Abstract
The chemistry of mechanically interlocked molecules (MIMs), in which two or more covalently linked components are held together by mechanical bonds, has led to the coining of the term mechanostereochemistry to describe a new field of chemistry that embraces many aspects of MIMs, including their syntheses, properties, topologies where relevant and functions where operative. During the rapid development and emergence of the field, the synthesis of MIMs has witnessed the forsaking of the early and grossly inefficient statistical approaches for template-directed protocols, aided and abetted by molecular recognition processes and the tenets of self-assembly. The resounding success of these synthetic protocols, based on templation, has facilitated the design and construction of artificial molecular switches and machines, resulting more and more in the creation of integrated functional systems. This review highlights (i) the range of template-directed synthetic methods being used currently in the preparation of MIMs; (ii) the syntheses of topologically complex knots and links in the form of stable molecular compounds; and (iii) the incorporation of bistable MIMs into many different device settings associated with surfaces, nanoparticles and solid-state materials in response to the needs of particular applications that are perceived to be fair game for mechanostereochemistry.
Keywords: catenanes, chemical topology, integrated functional systems, molecular switches, rotaxanes, template-directed synthesis
1. Introduction
One of the fundamental challenges in chemistry has been understanding and coming to terms with the manner in which atoms interact with one another to form molecules, i.e. how chemical bonding occurs. Even before the early twentieth century rationalizations (Lewis 1916; Langmuir 1919) of the covalent bond, chemists were able to bring atoms and subcomponents of atoms together to make molecules in a process we refer to as chemical synthesis today. The interactions that occur between molecules, more so than between atoms, are now a major focus of research in the broad area of supramolecular chemistry (Lehn 1995). Led by the Nobel Prize winning pioneers, Charles Pedersen (1988), Jean-Marie Lehn (1988) and Donald Cram (1988), chemists now understand and exploit the plethora of non-covalent bonding interactions that occur between molecules—including, but not limited to, metal coordination, π–π stacking, donor–acceptor interactions, hydrogen bonding, hydrophobic forces, van der Waals interactions and halogen bonding—in the self-assembly of complexes. Over the past 30 years, the mechanical bond (Stoddart 2009) has emerged as a consequence of the molecular recognition and self-assembly processes that are underpinned by non-covalent bonding interactions prior to different kinds of post-assembly covalent modifications. Molecular components can be mechanically interlocked (figure 1) in a manner such that the components cannot part company without the breaking of at least one covalent bond, giving rise to molecules (Schill 1971; Sauvage & Dietrich-Buchecker 1999) such as catenanes, where two or more rings are linked together as in a chain (catena is Latin for chain) and rotaxanes (rota is Latin for wheel, and axis is Latin for axle) where ring components are threaded onto rod whose termini are stoppered—forming dumbbell components—and thus blocking the rings from escaping. In many of these mechanically interlocked molecules (MIMs), the non-covalent bonding interactions between the components live on, endowing them with a unique kind of functionality. We have coined (Olson et al. 2010) the term mechanostereochemistry to encompass the chemistry of molecules with mechanical bonds, including their structure and topology as well as the coordinative, non-covalent and dynamic covalent bonding interactions that invariably exist between the components of MIMs.
Figure 1.

The creation of a mechanical bond, in the form of a catenane or a rotaxane, from a 1:1 complex that we call (Ashton et al. 1991) a pseudorotaxane, underlines the importance of non-covalent bonding interactions in the synthesis of MIMs, which, by definition, are molecules and not supermolecules because the parting of their components requires the breaking of a covalent bond.
This review will provide a discussion of the synthetic protocols (scheme 1) used in the preparation of MIMs, from the very early statistical approaches to the state-of-the-art template-directed syntheses (Diederich & Stang 1999) that are in vogue today. The different synthetic protocols will be highlighted with reference to seminal breakthroughs in the literature before discussing (scheme 1) some of the more topologically complex molecules prepared to date and their subsequent incorporation into integrated functional systems. Finally, some perspectives on what the future holds for this field of contemporary chemistry will be advanced.
Scheme 1.
A list of timelines illustrating the progress in the field of mechanically interlocked molecules (MIMs) relating to their structures, preparations and applications as well as providing the names of some of the key players.
2. Synthetic approaches to mechanically interlocked molecules
Initial attempts to prepare MIMs relied on statistical approaches, wherein a successful synthesis depends on the chance generation of rotaxanes or catenanes during macrocyclic ring formation. To the best of our knowledge, the first synthesis (figure 2a) and isolation of a catenane (Wasserman 1960) was reported by Wasserman. The introduction of a pentadeuterated C34-cycloalkane 2 to the reaction of diethyl tetratriacontanedioate 1, which can form a cyclic acyloin, resulted in the isolation of a deuterated product in less than 1 per cent yield. The product was identified as the [2]catenane 3 because, upon cleavage of the acyloin ring, the deuterated cycloalkane was set free and could once again be isolated and characterized. This synthesis heralded the beginning of the field of chemical topology (Frisch & Wasserman 1961)—i.e. the study of molecules with non-trivial topologies.
Figure 2.
Two statistical approaches to the synthesis of MIMs. (a) The purported synthesis of a [2]catenane 3 by carrying out a macrocyclization in the presence of a pre-formed ring (Wasserman 1960). (b) The synthesis of a [2]rotaxane 7 by repetitive threading and stoppering of a ring bound to a solid support, followed by its release (Harrison & Harrison 1967).
Harrison & Harrison (1967) followed this breakthrough with the statistical synthesis of a [2]rotaxane—rotaxanes are topologically trivial—employing (figure 2b) an ingenious solid-supported synthesis. The ring component, a cyclic ketone 4, was attached to a Merrifield resin by a pendant succinate linker, and loaded onto a column. The thread, decane-1,10-diol 5, and the stoppering reagent, triphenylmethyl chloride 6, were introduced in a solvent to the resin, generating trace amounts of appended [2]rotaxane molecules. Iterative repetitions of this process—up to a heroic 70 cycles—followed by cleavage of the product from the resin led to the isolation of the [2]rotaxane 7 in 6 per cent yield. Again, chemical methods were used to confirm the mechanically interlocked nature of the structure that the authors called a ‘hooplane’. Despite this combination of an inventive preparative approach, coupled with admirable perseverance, the very low yield of the [2]rotaxane illustrates the major disadvantage of the statistical approach—i.e. the tiny, or indeed non-existent, non-covalent bonding interactions between the components prior to mechanical interlocking.
Schill and co-workers realized that, if MIMs were ever going to be prepared on a larger scale in better yields, there had to be something to hold the components of the MIMs in place. In the days before the advent of supramolecular chemistry, locking the components together covalently during synthesis was proposed in the context of the so-called directed synthesis of MIMs. This covalent templation (figure 3) yielded a [2]catenane (Schill & Lüttringhaus 1964). After the bis-aldehyde 8 had been converted to the macrocycle 9 in a total of eight synthetic steps, three further steps resulted in the isolation of the amino-substituted ketal 10. Cyclization of 10 yielded the bis-ansa compound 11 in a manner such that the polymethylene chloride chains extending from the ketal react with the amino group on opposite faces of the benzene ring: this step is crucial in forming the crossover point, or node, of the mechanically interlocked product. Cleavage of the ketal group resulted in 12, and subsequent hydrolysis of the aniline moiety yielded the [2]catenane, a derivative 13 of which was isolated and shown by chromatography not to break down into the two constituent macrocycles. Rotaxanes were also prepared (Schill & Zollenkopf 1969) using similar approaches. The many ingenious and challenging directed synthetic routes to these MIMs are summed up in a seminal monograph entitled ‘Catenanes, rotaxanes and knots’ (Schill 1971).
Figure 3.
The significant transformations in a 20-step directed synthesis of a [2]catenane 13 wherein the two rings are held together by covalent bonds until the final steps (Schill & Lüttringhaus 1964) when these covalent bonds are cleaved.
The field of MIMs was transformed by Sauvage and co-workers in 1983, with the publication (Dietrich-Buchecker et al. 1983) of a seminal paper describing the synthesis (figure 4) of a new class of compounds, ‘Les Metallocatenanes’. Realizing that the arrangement of two 2,9-diphenyl-1,10-phenanthroline ligands, such as 14 and 15, around a tetrahedral Cu(I) centre, e.g. 16, was a chemical expression of the crossover point, or node, inherent to all knots and links, a compound wherein the pendant aromatic moieties, specifically para-phenols, could be linked covalently by oligoethylene glycol spacers was designed and synthesized. The resulting MIM 17 was designated a [2]catenate, rather than a [2]catenane, in recognition of the fact that the metal template used during synthesis remains coordinated to the structure, as demonstrated in a beautiful crystal structure of this remarkable MIM. Removal of the Cu(I) template, by addition of tetramethylammonium cyanide to a red solution of the catenate in H2O/MeCN, generated the colourless metal-free catenand 18—the term catenand refers to a catenane prepared by metal templation but with the metal cation subsequently removed—which was also characterized structurally in the solid state (Cesario et al. 1985). Circumrotation of the rings with respect to each other is clearly obvious, with the disparate phenanthroline chelating sites of the [2]catenand distanced at 11.2 Å apart, in stark contrast to the [2]catenate precursor.
Figure 4.
The Cu(I)-templated synthesis (Dietrich-Buchecker et al. 1983) of a [2]catenate comprising two 1,10-phenanthroline-based rings together with the crystal structures (Cesario et al. 1985) of both the Cu(I) [2]catenate 17 and its demetallated analogue, the [2]catenand 18.
The coordination of Cu(I) to 1,10-phenanthrolines and related ligands, including 2,2′-bipyridines, 2,2′:6′2′′-terpyridines and 3,3′-biisoquinolines, remains the dominant mode of transition metal templation used to prepare MIMs, resulting in switchable molecules based on rotaxanes, catenanes and daisy chains (Durot et al. 2010). A recent extension of this synthetic strategy has been the introduction of active metal templation, wherein the metal ion not only holds the precursors of the MIM together, but also acts as a catalyst in the formation of a covalent bond that locks the components in place and forms the MIM at one and the same time (Crowley et al. 2009). In the case of Cu(I), renowned for its role as a catalyst in the ubiquitous copper(I)-catalysed azide-alkyne cycloaddition (CuAAC) (Rostovtsev et al. 2002) ‘click’ reaction between alkynes and azides to form 1,2,3-triazoles, rotaxanes can be formed in more than 80 per cent yields from bipyridine-based macrocycles, azide- and alkyne-substituted half-dumbbells and sub-stoichiometric quantities of the active metal template. The Cu(I) cation holds each of the three components together while also catalysing the CuAAC reaction between the two halves of the dumbbell (Aucagne et al. 2006).
