Abstract
Proteolytic enzymes play a key role in the metastatic stage of gastric cancer (GC). In this study, we aimed to identify the serine proteases (SPs) and their inhibitors (serpins) as related to GC. The gene expression profiles of 40 cases of GC were initially detected by cDNA microarray. The results of the differentially expressed SPs and their inhibitor genes from the microarrays were confirmed by real-time PCR. The status of the immunohistochemical staining of the confirmed genes in patients with complete data was used to develop a survival prediction model. Finally, the prediction model was tested in different groups of GC patients. As a result, seven genes, SERPINB5, KLK10, KLK11, HPN, SPINK1, SERPINA5 and PRSS8, were considered as GC progression-related genes. A survival prediction model including the immunohistochemical scores of three genes and the tumor node metastasis (TNM) score was developed: Survival time (months) = 88.8607 + 2.6395 SERPINB5 − 12.0772 KLK10 + 13.7562 KLK11 − 7.0318 TNM. In conclusion, SERPINB5, KLK10, KLK11, HPN, SPINK1, SERPINA5 and PRSS8 were GC progression-related SPs or serpin genes. The model consisting of the expression profiles of three genes extracted from the microarray study accompanied by the TNM score accurately predicts surgery-related survival of GC patients.
Keywords: serine protease, serine protease inhibitor, gastric cancer, survival prediction model, microarray, immunohistochemistry
Introduction
There are 300,000 deaths and 400,000 new cases of gastric cancer in China every year (1). Gastric cancer (GC) is the fourth most common type of cancer worldwide. Although there has been a steady decline in the risk of GC incidence and mortality over several decades in most countries, it is the second most common cause of cancer-related death (700,000 deaths annually) (2). The current staging system for GC is inadequate for predicting the outcome of treatment.
The poor prognosis of patients with GC has been attributed mostly to metastases and relapse (3,4). Tumor cells utilize many cellular or biochemical mechanisms to complete metastatic spread, and proteolytic enzymes play a key role in the metastatic stage (5). Mammalian proteolytic enzymes are divided into five classes (aspartic, cysteine, metallo, serine and threonine), and the serine protease (SP) family is the largest (6). It is widely accepted that SPs degrade extracellular matrix and facilitate neoplastic progression. The urokinase plasminogen activator, one of the SPs, promotes tumor cell invasion (7) and the kallikrein prostate-specific antigen predicts prognosis of prostate cancer (8). KLK6 was found to be significantly up-regulated in tissues and sera from patients with colon cancer and was closely associated with a poor prognosis, suggesting that KLK6 may be used as a potential biomarker and a therapeutic target for colon cancer (9). SP inhibitors (serpins) are the largest family of protease inhibitors identified to date. Most serpins act as inhibitors of chymotrypsin-like SPs, others as cross-class serine protease inhibitors of papain-like cysteine proteases and caspases (10–13). Certain serpins have been identified to be involved in the progression of malignant tumor; these include SERPINB3 and SERPINB4 in squamous cell carcinoma (14,15), SERPINB5 in human breast cancer (16), SERPINB13 in head and neck squamous cell carcinoma (17), SERPINH1 in head and neck carcinomas (18), SERPINI2 in breast cancer (19), SERPINB2 and SERPINE1 in breast cancer and other cancers (20), and SERPINB2, SERPINB3, SERPINB4, SERPINB7, SERPINB11, SERPINB12 and SERPINB13 in oral squamous cell carcinoma (21).
Yet, which SPs and their inhibitors are associated with the progression of GC globally? Minimal research has been carried out concerning this issue. In the present study, we focused on SPs and serpins, the largest protease and protease inhibitor families, since they carry out diverse functions, including clotting, fibrinolytic cascades, complement activation, fertilization, pro-hormone conversion, apoptosis, extracellular matrix maintenance and remodeling, angiogenesis and tumor cell invasion (22,23). We aimed to compare the difference in gene expression profiles between cancerous and non-cancerous gastric tissues by cDNA microarrays, and to identify the SPs and serpins associated with the progression of GC. The results from the microarrays were confirmed by real-time PCR and immunohistochemistry (IHC). A survival prediction model was further developed using the IHC data.
Materials and methods
Patients and clinical features
Human GC tissues and their paired normal gastric tissues were obtained with informed consent from 140 patients who underwent radical resection for GC in 2000 and 2003 at the Department of Surgery, Ruijin Hospital, Shanghai, China. The corresponding non-cancerous gastric tissue was obtained at least 6 cm from the tumor. The diagnoses were confirmed by histopathology. Stage and grade were established using the tumor node metastases (TNM) and World Health Organization classification systems. For stage IIIB and stage IV by clinical staging, pre-operative chemotherapy was offered. All of the patients, except stage IA of surgical pathologic staging, received fluoropyrimidine-based chemoradiotherapy. All patients were followed up systematically. This follow-up included a complete history and physical examination every 4 months for 3 years, then annually thereafter, complete blood count, platelets, multichannel serum chemistry analysis and other investigations (such as endoscopy and other radiologic studies). Of these 140 patients, 100 were enrolled in 2000 and included in the IHC analyses; 40 were enrolled in 2003 and initially included in the microarray analyses. In total, 10 of the 40 patients in 2003 were later included in the real-time PCR analyses. In addition to the 10 patients, half of the 40 patients were involved in the IHC analyses.
