mice, O2-induced hyperoxia causes alveolar epithelial cell death by day 3 (reviewed in [37]) and repair is complete by day 14. The primary phase of hyperoxic lung toxicity consists of damage to the epithelium and endothelium that results in oedema and immune cell infiltration. The subsequent secondary phase consists of proliferation of AEC II, interstitial fibrosis and impaired gas exchange (reviewed in [50]). Inhaled SO2 causes destruction of the luminal cells of the pseudo-stratified tracheo-bronchial epithelium [11,51], the distal lung epithelium being spared [39].
Other agents that cause lung injury include nitric oxide, ozone, chlorine, polidocanol and particulates, among others [52–54]. Future work that determines in vivo the specific cells affected and those responsible for the repair to injury in these injury models may provide further tools to investigate adult lung stem cells.
4. The search for adult lung stem cells
Use of the murine models of lung injury described above has enlightened our understanding of lung regeneration and led to the identification of lung stem/progenitor cells. It is becoming evident that in mice diverse types of injury activate different signalling pathways, leading to the activation of different types of progenitor cells, and that different regions of the respiratory system (alveoli, bronchioles and upper airways, i.e. bronchi and trachea) have different kinds of progenitor cells for maintenance and repair [15].
Regeneration of the lung parenchyma after injury is thought to be dependent on SP-C+ AEC II cells that can proliferate and regenerate AEC I cells after injury [43,55,56]. Recent lineage tracing experiments in mice unequivocally showed that at steady state and in response to bleomycin injury, AEC I cells were generated from AEC II cells [45]. However, newly generated AEC II cells after injury were derived from SP-C− cells, suggesting the existence of an SP-C− alveolar progenitor population capable of regenerating the AEC II cells in the injured distal lung [29,45]. Chapman et al. [29] recently identified a population of CD49f+CD104+ (integrin-α6+β4+) alveolar epithelial cells in the murine distal lung enriched for SPC−CC10− cells. These cells have the capacity to give rise to SP-C+ and CC10+ cells in vitro or after transplantation under the kidney capsule when aggregated with embryonic lung cells. These progenitor cells may therefore be the precursors of differentiated AEC II SP-C+ cells [29]. Using additional cell surface markers, McQualter et al. [57] isolated three distinct subpopulations of mouse lung epithelial cells and evaluated their colony formation capacity in vitro. The EpCAMhiCD104+CD24lo subset is enriched in cells with colony-forming capacity, capable of self-renewal and forming colonies composed of airway, alveolar or mixed lung epithelial lineages in vitro. They may be similar to CD49f+CD104+ cells identified by Chapman et al. [29]. The EpCAMmedCD104− subset is enriched in alveolar cells and only a small fraction of these cells has alveolar-committed progenitor activity with the generation of saccular, AEC II-like colonies, whereas EpCAMhiCD104+CD24hi cells did not exhibit colony-forming capacity and were enriched in ciliated cells [57].
In the terminal bronchiole, cells located at the bronchio-alveolar ductal junction were proposed to be responsible for repair after injury in mice. Slow-cycling label-retaining cells expressing the Clara cell-specific marker CC10 were observed after naphthalene injury [58]. Subsequently, a population of putative bronchio-alveolar stem cells (BASCs) resistant to bronchiolar and alveolar damage was described [30]. These cells coexpressed CC10 and SP-C and expanded subtly after bronchiolar (naphthalene-induced) or alveolar (bleomycin-induced) injury. In vitro, BASCs had self-renewal capacity and when cultured on Matrigel could differentiate into Clara cells, AEC I and AEC II cells, but did not differentiate into ciliated cells. Cell surface markers to isolate the BASCs are still a controversial and unresolved question. Initial studies demonstrated an enrichment of BASCs in Sca-1+CD34+ cells [30]. But Teisanu et al. proposed that bronchiolar progenitor cells, resistant to naphthalene injury, were enriched in the Sca-1loCD34− subset and could be further separated from Clara cells based on their level of autofluorescence [59,60]. McQualter et al. demonstrated that mouse lung Sca-1+ cells were enriched in fibroblasts that could support the growth of epithelial progenitor cells, further indicating that Sca-1 is probably not a marker of epithelial progenitor cells [57,61]. Further refinement of the cell surface markers expressed by BASCs suggested that they were enriched in the EpCAMhiCD104+Sca-1loCD24lo subset [62]. These discrepancies in the cell surface markers described by different groups may be the result of distinct tissue processing, as well as analytical approaches. However, there is accumulating evidence to propose that the EpCAMhiCD104+CD49f+Sca-1loCD24lo subset is enriched in mouse lung progenitor cells [29,57,61,62], but whether these cells express SP-C and/or CC10 is still unresolved. Combining cell surface marker studies with lineage tracing experiments using a split-Cre approach, in which inactive ‘split-Cre’ fragments are controlled by two different promoters (e.g. N-cre controlled by SP-C promoter and C-cre controlled by CC10 promoter) and regain Cre activity when overlapping expression exists, will help resolve this question [63].
In the bronchioles, Clara cells are capable of self-regeneration and generate terminally differentiated ciliated cells in mice. In vivo lineage tracing experiments using CC10-creERTam mice showed that CC10-expressing Clara cells in the bronchioles self-renewed and generated ciliated cells during post-natal growth, adult homoeostasis and repair after bronchiolar injury [41]. Interestingly, damage of the alveolar compartment by hyperoxia in CC10-creERTam mice did not yield to the production of lineage-labelled AEC I and AEC II cells, suggesting that in this model CC10+ Clara cells in the bronchiole could not generate alveolar cells [41]. In contrast, lineage-labelled AEC II and AEC I cells were detected in fibrotic regions after bleomycin-induced alveolar injury in CC10-creERTam mice [45]. Although it is still unclear whether the lineage-labelled AEC II and AEC I cells observed following bleomycin injury in CC10-creERTam mice are derived from BASCs, lineage-labelled AEC II or SP-C negative alveolar cells, it would be of interest to identify the signals mediated by bleomycin, but not by hyperoxia, that can induce differentiation of CC10+ cells into alveolar lineages.