Alternative metal templates have been exploited to a lesser extent, but many synthetic routes involve the reversibility of dynamic coordinative and covalent bonds, with the MIM being the thermodynamic product of a multi-component self-assembly process. A prime example is Leigh's synthesis of benzylic imine catenates involving templation by octahedral metal cations such as Zn(II) and Fe(II) and the reversible formation of imine bonds (Leigh et al. 2001). By simply combining (figure 5a) two equivalents of 2,6-diformylpyridine 19 and two equivalents of a bis-benzylamine 20 linked by spacers of appropriate lengths with one equivalent of the divalent metal cation in MeOH, the [2]catenate, in which the metal template is bound in a six-coordinate environment by two diiminopyridyl ligands, is generated in a reversible self-assembly process, with yields being more than 50 per cent. The solid-state structure of the Zn(II)-templated [2]catenate 21 (figure 5b) reveals the combination of coordination bonds and π–π stacking interactions which stabilizes the MIM. Reduction of the imine bonds with NaBH4 followed by removal of the metal template employing EDTA chelation yields the analogous [2]catenand. This ‘all-in-one’ approach to the preparation of MIMs using dynamic covalent and coordinative chemistry under thermodynamic control stands in contrast to the stepwise approach, which relies on the sequential covalent bond formation under kinetic control. While stepwise syntheses have produced a wide variety of MIMs, all-in-one protocols are becoming increasingly popular because of their simplicity and efficiency, not to mention the topologically complex molecules that can be accessed (vide infra) using this approach.
Figure 5.
(a) The ‘all-in-one’ synthesis of a benzylic imine [2]catenate templated by transition metal coordination. (b) A tubular representation of the solid-state structure of a Zn(II)-templated benzylic imine [2]catenate illustrates the topological node generated by the meridional octahedral metal cation (Leigh et al. 2001).
An alternative method to the use of metal cations to template the synthesis of MIMs is to integrate the metal ions themselves into the mechanically interlocked structure, i.e. the MIM is held together by a mixture of covalent and coordinative bonds (Fujita 1999). The self-assembly of Pd(II)-containing molecules illustrates this concept extremely well. Extensive investigations within the Fujita group (Fujita et al. 2005) revealed that cis-protected Pd(II)-ethylenediamine ‘corners’ not only provide a route into engineering 90° joints into molecules, but also afford a perfect opportunity for self-assembly to occur because the coordination bonds between the square planar Pd(II) cation and the ethylenediamine (en) ligand are kinetically inert while bonds between the two further cis sites on the metal centre and aromatic non-chelating ligands (such as pyridine derivatives) are kinetically labile. These factors result in a right-angled coordination ‘synthon’, which has been extensively used as a building block in the construction of large metallosupramolecular architectures. The Pt(II) analogues are kinetically inert at room temperature but can be self-assembled thermodynamically at high temperatures and using solvents of higher polarities. They can also be isolated as kinetically inert structures on cooling—the so-called molecular lock concept (Fujita et al. 1995). It is now commonplace to see inert Pt(II)-templated compounds reported alongside their labile Pd(II)-templated siblings.
The Fujita group has led the way in the self-assembly of MIMs based on incorporating metal ions into their structures (Fujita et al. 1994). During the preparation (figure 6a) of the metallomacrocycle 22 by dissolving two equivalents each of [Pd(en)(NO3)2] and a bis-pyridyl ligand in D2O, it was found that, when the concentration of the reagents was raised, a second product was evident in the 1H NMR spectra recorded on reaction mixtures: while the metallomacrocycle is the sole product at a concentration of 1 mM, a second product dominates (more than 90%) the equilibrium at 50 mM. Examination of 1H NMR spectra of the second product led to its identification as the [2]catenane 23; upfield shifts of protons on the aromatic units housed inside each macrocycle and the commuted symmetry in comparison with the single macrocycle are characteristic of catenane formation. A prime example of the complexity that can be engineered by this approach is the subsequent triply interlocked [2]catenane prepared by Fujita from tripodal ligands (Fujita et al. 1999). This particular MIM can not only be self-assembled quantitatively in solution from two equivalents of each tripodal ligand plus six equivalents of [Pd(en)(NO3)2], but also, strikingly, by mixing solutions of two separate metallosupramolecular entities—a trigonal prism and an octahedron—which contain the appropriate ratio of precursors. The triply interlocked [2]catenane is the thermodynamic product of a 10-component reversible self-assembly process even if the reagents are already arranged in stable compounds by themselves. A Pt(II) analogue 24 could also be isolated and characterized in the solid state, with its crystal structure (figure 6b) illustrating beautifully its mechanically interlocked nature.
Figure 6.
(a) The equilibrium that exists in aqueous solution between the Pd(II)-templated metallomacrocycle 22 and the dimeric [2]catenane 23 (Fujita et al. 1994). The dynamic coordinative bonds between the Pd(II) cation and the pyridyl ligands allows the reversible self-assembly of the thermodynamic product. (b) A tubular representation of the crystal structure of a triply interlocked [2]catenane 24 prepared by Pt(II) templation (Fujita et al. 1999).
Subsequently, metallosupramolecular architectures held together by coordinative bonds have become commonplace (Leininger et al. 2000), and some contain mechanical bonds. An important class of such MIMs are those based on coordination to axial sites of metalated—often with Zn(II)—porphyrins (Faiz et al. 2009). Their size allows them to be used as stoppers in rotaxanes as a result of coordinating the termini of thread components to the metal centres of the porphyrins. In addition, the ease of modification of porphyrins allows their linkage covalently and coordinatively, generating metallomacrocycles that form components of rotaxanes and catenanes.
Building upon their experiences in the use of coordinative and covalent bonds to orient appropriately the precursors of MIMs during their synthesis, chemists began to use the numerous non-covalent bonding interactions gathered under the guise of supramolecular chemistry to prepared molecules with mechanical bonds. These protocols often take advantage of the fact that host–guest complexes, which underpin supramolecular chemistry, can be modified by either (i) attaching bulky stoppers to the end of the guest in a 1 : 1 complex to form a rotaxane, or (ii) connecting the ends of the guest in a 1 : 1 complex to form a catenane (figure 1). This latter approach was first attempted, although unsuccessfully, as long ago as 1958 (Lüttringhaus et al. 1958) with α-cyclodextrin as the host. The cyclodextrins, naturally occurring cyclic oligosaccharides, have a long-known propensity to encapsulate organic molecules within their hydrophobic interiors in aqueous solutions (Dodziuk 2006). Lüttringhaus and co-workers prepared a host–guest complex in aqueous solution between α-cyclodextrin (host) and a hydroquinone derivative with pendant alkanethiol groups (guest), and attempted to form a [2]catenane by oxidative coupling of the terminal thiol groups. While this attempt did not meet with success—in large measure because the experimental design was years ahead of its time—it established cyclodextrins as a readily available source of macrocycles to be used in the synthesis of MIMs (Nepogodiev & Stoddart 1998).
Most syntheses of MIMs containing cyclodextrins follow a host–guest strategy whereby a guest is encircled by the cyclodextrin (the host) to form a pseudorotaxane, and the ends of the guest are either joined together to form a catenane, or capped with stoppers to form a rotaxane. This threading-followed-by-stoppering approach yielded (figure 7a) the first cyclodextrin rotaxane 27 in 1981, when Ogino threaded 1,12-diaminododecane 26 through both α- and β-cyclodextrin and stoppered the pseudorotaxanes formed in each case by coordination of a Co(III) 25 to the terminal amino functions of the guest (Ogino 1981). The key to the success of this approach lies in the fact that all components are soluble in water, which also enhances the recognition of the thread by the cyclodextrin torus, and that the capping reaction is tolerant to aqueous conditions. These characteristics are present in Anderson's use of Pd-catalysed cross coupling reactions in the preparation (figure 7b) of discrete oligorotaxanes by Suzuki coupling of cyclodextrin-based pseudorotaxanes. Linear molecules, such as the stilbene derivative 29, have been coupled to water-soluble stoppers, e.g. 28, in the presence of α- or β-cyclodextrin to form [2]rotaxanes such as 30, which was characterized by X-ray crystallography (Stanier et al. 2001). This method is also well suited for the preparation of polyrotaxanes, which act as insulated molecular wires (Taylor et al. 2000). Subsequently, many rotaxanes and polyrotaxanes (Wenz et al. 2006) have been prepared using this threading-followed-by-stoppering protocol, often displaying unique properties. Examples of cyclodextrin-based catenanes, however, remain relatively rare (Armspach et al. 1993).
Figure 7.
The stoppering of cyclodextrin-based pseudorotaxanes to form [2]rotaxanes can be carried out through coordinative bonding (a) or by covalent bond formation (b).
More recently, other readily available macrocycles have been added into the mechanostereochemical toolkit on account of their favourable host–guest complexation properties. Cucurbiturils, cyclic oligomers of glycoluril units with binding properties similar to those of the cyclodextrins, have been exploited, particularly by Kim and co-workers, in the assembly of spectacular molecular necklaces and polyrotaxanes (Kim 2002). Calixarenes, cyclic oligomers of phenols linked by meta-methylene groups (Arduini et al. 2003, 2009), are also prevalent in the literature, while the recently discovered pillar[5]arenes, their para-linked siblings, have subsequently been incorporated into MIMs (Strutt et al. 2011).
The crown ethers are another category of macrocycle that have been incorporated into a wide variety of MIMs. Building upon the fact that crown ethers containing π-electron-rich aromatic moieties, such as bis-para-phenylene[34]crown-10 (33), are excellent hosts for π-electron-deficient species, e.g. viologens, as a result of the donor–acceptor interactions between the host and the guest, we reported the synthesis (figure 8a) of a donor–acceptor [2]catenane in 1989 (Ashton et al. 1989). Simply by mixing 33 with 1,4-bis(bromomethyl)benzene 32 and the bipyridinium dication 31 in MeCN, the [2]catenane 35 is formed in 70 per cent yield, presumably by a mechanism that involves the formation of a tricationic supramolecular precursor 34. The crystal structure (figure 8b) of 35 reveals the extent, not only of intramolecular, but also of intermolecular donor–acceptor interactions in the solid-state: the catenanes crystallize as one-dimensional alternating donor–acceptor stacks. Furthermore, [C−H⋅⋅⋅O] interactions between the hydrogen atoms α to the nitrogens in the cyclophane and the oxygen atoms in the polyether loops are an important source of stabilization within the catenane. This source of templation has since been demonstrated (Raymo et al. 2001) to be vital in the synthesis of many types of MIMs.
Figure 8.
(a) The template-directed synthesis of the donor–acceptor [2]catenane 35 comprising the macrocycle cyclobis(paraquat-para-phenylene) and bis-para-phenylene[34]crown-10 (Ashton et al. 1989). Tubular renderings of the solid-state structures of (b) the previously described [2]catenane 35, (c) a branched [7]catenane 36 (Amabilino et al. 1997) and (d) a folded [3]pseudorotaxane 37 (Basu et al. 2011).
Following on from this initial breakthrough, a multitude of donor–acceptor MIMs have been prepared (Griffiths & Stoddart 2008; Barin et al. 2012), many incorporating the electron-deficient cyclophane component of the prototype [2]catenane, cyclobis(paraquat-para-phenylene), or CBPQT4+ for short. These MIMs include (figure 8c,d) a branched [7]catenane 36 (Amabilino et al. 1997), well-defined oligorotaxanes 37 which fold in solution (Basu et al. 2011), and numerous switchable MIMs, based on the redox-active electron donor tetrathiafulvalene (TTF) (Asakawa et al. 1998). In addition, Sanders and co-workers have expanded the donor–acceptor toolkit, preparing MIMs with pyromellitic diimide and naphthalene diimide units as the electron-deficient components; these units have led to the synthesis of neutral donor–acceptor MIMs (Hamilton et al. 1998). This very same research group have described (Au-Yeung et al. 2009) more recently the preparation of various catenanes (Cougnon et al. 2011, 2012) based on neutral building blocks by employing dynamic combinatorial protocols in aqueous media. By mixing compounds containing electron-poor and electron-rich aromatic rings with appended thiol functions, a dynamic library of interconverting macrocycles and catenanes is generated through reversible oxidative dithiol coupling reactions where conditions can be altered to favour the self-assembly of the desired MIM(s). Furthermore, Peinador and Quintela have combined the templating effects of both donor–acceptor interactions and metal coordination to prepare a series of singly (Blanco et al. 2007) and doubly interlocked (Peinador et al. 2009) catenanes using dynamic reversible self-assembly protocols.
