Abstract
Anti-secretory drugs, particularly proton pump inhibitors (PPIs), are the preferred treatment agents for patients with gastroesophageal reflux disease (GERD). However, refractory GERD, which may manifest as an incomplete or lack of response to PPI therapy, is common. Despite the administration of PPIs for symptomatic control, duodenogastroesophageal reflux (DGER) containing bile is successfully controlled in only one-third of patients. It has previously been reported that the traditional Japanese herbal medicine rikkunshito, which has a prokinetic action on gastric emptying, exhibits clinically therapeutic effects against GERD and DGER that does not respond to PPIs. However, the precise mechanisms responsible for the effects of rikkunshito are still unknown. It has been suggested that the cytotoxicity of the bile salts in the gut lumen is important in GERD and DGER. The aim of the present study was to investigate whether rikkunshito is able to adsorb bile salts through the mechanism by which it ameliorates the symptoms of GERD and DGER. The binding capacities of rikkunshito for bile salts were measured using Langmuir’s method. The morphology of rikkunshito was also observed by light microscopy. Rikkunshito strongly adsorbed bile salts. The binding capabilities of rikkunshito were far beyond those of a typical dietary fiber, α-cellulose, or an oral adsorbent. In addition, rikkunshito had higher binding capacities for hydrophobic bile salts as compared with hydrophilic bile salts. In conclusion, rikkunshito has a great capacity to adsorb bile salts. This may be part of the mechanism(s) responsible for the therapeutic effects of rikkunshito in patients with GERD and DGER.
Keywords: rikkunshito, herbal medicine, dietary fiber, bile salt, adsorption
Introduction
In recent decades, gastroesophageal reflux disease (GERD) has become a common disorder in the USA and Western Europe (1). Anti-secretory therapies, especially proton pump inhibitors (PPIs), are the preferred treatment for patients with GERD. However, it has been estimated that between 10 and 40% of patients with GERD fail, either partially or completely, to respond symptomatically to standard doses of PPIs (2–5). The majority of bile reflux occurs concomitantly with acid reflux events and it is believed that the acid rather than the bile is the dominant factor responsible for the symptoms of GERD (6,7). However, certain studies have suggested that persistent typical and atypical GERD symptoms refractory to PPIs might be due to less acidic or non-acidic reflux (8). In addition, experimental data support a role for persistent bile acids in the refluxate as factors potentially involved in refractory heartburn. Although PPI therapy reduces the occurrence of acid as well as bile reflux (9), it has been shown that complete acid suppression does not guarantee the elimination of duodenogastroesophageal reflux (DGER) (10). Taken together, it is possible that bile reflux accounts for at least some of the non-acid reflux symptoms (11).
Previously, there were no data available concerning the value of administering a promotility drug to patients who have failed PPI therapy. However, in patients receiving PPI therapy who have delayed gastric emptying and persistent GERD symptoms, the use of a promotility drug is an attractive option. Over the years, the traditional Japanese herbal medicine rikkunshito, which is used to treat various disorders of the gastrointestinal tract, including functional dyspepsia, gastroesophageal reflux, dyspeptic symptoms of post-gastrointestinal surgery and chemotherapy-induced nausea, has been used to treat the symptoms of GERD and DGER and studies concerning its efficacy have been published (12–15). Rikkunshito has a prokinetic action on gastric emptying and its pharmacological action is closely correlated with an increase in plasma-active ghrelin levels, which stimulates gastric motility (16). However, it is unknown whether rikkunshito exerts its effects against GERD and DGER via prokinetic actions alone.
Rikkunshito is a type of dietary fiber derived from medicinal plants. In general, medicinal plants mainly consist of carbohydrates, insulin, fats, proteins, wax, mucus, gum resin, balsam resin, essential oils, triterpenes, saponins, tannins, lignin, lignans, glycosides, alkaloids and calcium salts. In recent decades, there has been an increased interest in dietary fiber due to its apparently beneficial effects on the human gastrointestinal tract, which include improving constipation, reducing serum cholesterol levels and excreting carcinogenic compounds into the feces (17,18). It is widely accepted that the beneficial effects of dietary fiber are mainly due to its binding or bulking characteristics (19). Dietary fiber refers to plant cell wall components and consists mainly of two types of fiber: soluble fiber (pectin, β-D-glucans, fructans, oligosaccharides, certain hemicelluloses, guar and gums) and insoluble fiber (hemicellulose, cellulose and lignin) (20). Dietary fibers cannot be digested by human or other mammalian digestive enzymes and can only be degraded by anaerobic bacteria located in the large intestine. We have previously focused on a certain type of dietary fiber, germinated barley foodstuff (GBF), as a therapeutic agent for inflammatory bowel disease (21–24). In the process of investigating the therapeutic mechanisms of GBF, we discovered that one of its major properties was its capacity to adsorb bile salts (25), thus eliminating bile salts from the gut lumen and ameliorating colitis.