The specimens were snap-frozen in liquid nitrogen immediately after resection and then stored at −80°C until use or fixed in 10% formalin and paraffin embedded by conventional techniques for IHC staining. The Ethics Committee of Ruijin Hospital approved the use of these tissues for research purposes.
RNA preparation
Total RNAs were extracted from each tissue sample using TRIzol reagent (Life Technologies, Grand Island, NY, USA) and further purified with an RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The quality of the total RNA samples was determined by electrophoresis through formaldehyde agarose gels, and the 18S and 28S RNA bands were visualized under ultraviolet light.
Microarray assays and statistical analysis
The cDNA microarray used in the present study consisted of 13,824 genes/ESTs; this microarray was the same as that used by Huang et al (24) and constructed by the National Engineering Center for Biochips at Shanghai (Shanghai, China). The microarray and the experimental procedure were confirmed to be feasible by previous studies (24,25). Thus, it was used instead of a new customized microarray containing SP and serpin genes only. The microarray contained 50 common SPs and serpin genes.
Total RNA (100 μg) was used to prepare fluorescent dye-labeled first-strand cDNA, using SuperScript™ II RNase H Reverse Transcriptase according to the relevant protocol (Invitrogen, Carlsbad, CA, USA). Cy3-dCTP and Cy5-dCTP (Amersham, Piscataway, NJ, USA) were used to label the GC specimens and the corresponding non-cancerous gastric specimen, respectively. The dye-labeled probes were purified by QIAquick Nucleotide Removal kit (Qiagen) according to the specified protocol. A total of 30 pmol of each probe was used in the two-color array hybridization. The hybridization and scanning procedures were identical as those described previously (24).
To avoid systematic error for each microarray sample, a space and intensity-dependent normalization based on a LOWESS program was employed (26) to normalize the logarithm transformed background corrected signal intensities for the dual-channel data. Thereafter, normalized data were withdrawn for each SP and serpin gene for each specimen. The difference in C (GC specimen) and N (corresponding non-cancerous gastric specimen) was estimated by the ln C-ln N and T test performed using SAS to compare the difference between the GC specimen and its corresponding non-cancerous gastric specimen transcript. All ratios were filtered using a P-value of ≤0.05.
Real-time PCR
Real-time PCR reactions were performed according to a previously reported protocol (27). The primer sequences were as follows: sense 5′-GTTCCAGACATTCT CGCTTC-3′ and anti-sense 5′-ATAGTAGCCTGAGCAT GTGC-3′ for SERPINB5 (107 bp); sense 5′-CCTGCTCCA GCATCACTATC-3′ and anti-sense 5′-GGTCCAGTCCAGC ACATATC-3′ for KLK10 (93 bp); sense 5′-AGCTCTCCAGC CTCATCATC-3′ and anti-sense 5′-CAACAGCCTTCTTCTG CATC-3′ for SERPINH1 (120 bp); sense 5′-CAGAAGTGTGA GAACGCCTAC-3′ and anti-sense 5′-CCTTGAAGAGA CTGGTTACAG-3′ for KLK11 (131 bp); sense 5′-GTCATCTC CGTGTGTGATTG-3′ and anti-sense 5′-TCATAGCGAA GGCTGACTTG-3′ for HPN (149 bp); sense 5′-AACGCCAG ACTTCTATCCTC-3′ and anti-sense 5′-CAACAATAAGGC CAGTCAGG-3′ for SPINK1 (102 bp); sense 5′-GATGAAGAA GAGAGTCGAGG-3′ and anti-sense 5′-GAAGAAGATGTT CTGGCTGG-3′ for SERPINA5 (124 bp); sense 5′-CAAGGAAG CCTATGAGGTCAAG-3′ and anti-sense 5′-TGAGTTGGA GGAGTGCAAT-3′ for PRSS8 (146 bp); sense 5′-AAGCAC TGTGCATCACCTTG-3′ and anti-sense 5′-CAGAGTTGG AGCACTTGCTG-3′ for TMPRSS2 (102 bp); sense 5′-GGA CCTGACCTGCCGTCTAG-3′ and anti-sense 5′-GTAGCC CAGGATGCCCTTGA-3′ for GAPDH (100 bp).
The results of the real-time PCR are presented as Ct values. The relative changes in gene expression were calculated by the ΔΔCt method (28).