The so-called amide catenanes whose formation is templated by hydrogen-bonding interactions were discovered independently in the early 1990s by Hunter (1992) and Vögtle (Vögtle et al. 1992). Their discovery has led to a new generation of neutral MIMs synthesized using the stabilization and directionality provided by hydrogen bonds (Schalley et al. 2004).
Both groups were investigating the recognition properties of macrocycles bearing isophthalimido units, and isolated very similar [2]catenanes as one of the products of the attempted stepwise syntheses of larger macrocycles. Simply reacting (figure 9a) the diacid chloride 38 and diamino precursors 39 (in a one-pot reaction) or 40 (in a stepwise synthesis) in a non-polar solvent gives the catenanes 41, among other products, in approximately 30 per cent yield: the templating amido groups are formed in a stepwise manner during the one-pot reaction. Leigh and co-workers subsequently uncovered a smaller, more compact [2]catenane 42 (Johnston et al. 1995), on replacing the diphenylenecyclohexylidene spacer (figure 9b) with a phenylene one, allowing for increased π–π stacking interactions between the two rings. This hydrogen-bonding motif was used in the successful syntheses (figure 9c) of rotaxanes, such as 44, in the presence of the dumbbell 43 (Johnston et al. 1996) and, subsequently, a large number of MIMs displaying dynamic features have been prepared (Kay & Leigh 2005) using amide hydrogen bonds as the source of templation.
Figure 9.
(a) The synthesis of the amide hydrogen-bonded [2]catenanes 41 discovered independently by Hunter (1992) and Vögtle et al. (1992). (b) A smaller [2]catenane 42 and (c) a [2]rotaxane 44 prepared using a similar strategy by Leigh and co-workers (Johnston et al. 1995, 1996).
Hydrogen bonding also underpins a mode of templation that involves the threading of secondary dialkylammonium ions ( through crown ethers. Pseudorotaxanes based on this binding motif were reported independently in 1995 by our research group (Ashton et al. 1995) and that of Busch (Kolchinski et al. 1995). Each group investigated the propensity for R2NH+2 ions to thread through crown ethers, and found that the cationic guests were clasped within the rings through a series of non-covalent bonding interactions—namely [+N−H⋅⋅⋅O] and [+N−C−H⋅⋅⋅O] short contacts—which are responsible for the stability of the resultant pseudorotaxanes. The importance of the hydrogen bonding is demonstrated, not only in the crystal structure (figure 10a) of the pseudorotaxane 47 formed between dibenzylammonium hexafluorophosphate (45) and dibenzo[24]crown-8 (46), but also in the fact that the binding constant between the crown ether and the
through crown ethers. Pseudorotaxanes based on this binding motif were reported independently in 1995 by our research group (Ashton et al. 1995) and that of Busch (Kolchinski et al. 1995). Each group investigated the propensity for R2NH+2 ions to thread through crown ethers, and found that the cationic guests were clasped within the rings through a series of non-covalent bonding interactions—namely [+N−H⋅⋅⋅O] and [+N−C−H⋅⋅⋅O] short contacts—which are responsible for the stability of the resultant pseudorotaxanes. The importance of the hydrogen bonding is demonstrated, not only in the crystal structure (figure 10a) of the pseudorotaxane 47 formed between dibenzylammonium hexafluorophosphate (45) and dibenzo[24]crown-8 (46), but also in the fact that the binding constant between the crown ether and the  ion relies on the polarity of the solvent in which it is measured. Increasing the polarity results in competition of the solvent for the hydrogen-bond donors and acceptors and a subsequent drop in association, culminating in no detectable complexation in dimethyl formamide (DMF) and dimethyl sulphoxide (DMSO). A wide variety of MIMs (Cantrill et al. 2000) of increasing complexity have emerged in the literature following on from these initial discoveries—a branched hetero[7]rotaxane being a recent example (Zhang et al. 2011)—with many of the MIMs exhibiting π–π interactions between the components to complement the hydrogen bonds.
ion relies on the polarity of the solvent in which it is measured. Increasing the polarity results in competition of the solvent for the hydrogen-bond donors and acceptors and a subsequent drop in association, culminating in no detectable complexation in dimethyl formamide (DMF) and dimethyl sulphoxide (DMSO). A wide variety of MIMs (Cantrill et al. 2000) of increasing complexity have emerged in the literature following on from these initial discoveries—a branched hetero[7]rotaxane being a recent example (Zhang et al. 2011)—with many of the MIMs exhibiting π–π interactions between the components to complement the hydrogen bonds.
Figure 10.
(a) The formation of a pseudorotaxane 47 between dibenzylammonium hexafluorophosphate (45) and dibenzo[24]crown-8 (46) with its solid-state structure portrayed alongside. (b) Reversible formation of a [2]rotaxane 51 by a clipping protocol wherein a diimino-crown ether self-assembles around a secondary dialkylammonium ion centre, which can then be kinetically trapped by reduction of its imine bonds to form the stable [2]rotaxane 52. Its crystal structure is displayed alongside.
The introduction (Glink et al. 2001) of a diimino-crown ether, based on 2,6-diformylpyridine 48 and a diamino-polyether chain 50 with a constitution similar to that of [24]crown-8, around the dialkylammonium dumbbell 49 has led to the reversible self-assembly of [2]rotaxane 51 under thermodynamic control in high yields (figure 10b). The reduction of the imine bonds has resulted in the formation of the [2]rotaxane 52, its crystal structure demonstrating its mechanically interlocked nature. Simply mixing the components of the ring, alongside a secondary dialkylammonium thread in acetonitrile results in the quantitative formation of well-defined linear oligorotaxanes up to a [20]rotaxane (Belowich et al. 2010, 2012).
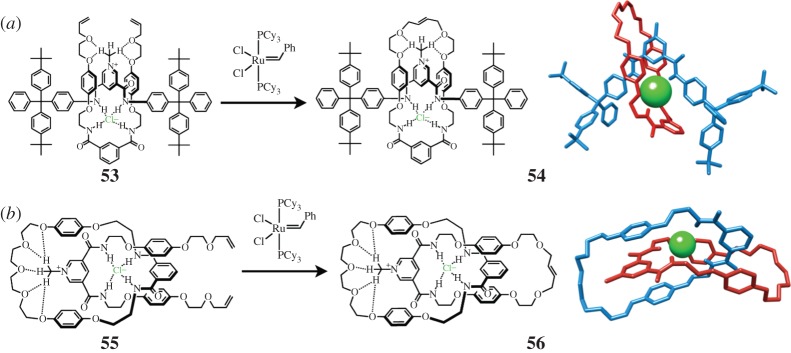
Because the binding of cations, be they metal ions or cationic organic compounds, has proved to be such a successful method of templation in the synthesis of MIMs, it is not surprising that anions are now also being exploited as templates (Vickers & Beer 2007). While anion binding is generally viewed (Bianchi et al. 1997) as being more challenging than cation coordination because of the larger size, more diffuse charge, greater solvation and wide variety of geometries prevalent in anions, significant progress has been made in this arena. In a historical perspective, Sessler and Vögtle described (Andrievsky et al. 1998), for the first time, the affinity of [2]catenanes incorporating pyrrole and amide functional groups towards anions, and the syntheses of [2]rotaxanes employing a templating interaction between the ring and an anionic portion of the rod were demonstrated (Hübner et al. 1999) by Vögtle. Subsequently, Beer and co-workers have used the tetrahedral geometry arranged around a chloride anion—acting as an external template—by an isophthalimide and a pyridinium based diamide, each providing (figure 11) two N–H hydrogen-bond donors, as a crossing point or node in a manner similar to that of the archetypal bis-phenanthrolino copper(I) synthon. When a dumbbell with bulky stoppers and a pyridinium diamido binding station is combined (Wisner et al. 2002) with an isophthalimido derivative terminated with alkene functions and one equivalent of chloride, the 1 : 1 : 1 complex 53 that is formed can be converted (figure 11a) to a [2]rotaxane 54 by ring-closing alkene metathesis. Similarly, when the dumbbell is replaced (Sambrook et al. 2004) by a macrocycle, thus resulting in the complex 55, a chloride-templated [2]catenane 56 can be synthesized (figure 11b). The crystal structures of these two anion-templated MIMs reveal not only the expected hydrogen bonds from the amide moieties to the chloride anion template, but also π–π stacking interactions between the two mechanically interlocked components and [C−H⋯ O] interactions from the pyridinium methyl groups to the polyether loops that are used as linkers. These initial examples of anion templated MIMs again underscore the fact that many non-covalent bonding forces operate in unison during the self-assembly of MIMs.
Figure 11.
The chloride-templated synthesis of (a) a [2]rotaxane 54 (Wisner et al. 2002), and (b) a [2]catenane 56 (Sambrook et al. 2004) with their respective crystal structures positioned alongside in tubular format with the chloride template rendered as a green sphere.
Subsequently, a large number of MIMs have been prepared (Vickers & Beer 2007) using a range of anions as templates, including Cl−, Br− and SO2−4, and most recently of all, the templation of MIMs involving halide anions has been invigorated by the newly discovered concept of halogen bonding (Kilah et al. 2010). The development of functional devices, for example optical chloride sensors (Gassensmith et al. 2010) based on anion-templated MIMs, underscores their potential for applications in the future.
One of the most recently uncovered modes of templation is the one that relies on radical–radical interactions (Spruell 2010). The radical organocations of TTF and methyl viologen (MV2+)—generated by oxidation and reduction, respectively—have been long known to interact with one another. They are capable of forming mixed-valence pairs—(TTF2)+⋅—and radical cation dimers (TTF+⋅)2 and (MV+⋅)2. In the early 2000s, Kim and co-workers demonstrated that the radical cation dimers of MV2+ (Jeon et al. 2002) and TTF (Ziganshinsa et al. 2004) could be stabilized significantly in a supramolecular context by their encapsulation in cucurbit[8]uril. We have extended (Spruell et al. 2010) this concept by preparing [3]catenanes, such as 574+ (figure 12) wherein TTF units are mechanically interlocked within the confines of a tetracationic cyclophane, and can be oxidized sequentially through their varying radical dimers 575+ and 576+. These ‘mechanically stabilized’ TTF radical dimers are sufficiently stable (Coskun et al. 2011) to allow their characterization using a suite of structural and analytical techniques, including single crystal X-ray diffraction, elucidating the extent of radical–radical interactions.
Figure 12.
Structural representations (a) and solid-state structures (b) of the ground, mixed-valence and radical cation dimer states, in turn, of the [3]catenane 574+ (Spruell et al. 2010).