Therefore, the aim of the present study was to investigate whether rikkunshito is able to adsorb bile salts in vitro and, if so, to establish whether this capability contributes to its therapeutic effects in reducing the symptoms of patients with GERD and DGER.
Materials and methods
Chemicals
Dicyclohexano-18-crown-6, 4-bromomethyl-6,7-dimethoxycoumarin, cholate (CA), taurocholate (T-CA), deoxycholate (DCA), taurodeoxycholate (T-DCA) and α-cellulose were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). All these bile salts were of analytical grade.
Rikkunshito
Rikkunshito was used in the form of a powdered mixture of eight types of crude herbs, sojutsu (Atractylodis lanceae rhizoma), ninjin (Ginseng radix), hange (Pinelliae tuber), bukuryo (Hoelen), taiso (Zizyphi fructus), chinpi (Aurantii nobilis pericarpium), kanzo (Glycyrrhizae radix) and shokyo (Zingiberis rhizoma). Rikkunshito was supplied by Tsumura and Co. (Tokyo, Japan).
Microscopic observations of rikkunshito
We observed the morphology of the two dietary fibers, rikkunshito and α-cellulose, microscopically. The morphological differences between the dry and the wet forms are important. Therefore, when observing the dry form, we inspected the fibers directly. In the observation of the wet form, 50 mg of each dietary fiber was immersed in 10 ml of distilled water for 5 min and was then set under a cover glass for direct inspection. We used the light microscope Olympus BX 50 (Olympus Optical Co., Ltd., Tokyo, Japan) to carry out these observations.
Binding capacities of rikkunshito for bile salts
We used bile salt solutions of concentrations ranging from 100 µM to 1 mM in the binding experiment. In addition, α-cellulose was used in order to compare the binding capacity of rikkunshito with other types of fiber. The binding experiment was carried out according to our previous method (25). Briefly, 50 mg of rikkunshito or α-cellulose was placed into a glass-stoppered conical flask with 5 ml of water. The flasks were then closed securely, mechanically agitated at 25°C for 30 min and the supernatant subjected to filtration (0.45-μm pore size). The bile salts in the supernatants were analyzed as their fluorescent dimethoxy coumarin esters using high-performance liquid chromatography (HPLC) according to our previous method. Briefly, following the drying of the supernatants using a vacuum pump, a 100 μl aliquot of acetonitrile was added, followed by 40 μl of dicyclohexano-18-crown-6 (1.5 mg/ml acetonitrile) and 40 μl of 4-bromomethyl-6,7-dimethoxycoumarin (3.0 mg/ml). The tubes were then sealed with parafilm and placed in a heated water bath at 60°C for 30 min. Next, the solution was centrifuged at 14,000 rpm for 10 min and 5 μl of the supernatant was injected into the HPLC column. We used a reverse-phase column (Cosmosil 5C18-MS, 4.6 mm IDx15 cm long, NacalaiTesque Inc.) and two solvents as the mobile phase. Solvent A was a mixture of water, acetonitrile and methanol at a ratio of 3:2:1. Solvent B was a mixture of acetonitrile and methanol at a ratio of 2:1. A 1 ml aliquot of 7.6 M ammonium acetate was added to each 500 ml of solvents A and B. The flow rate was constant at 0.6 ml/min. The gradient elution program began with 100% solvent A and the proportion of solvent B was gradually increased from 0 to 95% over a 45-min period, then kept constant for an additional 10 min prior to recycling to the initial conditions. A fluorescence detector RF-535 (Shimadzu, Kyoto, Japan) was set at an excitation wavelength of 340 nm and an emission wavelength of 430 nm. The bile salt peaks were quantified by comparing the areas to standard curves produced by chromatographing known quantities of bile salt standards under similar conditions.