Immunohistochemistry
IHC was performed according to our previously reported protocol (29). The primary antibodies used in the present study were Hepsin polyclonal antibody (Abcam, Cambridge, MA, USA), anti-human Kallikrein 11 antibody (R&D Systems, Minneapolis, MN, USA), goat polyclonal antibody against KLK10 (Santa Cruz, Santa Cruz, CA USA) and mouse monoclonal antibody to SERPINB5 (Novocastra, Newcastle-upon-Tyne, UK). Negative control slides were treated without the primary antibody under equivalent conditions. For the secondary developing reagents, EnVision™ System labeled Polymer-HRP/M/R (DakoCytomation, Glostrup, Denmark) and UltraSensitive™ S-P (Goat) kit (Maixin Bio, Fuzhou, China) were used. Slides were developed with diaminobenzidine (DAB; DakoCytomation) and counterstained with hematoxylin.
Pathologists without knowledge of the patient outcomes scored the immunostained slides independently as previously described (30). In brief, a proportion (proportion of positive-staining tumor cells) score (0, none; 1, <1/100; 2, 1/100-1/10; 3, 1/10-1/3; 4, 1/3-2/3; and 5, >2/3), and an intensity score (0, none; 1, weak; 2, intermediate; and 3, strong) were assigned. These two scores were then added to obtain a total score for each slide.
Statistical analysis
Results of the real-time PCR were evaluated by the Wilcoxon signed rank test. Associations between gene expression profiles as assessed by real-time PCR and IHC were analyzed using non-parametric Spearman rank correlation coefficients. Survival curves were computed with the Kaplan-Meier method and were compared using the log-rank test. A two-sided Fisher's exact test was used in univariate analysis of potential prognostic factors regarding overall survival. Stepwise regression analysis and the best subset regression were used to develop a prediction model of survival. A P-value <0.05 was taken as the level of significance. Statistical analyses were performed using the SAS 6.12 software (SAS Inc., Cary, NC, USA) or GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Selection and confirmation of the serine protease-related genes for the prediction model
Nine serpins or SP genes were determined to be differentially expressed by the microarray experiments. The gene list and fold changes are shown in Table I; it includes three up-regulated and six down-regulated genes. The up-regulated genes included two serpins (SERPINB5 and SERPINH1) and one SP (KLK10); the six down-regulated genes included two Serpins (SPINK1 and SERPINA5) and four SPs (KLK11, HPN, PRSS8 and TMPRSS2).
Table I.
Differentially expressed genes as determined by the microarray.
| Gene name | GeneBank no. | Fold changea |
|---|---|---|
| Up-regulated | ||
| KLK10 | NM_002776 | 7.181442890 |
| SERPINB5 | NM_002639 | 5.827452523 |
| SERPINH1 | NM_001235 | 4.313578371 |
| Down-regulated | ||
| TMPRSS2 | NM_005656 | 0.823943603 |
| PRSS8 | NM_002773 | 0.659704511 |
| HPN | NM_002151 | 0.527439252 |
| SERPINA5 | NM_000624 | 0.392424461 |
| SPINK1 | NM_003122 | 0.381989951 |
| KLK11 | NM_006853 | 0.193303929 |
GC specimen/the corresponding non-cancerous gastric specimen.
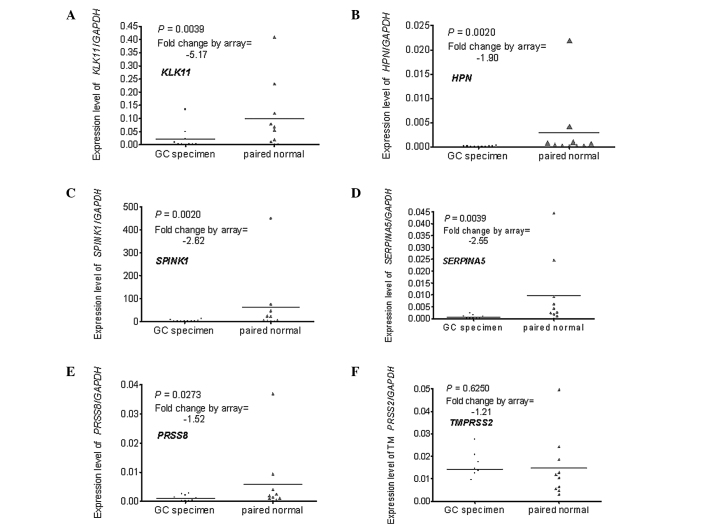
Real-time PCR was used to verify the differential expression of the genes selected by the microarray. A total of 10 pairs of RNA stock used in the real-time PCR were the same as those in the microarray, the remaining 30 had no remaining RNA or the RNA was of inadequate quality. The results of the real-time PCR were compared to those of the microarray. One of the three up-regulated genes (SERPINH1) was excluded as it was down-regulated in the real-time PCR assay (Fig. 1A–C). One of the six down-regulated genes (TMPRSS2) was excluded as the difference did not reach statistical significance (P=0.6250) (Fig. 2A–F).