In some recently published research, we have illustrated (Trabolsi et al. 2010) the power of radical recognition in assembling a tris-radical host–guest complex composed of CBPQT4+, which contains two bipyridinium radical cations and methyl viologen as the guest. In their respective tetracationic and dicationic ground states, no association between the components is detectable. Upon oxidation of the three bipyridinium units of the host CBPQT4+ and the guest 58 to their radical cationic states, radical–radical interactions overcome the Coulombic repulsion between the now dicationic host and monocationic guest and the inclusion complex 59 is formed. Combining this source of radical templation with light-induced oxidation and copper-free 1,3-dipolar azide-alkyne cycloaddition, [2]rotaxanes such as 60 (figure 13a) can be synthesized (Li et al. 2010a), which show, upon aerobic oxidation to their non-radical ground states, little or no recognition between the thread and ring components. The recently solved solid-state structure (figure 13b) of the tris-radical complex 61 formed between CBPQT2+⋅ and MV+⋅ reveals significant radical–radical interactions (Fahrenbach et al. 2012). This new departure for CBPQT4+—well used as a host for electron-rich aromatic guests for over 20 years—as a radical recognition unit will no doubt lead to many further MIMs endowed with novel switching properties.
Figure 13.
(a) The synthesis of a frustrated [2]rotaxane 60 based on the recognition of a monocationic bipyridine-based radical thread by the dicationic diradical CBPQT2+, followed by copper-free click chemistry (Li et al. 2010a). (b) Tubular representation of the crystal superstructure of the tris-radical host–guest complex 61 formed between CBPQT2+⋅ and MV+⋅ (Fahrenbach et al. 2012).
These seminal examples of template-directed synthetic protocols for the production of MIMs reflect a booming area of research, and are intended to give the general reader an introduction to the methods of templation that can be used to prepare MIMs. It should be noted that it is very rarely only one mode of templation that is used in a synthesis, more often than not a variety of non-covalent and also coordinative interactions are used in these syntheses. The toolkit that is now available to chemists for making mechanical bonds is a large one, and it is being exploited in many ingenious ways to form MIMs endowed both with topological complexity and with functionality, two research areas that will now be addressed in turn.
3. Chemical topology
From the very early days of chemical topology, the chemists involved have speculated on the potential synthesis of molecular versions of the highly complex knots and links that were already well-studied by mathematicians (Rolfsen 1976; Adams 1994). Moving forward from singly interlocked catenanes and rotaxanes, the synthetic difficulty in arranging the crossover points, or nodes, inherent to knots and links, in concert with the formation of one or more covalent bonds in an appropriate manner to prepare the target compound, has limited the extent to which these species have been isolated to date (Forgan et al. 2011). The Trefoil knot, the simplest knot and composed of a torus with three crossing points, has been expressed chemically in a number of examples, the first (Dietrich-Buchecker & Sauvage 1989) being an extension of the protocol used by Sauvage et al. to prepare metal-templated catenanes and rotaxanes (figure 14a). In this case, a dicopper(I) double helical complex 63 was prepared from bis-phenanthrolino ligands 62, providing two crossover points, and was then cyclized with polyether loops to form the molecular Trefoil knot 64. It should be noted that Trefoil knots are intrinsically topologically chiral—two non-superimposable mirror images exist—and both topological enantiomers were observed (Dietrich-Buchecker et al. 1990) as a result of their spontaneous resolution during crystallization. Each crystal isolated from vapour diffusion of CH2Cl2 into a benzene solution contained only one enantiomer, i.e. the compound crystallized as a conglomerate. Taking this approach even further, the cyclization of a related tricopper(I) double helicate formed from extended phenanthroline ligands allowed access to a molecular Solomon Link (Nierengarten et al. 1994), and the cyclization of helical complexes has proved to be a successful methodology in preparing molecular knots and links. A series of Trefoil knots have also been prepared (Lukin & Vögtle 2005) by serendipitous neutral hydrogen-bond templation, while metal cations have also adopted a significant role in the preparation of topologically non-trivial knots and links (Dietrich-Buchecker et al. 2005).
Figure 14.
(a) A stepwise synthesis under kinetic control of a molecular Trefoil knot 64 by cyclization of a dinuclear double helical complex 63, with the solid-state structure of one of the topological enantiomers displayed (Dietrich-Buchecker & Sauvage 1989; Dietrich-Buchecker et al. 1990). (b) All-in-one synthesis under thermodynamic control of a molecular Borromeate 67 displayed as its solid-state structure (Chichak et al. 2004).
As the topological complexity of the target molecule increases, the synthesis often relies on an ‘all-in-one’ strategy under thermodynamic control rather than a stepwise one under kinetic control. A prime example is the preparation of molecular Borromean rings (figure 14b), three interlocked but non-catenated rings, in an 18-component reversible self-assembly process in nearly quantitative yields (Chichak et al. 2004) When six equivalents each of the dialdehyde 65, the diamine 66 and the Zn(II) template are heated in methanol, the molecular Borromeate 67 is obtained in 95 per cent isolated yield, clearly demonstrating the efficacy of thermodynamic, all-in-one preparative approaches. Interestingly, subtle changes to reaction conditions result in the isolation of a molecular Solomon Link from the same ligand system (Meyer et al. 2010). In dynamic systems such as this one, serendipity often plays a role in the synthesis of topologically complex MIMs.
It would be remiss of us to discuss topologically interesting molecules without mentioning the significant contributions made by those involved in the field of biochemical topology—the study of knotted and linked biological entities. Knots occur naturally in both DNA (Gellert 1981) and proteins (Taylor & Lin 2003), while knotting enzymes—DNA topoisomerases—can be used (Dean et al. 1985) to prepare a whole gamut of knots and links in DNA, although in a random manner. The reproducibility and programmability of the Watson–Crick base pairs of DNA can be exploited (Seeman 1998) to design wholly synthetic strands of DNA, which, under the appropriate conditions, can be ligated and cyclized to form knots and links. The group of Seeman have blazed a trail in this area, with some highlights being DNA versions of (i) a 790 kDa-truncated octahedron that corresponds to a [14]catenane (Zhang & Seeman 1994), (ii) the Borromean rings (Mao et al. 1997), and (iii) trefoil and figure of eight knots, prepared from one single strand of DNA ligated under different conditions (Du et al. 1995). These examples are only a selection of a series of remarkable synthetic accomplishments, which are not, however, scalable in the manner of the chemically derived species listed previously.
4. Integrated functional systems
With the full force of template-directed synthesis of MIMs in place, the field of mechanostereochemistry has started to witness during the past decade the addressing of the preparation of integrated functional systems with the ultimate goal of producing molecular machines. The intriguing possibility of building synthetic molecular machines (Kay et al. 2007; Coskun et al. 2012) similar to biological ones has been raised to another level of sophistication by the development of molecular switches in the form of MIMs. A molecular switch can be achieved by the relative movement of components in a MIM that results in two or more stable states. In such a system, the ring component is located on one of the two different binding sites, and its position can be varied reversibly on applying external stimuli, e.g. redox, pH, light, temperature, etc. The presence of two or more binding sites, on one ring component in a catenane, or on the dumbbell in a rotaxane, results in a distribution of states in which the moving ring component occupies these sites to a certain extent depending on the relative binding affinities, leading to bistability. Employing an external stimulus allows the manipulation of binding affinities and, therefore, can change the location of the ring component. An example of a switchable molecular shuttle 68 was demonstrated (Bissell et al. 1994) by our group in a bistable [2]rotaxane (figure 15) in 1994. In this system, a CBPQT4+ ring resides on the benzidine (orange) station of the dumbbell component with an 84 per cent preference over the biphenol (red) station at 229 K.
Figure 15.
A switchable donor–acceptor molecular shuttle 68 (Bissell et al. 1994). The CBPQT4+ ring prefers to be located on the benzidine (orange) station until its oxidation or protonation moves the ring to the biphenol (red) station. Deprotonation or reduction results in the return of the ring to the benzidine station.
The oxidation or protonation of the benzidine unit results in a loss of its affinity towards the CBPQT4+ ring on account of Coulombic repulsion, and so the ring component migrates onto the biphenol station. The process can be reversed upon reduction or deprotonation, resulting in the recovery of the initial state. Electroactive TTF derivatives have been investigated (Choi et al. 2006) extensively in the construction of bistable MIMs in order to achieve higher selectivities between the stations, as well as to benefit from the rich redox properties of the TTF units.
Although many examples of molecular switches based on MIMs have been reported (Balzani et al. 2008), it has not been an easy task to construct molecular machines from these switches. A recent review (Coskun et al. 2012) draws a distinction between molecular switches and machines, and outlines what is required for a molecular switch to function as a machine. Extracting useful work or energy using molecular switches has not yet truly been achieved either on the nano- or macroscale, although there are few examples in which the outcome of switching of a MIM results in measurable work. Leigh and co-workers (Berná et al. 2005) demonstrated that the millimetre-scale directional transport of a liquid droplet can be achieved by using the biased Brownian motion of a molecular shuttle which results in a change in surface tension. The ring component resides initially on the fumaramide (green) station in the bistable [2]rotaxane 69 (figure 16a). (E) to (Z) Photoisomerization [(E)→(Z)] of fumaramide to maleimide (blue) results in the disruption of hydrogen-bonding interactions and the ring component shuttles onto the tetrafluorosuccinimide (orange) station. The concealment of the short fluoroalkane segment by the ring changes the surface properties such that it becomes polarophilic. This fact results in the movement of a diiodomethane droplet upon illumination of UV light on one side of the droplet. Photoisomerization-induced shuttling motion in the [2]rotaxane molecules can even move the droplet several millimetres up a 12° incline successfully.
Figure 16.
(a) A photoswitchable [2]rotaxane 69 that changes its physical properties upon switching, from fluorophilic to polarophilic. When 69 is (b) deposited on a surface and covered with droplets of CH2I2, photo-induced switching moves the droplet uphill, constituting an example of a nanoscale molecular machine carrying out work on the macroscale (Berná et al. 2005).
In another example of a molecular machine, we have demonstrated (Liu et al. 2005; Juluri et al. 2009) the bending of a microcantilever using molecular switches in a palindromic, doubly bistable [3]rotaxane. The [3]rotaxane molecules are assembled onto the gold cantilever surface via the attachment of two CBPQT4+ ring components, which reside upon two TTF stations located near the termini of the thread components. Upon oxidation of the outer TTF stations on the dumbbell, the CBPQT4+ rings move to encircle the inner dioxynaphthalene (DNP) stations, resulting in a contraction at molecular level, which, in turn, causes the bending of the microcantilever. This bending process can be performed chemically or electrochemically, and has been shown to be reversible.
Besides the goal of building artificial molecular machines, MIMs have been used as active components in molecular electronic devices. In a recent demonstration (Green et al. 2007), a monolayer of bistable [2]rotaxane molecules was incorporated into a molecular electronic memory circuit as the data storage elements (figure 17). This approach uses ‘bottom-up’ assembly, thus allowing the required decrease in dimensions for improved integrated circuit technology. A 160 000-bit molecular electronic memory chip was fabricated at a density of 1011 bits cm−2 using the bistable [2]rotaxane 70, once again composed of a CBPQT4+ ring and a thread with TTF and DNP components. The crossbar memory was assembled by sandwiching a monolayer of bistable [2]rotaxane molecules between Si bottom-nanowire and Ti top-nanowire electrodes. Each crossing between electrodes corresponds to an individual molecular switch tunnel junction, i.e. 1 bit, and comprises approximately 200 molecules of 70.