Following the measurement of the bile salt concentrations, we obtained adsorption isotherms for the binding of individual bile salts to rikkunshito, according to a previous method using a Langmuir-type equation (26,27):
| Equation 1: |
| Equation 2: |
where Ceq is the concentration of the bile salt remaining in solution at equilibrium, x is the amount of bile salt bound to the rikkunshito and m is the amount of rikkunshito used. A plot of Ceq/(x/m) versus Ceq should yield a straight line, from which we may obtain the constants k1 (the adsorption coefficient) and k2 (the maximum binding capacity).
Results
Macroscopic and microscopic observations of rikkunshito
Fig. 1A shows the macroscopic appearance of rikkunshito, which is a yellow and fairly rough powder. Fig. 1B shows the macroscopic appearance of α-cellulose, which is a white and smooth powder. Fig. 2A shows the microscopic appearance of rikkunshito. Rikkunshito has an amorphous structure of spherical particles ∼40–80 µm in diameter. Fig. 2B shows the microscopic appearance of α-cellulose, which has an amorphous structure of long, rod-shaped particles of ∼20 µm by 40–240 μm. Notably, if the rikkunshito was immersed in distilled water, the particles swelled rapidly and their diameter increased ∼3-fold (Fig. 2C). However, α-cellulose did not swell in distilled water to any appreciable extent (Fig. 2D).
Figure 1.
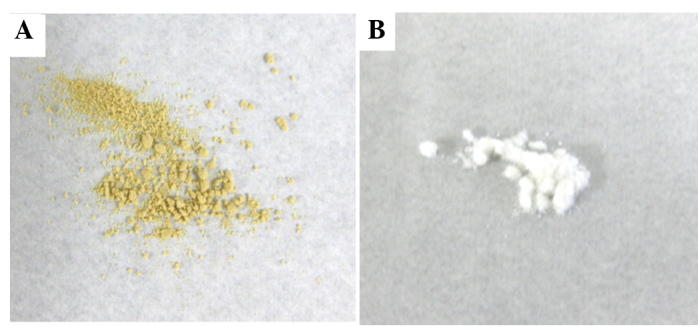
Macroscopic observation of rikkunshito and α-cellulose. (A) Macroscopic observation of rikkunshito. Rikkunshito is a yellow and rough powder. (B) Macroscopic observation of α-cellulose. α-cellulose is a white and fairly smooth powder.
Figure 2.
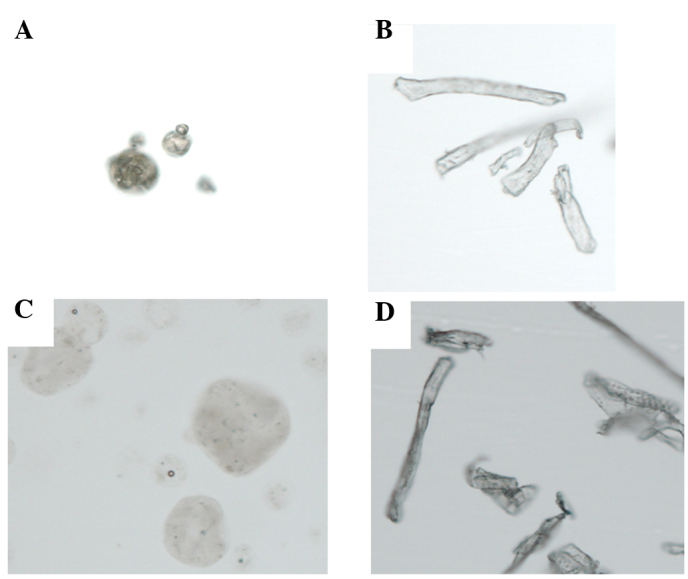
Microscopic observation of rikkunshito and α-cellulose (x100 magnification). (A) Microscopic observation of rikkunshito. Rikkunshito has an amorphous structure of spherical particles of ∼40–80 µm in diameter. (B) Microscopic observation of α-cellulose. α-cellulose has an amorphous structure of long rod-shaped particles of ∼20 µm by 40–240 µm. (C) When rikkunshito was immersed in distilled water, the particles swelled and increased ∼3-fold in diameter. (D) α-cellulose did not swell when immersed in distilled water.