Figure 1.
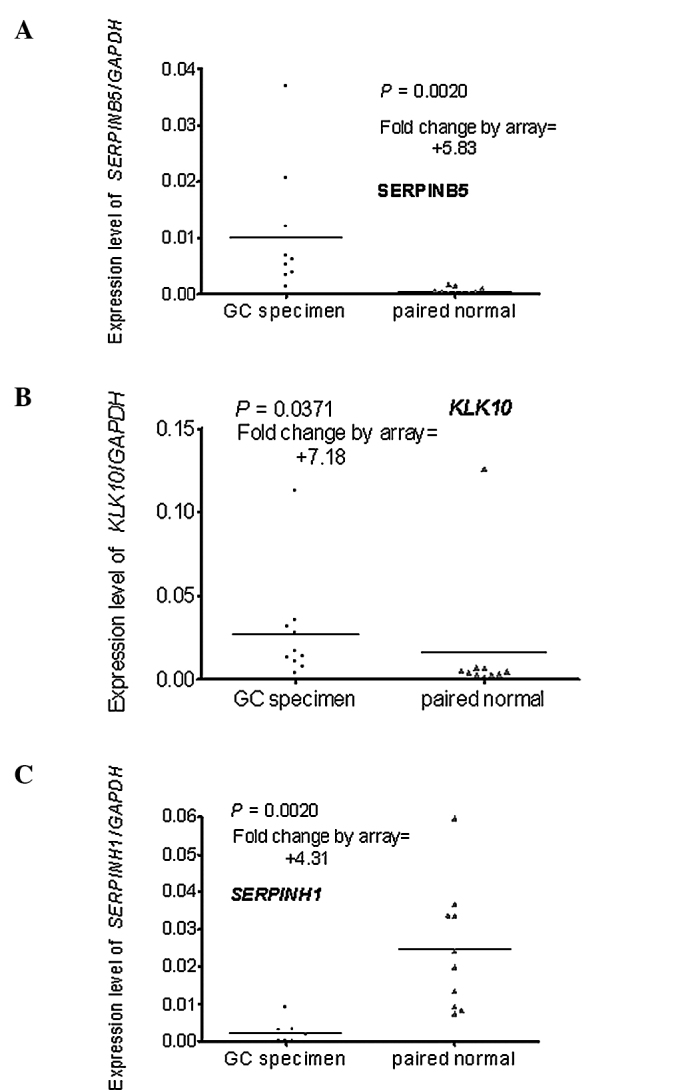
Expression profiles of the three up-regulated genes in the microarray assay as determined by real-time PCR. (A) SERPINB5 was up-regulated with a 5.83-fold change in the GC specimens by microarray and demonstrated the same expression profile upon real-time PCR. (B) KLK10 was up-regulated with a 7.18-fold change in the GC specimens by microarray and demonstrated the same expression profile upon real-time PCR. (C) SERPINH1 showed a contradictory expression profile between the microarray and real-time PCR.
Figure 2.
Expression profiles of the down-regulated genes in the microarray assay as determined by real-time PCR. (A) KLK11, (B) HPN, (C) SPINK1, (D) SERPINA5 and (E) PRSS8 were all down-regulated in the GC specimens by real-time PCR and exhibited the same expression profiles as the microarray. (F) TMPRSS2 was excluded as the difference did not reach statistical significance (P=0.6250) by real-time PCR.
Therefore, seven genes were further analyzed. Four genes (SERPINB5, KLK10, KLK11 and HPN), which exhibited the highest differential fold change and for which a primary antibody for IHC was commercially available, were included in the IHC assay.
Correlation of the RNA and protein expression profiles of SERPINB5, KLK10, KLK11 and HPN
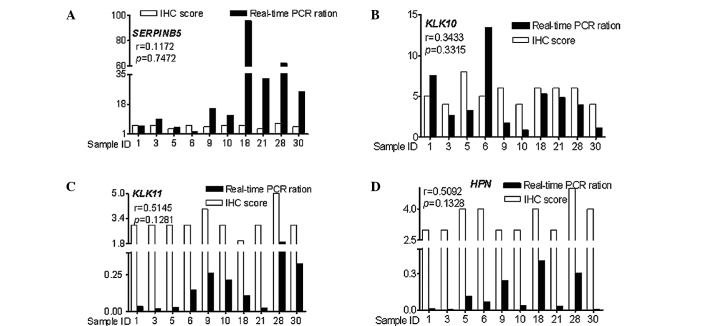
The expression profiles of the aforementioned four genes were assayed by real-time PCR and IHC. The RNA expression profiles were positively correlated with the protein expression profiles (r=0.1172 for SERPINB5, r= 0.3433 for KLK10, r= 0.5145 for KLK11, r=0.5092 for HPN), but the correlations did not reach statistical significance (P=0.7472 for SERPINB5, P=0.3315 for KLK10, P=0.1281 for KLK11, P=0.1328 for HPN) (Fig. 3).