In the upper airways, basal cells and not Clara cells were found to be the precursors of the tracheal lineages in mice. Lineage tracing of CC10-labelled cells during ontogeny in the trachea showed an initial increase in labelled ciliated cells followed by a decrease in the number of labelled Clara cells and ciliated cells over time. After SO2-induced tracheal injury, proliferation of CC10+ cells was observed, but the majority of the newly formed tracheal epithelium was unlabelled. These results suggested the existence of a CC10− epithelial progenitor population responsible for maintenance of the tracheal luminal epithelium during post-natal growth, adult homeostasis and repair [41]. Lineage-tracing experiments of the basal cells using keratin 5-creERTam or keratin 14-creERTam strains showed that these cells in the mouse trachea have the potential to self-renew and generate both Clara cells and ciliated cells in vivo during post-natal growth and after injury, placing the basal cells at the apex of the cellular hierarchy to generate and repair the tracheal epithelium [14,64]. These cells were further isolated from the mouse trachea and human airways based on the expression of CD49f (integrin α6) and NGFR, and formed ‘tracheospheres’ or ‘bronchospheres’ in in vitro culture [14]. At steady state, basal cells are only detected in the trachea of the mouse, in contrast with the human airway epithelium where keratin-5+/keratin-14+ cells are also detected in the bronchi and bronchioles [12]. However, two mouse models of lung injury demonstrated the emergence of basal cells in the mouse bronchi and the distal lung, suggesting that basal cells could play a transient role in mouse distal lung regeneration after injury [65]. Proliferative keratin-14+ cells were detected 1 day following naphthalene injury in the mouse bronchi, and gave rise to Clara cells and ciliated cells [13,65]. Using a different mouse model of injury, Kumar et al. [13] recently described a population of p63+, keratin 5+ basal cells that appear after influenza A virus (H1N1) sub-lethal injury in the distal lung that was not detected after bleomycin injury. Gene expression profiling and lineage tracing experiments suggested that these cells participated in the restoration of the injured alveoli after H1N1 infection and expressed high levels of angiogenic factors to promote neo-capillary formation [13]. Kumar et al. proposed that the keratin-5+ cells observed after H1N1 injury originated from a rare population of basal cells present in the distal mouse lung. However, we (M.-L. Asselin-Labat 2012, unpublished data) and others [14] were not able to detect keratin-5 positive cells in the distal lung by immunohistochemistry. Lineage-tracing experiments may help define the origin of the basal cells observed after influenza-induced injury and define a population of early progenitor cells.
These studies highlight how different mouse models of lung injury have been used to identify progenitor cells in the lung. Each model activates different regenerative properties. Interestingly, chemical injury (bleomycin), hyperoxia and viral infection (H1N1), although all damaging the distal lung, appear to stimulate distinct signalling pathways, leading to the activation of different types of progenitor cells to induce lung regeneration. Comparison of those different signals would generate insights into the processes responsible for the activation of a specific progenitor cell type. A caveat of murine models of the lung is that there are well-described differences between mouse and human lung. These include the absence of respiratory bronchioles and a reduction in both the number of airway generations and submucosal glands in the mouse, as well as absence or limited number of basal cells in mouse airways compared with human airways [12,66]. Translating the results described in mice to the human lung is critical for understanding human lung biology and pathology. However, the search for human adult lung stem cells has proved a lot more difficult and only minor advancement has been made. Kajstura et al. recently published a controversial study [67,68] claiming the identification of human lung stem cells based on the expression of c-kit [69]. The most surprising finding in this work is the unprecedented identification of cells that can give rise to both endodermal and mesodermal lineages. Thorough replication of this work will be necessary to confirm their claim. The gold standard assay to assess stem cell property is in vivo repopulation after challenge of the environment to generate a stem cell niche. Developing such an assay in the lung constitutes a major challenge, but will be instrumental to demonstrate the existence of mouse and human lung stem cells. Combining mouse lineage tracing experiments with prospective isolation of lung stem cells with cell surface markers will enable further delineation of their molecular characteristics to identify genetic and epigenetic factors regulating their function in normal lung and diseased lung, including lung cancer.
5. Regulators of adult lung stem/progenitor cells
Pathways regulating embryonic lung development have been well studied (reviewed in [70]), but signalling pathways regulating cell proliferation, self-renewal or differentiation in the adult lung are still largely underexplored, in large part due to the paucity of markers available to prospectively isolate lung stem and progenitor cells. In the mouse lung subpopulations described earlier, the proportion of progenitor cells remains low, with only approximately 5 per cent of EpCAMhiCD104+CD24lo cells having colony-forming capacity in vitro [57], while limiting dilution studies showed that 1 in 110 cells in the BASC-enriched population had colony-forming potential [71]. This presents a major limitation for the use of gene profiling studies to identify pathways regulating stem/progenitor cells.
Recent studies have relied on gain or loss of function of genes known to be regulators of self-renewal in other stem cell systems, such as Bmi-1, β-catenin or Notch. Bmi-1 regulates stem cell self-renewal and cancer progression in many organs, including the haematopoietic and neural systems and the breast [72–74]. Bmi-1 is also involved in chromatin remodelling and is upregulated during lung organogenesis, where it may play a role in enhancing the accessibility of transcription factor binding sites [75]. In Bmi-1-deficient mice, lung repair after naphthalene-injury was impaired [71]. Bmi-1-deficient BASCs were less proliferative than wild-type BASCs in vitro and failed to self-renew [71]. Loss of Bmi-1-target genes p16/p19 only partially rescued the self-renewal capacity of Bmi1-deficient BASCs in vitro, whereas loss of the imprinted gene p57 largely reactivated Bmi-1-deficient BASCs self-renewal capacity [62,71]. β-catenin, a downstream target of the Wnt pathway, regulates stem cell self-renewal [76,77]. Stabilization of β-catenin in Clara cells resulted in an accumulation of progenitor cells resistant to naphthalene injury, leading to increased cell proliferation and earlier lung repair [78]. The Notch pathway plays an important role in embryonic lung development to maintain the balance of proximal–distal cells at early stages and in cell fate decision later in development. In particular, Notch favours a non-neuroendocrine fate, and promotes mucous cell differentiation at the expense of ciliated cells of the conducting airways and alveolar cells of the distal airways [79–82]. In the adult, family members of the Notch signalling pathway are expressed in the basal epithelial cells of the adult mouse trachea [51]. Notch activation in the basal cells of the adult mouse trachea resulted in their differentiation into the secretory luminal lineage following SO2 injury [51]. Conversely, loss of Notch function resulted in a significant reduction in the number of luminal cells in SO2-injured trachea. Mouse basal cells treated with the γ-secretase inhibitor dibenzazepine (inhibitor of Notch signalling) led to the formation of p63+ tracheospheres that did not express luminal cell markers, indicating that although Notch is not required at steady state in the trachea, it is required for basal cell differentiation into luminal cells after tracheal injury [51].