Binding capacities of rikkunshito for bile salts
Figs. 3 and 4 show the adsorption isotherms of rikkunshito for individual conjugated and unconjugated bile salts according to equations 1 and 2, respectively. The curves had a tendency to reach a plateau at high Ceq values. The adsorption constants k1 and k2, obtained from the intercept and slope values of Figs. 3 and 4, are listed in Table I. Rikkunshito adsorbed individual conjugated and unconjugated bile salts strongly compared with α-cellulose. In particular, unconjugated CA and DCA were more strongly adsorbed by rikkunshito than their conjugated counterparts, T-CA and T-DCA. CA and DCA had maximum binding capacities of 748.0×10−6 and 752.0×10−6 mol/g, respectively. However, T-CA and T-DCA both had maximum binding capacities of 89.0×10−6 mol/g. These results suggest that rikkunshito has high binding capacities for more hydrophobic bile salts compared with hydrophilic bile salts.
Figure 3.

Langmuir adsorption isotherms for the binding of bile salts. The Langmuir adsorption isotherms for the binding of unconjugated or conjugated bile salts to rikkunshito at 25°C are shown. The isotherms were calculated using equation 1 (described in Materials and methods). CA, cholate; DCA, deoxycholate; T-CA, taurocholate; T-DCA, taurodeoxycholate.
Figure 4.
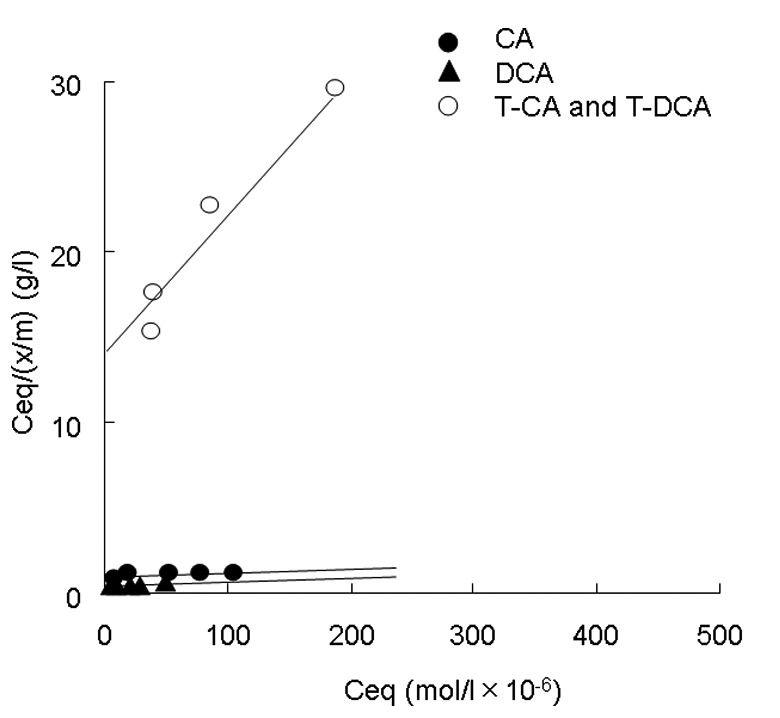
Langmuir adsorption isotherms for the binding of bile salts. The Langmuir adsorption isotherms for the binding of unconjugated or conjugated bile salts to rikkunshito at 25°C are shown. The isotherms were calculated using equation 2 (described in Materials and methods). CA, cholate; DCA, deoxycholate; T-CA, taurocholate; T-DCA, taurodeoxycholate.
Table I.
Langmuir adsorption constants for the binding of bile salts.
| Fiber | Bile salt | k1 (l/mol×104) | k2 (mol/g×10−6) |
|---|---|---|---|
| Rikkunshito | Cholate | 0.14 | 748.0 |
| Deoxycholate | 0.25 | 752.0 | |
| Taurodeoxycholate (Taurocholate) | 0.079 | 89.0 | |
| Cellulose | Deoxycholate | 0.31 | 5.23 |
Under our HPLC conditions, the hydrophobicity indices were DCA>CA>T-DCA or T-CA.
Discussion
Rikkunshito has been shown to promote adaptive gastric relaxation (28) and to facilitate gastric emptying (29). In addition, other pharmacological properties of rikkunshito have been reported, including reducing distal esophageal acid exposure by improving esophageal acid clearance (30,31) and promoting adaptive relaxation (28,30). This herbal drug may also aid the amelioration of GERD symptoms and these effects could be considered to be caused by the prokinetic actions of rikkunshito. One possible mechanism for these therapeutic effects is an increase in the plasma-active ghrelin levels, which stimulate gastric motility (16). However, it is possible that there are other mechanisms whereby rikkunshito improves GERD or DGER symptoms, possibly involving the bile acids.