Figure 3.
Correlation of the RNA and protein expression profiles of SERPINB5, KLK10, KLK11 and HPN. The RNA expression profiles were positively correlated with the protein expression profiles, yet the correlations did not reach statistical significance: (A) r=0.1172, P=0.7472 for SERPINB5; (B) r=0.3433, P=0.3315 for KLK10; (C) r=0.5145, P=0.1281 for KLK11; (D) r=0.5092, P=0.1328 for HPN.
Prediction of survival using the IHC score and clinical and pathological characteristics
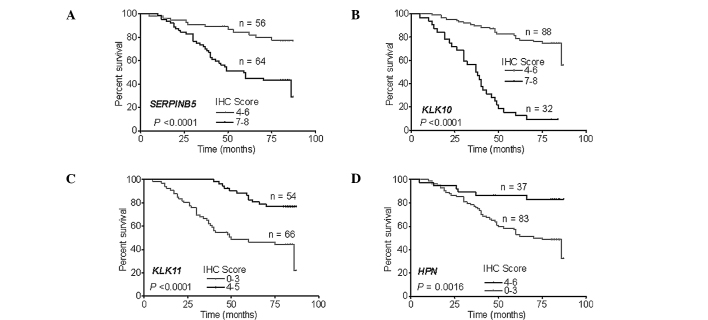
IHC was performed in 120 patients, including 100 patients who underwent GC surgery in 2000 and 20 patients in 2003 who were enrolled in the microarray. An IHC score <3 was recognized as negative, 4–6 as weakly positive and 7–8 as positive. SERPINB5 and KLK10 were positively expressed in all of the patients (IHC score >4), while the patients with weak positive expression of SERPINB5 and KLK10 had a more favorable prognosis (Fig. 4A and B). KLK11 and HPN were negatively expressed in most of the patients. The number of patients with negative expression of KLK11 and HPN was 66 and 83, respectively, and negative expression of these genes indicated a worse survival (Fig. 4C and D).
Figure 4.
The survival curves of the patients were assessed according to the IHC score. The survival rate of the entire test group of patients was divided according to the IHC scores of (A) SERPINB5 and (B) KLK10. Results showed that SERPINB5 and KLK10 were positively expressed in all patients and the patients with positive expression (IHC scores 7 and 8) exhibited a worse prognosis. The survival rate of the entire test group of patients was divided according to the IHC scores of (C) KLK11 and (D) HPN. KLK11 and HPN were negatively expressed in most of the patients, while the patients with negative expression of KLK11 and HPN demonstrated worse survival rates.
To elucidate the factors contributing to improved survival, an analysis was carried out to identify the prognostic factors for overall survival using univariate analyses. A total of 10 factors were considered in the analyses, including patient age and gender, tumor size, WHO classification and differentiation of the tumor, TNM score (e.g., score of T3N3M0 = 3+3+0 = 6), and IHC scores for SERPINB5, KLK10, KLK11 and HPN. Tumor size, TNM score, IHC scores for SERPINB5, KLK10, KLK11 and HPN were shown to be indicative of patient survival (Table II); these six factors were used in further analyses.
Table II.
Univariate analysis of significant prognostic factors for overall survival in the GC patients.
| Variable | Overall survival
|
||
|---|---|---|---|
| n | Events | P-value | |
| Age at diagnosis (years) | |||
| ≤65 | 87 | 31 | 0.311 |
| >65 | 33 | 17 | |
| Gender | |||
| Male | 77 | 31 | 0.959 |
| Female | 43 | 17 | |
| Tumor size (cm) | |||
| ≤5 | 86 | 26 | 0.03 |
| >5 | 34 | 22 | |
| WHO classification | |||
| 1 | 104 | 38 | 0.469 |
| 2 | 7 | 4 | |
| 3 | 9 | 6 | |
| TNM score | |||
| 1 | 14 | 2 | 0.006 |
| 2 | 31 | 4 | |
| 3 | 22 | 5 | |
| 4 | 32 | 19 | |
| 5 | 16 | 13 | |
| 6 | 5 | 5 | |
| Differentiation | |||
| 1 | 13 | 3 | 0.612 |
| 2 | 63 | 25 | |
| 3 | 44 | 20 | |
| SERPINB5 | |||
| Weakly positive | 56 | 11 | 0.005 |
| Positive | 64 | 37 | |
| KLK10 | |||
| Weakly positive | 88 | 19 | 0.001 |
| Positive | 32 | 29 | |
| KLK11 | |||
| Negative | 66 | 36 | 0.016 |
| Positive | 56 | 12 | |
| HPN | |||
| Negative | 84 | 42 | 0.014 |
| Positive | 36 | 6 | |
Translation of expression of SERPINB5, KLK10, KLK11 and HPN together with tumor size and TNM score in the prediction model of survival
Complete data from 42 patients who underwent GC surgery in 2000 were used to develop a prediction model of survival by stepwise regression analysis and the best subset regression. It identified 63 models totally using the data of expression of SERPINB5, KLK10, KLK11 and HPN together with tumor size and TNM score. The following was identified as the most accurate model: Survival time (months) = 88.8607 + 2.6395 SERPINB5 − 12.0772 KLK10 + 13.7562 KLK11 − 7.0318 TNM, where SERPINB5, KLK10 and KLK11 are the IHC scores of these genes and TNM indicates the TNM score. This model had the highest adjusted R2 value (0.9556) and the least CP value (4.4748).