Figure 17.
(a) A switchable [2]rotaxane 70 that can be incorporated into (b) a 160000-bit molecular memory chip (Adapted from Nature Publishing Group). (c) The read/write cycle is achieved by oxidation of the green TTF station of 70, upon which the blue CBPQT4+ macrocycle moves to the red DNP station. Re-reduction of the TTF unit generates the metastable state co-conformation (MSCC), which represents the ‘1’ state, and subsequent relaxation to the ground state co-conformation (GSCC) regenerates the ‘0’ state (Green et al. 2007).
The hydrophilic stopper (light blue) is in contact with the Si electrode. In the ground state co-conformation (GSCC), the CBPQT4+ ring encircles the TTF site that corresponds to the low-conductance ‘0’ state. The oxidation of TTF to its radical cation or dication results in the translation of the CBPQT4+ ring to the DNP site. The subsequent reduction of the TTF radical cation back to its neutral state forms the so-called metastable state co-conformation (MSCC)—the CBPQT4+ ring resides on the DNP unit despite having a higher affinity for the newly generated neutral TTF moiety: this MSCC corresponds to the high conductance ‘1’ state. The MSCC then relaxes back to the GSCC as the CBPQT4+ ring returns to the TTF station with a half-life of about an hour in the device environment. This fact renders the memory volatile, and current efforts in the field are devoted to adjusting the relaxation kinetics in order to obtain non-volatile memory devices by increasing the lifetime of the MSCC while searching for ways to increase the robustness of the devices.
MIMs have also been proved (Cotí et al. 2009; Ambrogio et al. 2011; Li et al. 2012) to be promising active components for drug delivery and controlled release purposes in the form of mechanized silica nanoparticles (MSNPs). The MSNPs are fabricated by incorporating stimulus-responsive MIMs, i.e. pseudorotaxanes and rotaxanes, onto the surface of mesoporous silica nanoparticles (figure 18). These molecules act as gatekeepers at the entrances of the pores of the nanoparticles, preventing the leakage of drugs or imaging agents which have been loaded inside them. Employing an appropriate stimulus (redox, pH, light, biological triggers) initiates the mechanical motion of the ring component—a decomplexation process in a pseudorotaxane and a switching process in a bistable rotaxane—and results in the release of the cargo from inside silica nanoparticles.
Figure 18.

A redox-active mechanized silica nanoparticle (MSNP) decorated with bistable rotaxanes. Target molecules are loaded (step 1) in the ‘open’ state of the rotaxane, and the valve closed (step 2) by switching the rotaxane to the ‘closed’ state. Switching back to the ‘open’ state (step 3) under specific stimulus results in release (step 4) of the target molecule (Nguyen et al. 2005).
In an early breakthrough (Nguyen et al. 2005) in this area, the decoration of silica nanoparticles with bistable [2]rotaxanes, similar in constitution to those used in the previously described molecular flash memory, allowed the construction of redox-active nanovalves on MSNPs that can be opened and closed reversibly (figure 18). Initially, the CBPQT4+ ring sits on the TTF unit away from the pore openings and, therefore, cargo molecules can be loaded into the nanoparticle under a concentration gradient in this ‘open’ state. The chemical oxidation of the TTF unit causes the movement of the CBPQT4+ ring onto the DNP site which now forms the ‘closed’ state. The opening of the pores can be achieved upon addition of a reducing agent that regenerates the neutral TTF unit that is then encircled by the CBPQT4+ ring. Although early proof-of-concept studies were carried out in organic solvents, subsequent research (Ambrogio et al. 2011) has focused on systems that operate in aqueous environment, as well as developing highly responsive systems that act under mild stimuli such as light, pH and enzymatic activators.
Drug release systems based on pH activation have been developed (Angelos et al. 2008) using cyclodextrins and cucurbiturils as the ring components in a [2]pseudorotaxane assembly, offering the advantages of biocompatibility and water solubility. Azobenzenes have been used in the construction of light-activated drug delivery systems in the form of impellers (Sierocki et al. 2006) and nanovalves (Ferris et al. 2009). Finally, enzymatic activation can be achieved (Patel et al. 2008) using MSNPs functionalized with [2]rotaxanes incorporating stopper units, which can be excised upon exposure to a specific enzyme. The cleavage of the stopper is followed by the dethreading of the ring component, resulting in the opening of pores.
Integration of MIMs into robust platforms has been extended (Loeb 2005; Deng et al. 2010) recently to metal-organic frameworks (MOFs), highly ordered three-dimensional networks of organic struts, linked by transition metal clusters which exhibit permanent porosity and striking uptake properties. The marriage of MIM and MOF chemistries—so-called ‘robust dynamics’—leads to the incorporation of the dynamics associated with MIMs into the robust extended structures that are typical of MOFs. Furthermore, MOFs provide the opportunity for the precise location of molecular switches within their extended structures as well as addressability of the dynamic MIMs individually on account of their ultrahigh porosities. Such features are not readily available in solution or in condensed phases.
A prime example (Li et al. 2009) is the formation of a cubic repeating structure (figure 19a) using an approximately 2 nm long organic strut 71 incorporating a bisparaphenylene[34]crown-10 receptor that is known to be capable of binding electron-deficient aromatic guests, e.g. methyl viologen, to form [2]pseudorotaxanes. When this MOF is exposed to methyl viologen dications in acetonitrile, the openness of the structure allows the entry into the framework of the guest, which is bound subsequently by the macrocyclic polyether component, without compromising the structural integrity of the material. This research demonstrates the feasibility of carrying out molecular recognition in porous crystals.
Figure 19.
(a) A 2 nm MOF strut 71 containing electron-rich crown ethers can generate a cubic MOF capable of binding electron-poor molecules, such as methyl viologen, within its pores (from Li et al. 2009). Adapted from AAAS. (b) A related strut 72 wherein the crown ether is now catenated generates a dense two-dimensional network of catenanes in the solid state (Li et al. 2010c) (adapted from The Royal Society of Chemistry), whereas (c) a longer derivative 73 results in a three-dimensional array of MIMs (Li et al. 2010b).
Encouraged by the successful formation of supramolecular [2]pseudorotaxane molecules inside a MOF, organic struts comprising [2]catenanes on their side chain, 72 (figure 19b) and 73 (figure 19c), have been prepared and shown to form highly ordered two-dimensional (Li et al. 2010c) and three-dimensional (Li et al. 2010b) frameworks. In the assembly of the three-dimensional framework, a strut of exceptional length (3.3 nm) has been used, resulting in a unit cell containing 7524 atoms. In this extended structure, catenation is expressed both in the struts and in the framework itself, i.e. interpenetration is observed. In a recent report (Gong et al. 2011), Sessler and co-workers have shown that a one-step, self-assembly based protocol can be used in the construction of three-dimensional metal-organic rotaxane frameworks (MORFs). In this approach, the struts are composed of pseudorotaxanes formed between a large tetracationic imidazolium macrocycle and 2,6-naphthalene dicarboxylate dianions, whose formation is driven by anion complexation prior to MORF assembly, a situation that is enacted by familiar metal ion bonding interactions. Similarly, three-dimensional MORFs have been reported by Loeb and co-workers (Hoffart & Loeb 2005) employing [2]pseudorotaxanes—composed of a dibenzo[24]crown-8 ether wheel and a dipyridinium N-oxide axle—and transition metals.
These results pave the way for the incorporation of molecular switches into three-dimensional arrays, an anticipated development that could ultimately yield functional materials for ultrahigh-density electronics applications. Moreover, the well-defined arrangement of many molecular switches in a robust environment could potentially provide macroscopic integrated functional systems as a result of their synchronized movements.
5. Conclusions
In the more than 50 years since the field of chemical topology (Frisch & Wasserman 1961) was defined, considerable advances have been made in the preparation of MIMs, not least of all in the area of template-directed synthesis. Be the synthetic strategy a stepwise one under kinetic control or an all-in-one reversible self-assembly process under thermodynamic control, supramolecular chemistry plays a significant role—through metal cation coordination, anion binding, hydrogen bonding, host–guest chemistry, radical–radical interactions and more—endowing MIMs with functionality that lives on well after their synthesis. Mechanostereochemistry—the study of MIMs and the non-covalent bonding interactions which define their properties—has led to the development of molecular switches and machines that show great promise in the bottom-up nanofabrication of devices. While the majority of work into the characterization of dynamic MIMs has been carried out in solution, it is clear that many applications result from taking MIMs out of solution and onto surfaces, into interfaces, onto nanoparticles and, more recently, into well-ordered solid-state materials such as MOFs. With their potential starting to be realized in device settings, and their synthesis continuing to be revolutionized by newly discovered methods of templation, MIMs and the field of mechanostereochemistry are beginning to reveal not only the wonders, but also the promise of the mechanical bond in chemistry and molecular nanotechnology.
Acknowledgements
During the writing of this article, G.B. and J.F.S. were visitors to the Korea Advanced Institute of Science and Technology (KAIST) under the auspices of the World Class University (WCU) Program (NRF R-31-2008-000-10055-0) funded by the Ministry of Education, Science and Technology in Korea. G.B. was supported by a Fellowship from the Non-Equilibrium Research Center (NERC) which is an Energy Frontier Research Center (EFRC) funded by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under award no. DE-SC0000989. He also thanks the International Center for Diffraction Data for the award of a 2012 Ludo Frevel Crystallography Scholarship.