Rikkunshito is a type of dietary fiber derived from medicinal plants. Early evidence that dietary fiber binds to cytotoxic bile salts was presented by Eastwood and Hamilton (32). It has since been well-documented that fiber is able to adsorb bile salts and thus eliminate bile salts from the digestive tract.
We have studied the role of bile salts in the gut lumen when considering the pathogenesis and disease-promoting factors for experimental and clinical gastrointestinal diseases (25,26,33–37). In the course of these investigations, it has been revealed that bile salts exhibit mainly cytotoxic but also certain stimulatory effects towards the intestinal epithelium (38). In addition, previous clinical studies have suggested that toxic secondary bile acid fractions were detected more frequently in patients with symptoms of GERD. This study also indicated that reflux mixed with gastric acid and bile acid is more harmful than gastric acid reflux alone, with a possible toxic synergism (39). However, the effects of bile salts on the esophageal mucosa are less well understood. Therefore, we investigated whether rikkunshito could adsorb bile salts.
In the present study, we found that rikkunshito swells in distilled water. This phenomenon was accompanied by a high capacity to adsorb bile salts compared with α-cellulose (∼150-fold greater; Table I). In general, hydrophobic bile salts bind preferentially to fiber (40). As expected, rikkunshito exhibited high binding capacities for hydrophobic bile salts (DCA) compared with hydrophilic bile salts (T-DCA) (∼8.5-fold greater).
In this study, we compared the binding capacity of rikkunshito with that of cholestyramine. Cholestyramine is an anion-exchange resin and is used clinically to achieve anti-hypercholesterolemia effects. Since cholestyramine has a high binding capacity for bile salts (27), it is able to eliminate bile salts present in the digestive tract. As bile salts are biosynthesized from cholesterol, serum cholesterol levels are eventually reduced. The maximum binding capacities of rikkunshito for DCA reached ∼20% of that of cholestyramine. In addition, according to our previous study rikkunshito has 30 times the maximum binding capacity for DCA as the clinically-used adsorbent AST-120 (26).
Taken together, it is possible that rikkunshito is not only involved in gastric emptying through its prokinetic action, but may also reduce the bile acid exposure of the esophageal mucosa by adsorbing bile salts. Therefore, rikkunshito may be an effective drug for the treatment of refractory GERD and DGER. This effect could be beneficial for other diseases, including esophageal cancer, as it has been suggested that duodenal juice, including bile salts, stimulates esophageal stem cells to induce Barrett’s esophagus and esophageal adenocarcinomas in experimental models (41).
In conclusion, it has become clear for the first time that rikkunshito exhibits a high adsorbing capacity for bile salts, especially hydrophobic and cytotoxic bile salts including DCA and CA. This adsorbing capacity may contribute in part to the therapeutic efficacy of rikkunshito in the treatment of GERD and DGER patients.
References
- 1.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple-to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol. 2003;98:1940–1944. doi: 10.1111/j.1572-0241.2003.07665.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson R, Dent J, Watts R, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119–124. [PubMed] [Google Scholar]
- 4.Crawley JA, Schmitt CM. How satisfied are chronic heartburn sufferers with their prescription medications? Results of the patient unmet needs study. J Clin Outcomes Manag. 2000;7:29–34. [Google Scholar]
- 5.The Gallup Organization . The 2000 Gallup Study of Consumers’ Use of Stomach Relief Products. Princeton: Gallup Organization; 2000. [Google Scholar]
- 6.Vaezi MF, Lacamera RG, Richter JE. Validation studies of Bilitec 2000: an ambulatory duodenogastric reflux monitoring system. Am J Physiol. 1994;267:G1050–1057. doi: 10.1152/ajpgi.1994.267.6.G1050. [DOI] [PubMed] [Google Scholar]