Development of the embryonic lung is regulated by endodermal–mesenchymal cross-talk, and alveolarization of the embryonic lung is highly controlled by parallel blood vessel formation [70,83–85]. Similarly, in the adult lung, progenitor activity is tightly controlled by autocrine and paracrine signals released by other cells types. Co-culture of mouse lung epithelial progenitor cells with mouse lung fibroblasts was required to induce multi-lineage differentiation of the epithelial cells in vitro [57]. Recent evidence showed the importance of interactions between the lung epithelium and the vasculature for adult mouse lung regeneration [36]. Stimulation of pulmonary capillary endothelial cells after PNX led to the production of angiocrine factors, including MMP14 in a VEGFR2/FGFR1-dependent manner. Expression of VEGFR2 and FGFR1 in the endothelium was required to stimulate pulmonary capillary endothelial cells to support neo-angiogenesis. This resulted in expansion of BASCs and AEC II amplification, as demonstrated by the use of endothelium-specific vegfr2/fgfr1 deletion in mice [36]. Similarly, genes involved in angiogenesis and endothelin signalling were detected in regions of lung repair after H1N1 infection [13], further indicating that endothelial–epithelial interactions are involved in repair of the lung in response to varied injurious stimuli.
6. Cells of origin in lung cancer
Identifying distinct populations of stem or progenitor cells in the lung has important implications for a better understanding of normal lung function and lung disease processes [9]. It is also key to better understand lung cancer pathology, and to determine the cell of origin of different subtypes of lung cancer [86]. The target cell of transformation for most cancers is unknown. Although there is evidence that certain types of leukaemia arise from mutations that accumulate in haematopoietic stem cells, more recent work suggests that the cell of origin of acute myeloid leukaemia or the basal type of breast cancer may reside in committed progenitor rather than stem cell populations [87,88]. Gene profiling studies of lung cancers led to further stratification of the histopathological subtypes of lung cancer into distinct molecular subgroups [5,89,90]. This heterogeneity probably reflects different oncogenic transformations occurring in different cell types. Until now, insights into the cell of origin have come from genetically defined mouse models of lung cancer.
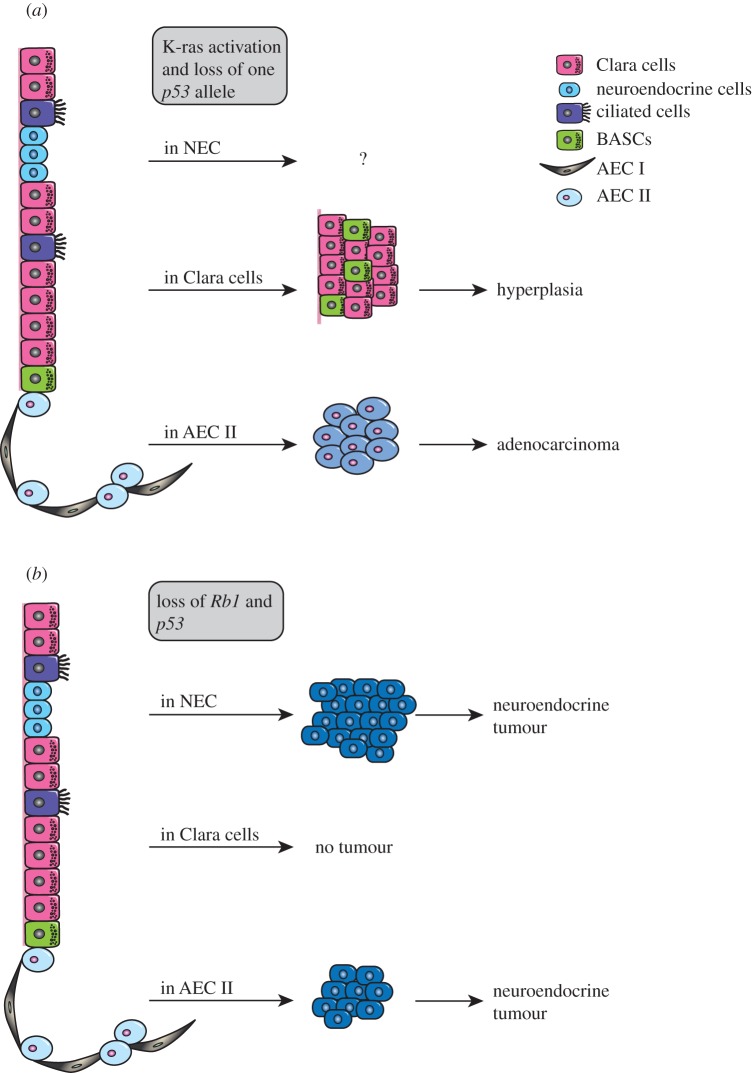
K-ras is mutated in 15 to 20 per cent of NSCLC [91]. Mouse models with oncogenic K-ras (K-rasG12D) expression have been developed to mimic human lung cancer [92]. Conditional expression of oncogenic K-rasG12D after intratracheal or intranasal administration of adenovirus-cre in mice results in the formation of lung adenocarcinoma [93–95]. Additional p53 mutation accelerates tumour formation and increases the metastatic properties of tumour cells, making it a mouse model more similar to human advanced lung adenocarcinoma [92,96]. These models have been successfully used to predict response to treatment [97], but also to study the cells of origin in lung adenocarcinoma. Treatment of mice with naphthalene, a component of cigarette smoke, accelerated tumour formation in K-rasG12D mice, and expansion of BASCs was reported in K-rasG12D mice [30]. These results and the observations that BASCs are expanded after naphthalene injury suggested that CC10+SP-C+ BASCs may be the cell of origin of K-rasG12D-driven lung adenocarcinomas. However, more recent work in which activation of the oncogenic K-ras and loss of one allele of p53 was specified to CC10+ cells resulted in hyperplasia at the bronchio-alveolar ductal junction that did not evolve to adenocarcinoma [98] (figure 2a). Conversely, K-rasG12D activation and p53 heterozygosity in SP-C+ AEC II cells led to the formation of adenocarcinoma in the alveolar region of the lung. The survival of SP-C-cre;K-rasG12Dp53f/+ mice was reduced by 10 weeks compared with CC10-cre;K-rasG12Dp53f/+ mice, suggesting that SP-C+ cells may be the cells of origin in these genetically defined tumours [98]. Activation of K-rasG12D exclusively in SP-C+CC10+ putative BASCs will help to resolve the role of this rare population in K-ras-driven lung cancers. Interestingly, K-ras-mutated lung adenocarcinomas in humans seem to be more prevalent in the distal lung than the proximal airways [7], further suggesting that AEC II cells may play an important role in the initiation of this subtype of lung tumours. Using a similar approach of specific activation of K-rasG12D in other lung epithelial cell types such as the neuroendocrine cells may help further understand the connection between the cell of origin, oncogenic mutation and subtypes of lung cancers.