Survival prediction for the different groups of GC patients
The prediction model consisting of SERPINB5, KLK10, KLK11 and TNM was applied to the different test groups of GC patients. This was considered to be accurately predictive, for complete data, when the prediction value was in the range of actual survival time ± 5 months; for censored data, the prediction value was higher than the actual survival time. Survival of 47 patients out of 58 patients of 2000 was correctly predicted, and yielded a sensitivity of 0.8103. In particular, this prediction model correctly predicted 5 death events and 14 survivals for 20 patients of 2003 (Table III). A highly predictive power was achieved for this prediction model for the GC patients in the independent test group.
Table III.
Frequency distribution of accuracy in all of the patients of 2003 (n=20).
| Predicted status | Clinical status
|
Total | |
|---|---|---|---|
| Death | Survival | ||
| Death | 5 | 0 | 5 |
| Survival | 1 | 14 | 15 |
| Total | 6 | 14 | 20 |
Sensitivity, 83.33%; specificity, 100%; negative predictive value, 93.33%; positive predictive value, 100%.
Discussion
Microarray technology has rapidly evolved during the past decade. The main objectives of microarray studies are i) to identify homogeneous subtypes of a disease on the basis of gene expression, ii) to find genes that are differentially expressed in tumors with different characteristics, or iii) to develop a rule on the basis of gene expression allowing the prediction of patient prognosis or of the response to a particular treatment (31). Using cDNA microarray, a classifier containing 153 genes with weights was generated (32). Microarrays can also be used to identify leukemia subtypes (33). However, these significant results have seldom been put into clinical application as they rely on massive data output gathered from a relatively large number of genes, sophisticated algorithms for analyzing the data and costly experiment platforms, let alone the technical variance in the hybridization-based systems. Studies have demonstrated that the combination of microarray and RT-PCR technologies is a highly efficient and reliable approach for the identification of clinically important diagnostic and prognostic biomarkers, as well as for the identification of novel therapeutic target candidates in pancreatic cancer (34). However, protein is mainly the effective molecule in cells. We found that the correlations between the RNA and protein expression profiles did not reach statistical significance. In our study, the cDNA microarray was used to identify differentially expressed genes, and a survival prediction model of clinical applicability was developed using the IHC data of these genes.
Nine SPs and serpin genes were found to be differentially expressed between the GC and non-cancerous gastric tissues after the microarray assay, but two genes were excluded after confirmation by real-time PCR. Therefore, seven genes were further analyzed: SERPINB5, KLK10, KLK11, HPN, SPINK1, SERPINA5 and PRSS8. The first four genes were included in the IHC assay. Finally, three, SERPINB5, KLK10 and KLK11, were identified to be involved in the most effective prediction model.
SERPINB5 (maspin, mammary serine protease inhibitor) was identified in 1994 by subtractive hybridization analysis of normal mammary tissue and breast cancer cell lines (16). SERPINB5 regulates the invasive activity of tumor cells (35), inhibits angiogenesis (36), primary tumor growth as well as invasion and metastasis (37). However, high expression of maspin is associated with early tumor relapse in breast cancer (38), and SERPINB5 may contribute to gastric carcinogenesis and have a potential role in tumor metastasis in GC (39,40). Our microarray data, real-time PCR and IHC assay demonstrated that the expression of SERPINB5 was up-regulated at the RNA and protein levels in the GC tissues. These may indicate that SerpineB5 is a ‘bad’ gene for survival. However, the final model indicated expression of SerpineB5 to be a good predictor for survival. The predictive effectiveness of SerpineB5 may be poor; further studies are required to explain this discrepancy.