- 7.Sifrim D. Acid, weakly acidic and non-acid gastro-oesophageal reflux: differences, prevalence and clinical relevance. Eur J Gastroenterol Hepatol. 2004;16:823–830. doi: 10.1097/00042737-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Sifrim D, Castell DO, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netzer P, Gut A, Brundler R, et al. Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in healthy control subjects. Aliment Pharmacol Ther. 2001;15:1375–1384. doi: 10.1046/j.1365-2036.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 10.Todd JA, Basu KK, de Caestecker JS. Normalization of oesophageal pH does not guarantee control of duodenogastrooesophageal reflux in Barrett’s oesophagus. Aliment Pharmacol Ther. 2005;21:969–975. doi: 10.1111/j.1365-2036.2005.02406.x. [DOI] [PubMed] [Google Scholar]
- 11.Pace F, Sangaletti O, Pallotta S, et al. Biliary reflux and non-acid reflux are two distinct phenomena: a comparison between 24-hour multichannel intraesophageal impedance and bilirubin monitoring. Scand J Gastroenterol. 2007;42:1031–1039. doi: 10.1080/00365520701245645. [DOI] [PubMed] [Google Scholar]
- 12.Tatsuta M, Iishi H. Effect of treatment with Liu-Jun-Zi-Tang (TJ-43) on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther. 1993;7:459–462. doi: 10.1111/j.1365-2036.1993.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 13.Tomono H, Ito Y, Watanabe T. Successful antiemetic treatment of Tsumura rikkunshi-to extract granules for ethical use in addition to other antiemetic agents in neoadjuvant chemotherapy for an advanced breast cancer patient. Jpn J Cancer Chemother. 2006;33:1129–1131. [PubMed] [Google Scholar]
- 14.Oka T, Tamagawa Y, Tamagawa Y, Hayashida S, Kaneda Y, Kodama N, et al. Rikkunshi-to attenuates adverse gastrointestinal symptoms induced by fluvoxamine. Biopsychosoc Med. 2007;15:1–21. doi: 10.1186/1751-0759-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahara H, Kubota A, Hasegawa T, Okuyama H, Ueno T, Ida S, et al. Effects of rikkunshito on the clinical symptoms and esophageal acid exposure in children with symptomatic gastroesophageal reflux. Pediatr Surg Int. 2007;23:1001–1005. doi: 10.1007/s00383-007-1986-7. [DOI] [PubMed] [Google Scholar]
- 16.Hattori T. Rikkunshito and ghrelin. Int J Pept. 2010 Jan 26;:pii 283549. doi: 10.1155/2010/283549. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings JH, Hill MJ, Jenkins DJA. Changes in fecal composition and colonic function due to cereal fiber. Am J Clin Nutr. 1976;29:1473–1486. doi: 10.1093/ajcn/29.12.1468. [DOI] [PubMed] [Google Scholar]
- 18.Kanauchi O, Hitomi Y, Agata K. Germinated barley foodstuff improves constipation induced by loperamide in rats. Biosci Biotechnol Biochem. 1998;62:1788–1790. doi: 10.1271/bbb.62.1788. [DOI] [PubMed] [Google Scholar]
- 19.Schneeman BO, Gallaher DD. Dietary fiber. In: Ziegler EE, Filer LJ, editors. Present Knowledge in Nutrition. 7th edition. International Life Science Institute Nutritional Foundation; Washington: 1996. pp. 87–97. [Google Scholar]
- 20.Oakenfull DG, Fenwick DE. Adsorption of bile salts from aqueous solution by plant fiber and cholestyramine. Br J Nutr. 1978;40:299–309. doi: 10.1079/bjn19780126. [DOI] [PubMed] [Google Scholar]
- 21.Araki Y, Kanauchi O, Sugihara H, Fujiyama Y, Hattori T. Germinated barley foodstuff suppresses dextran sulfate experimental colitis in rats: The role of mast cells. Int J Mol Med. 2007;19:257–262. [PubMed] [Google Scholar]
- 22.Kanauchi O, Fujiyama Y, Mitsuyama K, Araki Y, Ishii T, Nakamura T, Hitomi Y, Agata K, Saiki T, Andoh A, Toyonaga A, Bamba T. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int J Mol Med. 1999;3:175–179. doi: 10.3892/ijmm.3.2.175. [DOI] [PubMed] [Google Scholar]
- 23.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, Naganuma M, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–704. [PubMed] [Google Scholar]
- 24.Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I, Araki Y, Fujiyama Y, Toyonaga A, Sata M, et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643–647. [PubMed] [Google Scholar]