Figure 2.
Cell of origin of lung cancers. (a) Models for adenocarcinoma formation in K-rasG12Dp53f/+ mice. Alveolar epithelial type II cells (AEC II) are the most probable cells of origin of adenocarcinoma in these mice. (b) Models for SCLC formation in Rb1f/f;p53f/f mice. Neuroendocrine cells are the most probable cells of origin of neuroendocrine tumour in this mouse model.
SCLCs have the phenotypic characteristics of neuroendocrine tumours, expressing neural cell adhesion molecule, synaptophysin and calcitonin gene-related peptide (CGRP). Rb1 and p53 loss of heterozygosity or mutations are present in 70 per cent of SCLCs [91]. This double inactivating mutation was reproduced in mice to generate a mouse model of SCLC that recapitulated the human phenotype [99]. Naphthalene injury did not accelerate tumour burden in this SCLC model [100]. To evaluate the cell of origin of SCLC, inactivation of Rb1 and p53 in different cellular compartments of the lung using cell-specific promoter (CC10-cre, SP-C-cre and CGRP-cre) adenoviral cre administration in Rb1f/f;p53f/f mice was performed [100] (figure 2b). These experiments showed that neuroendocrine cells were the most probable cell of origin of SCLC. CGRP-cre-driven Rb1/p53 loss resulted in tumour development in all animals, with a median tumour latency of a year. Interestingly, SP-C-cre driven deletion of Rb1/p53 resulted in neuroendocrine tumour with the same phenotype as CGRP-cre-driven tumours in half of the animals, but with an extended median tumour latency [100]. Inactivation of Rb1 and p53 in CC10-positive Clara cells only yielded rare tumours in animals over 18 months old, indicating that Clara cells do not contribute to SCLC formation in this model (figure 2b).
In these genetically defined models (K-rasG12D/p53 and Rb1/p53), Clara cells do not appear to be the cells of origin of lung tumours. It remains to be explored whether any other genetic changes occurring in Clara cells would lead to cell transformation and the appearance of a distinct subtype of lung cancer, or whether Clara cells, although playing a role in lung repair after injury, may play a limited role as the cells of origin of lung cancers. The role of basal cells as the cell of origin of lung tumour was not evaluated in these models. Given that basal cells appear to sit at the top of the mouse lung epithelial hierarchy in the trachea [14] and are activated in the distal lung after injury [13,65], their role in lung tumourigenesis would be worthy of further investigation. Such work has been hampered due to the lack of specificity of basal cell markers in the upper airways (p63, Keratin-5, Keratin-14). These markers are also expressed in other epithelial organs, including the skin, limiting their use to induce oncogenic transformation in the lung. Identification of lung basal cell-specific markers will be necessary to evaluate their role in lung tumour initiation. In vivo cell-specific activation of oncogenes or inactivating mutation of tumour suppressor genes can only be performed in mouse models. Other approaches will be required to decipher the cell of origin in the different subtypes of human lung cancers. One question that remains is this: what are the phenotypic characteristics of the cells acquiring the first oncogenic transformation in these mouse models? How do they relate to stem/progenitor cells described above? Identification of the cell surface markers expressed by these different cell types will enable identification of key factors driving their proliferation. These cell surface markers may also be translated to human lung tumours and provide insights into the cell of origin of human lung cancers.
7. Cancer stem cells
The cancer stem cell model is based on the hypothesis that tumours are organized in a hierarchical way, and only a small proportion of cells with stem-like properties has the capacity to propagate the tumour and generate the different cell types constituting the tumour [101]. The origin of the cancer stem cell is not necessarily the normal stem cell but could be a committed progenitor cell that reverts to a stem-like phenotype during transformation. Evidence exists in the literature that leukaemia as well as some solid tumours may follow a cancer stem cell model while other tumour types such as melanoma follow the clonal evolution model in which all undifferentiated cells have the same tumourigenic capacity [101–103]. The heterogeneity of lung tumours suggests that they may follow a cancer stem cell model, but only a functional assay will definitely prove this hypothesis [104].
In human lung, CD133 was first suggested as a marker of cancer stem cells [105–107]. A small proportion of CD133+ cells were observed in primary SCLC and NSCLC, and were shown to have higher sphere-forming capacity in vitro than CD133− cells [106]. Freshly isolated CD133+ cells from NSCLC have a higher tumourigenic potential than CD133− cells after subcutaneous transplantation in immunocompromised animals and had self-renewal properties [106–108]. Treatment of the xenografted mice with cisplatin resulted in reduction of the tumour burden although CD133+ cells remained, suggesting that CD133+ cells may be a population of cancer stem cells resistant to standard chemotherapy [107]. More recently, CD166 was found to enrich for tumour-propagating cells in human lung adenocarcinomas. Transplantation of CD166-positive cells in immuno-compromised mice gave rise to tumours that recapitulated the heterogeneity of the primary tumour [109]. Increased metabolic activity in the glycine/serine metabolism enzyme pathway was observed in CD166+ cells and shown to induce oncogenesis [109]. Significantly, NSCLC patients with high expression of glycine decarboxylase, a glycine/serine metabolism enzyme overexpressed in CD166+ tumour cells, had the worst survival prognosis [109]. It is unclear whether CD133 and CD166 mark the same population of tumour-propagating cells. Genes that regulate cancer stem cell activity are still underexplored. It was suggested that the stem cell gene Oct-4 may be an important regulator of the cancer stem cell properties of CD133-positive cells [108].
In mouse models, Sca-1 appears to segregate distinct tumour-propagating cells in some mouse models of lung adenocarcinomas, but not in others [110]. Sca-1+ cells were enriched in cancer-propagating cells in K-RasG12Dp53f/f tumours, but not in K-RasG12D mice. Conversely, only Sca-1− cells had tumour-propagating activity in EGFRL858R mouse model [110]. Genetic mutation status of mouse lung cancer therefore appears to change the phenotype of the tumour-propagating cells. It remains to be evaluated whether, as in the mouse, markers of cancer stem cells in human lung tumours differ according to the genetic mutation status of individual tumours.