KLK10 and KLK11 belong to the kallikreins which are enzymes which historically release vasoactive peptides from high molecular weight precursors. In humans, there are two categories of kallikreins: plasma and tissue kallikreins. Human tissue kallikreins have attracted increased attention due to their role as biomarkers for the screening, diagnosis, prognosis and monitoring of various types of cancers, including those of the prostate, ovarian, breast, testicular and lung (41). KLK10 is significantly up-regulated in pancreatic, colon, ovarian and gastric cancer (42,43); this is consistent with our findings concerning KLK10. KLK11 is down-regulated in renal cell carcinoma and prostate cancer (44,45), similar to our findings in GC. It appears that these genes have only limited predictive power in isolation, but we get a more efficient survival prediction model combining the expression profiles of these genes with the TNM status of the tumor.
The prognostic model presented here may predict the survival of GC patients after radical resection; this may influence the clinical treatment of GC patients by aiding in the selection of patients for adjuvant therapy and developing meaningful clinical trials for new regimens.
Although the genes in our model were selected by microarray, the model is independent of the microarray platform. It requires only IHC, which is a common assay and can be performed easily in any laboratory or pathological department of any hospital. Certainly, our model is developed from the data of a relatively small population of patients and from one digestive surgery center, therefore it may require various modifications in future practice.
Acknowledgments
The authors would like to thank Mr Qi Li and Mr Xi-Jian Chen from the Geminix Informatics Co., Ltd., Shanghai, China, for the assistance in the microarray statistical analysis; Ms. Qu Cai from the Shanghai Institute of Digestive Surgery for the assistance in the real-time PCR experiments; Ms. Yue-Mei Sun from the Shanghai Institute of Digestive Surgery for the assistance in the patient follow-up; Mr Jun Ji from the Shanghai Institute of Digestive Surgery for the assistance in the IHC experiments; Mr Kang Chen from the Department of Pathology, Ruijin Hospital, for the assistance in the preparation of the sections for the IHC experiments. This study was supported in part by the China National ‘863’ R&D High-Tech Key Project (2006AA02A301 and 2007AA02Z179), and the National Natural Science Foundation of China (30772107).
References
- 1.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer – pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 4.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 5.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings ND, Morton FR, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2006;34:D270–D272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quemener C, Gabison EE, Naimi B, et al. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007;67:9–15. doi: 10.1158/0008-5472.CAN-06-2448. [DOI] [PubMed] [Google Scholar]
- 8.Obiezu CV, Diamandis EP. Human tissue kallikrein gene family: applications in cancer. Cancer Lett. 2005;224:1–22. doi: 10.1016/j.canlet.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Kim JT, Song EY, Chung KS, et al. Up-regulation and clinical significance of serine protease kallikrein 6 in colon cancer. Cancer. 2011;117:1–12. doi: 10.1002/cncr.25841. [DOI] [PubMed] [Google Scholar]
- 10.Whisstock JC, Bottomley SP, Bird PI, Pike RN, Coughlin P. Serpins 2005 – fun between the beta-sheets. Meeting report based upon presentations made at the 4th International Symposium on Serpin Structure, Function and Biology (Cairns, Australia) FEBS J. 2005;272:4868–4873. doi: 10.1111/j.1742-4658.2005.04927.x. [DOI] [PubMed] [Google Scholar]
- 11.Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- 12.Irving JA, Shushanov SS, Pike RN, et al. Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation. J Biol Chem. 2002;277:13192–13201. doi: 10.1074/jbc.M108460200. [DOI] [PubMed] [Google Scholar]
- 13.Schick C, Bromme D, Bartuski AJ, Uemura Y, Schechter NM, Silverman GA. The reactive site loop of the serpin SCCA1 is essential for cysteine proteinase inhibition. Proc Natl Acad Sci USA. 1998;95:13465–13470. doi: 10.1073/pnas.95.23.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suminami Y, Nagashima S, Murakami A, et al. Suppression of a squamous cell carcinoma (SCC)-related serpin, SCC antigen, inhibits tumor growth with increased intratumor infiltration of natural killer cells. Cancer Res. 2001;61:1776–1780. [PubMed] [Google Scholar]
- 15.Suminami Y, Nagashima S, Vujanovic NL, Hirabayashi K, Kato H, Whiteside TL. Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer. 2000;82:981–989. doi: 10.1054/bjoc.1999.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 17.Shellenberger TD, Mazumdar A, Henderson Y, et al. Headpin: a serpin with endogenous and exogenous suppression of angiogenesis. Cancer Res. 2005;65:11501–11509. doi: 10.1158/0008-5472.CAN-05-2262. [DOI] [PubMed] [Google Scholar]