References
- Adams C. C. 1994. The knot book. New York, NY: Freeman [Google Scholar]
- Amabilino D. B., Ashton P. R., Boyd S. E., Lee J. Y., Menzer S., Stoddart J. F., Williams D. J. 1997. The five-stage self-assembly of a branched heptacatenane. Angew. Chem. Int. Ed. Engl. 36, 2070–2072 10.1002/anie.199720701 (doi:10.1002/anie.199720701) [DOI] [Google Scholar]
- Ambrogio M. W., Thomas C. R., Zhao Y.-L., Zink J. I., Stoddart J. F. 2011. Mechanized silica nanoparticles: a new frontier in theranostic nanomedicine. Acc. Chem. Res. 44, 903–913 10.1021/ar200018x (doi:10.1021/ar200018x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrievsky A., Ahuis F., Sessler J. L., Vögtle F., Gudat D., Moini M. 1998. Bipyrrole-based [2]catenane: a new type of anion receptor. J. Am. Chem. Soc. 120, 9712–9713 10.1021/ja980755u (doi:10.1021/ja980755u) [DOI] [Google Scholar]
- Angelos S., Yang Y.-W., Patel K., Stoddart J. F., Zink J. I. 2008. pH-responsive supramolecular nanovalves based on cucurbit[6]uril pseudorotaxanes. Angew. Chem. Int. Ed. 47, 2222–2226 10.1002/anie.200705211 (doi:10.1002/anie.200705211) [DOI] [PubMed] [Google Scholar]
- Arduini A., Calzavacca F., Pochini A., Secchi A. 2003. Unidirectional threading of triphenylureidocalix[6]arene-based wheels: oriented pseudorotaxane synthesis. Chem. Eur. J. 9, 793–799 10.1002/chem.200390089 (doi:10.1002/chem.200390089) [DOI] [PubMed] [Google Scholar]
- Arduini A., et al. 2009. Towards controlling the threading direction of a calix[6]arene wheel by using nonsymmetric axles. Chem. Eur. J. 15, 3230–3242 10.1002/chem.200801926 (doi:10.1002/chem.200801926) [DOI] [PubMed] [Google Scholar]
- Armspach D., Ashton P. R., Moore C. P., Spencer N., Stoddart J. F., Wear T. J., Williams D. J. 1993. The self-assembly of catenated cyclodextrins. Angew. Chem. Int. Ed. Engl. 32, 854–858 10.1002/anie.199308541 (doi:10.1002/anie.199308541) [DOI] [Google Scholar]
- Asakawa M., et al. 1998. A chemically and electrochemically switchable [2]catenane incorporating a tetrathiafulvalene unit. Angew. Chem. Int. Ed. 37, 333–337 (doi:10.1002/(SICI)1521-3773(19980216)37:3<333::AID-ANIE333>3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- Ashton P. R., Goodnow T. T., Kaifer A. E., Reddington M. V., Slawin A. M. Z., Spencer N., Stoddart J. F., Vicent C., Williams D. J. 1989. A [2]catenane made to order. Angew. Chem. Int. Ed. Engl. 28, 1396–1399 10.1002/anie.198913961 (doi:10.1002/anie.198913961) [DOI] [Google Scholar]
- Ashton P. R., Philp D., Reddington M. V., Slawin A. M. Z., Spencer N., Stoddart J. F., Williams D. J. 1991. The self-assembly of complexes with [2]pseudorotaxane superstructures. J. Chem. Soc. Chem. Commun. 1991, 1680–1683 10.1039/c39910001680 (doi:10.1039/c39910001680) [DOI] [Google Scholar]
- Ashton P. R., et al. 1995. Dialkylammonium ion/crown ether complexes: the forerunners of a new family of interlocked molecules. Angew. Chem. Int. Ed. Engl. 34, 1865–1869 10.1002/anie.199518651 (doi:10.1002/anie.199518651) [DOI] [Google Scholar]
- Aucagne V., Hänni K. D., Leigh D. A., Lusby P. J., Walker D. B. 2006. Catalytic click rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128, 2186–2187 10.1021/ja056903f (doi:10.1021/ja056903f) [DOI] [PubMed] [Google Scholar]
- Au-Yeung H. Y., Pantoş G. D., Sanders J. K. M. 2009. Dynamic combinatorial synthesis of a catenane based on donor–acceptor interactions in water. In Proc. Natl Acad. Sci. USA 106, 10466–10470 10.1073/pnas.0809934106 (doi:10.1073/pnas.0809934106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzani V., Credi A., Venturi M. 2008. Molecular devices and machines. Weinheim, Germany: Wiley VCH [Google Scholar]
- Barin G., Coskun A., Fouda M. M. G., Stoddart J. F. 2012. Mechanically interlocked molecules assembled by π–π recognition. ChemPlusChem. 77, 159–185 10.1002/cplu.201100075 (doi:10.1002/cplu.201100075) [DOI] [Google Scholar]
- Basu S., et al. 2011. Donor–acceptor oligorotaxanes made to order. Chem. Eur. J. 17, 2107–2119 10.1002/chem.201001822 (doi:10.1002/chem.201001822) [DOI] [PubMed] [Google Scholar]
- Belowich M. E., Valente C., Stoddart J. F. 2010. Template-directed syntheses of rigid oligorotaxanes under thermodynamic control. Angew. Chem. Int. Ed. 49, 7208–7212 10.1002/anie.201004304 (doi:10.1002/anie.201004304) [DOI] [PubMed] [Google Scholar]
- Belowich M. E., Valente C., Smaldone R. A., Friedman D. C., Thiel J., Cronin L., Stoddart J. F. 2012. Positive cooperativity in the template-directed synthesis of monodisperse macromolecules. J. Am. Chem. Soc. 134, 5243–5261 10.1021/ja2107564 (doi:10.1021/ja2107564) [DOI] [PubMed] [Google Scholar]
- Berná J., Leigh D. A., Lubomska M., Mendoza S. M., Perez E. M., Rudolf P., Teobaldi G., Zerbetto F. 2005. Macroscopic transport by synthetic molecular machines. Nat. Mater. 4, 704–710 10.1038/Nmat1455 (doi:10.1038/Nmat1455) [DOI] [PubMed] [Google Scholar]
- Bianchi A., Bowman-James K., Garcia-Espana E. 1997. Supramolecular chemistry of anions. New York, NY: Wiley-VCH [Google Scholar]
- Bissell R. A., Córdova E., Kaifer A. E., Stoddart J. F. 1994. A chemically and electrochemically switchable molecular shuttle. Nature. 369, 133–137 10.1038/369133a0 (doi:10.1038/369133a0) [DOI] [Google Scholar]
- Blanco V., Chas M., Abella D., Peinador C., Quintela J. M. 2007. Molecular catenation via metal-directed self-assembly and π-donor/π-acceptor interactions: efficient one-pot synthesis, characterization, and crystal structures of [3]catenanes based on Pd or Pt dinuclear metallocycles. J. Am. Chem. Soc. 129, 13978–13986 10.1021/ja074721a (doi:10.1021/ja074721a) [DOI] [PubMed] [Google Scholar]
- Cantrill S. J., Pease A. R., Stoddart J. F. 2000. A molecular meccano kit. J. Chem. Soc. Dalton Trans. 2000, 3715–3734 10.1039/b003769i (doi:10.1039/b003769i) [DOI] [Google Scholar]
- Cesario M., Dietrich-Buchecker C. O., Guilhem J., Pascard C., Sauvage J.-P. 1985. Molecular-structure of a catenand and its copper(I) catenate—complete rearrangement of the interlocked macrocyclic ligands by complexation. J. Chem. Soc. Chem. Commun. 1985, 244–247 10.1039/c39850000244 (doi:10.1039/c39850000244) [DOI] [Google Scholar]
- Chichak K. S., Cantrill S. J., Pease A. R., Chiu S.-H., Cave G. W. V., Atwood J. L., Stoddart J. F. 2004. Molecular Borromean rings. Science. 304, 1308–1312 10.1126/science.1096914 (doi:10.1126/science.1096914) [DOI] [PubMed] [Google Scholar]
- Choi J. W., et al. 2006. Ground-state equilibrium thermodynamics and switching kinetics of bistable [2]rotaxanes switched in solution, polymer gels, and molecular electronic devices. Chem. Eur. J. 12, 261–279 10.1002/chem.200500934 (doi:10.1002/chem.200500934) [DOI] [PubMed] [Google Scholar]
- Coskun A., et al. 2011. Mechanically stabilized tetrathiafulvalene radical dimers. J. Am. Chem. Soc. 133, 4538–4547 10.1021/ja110584c (doi:10.1021/ja110584c) [DOI] [PubMed] [Google Scholar]
- Coskun A., Banaszak M., Astumian R. D., Stoddart J. F., Grzybowski B. A. 2012. Great expectations: Can artificial molecular machines deliver on their promise?. Chem. Soc. Rev. 41, 19–30 10.1039/c1cs15262a (doi:10.1039/c1cs15262a) [DOI] [PubMed] [Google Scholar]
- Cotí K. K., Belowich M. E., Liong M., Ambrogio M. W., Lau Y. A., Khatib H. A., Zink J. I., Khashab N. M., Stoddart J. F. 2009. Mechanised nanoparticles for drug delivery. Nanoscale. 1, 16–39 10.1039/b9nr00162j (doi:10.1039/b9nr00162j) [DOI] [PubMed] [Google Scholar]
- Cougnon F. B. L., Au-Yeung H. Y., Pantoş G. D., Sanders J. K. M. 2011. Exploring the formation pathways of donor–acceptor catenanes in aqueous dynamic combinatorial libraries. J. Am. Chem. Soc. 133, 3198–3207 10.1021/ja111407m (doi:10.1021/ja111407m) [DOI] [PubMed] [Google Scholar]
- Cougnon F. B. L., Jenkins N. A., Pantoş G. D., Sanders J. K. M. 2012. Templated dynamic synthesis of a [3]catenane. Angew. Chem. Int. Ed. 51, 1443–1447 10.1002/anie.201106885 (doi:10.1002/anie.201106885) [DOI] [PubMed] [Google Scholar]
- Cram D. J. 1988. The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew. Chem. Int. Ed. Engl. 27, 1009–1020 10.1002/anie.198810093 (doi:10.1002/anie.198810093) [DOI] [Google Scholar]
- Crowley J. D., Goldup S. M., Lee A.-L., Leigh D. A., McBurney R. T. 2009. Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 38, 1530–1541 10.1002/anie.198810093 (doi:10.1002/anie.198810093) [DOI] [PubMed] [Google Scholar]
- Dean F. B., Stasiak A., Koller T., Cozzarelli N. R. 1985. Duplex DNA knots produced by Escherichia coli topoisomerase-I: structure and requirements for formation. J. Biol. Chem. 260, 4975–4983 [PubMed] [Google Scholar]
- Deng H., Olson M. A., Stoddart J. F., Yaghi O. M. 2010. Robust dynamics. Nature Chem. 2, 439–443 10.1038/nchem.654 (doi:10.1038/nchem.654) [DOI] [PubMed] [Google Scholar]
- Diederich F., Stang P. J. 1999. Templated organic synthesis. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Dietrich-Buchecker C. O., Sauvage J.-P. 1989. A synthetic molecular Trefoil knot. Angew. Chem. Int. Ed. Engl. 28, 189–192 10.1002/anie.198901891 (doi:10.1002/anie.198901891) [DOI] [Google Scholar]
- Dietrich-Buchecker C., Sauvage J.-P., Kintzinger J.-P. 1983. Une nouvelle famille de molecules: Les metallo-catenane. Tetrahedron Lett. 24, 5095–5098 10.1016/S0040-4039(00)94050-4 (doi:10.1016/S0040-4039(00)94050-4) [DOI] [Google Scholar]