8. Conclusion
Works in other tumour types have highlighted the importance of dissecting the cellular hierarchy in normal tissue in order to understand potential cells of origin in cancers [87,88]. In the lung, prospective isolation of lung stem and progenitor cells has been hindered by the lack of in vivo repopulation stem cell assays. Bioengineering strategies to develop a decellularized rodent lung matrix bioreactor system in which a lung scaffold is concurrently seeded with microvasculature cells and connected to a ventilation system could potentially constitute a surrogate assay to evaluate adult lung stem cell function [111–113]. A similar strategy was recently used to demonstrate that lung progenitor cells derived from embryonic stem cells could repopulate a decellularized lung [114]. Until now, in vitro culture of sorted cell populations and lineage-tracing strategies have provided some insights into the organization of the mouse lung and the identification of progenitor cells. Prospective isolation of these cells in human and in genetically engineered mouse models will enable the dissection of molecular mechanisms regulating self-renewal and differentiation at steady state and in lung repair after injury.
The cell of origin of most cancers remains unknown. Genetically defined mouse models of lung cancer have given insights into the possible cell of origin of K-rasG12D-induced adenocarcinomas and SCLC (Rb1/p53 loss) (figure 2). Other oncogenic driver mutations have been discovered in NSCLC, although at a lower frequency than K-ras mutations [115–120]. Development of mouse models in which these genetic transformations occur as well as use of genetically characterized human lung tumours will allow a better understanding of the contribution of oncogenic driver mutations and cells of origin to lung tumour heterogeneity [8]. Establishing a link between the first cell in which a specific mutation occurs and the molecular subtypes of lung cancer will enable better stratification of patients for improved therapeutic strategies.
9. Acknowledgements
The authors thank Prof. A. Jane Visvader, Assoc. Prof. Ivan Bertoncello and Prof. Robert Williamson for their critical reading of the manuscript. M.-L.A.-L. is supported by an Australian Research Council Queen Elizabeth II Fellowship. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
References
- 1.WHO 2008. Health statistics 2008. Geneva, Switzerland: World Health Organization.
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 10.3322/caac.20107 (doi:10.3322/caac.20107) [DOI] [PubMed] [Google Scholar]
- 3.Sun S, Schiller JH, Gazdar AF. 2007. Lung cancer in never smokers: a different disease. Nat Rev Cancer 7, 778–790 10.1038/nrc2190 (doi:10.1038/nrc2190) [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee A, et al. 2001. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl Acad. Sci. USA 98, 13 790–13 795 10.1073/pnas.191502998 (doi:10.1073/pnas.191502998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes DN, Monti S, Parmigiani G, Gilks CB, Naoki K, Bhattacharjee A, Socinski MA, Perou C, Meyerson M. 2006. Gene expression profiling reveals reproducible human lung adenocarcinoma subtypes in multiple independent patient cohorts. J. Clin. Oncol. 24, 5079–5090 10.1200/JCO.2005.05.1748 (doi:10.1200/JCO.2005.05.1748) [DOI] [PubMed] [Google Scholar]
- 6.Travis WD. 2002. Pathology of lung cancer. Clin. Chest Med. 23, 65–81 10.1016/S0272-5231(03)00061-3 (doi:10.1016/S0272-5231(03)00061-3) [DOI] [PubMed] [Google Scholar]
- 7.Giangreco A, Groot KR, Janes SM. 2007. Lung cancer and lung stem cells: strange bedfellows? Am. J. Respir. Crit. Care Med. 175, 547–553 10.1164/rccm.200607-984PP (doi:10.1164/rccm.200607-984PP) [DOI] [PubMed] [Google Scholar]
- 8.Farago AF, Snyder EL, Jacks T. 2012. SnapShot: lung cancer models. Cell 149, 246–246 e241 [DOI] [PubMed] [Google Scholar]
- 9.Bertoncello I, McQualter JL. 2010. Endogenous lung stem cells: what is their potential for use in regenerative medicine? Expert Rev. Respir. Med. 4, 349–362 10.1586/ers.10.21 (doi:10.1586/ers.10.21) [DOI] [PubMed] [Google Scholar]
- 10.Rawlins EL, Hogan BL. 2008. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L231–L234 10.1152/ajplung.90209.2008 (doi:10.1152/ajplung.90209.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. 2007. Lung development and repair: contribution of the ciliated lineage. Proc. Natl Acad. Sci. USA 104, 410–417 10.1073/pnas.0610770104 (doi:10.1073/pnas.0610770104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock JR, Randell SH, Hogan BL. 2010. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model Mech. 3, 545–556 10.1242/dmm.006031 (doi:10.1242/dmm.006031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar PA, et al. 2011. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147, 525–538 10.1016/j.cell.2011.10.001 (doi:10.1016/j.cell.2011.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. 2009. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12 771–12 775 10.1073/pnas.0906850106 (doi:10.1073/pnas.0906850106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawlins EL, Hogan BL. 2006. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133, 2455–2465 10.1242/dev.02407 (doi:10.1242/dev.02407) [DOI] [PubMed] [Google Scholar]
- 16.Barker N, et al. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 10.1038/nature06196 (doi:10.1038/nature06196) [DOI] [PubMed] [Google Scholar]
- 17.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat M-L, Wu L, Lindeman GJ, Visvader JE. 2006. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 10.1038/nature04372 (doi:10.1038/nature04372) [DOI] [PubMed] [Google Scholar]
- 18.Stingl J.et al. 2006. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 [DOI] [PubMed] [Google Scholar]
- 19.Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. 2007. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 10.1038/nature05574 (doi:10.1038/nature05574) [DOI] [PubMed] [Google Scholar]
- 20.Jones P, Simons BD. 2008. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat. Rev. Mol. Cell Biol. 9, 82–88 10.1038/nrm2292 (doi:10.1038/nrm2292) [DOI] [PubMed] [Google Scholar]
- 21.Jones PH, Simons BD, Watt FM. 2007. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell 1, 371–381 10.1016/j.stem.2007.09.014 (doi:10.1016/j.stem.2007.09.014) [DOI] [PubMed] [Google Scholar]
- 22.Sigal SH, Brill S, Fiorino AS, Reid LM. 1992. The liver as a stem cell and lineage system. Am. J. Physiol. 263, G139–G148 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. 2003. The origin and liver repopulating capacity of murine oval cells. Proc. Natl Acad. Sci. USA 100(Suppl. 1), 11 881–11 888 10.1073/pnas.1734199100 (doi:10.1073/pnas.1734199100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dor Y, Brown J, Martinez OI, Melton DA. 2004. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 10.1038/nature02520 (doi:10.1038/nature02520) [DOI] [PubMed] [Google Scholar]