- 18.Ollins GJ, Nikitakis N, Norris K, Herbert C, Siavash H, Sauk JJ. The production of the endostatin precursor collagen XVIII in head and neck carcinomas is modulated by CBP2/Hsp47. Anticancer Res. 2002;22:1977–1982. [PubMed] [Google Scholar]
- 19.Xiao G, Liu YE, Gentz R, et al. Suppression of breast cancer growth and metastasis by a serpin myoepithelium-derived serine proteinase inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci USA. 1999;96:3700–3705. doi: 10.1073/pnas.96.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy MJ, Duggan C. The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin Biochem. 2004;37:541–548. doi: 10.1016/j.clinbiochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Shiiba M, Nomura H, Shinozuka K, et al. Down-regulated expression of SERPIN genes located on chromosome 18q21 in oral squamous cell carcinomas. Oncol Rep. 2010;24:241–249. doi: 10.3892/or_00000852. [DOI] [PubMed] [Google Scholar]
- 22.Silverman GA, Bird PI, Carrell RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 23.Yousef GM, Diamandis EP. Tissue kallikreins: new players in normal and abnormal cell growth? Thromb Haemost. 2003;90:7–16. [PubMed] [Google Scholar]
- 24.Huang J, Sheng HH, Shen T, et al. Correlation between genomic DNA copy number alterations and transcriptional expression in hepatitis B virus-associated hepatocellular carcinoma. FEBS Lett. 2006;580:3571–3581. doi: 10.1016/j.febslet.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Xu XR, Huang J, Xu ZG, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA. 2001;98:15089–15094. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang YH, Dudoit S, Luu P, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei KF, Wang YF, Zhu XQ, et al. Identification of MSRA gene on chromosome 8p as a candidate metastasis suppressor for human hepatitis B virus-positive hepatocellular carcinoma. BMC Cancer. 2007;7:172. doi: 10.1186/1471-2407-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Qu Y, Li JF, Cai Q, et al. Over-expression of FRZB in gastric cancer cells suppresses proliferation and induces differentiation. J Cancer Res Clin Oncol. 2008;134:353–364. doi: 10.1007/s00432-007-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 31.Michiels S, Koscielny S, Hill C. Interpretation of microarray data in cancer. Br J Cancer. 2007;96:1155–1158. doi: 10.1038/sj.bjc.6603673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 33.Yeoh EJ, Ross ME, Shurtleff SA, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 34.Streit S, Michalski CW, Erkan M, Friess H, Kleeff J. Confirmation of DNA microarray-derived differentially expressed genes in pancreatic cancer using quantitative RT-PCR. Pancreatology. 2009;9:577–582. doi: 10.1159/000212084. [DOI] [PubMed] [Google Scholar]
- 35.Dokras A, Gardner LM, Kirschmann DA, Seftor EA, Hendrix MJ. The tumour suppressor gene maspin is differentially regulated in cytotrophoblasts during human placental development. Placenta. 2002;23:274–280. doi: 10.1053/plac.2001.0784. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 37.Shi HY, Zhang W, Liang R, et al. Modeling human breast cancer metastasis in mice: maspin as a paradigm. Histol Histopathol. 2003;18:201–206. doi: 10.14670/HH-18.201. [DOI] [PubMed] [Google Scholar]
- 38.Joensuu KM, Leidenius MH, Andersson LC, Heikkila PS. High expression of maspin is associated with early tumor relapse in breast cancer. Hum Pathol. 2009;40:1143–1151. doi: 10.1016/j.humpath.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Son HJ, Sohn TS, Song SY, Lee JH, Rhee JC. Maspin expression in human gastric adenocarcinoma. Pathol Int. 2002;52:508–513. doi: 10.1046/j.1440-1827.2002.01381.x. [DOI] [PubMed] [Google Scholar]
- 40.Terashima M, Maesawa C, Oyama K, et al. Gene expression profiles in human gastric cancer: expression of maspin correlates with lymph node metastasis. Br J Cancer. 2005;92:1130–1136. doi: 10.1038/sj.bjc.6602429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paliouras M, Borgono C, Diamandis EP. Human tissue kallikreins: the cancer biomarker family. Cancer Lett. 2007;249:61–79. doi: 10.1016/j.canlet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Yousef GM, Borgono CA, Popalis C, et al. In-silico analysis of kallikrein gene expression in pancreatic and colon cancers. Anticancer Res. 2004;24:43–51. [PubMed] [Google Scholar]
- 43.Yousef GM, White NM, Michael IP, et al. Identification of new splice variants and differential expression of the human kallikrein 10 gene, a candidate cancer biomarker. Tumour Biol. 2005;26:227–235. doi: 10.1159/000087377. [DOI] [PubMed] [Google Scholar]
- 44.Petraki CD, Gregorakis AK, Vaslamatzis MM, et al. Prognostic implications of the immunohistochemical expression of human kallikreins 5, 6, 10 and 11 in renal cell carcinoma. Tumour Biol. 2006;27:1–7. doi: 10.1159/000090150. [DOI] [PubMed] [Google Scholar]
- 45.Bi X, He H, Ye Y, et al. Association of TMPRSS2 and KLK11 gene expression levels with clinical progression of human prostate cancer. Med Oncol. 2010;27:145–151. doi: 10.1007/s12032-009-9185-0. [DOI] [PubMed] [Google Scholar]