- Dietrich-Buchecker C. O., Guilhem J., Pascard C., Sauvage J.-P. 1990. Structure of a synthetic Trefoil knot coordinated to two copper(I) centers. Angew. Chem. Int. Ed. Engl. 29, 1154–1156 10.1002/anie.199011541 (doi:10.1002/anie.199011541) [DOI] [Google Scholar]
- Dietrich-Buchecker C., Colasson B. X., Sauvage J.-P. 2005. Molecular knots. Top. Curr. Chem. 249, 261–283 10.1007/b104331 (doi:10.1007/b104331) [DOI] [Google Scholar]
- Dodziuk H. 2006. Cyclodextrins and their complexes. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Du S. M., Stollar B. D., Seeman N. C. 1995. A synthetic DNA molecule in three knotted topologies. J. Am. Chem. Soc. 117, 1194–1200 10.1021/ja00109a002 (doi:10.1021/ja00109a002) [DOI] [Google Scholar]
- Durot S., Reviriego F., Sauvage J. P. 2010. Copper-complexed catenanes and rotaxanes in motion: 15 years of molecular machines. Dalton Trans. 39, 10557–10570 10.1039/c0dt00457j (doi:10.1039/c0dt00457j) [DOI] [PubMed] [Google Scholar]
- Fahrenbach A. C., et al. 2012. Solution-phase mechanistic study and solid-state structure of a tris(bipyridinium radical cation) inclusion complex. J. Am. Chem. Soc. 134, 3061–3072 10.1021/ja2089603 (doi:10.1021/ja2089603) [DOI] [PubMed] [Google Scholar]
- Faiz J. A., Heitz V., Sauvage J.-P. 2009. Design and synthesis of porphyrin-containing catenanes and rotaxanes. Chem. Soc. Rev. 38, 422–442 10.1039/b710908n (doi:10.1039/b710908n) [DOI] [PubMed] [Google Scholar]
- Ferris D. P., Zhao Y.-L., Khashab N. M., Khatib H. A., Stoddart J. F., Zink J. I. 2009. Light-operated mechanized nanoparticles. J. Am. Chem. Soc. 131, 1686–1688 10.1021/ja807798g (doi:10.1021/ja807798g) [DOI] [PubMed] [Google Scholar]
- Forgan R. S., Sauvage J.-P., Stoddart J. F. 2011. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111, 5434–5464 10.1021/cr200034u (doi:10.1021/cr200034u) [DOI] [PubMed] [Google Scholar]
- Frisch H. L., Wasserman E. 1961. Chemical topology. J. Am. Chem. Soc. 83, 3789–3974 10.1021/ja01479a015 (doi:10.1021/ja01479a015) [DOI] [Google Scholar]
- Fujita M. 1999. Self-assembly of [2]catenanes containing metals in their backbones. Acc. Chem. Res. 32, 53–61 10.1021/ar9701068 (doi:10.1021/ar9701068) [DOI] [Google Scholar]
- Fujita M., Ibukuro F., Hagihara H., Ogura K. 1994. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature. 367, 720–723 10.1038/367720a0 (doi:10.1038/367720a0) [DOI] [Google Scholar]
- Fujita M., Ibukuro F., Yamaguchi K., Ogura K. 1995. A molecular lock. J. Am. Chem. Soc. 117, 4175–4176 10.1021/ja00119a036 (doi:10.1021/ja00119a036) [DOI] [Google Scholar]
- Fujita M., Fujita N., Ogura K., Yamaguchi K. 1999. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature. 400, 52–55 10.1038/21861 (doi:10.1038/21861) [DOI] [Google Scholar]
- Fujita M., Tominaga M., Hori A., Therrien B. 2005. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 38, 369–378 10.1021/ar040153h (doi:10.1021/ar040153h) [DOI] [PubMed] [Google Scholar]
- Gassensmith J. J., Matthys S., Lee J.-J., Wojcik A., Kamat P. V., Smith B. D. 2010. Squaraine rotaxane as a reversible optical chloride sensor. Chem. Eur. J. 16, 2916–2921 10.1002/chem.200902547 (doi:10.1002/chem.200902547) [DOI] [PubMed] [Google Scholar]
- Gellert M. 1981. DNA topoisomerases. Annu. Rev. Biochem. 50, 879–910 [DOI] [PubMed] [Google Scholar]
- Glink P. T., Oliva A. I., Stoddart J. F., White A. J. P., Williams D. J. 2001. Template-directed synthesis of a [2]rotaxane by the clipping under thermodynamic control of a crown ether like macrocycle around a dialkylammonium ion. Angew. Chem. Int. Ed. 40, 1870–1875 (doi:10.1002/1521-3773(20010518)40:10<1870::aid-anie1870>3.3.co;2-q) [DOI] [PubMed] [Google Scholar]
- Gong H.-Y., Rambo B. M., Cho W., Lynch V. M., Oh M., Sessler J. L. 2011. Anion-directed assembly of a three-dimensional metal–organic rotaxane framework. Chem. Commun. 47, 5973–5975 10.1039/C1CC10272A (doi:10.1039/C1CC10272A) [DOI] [PubMed] [Google Scholar]
- Green J. E., et al. 2007. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature. 445, 414–417 10.1038/nature05462 (doi:10.1038/nature05462) [DOI] [PubMed] [Google Scholar]
- Griffiths K. E., Stoddart J. F. 2008. Template-directed synthesis of donor/acceptor [2]catenanes and [2]rotaxanes. Pure Appl. Chem. 80, 485–506 10.1351/pac200880030485 (doi:10.1351/pac200880030485) [DOI] [Google Scholar]
- Hamilton D. G., Davies J. E., Prodi L., Sanders J. K. M. 1998. Synthesis, structure and photophysics of neutral π-associated [2]catenanes. Chem. Eur. J. 4, 608–620 (doi:10.1002/(SICI)1521-3765(19980416)4:4<608::AID-CHEM608>3.3.CO;2-3) [DOI] [Google Scholar]
- Harrison I. T., Harrison S. 1967. Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89, 5723–5724 10.1021/ja00998a052 (doi:10.1021/ja00998a052) [DOI] [Google Scholar]
- Hoffart D. J., Loeb S. J. 2005. Metal–organic rotaxane frameworks: three-dimensional polyrotaxanes from lanthanide-ion nodes, pyridinium N-oxide axles, and crown ether wheels. Angew. Chem. Int. Ed. 44, 901–904 10.1002/anie.200461707 (doi:10.1002/anie.200461707) [DOI] [PubMed] [Google Scholar]
- Hübner G. M., Gläser J., Seel C., Vögtle F. 1999. High-yielding rotaxane synthesis with an anion template. Angew. Chem. Int. Ed. 38, 383–386 (doi:10.1002/(SICI)1521-3773(19990201)38:3<383::AID-ANIE383>3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- Hunter C. A. 1992. Synthesis and structure elucidation of a new [2]catenane. J. Am. Chem. Soc. 114, 5303–5311 10.1021/ja00039a047 (doi:10.1021/ja00039a047) [DOI] [Google Scholar]
- Jeon W. S., Kim H.-J., Lee C., Kim K. 2002. Control of the stoichiometry in host–guest complexation by redox chemistry of guests: inclusion of methylviologen in cucurbit[8]uril. Chem. Commun. 2002, 1828–1829 10.1039/B202082C (doi:10.1039/B202082C) [DOI] [PubMed] [Google Scholar]
- Johnston A. G., Leigh D. A., Pritchard R. J., Deegan M. D. 1995. Facile synthesis and solid-state structure of a benzylic amide [2]catenane. Angew. Chem. Int. Ed. Engl. 34, 1209–1212 10.1002/anie.199512091 (doi:10.1002/anie.199512091) [DOI] [Google Scholar]
- Johnston A. G., Leigh D. A., Murphy A., Smart J. P., Deegan M. D. 1996. The synthesis and solubilization of amide macrocycles via rotaxane formation. J. Am. Chem. Soc. 118, 10662–10663 10.1021/ja962046r (doi:10.1021/ja962046r) [DOI] [Google Scholar]
- Juluri B. K., et al. 2009. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano. 3, 291–300 10.1021/nn8002373 (doi:10.1021/nn8002373) [DOI] [PubMed] [Google Scholar]
- Kay E. R., Leigh D. A. 2005. Hydrogen bond-assembled synthetic molecular motors and machines. Top. Curr. Chem. 262, 133–177 10.1007/128_011 (doi:10.1007/128_011) [DOI] [Google Scholar]
- Kay E. R., Leigh D. A., Zerbetto F. 2007. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 10.1002/anie.200504313 (doi:10.1002/anie.200504313) [DOI] [PubMed] [Google Scholar]
- Kilah N. L., Wise M. D., Serpell C. J., Thompson A. L., White N. G., Christensen K. E., Beer P. D. 2010. Enhancement of anion recognition exhibited by a halogen-bonding rotaxane host system. J. Am. Chem. Soc. 132, 11893–11895 10.1021/ja105263q (doi:10.1021/ja105263q) [DOI] [PubMed] [Google Scholar]
- Kim K. 2002. Mechanically interlocked molecules incorporating cucurbituril and their supramolecular assemblies. Chem. Soc. Rev. 31, 96–107 10.1039/a900939f (doi:10.1039/a900939f) [DOI] [PubMed] [Google Scholar]
- Kolchinski A. G., Busch D. H., Alcock N. W. 1995. Gaining control over molecular threading—benefits of 2nd coordination sites and aqueous-organic interfaces in rotaxane synthesis. J. Chem. Soc. Chem. Commun. 1995, 1289–1291 10.1039/c39950001289 (doi:10.1039/c39950001289) [DOI] [Google Scholar]
- Langmuir I. 1919. The arrangement of electrons in atoms and molecules. J. Am. Chem. Soc. 41, 868–934 10.1021/ja02227a002 (doi:10.1021/ja02227a002) [DOI] [Google Scholar]
- Lehn J.-M. 1995. Supramolecular chemistry: concepts and perspectives. Weinheim, Germany: Wiley VCH [Google Scholar]
- Lehn J.-M. 1988. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices. Angew. Chem. Int. Ed. Engl. 27, 89–112 10.1002/anie.198800891 (doi:10.1002/anie.198800891) [DOI] [Google Scholar]
- Leigh D. A., Lusby P. J., Teat S. J., Wilson A. J., Wong J. K. Y. 2001. Benzylic imine catenates: readily accessible octahedral analogues of the Sauvage catenates. Angew. Chem. Int. Ed. 40, 1538–1543 (doi:10.1002/1521-3757(20010417)113:8<1586::AID-ANGE1586>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- Leininger S., Olenyuk B., Stang P. J. 2000. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev. 100, 853–907 10.1021/cr9601324 (doi:10.1021/cr9601324) [DOI] [PubMed] [Google Scholar]
- Lewis G. N. 1916. The atom and the molecule. J. Am. Chem. Soc. 38, 762–785 10.1021/ja02261a002 (doi:10.1021/ja02261a002) [DOI] [Google Scholar]
- Li Q., Zhang W., Miljanić O. Š., Sue C.-H., Zhao Y.-L., Liu L., Knobler C. B., Stoddart J. F., Yaghi O. M. 2009. Docking in metal-organic frameworks. Science. 325, 855–859 10.1126/science.1175441 (doi:10.1126/science.1175441) [DOI] [PubMed] [Google Scholar]
- Li H., Fahrenbach A. C., Dey S. K., Basu S., Trabolsi A., Zhu Z., Botros Y. Y., Stoddart J. F. 2010a Mechanical bond formation by radical templation. Angew. Chem. Int. Ed. 49, 8260–8265 10.1002/anie.201004488 (doi:10.1002/anie.201004488) [DOI] [PubMed] [Google Scholar]