- 25.Xu X, et al. 2008. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 10.1016/j.cell.2007.12.015 (doi:10.1016/j.cell.2007.12.015) [DOI] [PubMed] [Google Scholar]
- 26.Dor Y, Melton DA. 2008. Facultative endocrine progenitor cells in the adult pancreas. Cell 132, 183–184 10.1016/j.cell.2008.01.004 (doi:10.1016/j.cell.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 27.Rosler ES, Brandt JE, Chute J, Hoffman R. 2000. An in vivo competitive repopulation assay for various sources of human hematopoietic stem cells. Blood 96, 3414–3421 [PubMed] [Google Scholar]
- 28.Adamson IY, Bowden DH. 1979. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am. J. Pathol. 96, 531–544 [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. 2011. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 10.1172/JCI57673 (doi:10.1172/JCI57673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835 10.1016/j.cell.2005.03.032 (doi:10.1016/j.cell.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 31.Li L, Wei Y, Van Winkle L, Zhang Q-Y, Zhou X, Hu J, Xie F, Kluetzman K, Ding X. 2011. Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J. Pharmacol. Exp. Ther. 339, 62–71 10.1124/jpet.111.184671 (doi:10.1124/jpet.111.184671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckpitt A, et al. 1995. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol. Pharmacol. 47, 74–81 [PubMed] [Google Scholar]
- 33.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. 2000. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L1256–L1263 [DOI] [PubMed] [Google Scholar]
- 34.Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. 2004. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L1256–L1265 10.1152/ajplung.00203.2004 (doi:10.1152/ajplung.00203.2004) [DOI] [PubMed] [Google Scholar]
- 35.Londhe VA, Maisonet TM, Lopez B, Jeng J-M, Li C, Minoo P. 2011. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am. J. Respir. Cell Mol. Biol. 44, 804–812 10.1165/rcmb.2009-0429OC (doi:10.1165/rcmb.2009-0429OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding BS, et al. 2011. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 10.1016/j.cell.2011.10.003 (doi:10.1016/j.cell.2011.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagano A, Barazzone-Argiroffo C. 2003. Alveolar cell death in hyperoxia-induced lung injury. Ann. N Y Acad. Sci. 1010, 405–416 10.1196/annals.1299.074 (doi:10.1196/annals.1299.074) [DOI] [PubMed] [Google Scholar]
- 38.Bhandari V, Choo-Wing R, Harijith A, Sun H, Syed MA, Homer RJ, Elias JA. 2012. Increased hyperoxia-induced lung injury in nitric oxide synthase 2 null mice is mediated via angiopoietin 2. Am. J. Respir. Cell Mol. Biol. 46, 668–676 10.1165/rcmb.2011-0074OC (doi:10.1165/rcmb.2011-0074OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. 2001. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 662–670 [DOI] [PubMed] [Google Scholar]
- 40.Raiser DM, Zacharek SJ, Roach RR, Curtis SJ, Sinkevicius KW, Gludish DW, Kim CF. 2008. Stem cell biology in the lung and lung cancers: using pulmonary context and classic approaches. Cold Spring Harb. Symp. Quant. Biol. 73, 479–490 10.1101/sqb.2008.73.036 (doi:10.1101/sqb.2008.73.036) [DOI] [PubMed] [Google Scholar]
- 41.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BLM. 2009. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 10.1016/j.stem.2009.04.002 (doi:10.1016/j.stem.2009.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adamson IY. 1976. Pulmonary toxicity of bleomycin. Environ. Health Perspect. 16, 119–126 10.1289/ehp.7616119 (doi:10.1289/ehp.7616119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aso Y, Yoneda K, Kikkawa Y. 1976. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab. Invest. 35, 558–568 [PubMed] [Google Scholar]
- 44.Starcher B, Kuhn C. 2003. Combining histology and biochemical measurements of connective tissue components in small samples of lung: application to bleomycin-induced fibrosis in the mouse. Exp. Lung Res. 29, 179–194 10.1080/01902140303771 (doi:10.1080/01902140303771) [DOI] [PubMed] [Google Scholar]
- 45.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BLM. 2011. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl Acad. Sci. USA 108, E1475–E1483 10.1073/pnas.1117988108 (doi:10.1073/pnas.1117988108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rannels DE, Rannels SR. 1988. Compensatory growth of the lung following partial pneumonectomy. Exp. Lung Res. 14, 157–182 10.3109/01902148809115122 (doi:10.3109/01902148809115122) [DOI] [PubMed] [Google Scholar]
- 47.Cowan MJ, Crystal RG. 1975. Lung growth after unilateral pneumonectomy: quantitation of collagen synthesis and content. Am. Rev. Respir. Dis. 111, 267–277 [DOI] [PubMed] [Google Scholar]
- 48.Derks CM, De Francquen P. 1980. Distribution of ventilation-perfusion ratios in pneumonectomized dogs. Respiration 39, 61–74 10.1159/000194199 (doi:10.1159/000194199) [DOI] [PubMed] [Google Scholar]
- 49.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179, 199–210 10.1016/j.ajpath.2011.03.013 (doi:10.1016/j.ajpath.2011.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberger B, Laskin DL, Heck DE, Laskin JD. 2002. Oxygen toxicity in premature infants. Toxicol. Appl. Pharmacol. 181, 60–67 10.1006/taap.2002.9387 (doi:10.1006/taap.2002.9387) [DOI] [PubMed] [Google Scholar]
- 51.Rock JR, Gao X, Xue Y, Randell SH, Kong Y-Y, Hogan BLM. 2011. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8, 639–648 10.1016/j.stem.2011.04.003 (doi:10.1016/j.stem.2011.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barth PJ, Muller B, Wagner U, Bittinger A. 1995. Quantitative analysis of parenchymal and vascular alterations in NO2-induced lung injury in rats. Eur. Respir. J. 8, 1115–1121 10.1183/09031936.95.08071115 (doi:10.1183/09031936.95.08071115) [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Machida M, Adachi K, Otabe K, Sugimoto T, Hayashi M, Awazu S. 2000. Histopathological study of the effects of a single intratracheal instillation of surface active agents on lung in rats. J. Toxicol. Sci. 25, 49–55 10.2131/jts.25.49 (doi:10.2131/jts.25.49) [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Wang S, Manzer R, McConville G, Mason RJ. 2006. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic. Biol. Med. 40, 1914–1928 10.1016/j.freeradbiomed.2006.01.017 (doi:10.1016/j.freeradbiomed.2006.01.017) [DOI] [PubMed] [Google Scholar]
- 55.Adamson IY, Bowden DH. 1974. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Invest. 30, 35–42 [PubMed] [Google Scholar]
- 56.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. 1975. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 22, 142–150 10.1016/0014-4800(75)90059-3 (doi:10.1016/0014-4800(75)90059-3) [DOI] [PubMed] [Google Scholar]
- 57.McQualter JL, Yuen K, Williams B, Bertoncello I. 2010. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl Acad. Sci. USA 107, 1414–1419 10.1073/pnas.0909207107 (doi:10.1073/pnas.0909207107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giangreco A, Reynolds SD, Stripp BR. 2002. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 161, 173–182 10.1016/S0002-9440(10)64169-7 (doi:10.1016/S0002-9440(10)64169-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teisanu RM, Lagasse E, Whitesides JF, Stripp BR. 2009. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells 27, 612–622 10.1634/stemcells.2008-0838 (doi:10.1634/stemcells.2008-0838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, Bertoncello I, Stripp BR. 2011. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am. J. Respir. Cell Mol. Biol. 44, 794–803 10.1165/rcmb.2010-0098OC (doi:10.1165/rcmb.2010-0098OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. 2009. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 27, 623–633 10.1634/stemcells.2008-0866 (doi:10.1634/stemcells.2008-0866) [DOI] [PubMed] [Google Scholar]
- 62.Zacharek SJ, et al. 2011. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell 9, 272–281 10.1016/j.stem.2011.07.007 (doi:10.1016/j.stem.2011.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirrlinger J, et al. 2009. Split-cre complementation indicates coincident activity of different genes in vivo. PLoS ONE 4, e4286. 10.1371/journal.pone.0004286 (doi:10.1371/journal.pone.0004286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. 2004. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell Mol. Physiol. 286, L643–649 10.1152/ajplung.00155.2003 (doi:10.1152/ajplung.00155.2003) [DOI] [PubMed] [Google Scholar]
- 65.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. 2004. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164, 577–588 10.1016/S0002-9440(10)63147-1 (doi:10.1016/S0002-9440(10)63147-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ware LB. 2008. Modeling human lung disease in animals. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L149–150 10.1152/ajplung.00472.2007 (doi:10.1152/ajplung.00472.2007) [DOI] [PubMed] [Google Scholar]
- 67.Hogan BL, Stripp BR, Thannickal VJ. 2011. Lung stem cells: looking beyond the hype. Nat. Med. 17, 788–789 10.1038/nm0711-788 (doi:10.1038/nm0711-788) [DOI] [PubMed] [Google Scholar]
- 68.Chapman HA. 2011. Toward lung regeneration. N Engl. J. Med. 364, 1867–1868 10.1056/NEJMe1101800 (doi:10.1056/NEJMe1101800) [DOI] [PubMed] [Google Scholar]
- 69.Kajstura J, et al. 2011. Evidence for human lung stem cells. N Engl. J. Med. 364, 1795–1806 10.1056/NEJMoa1101324 (doi:10.1056/NEJMoa1101324) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Cardoso WV. 2001. Molecular regulation of lung development. Annu. Rev. Physiol. 63, 471–494 10.1146/annurev.physiol.63.1.471 (doi:10.1146/annurev.physiol.63.1.471) [DOI] [PubMed] [Google Scholar]
- 71.Dovey JS, Zacharek SJ, Kim CF, Lees JA. 2008. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc. Natl Acad. Sci. USA 105, 11 857–11 862 10.1073/pnas.0803574105 (doi:10.1073/pnas.0803574105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardal R, Molofsky AV, He S, Morrison SJ. 2005. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harb. Symp. Quant. Biol. 70, 177–185 10.1101/sqb.2005.70.057 (doi:10.1101/sqb.2005.70.057) [DOI] [PubMed] [Google Scholar]
- 73.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. 2005. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 19, 1432–1437 10.1101/gad.1299505 (doi:10.1101/gad.1299505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S, Dontu G, Wicha MS. 2005. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 7, 86–95 10.1186/bcr1021 (doi:10.1186/bcr1021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Millien G, Beane J, Lenburg M, Tsao P-N, Lu J, Spira A, Ramirez MI. 2008. Characterization of the mid-foregut transcriptome identifies genes regulated during lung bud induction. Gene Expr. Patterns 8, 124–139 10.1016/j.modgep.2007.09.003 (doi:10.1016/j.modgep.2007.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asselin-Labat ML, Vaillant F, Shackleton M, Bouras T, Lindeman GJ, Visvader JE. 2008. Delineating the epithelial hierarchy in the mouse mammary gland. Cold Spring Harb. Symp. Quant. Biol. 73, 469–478 10.1101/sqb.2008.73.020 (doi:10.1101/sqb.2008.73.020) [DOI] [PubMed] [Google Scholar]
- 77.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 10.1038/nature01593 (doi:10.1038/nature01593) [DOI] [PubMed] [Google Scholar]
- 78.Reynolds SD, et al. 2008. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 26, 1337–1346 10.1634/stemcells.2008-0053 (doi:10.1634/stemcells.2008-0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. 2009. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307 10.1242/dev.034884 (doi:10.1242/dev.034884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsao PN, et al. 2008. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J. Biol. Chem. 283, 29 532–29 544 10.1074/jbc.M801565200 (doi:10.1074/jbc.M801565200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. 2009. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136, 1751–1759 10.1242/dev.029249 (doi:10.1242/dev.029249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morimoto M, Liu Z, Cheng H-T, Winters N, Bader D, Kopan R. 2010. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell Sci. 123, 213–224 10.1242/jcs.058669 (doi:10.1242/jcs.058669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Metzger RJ, Klein OD, Martin GR, Krasnow MA. 2008. The branching programme of mouse lung development. Nature 453, 745–750 10.1038/nature07005 (doi:10.1038/nature07005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White AC, Lavine KJ, Ornitz DM. 2007. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development 134, 3743–3752 10.1242/dev.004879 (doi:10.1242/dev.004879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Filby CE, Hooper SB, Wallace MJ. 2010. Partial pulmonary embolization disrupts alveolarization in fetal sheep. Respir. Res. 11, 42. 10.1186/1465-9921-11-42 (doi:10.1186/1465-9921-11-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutherland KD, Berns A. 2010. Cell of origin of lung cancer. Mol. Oncol. 4, 397–403 10.1016/j.molonc.2010.05.002 (doi:10.1016/j.molonc.2010.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goardon N, et al. 2011. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 19, 138–152 10.1016/j.ccr.2010.12.012 (doi:10.1016/j.ccr.2010.12.012) [DOI] [PubMed] [Google Scholar]