- Li Q., et al. 2010b A catenated strut in a catenated metal–organic framework. Angew. Chem. and Int. Ed. 49, 6751–6755 10.1002/anie.201003221 (doi:10.1002/anie.201003221) [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang W., Miljanić O. Š., Knobler C. B., Stoddart J. F., Yaghi O. M. 2010c A metal-organic framework replete with ordered donor–acceptor catenanes. Chem. Commun. 46, 380–382 10.1039/b919923c (doi:10.1039/b919923c) [DOI] [PubMed] [Google Scholar]
- Li Z., Barnes J. C., Bosoy A., Stoddart J. F., Zink J. I. 2012. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 41, 2590–2605 10.1039/c1cs15246g (doi:10.1039/c1cs15246g) [DOI] [PubMed] [Google Scholar]
- Liu Y., et al. 2005. Linear artificial molecular muscles. J. Am. Chem. Soc. 127, 9745–9759 10.1021/ja051088p (doi:10.1021/ja051088p) [DOI] [PubMed] [Google Scholar]
- Loeb S. J. 2005. Metal-organic rotaxane frameworks; MORFs. Chem. Commun. 2005, 1511–1518 10.1039/b416609d (doi:10.1039/b416609d) [DOI] [PubMed] [Google Scholar]
- Lukin O., Vögtle F. 2005. Knotting and threading of molecules: chemistry and chirality of molecular knots and their assemblies. Angew. Chem. Int. Ed. 44, 1456–1477 10.1002/anie.200460312 (doi:10.1002/anie.200460312) [DOI] [PubMed] [Google Scholar]
- Lüttringhaus A., Cramer F., Prinzbach H., Henglein F. M. 1958. Cyclisationen von langkettigen dithiolen—versuche zur darstellung sich umfassender ringe mit hilfe von einschlussyerbindungen. Leibigs Ann. Chem. 613, 185–198 10.1002/jlac.19586130120 (doi:10.1002/jlac.19586130120) [DOI] [Google Scholar]
- Mao C. D., Sun W. Q., Seeman N. C. 1997. Assembly of Borromean rings from DNA. Nature. 386, 137–138 10.1038/386137b0 (doi:10.1038/386137b0) [DOI] [PubMed] [Google Scholar]
- Meyer C. D., Forgan R. S., Chichak K. S., Peters A. J., Tangchaivang N., Cave G. W. V., Khan S. I., Cantrill S. J., Stoddart J. F. 2010. The dynamic chemistry of molecular Borromean rings and Solomon knots. Chem. Eur. J. 16, 12. 10.1002/chem.201001806 (doi:10.1002/chem.201001806) [DOI] [PubMed] [Google Scholar]
- Nepogodiev S. A., Stoddart J. F. 1998. Cyclodextrin-based catenanes and rotaxanes. Chem. Rev. 98, 1959–1976 10.1021/cr970049w (doi:10.1021/cr970049w) [DOI] [PubMed] [Google Scholar]
- Nguyen T. D., Tseng H.-R., Celestre P. C., Flood A. H., Liu Y., Stoddart J. F., Zink J. I. 2005. A reversible molecular valve. In Proc. Natl Acad. Sci. USA 102, 10029–10034 10.1073/pnas.0504109102 (doi:10.1073/pnas.0504109102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierengarten J.-F., Dietrich-Buchecker C. O., Sauvage J.-P. 1994. Synthesis of a doubly interlocked [2]catenane. J. Am. Chem. Soc. 116, 375–376 10.1021/ja00080a045 (doi:10.1021/ja00080a045) [DOI] [PubMed] [Google Scholar]
- Ogino H. 1981. Relatively high-yield syntheses of rotaxanes—syntheses and properties of compounds consisting of cyclodextrins threaded by α,ω-diaminoalkanes coordinated to cobalt(III) complexes. J. Am. Chem. Soc. 103, 1303–1304 10.1021/ja00395a091 (doi:10.1021/ja00395a091) [DOI] [Google Scholar]
- Olson M. A., Botros Y. Y., Stoddart J. F. 2010. Mechanostereochemistry. Pure Appl. Chem. 82, 1569–1574 10.1351/PAC-CON-10-02-09 (doi:10.1351/PAC-CON-10-02-09) [DOI] [Google Scholar]
- Patel K., Angelos S., Dichtel W. R., Coskun A., Yang Y.-W., Zink J. I., Stoddart J. F. 2008. Enzyme-responsive snap-top covered silica nanocontainers. J. Am. Chem. Soc. 130, 2382–2383 10.1021/ja0772086 (doi:10.1021/ja0772086) [DOI] [PubMed] [Google Scholar]
- Pedersen C. J. 1988. The discovery of crown ethers (Nobel lecture). Angew. Chem. Int. Ed. Engl. 27, 1021–1027 10.1002/anie.198810211 (doi:10.1002/anie.198810211) [DOI] [Google Scholar]
- Peinador C., Blanco V., Quintela J. M. 2009. A new doubly-interlocked [2]catenane. J. Am. Chem. Soc. 131, 920–921 10.1021/ja8088372 (doi:10.1021/ja8088372) [DOI] [PubMed] [Google Scholar]
- Raymo F. M., Bartberger M. D., Houk K. N., Stoddart J. F. 2001. The magnitude of [C–H···O] hydrogen bonding in molecular and supramolecular assemblies. J. Am. Chem. Soc. 123, 9264–9267 10.1021/ja010443i (doi:10.1021/ja010443i) [DOI] [PubMed] [Google Scholar]
- Rolfsen D. 1976. Knots and links. Berkeley, CA: Publish or Perish Inc [Google Scholar]
- Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B. 2002. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 10. 10.1002/1521-3773(0715)41:14%3C2596::AID-ANIE2596%3E3.0.CO;2-4 (doi:10.1002/1521-3773(0715)41:14<2596::AID-ANIE2596>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- Sambrook M. R., Beer P. D., Wisner J. A., Paul R. L., Cowley A. R. 2004. Anion-templated assembly of a [2]catenane. J. Am. Chem. Soc. 126, 15364–15365 10.1021/ja045080b (doi:10.1021/ja045080b) [DOI] [PubMed] [Google Scholar]
- Sauvage J.-P., Dietrich-Buchecker C. 1999. Catenanes, rotaxanes, and knots—a journey through the world of molecular topology. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Schalley C. A., Weilandt T., Brüggemann J., Vögtle F. 2004. Hydrogen-bond-mediated template synthesis of rotaxanes, catenanes, and knotanes. Top. Curr. Chem. 248, 141–200 10.1007/b99913 (doi:10.1007/b99913) [DOI] [Google Scholar]
- Schill G. 1971. Catenanes, rotaxanes and knots. New York, NY: Academic Press [Google Scholar]
- Schill G., Lüttringhaus A. 1964. Preparation of catena compounds by directed synthesis (1). Angew. Chem. Int. Ed. 3, 546–547 10.1002/anie.196405461 (doi:10.1002/anie.196405461) [DOI] [Google Scholar]
- Schill G., Zollenkopf H. 1969. Rotaxane compounds (1). Leibigs Ann. Chem. 721, 53–74 10.1002/jlac.19697210109 (doi:10.1002/jlac.19697210109) [DOI] [Google Scholar]
- Seeman N. C. 1998. Nucleic acid nanostructures and topology. Angew. Chem. Int. Ed. 37, 3220–3238 (doi:10.1002/(SICI)1521-3773(19981217)37:23<3220::AID-ANIE3220>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- Sierocki P., Maas H., Dragut P., Richardt G., Vögtle F., De Cola L., Brouwer F., Zink J. I. 2006. Photoisomerization of azobenzene derivatives in nanostructured silica. J. Phys. Chem. B. 110, 24390–24398 10.1021/jp0641334 (doi:10.1021/jp0641334) [DOI] [PubMed] [Google Scholar]
- Spruell J. M. 2010. Molecular recognition and switching via radical dimerization. Pure Appl. Chem. 82, 2281–2294 10.1351/pac-con-10-08-03 (doi:10.1351/pac-con-10-08-03) [DOI] [Google Scholar]
- Spruell J. M., et al. 2010. Highly stable tetrathiafulvalene radical dimers in [3]catenanes. Nat. Chem. 2, 870–879 10.1038/nchem.749 (doi:10.1038/nchem.749) [DOI] [PubMed] [Google Scholar]
- Stanier C. A., O'Connell M. J., Clegg W., Anderson H. L. 2001. Synthesis of fluorescent stilbene and tolan rotaxanes by Suzuki coupling. Chem. Commun. 2001, 493–494 10.1039/b010015n (doi:10.1039/b010015n) [DOI] [Google Scholar]
- Stoddart J. F. 2009. The chemistry of the mechanical bond. Chem. Soc. Rev. 38, 1802–1820 10.1039/b819333a (doi:10.1039/b819333a) [DOI] [PubMed] [Google Scholar]
- Strutt N. L., Forgan R. S., Spruell J. M., Botros Y. Y., Stoddart J. F. 2011. Monofunctionalized pillar[5]arene as a host for alkanediamines. J. Am. Chem. Soc. 133, 5668–5671 10.1021/ja111418j (doi:10.1021/ja111418j) [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Lin K. 2003. Protein knots: a tangled problem. Nature. 421, 25. 10.1038/421025a (doi:10.1038/421025a) [DOI] [PubMed] [Google Scholar]
- Taylor P. N., O'Connell M. J., McNeill L. A., Hall M. J., Aplin R. T., Anderson H. L. 2000. Insulated molecular wires: synthesis of conjugated polyrotaxanes by Suzuki coupling in water. Angew. Chem. Int. Ed. 39, 3456–3460 (doi:10.1002/1521-3757(20001002)112:19<3598::AID-ANGE3598>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- Trabolsi A., et al. 2010. Radically enhanced molecular recognition. Nat. Chem. 2, 42–49 10.1038/nchem.479 (doi:10.1038/nchem.479) [DOI] [PubMed] [Google Scholar]
- Vickers M. S., Beer P. D. 2007. Anion templated assembly of mechanically interlocked structures. Chem. Soc. Rev. 36, 211–225 10.1039/b518077p (doi:10.1039/b518077p) [DOI] [PubMed] [Google Scholar]
- Vögtle F., Meier S., Hoss R. 1992. One-step synthesis of a fourfold functionalized catenane. Angew. Chem. Int. Ed. Engl. 31, 1619–1622 10.1002/anie.199216191 (doi:10.1002/anie.199216191) [DOI] [Google Scholar]
- Wasserman E. 1960. The preparation of interlocking rings: a catenane. J. Am. Chem. Soc. 82, 4433–4434 10.1021/ja01501a082 (doi:10.1021/ja01501a082) [DOI] [Google Scholar]
- Wenz G., Han B. H., Müller A. 2006. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 106, 782–817 10.1021/cr970027%2B (doi:10.1021/cr970027+) [DOI] [PubMed] [Google Scholar]
- Wisner J. A., Beer P. D., Drew M. G. B., Sambrook M. R. 2002. Anion-templated rotaxane formation. J. Am. Chem. Soc. 124, 12469–12476 10.1021/ja027519a (doi:10.1021/ja027519a) [DOI] [PubMed] [Google Scholar]
- Zhang Y. W., Seeman N. C. 1994. Construction of a DNA-truncated octahedron. J. Am. Chem. Soc. 116, 1661–1669 10.1021/ja00084a006 (doi:10.1021/ja00084a006) [DOI] [Google Scholar]
- Zhang Z.-J., Zhang H.-Y., Wang H., Liu Y. 2011. A twin-axial hetero[7]rotaxane. Angew. Chem. Int. Ed. 50, 10834–10838 10.1002/anie.201105375 (doi:10.1002/anie.201105375) [DOI] [PubMed] [Google Scholar]
- Ziganshinsa A. Y., Ko Y. H., Jeon W. S., Kim K. 2004. Stable π-dimer of a tetrathiafulvalene cation radical encapsulated in the cavity of cucurbit[8]uril. Chem. Commun. 2004, 806–807 10.1039/b316651a (doi:10.1039/b316651a) [DOI] [PubMed] [Google Scholar]