- 88.Lim E, et al. 2009. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913 10.1038/nm.2000 (doi:10.1038/nm.2000) [DOI] [PubMed] [Google Scholar]
- 89.Hassan KA, Chen G, Kalemkerian GP, Wicha MS, Beer DG. 2009. An embryonic stem cell-like signature identifies poorly differentiated lung adenocarcinoma but not squamous cell carcinoma. Clin. Cancer Res. 15, 6386–6390 10.1158/1078-0432.CCR-09-1105 (doi:10.1158/1078-0432.CCR-09-1105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilkerson MD, et al. 2010. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 16, 4864–4875 10.1158/1078-0432.CCR-10-0199 (doi:10.1158/1078-0432.CCR-10-0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meuwissen R, Berns A. 2005. Mouse models for human lung cancer. Genes Dev. 19, 643–664 10.1101/gad.1284505 (doi:10.1101/gad.1284505) [DOI] [PubMed] [Google Scholar]
- 92.Kim CF, et al. 2005. Mouse models of human non-small-cell lung cancer: raising the bar. Cold Spring Harb. Symp. Quant. Biol. 70, 241–250 10.1101/sqb.2005.70.037 (doi:10.1101/sqb.2005.70.037) [DOI] [PubMed] [Google Scholar]
- 93.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. 2001. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 10.1038/35074129 (doi:10.1038/35074129) [DOI] [PubMed] [Google Scholar]
- 94.Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. 2001. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene 20, 6551–6558 10.1038/sj.onc.1204837 (doi:10.1038/sj.onc.1204837) [DOI] [PubMed] [Google Scholar]
- 95.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. 2001. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 10.1101/gad.943001 (doi:10.1101/gad.943001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jackson EL, et al. 2005. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 65, 10 280–10 288 10.1158/0008-5472.CAN-05-2193 (doi:10.1158/0008-5472.CAN-05-2193) [DOI] [PubMed] [Google Scholar]
- 97.Chen Z, et al. 2012. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483, 613–617 10.1038/nature10937 (doi:10.1038/nature10937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BLM, Onaitis MW. 2012. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc. Natl Acad. Sci. USA 109, 4910–4915 10.1073/pnas.1112499109 (doi:10.1073/pnas.1112499109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meuwissen R, Linn SC, Linnoila R, Zevenhoven J, Mooi WJ, Berns A. 2003. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4, 181–189 10.1016/S1535-6108(03)00220-4 (doi:10.1016/S1535-6108(03)00220-4) [DOI] [PubMed] [Google Scholar]
- 100.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song J-Y, Berns A. 2011. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 19, 754–764 10.1016/j.ccr.2011.04.019 (doi:10.1016/j.ccr.2011.04.019) [DOI] [PubMed] [Google Scholar]
- 101.Visvader JE, Lindeman GJ. 2008. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 10.1038/nrc2499 (doi:10.1038/nrc2499) [DOI] [PubMed] [Google Scholar]
- 102.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. 2010. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 18, 510–523 10.1016/j.ccr.2010.10.012 (doi:10.1016/j.ccr.2010.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. 2008. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 10.1038/nature07567 (doi:10.1038/nature07567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berns A. 2005. Stem cells for lung cancer? Cell 121, 811–813 10.1016/j.cell.2005.06.004 (doi:10.1016/j.cell.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 105.Jiang F, et al. 2009. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 7, 330–338 10.1158/1541-7786.MCR-08-0393 (doi:10.1158/1541-7786.MCR-08-0393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eramo A, et al. 2008. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 15, 504–514 10.1038/sj.cdd.4402283 (doi:10.1038/sj.cdd.4402283) [DOI] [PubMed] [Google Scholar]
- 107.Bertolini G, et al. 2009. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl Acad. Sci. USA 106, 16 281–16 286 10.1073/pnas.0905653106 (doi:10.1073/pnas.0905653106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen YC, et al. 2008. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE 3, e2637. 10.1371/journal.pone.0002637 (doi:10.1371/journal.pone.0002637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang WC, et al. 2012. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 148, 259–272 10.1016/j.cell.2011.11.050 (doi:10.1016/j.cell.2011.11.050) [DOI] [PubMed] [Google Scholar]
- 110.Curtis SJ, et al. 2010. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell 7, 127–133 10.1016/j.stem.2010.05.021 (doi:10.1016/j.stem.2010.05.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. 2010. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. Part A 16, 2581–2591 10.1089/ten.tea.2009.0659 (doi:10.1089/ten.tea.2009.0659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cortiella J, et al. 2010. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. A 16, 2565–2580 10.1089/ten.tea.2009.0730 (doi:10.1089/ten.tea.2009.0730) [DOI] [PubMed] [Google Scholar]
- 113.Petersen TH, et al. 2010. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 10.1126/science.1189345 (doi:10.1126/science.1189345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Longmire TA, et al. 2012. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411 10.1016/j.stem.2012.01.019 (doi:10.1016/j.stem.2012.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heist RS, Engelman JA. 2012. SnapShot: non-small cell lung cancer. Cancer Cell 21 [DOI] [PubMed] [Google Scholar]
- 116.Takeuchi K, et al. 2012. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 18, 378–381 10.1038/nm.2658 (doi:10.1038/nm.2658) [DOI] [PubMed] [Google Scholar]
- 117.Kohno T, et al. 2012. KIF5B-RET fusions in lung adenocarcinoma. Nat. Med. 18, 375–377 10.1038/nm.2644 (doi:10.1038/nm.2644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lipson D, et al. 2012. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 18, 382–384 10.1038/nm.2673 (doi:10.1038/nm.2673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaw AT, et al. 2009. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 27, 4247–4253 10.1200/JCO.2009.22.6993 (doi:10.1200/JCO.2009.22.6993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiss J, et al. 2010. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci. Transl. Med. 2, 62ra93. 10.1126/scitranslmed.3001451 (doi:10.1126/scitranslmed.3001451) [DOI] [PMC free article] [PubMed] [Google Scholar]