- 25.Araki Y, Andoh A, Fujiyama Y, Kanauchi O, Takenaka K, Higuchi A, Bamba T. Germinated barley foodstuff exhibits different adsorption properties for hydrophilic versus hydrophobic bile acids. Digestion. 2001;64:248–254. doi: 10.1159/000048869. [DOI] [PubMed] [Google Scholar]
- 26.Araki A, Tsujikawa T, Andoh A, Sasaki M, Fujiyama Y, Bamba T. The therapeutic effects of an oral adsorbent on the acute dextran sulfate sodium-induced colitis and its recovery phase in rats, especially effects of the elimination of bile acids in gut lumen. Dig Liver Dis. 2000;32:691–698. doi: 10.1016/s1590-8658(00)80332-1. [DOI] [PubMed] [Google Scholar]
- 27.Johns WH, Bates TR. Quantification of the binding tendencies of cholestyramine. I. Effect of structure and added electrolytes on the binding of unconjugated and conjugated bile-salt anions. J Pharm Sci. 1969;58:179–183. doi: 10.1002/jps.2600580206. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa T, Arakawa T, Kase Y, Akiyama S, Ishige A, Takeda S, et al. Liu-Jun-Zi-Tang, a kampo medicine, promotes adaptive relaxation in isolated guinea pig stomachs. Drugs Exp Clin Res. 1999;25:211–218. [PubMed] [Google Scholar]
- 29.Kido T, Nakai Y, Kase Y, Sakakibara I, Nomura M, Takeda S, et al. Effects of rikkunshi-to, a traditional Japanese medicine, on the delay of gastric emptying induced by N(G)-nitro-L-arginine. J Pharmacol Sci. 2005;98:161–167. doi: 10.1254/jphs.fpj04056x. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Inadomi JM, Hibi T. Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:688–696. doi: 10.1111/j.1365-2982.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawahara H, Mitani Y, Nomura M, et al. Impact of rikkunshito, an herbal medicine, on delayed gastric emptying in profoundly handicapped patients. Pediatr Surg Int. 2009;25:987–990. doi: 10.1007/s00383-009-2453-4. [DOI] [PubMed] [Google Scholar]
- 32.Eastwood M, Hamilton D. Studies on the adsorption of bile salts to non-absorbed components of the diet. Biochim Biophys Acta. 1968;152:165–173. doi: 10.1016/0005-2760(68)90018-0. [DOI] [PubMed] [Google Scholar]
- 33.Araki Y, Andoh A, Tsujikawa T, Fujiyama Y, Bamba T. Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium-induced experimental acute colitis in rats. Eur J Gastroenterol Hepatol. 2001;3:107–112. doi: 10.1097/00042737-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Araki Y, Fujiyama Y, Andoh A, Nakamura F, Shimada M, Takaya H, Bamba T. Hydrophilic and hydrophobic bile acids exhibit different cytotoxicities through cytolysis, interleukin-8 synthesis and apoptosis in the intestinal epithelial cell lines. IEC-6 and Caco-2 cells. Scand J Gastroenterol. 2001;36:533–539. doi: 10.1080/003655201750153430. [DOI] [PubMed] [Google Scholar]
- 35.Araki Y, Andoh A, Bamba H, Yoshikawa K, Doi H, Komai Y, Higuchi A, Fujiyama Y. The cytotoxicity of hydrophobic bile acids is ameliorated by more hydrophilic bile acids in intestinal cell lines IEC-6 and Caco-2. Oncol Rep. 2003;10:1931–1936. [PubMed] [Google Scholar]
- 36.Araki Y, Katoh T, Ogawa A, Bamba S, Andoh A, Koyama S, Fujiyama Y, Bamba T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic Biol Med. 2005;39:769–780. doi: 10.1016/j.freeradbiomed.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Araki Y, Mukaisyo KI, Sugihara H, Fujiyama Y, Hattori T. Detection of N-nitroso-bile acids at 285 nm in reverse-phase HPLC. J Sep Sci. 2008;31:2827–2830. doi: 10.1002/jssc.200800230. [DOI] [PubMed] [Google Scholar]
- 38.Araki Y, Andoh A, Sasaki A, Shimada M, Bamba S, Fujino S, Fujiyama Y. Dietary bile acids inhibit potentially elemental diet-induced small intestinal atrophy in rats. Int J Mol Med. 2002;10:623–626. [PubMed] [Google Scholar]
- 39.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;45:598–602. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kern F, Birkner HJ, Ostrower VS. Binding of bile acids by dietary fiber. Am J Clin Nutr. 1978;31:S175–179. doi: 10.1093/ajcn/31.10.S175. [DOI] [PubMed] [Google Scholar]
- 41.Miyashita T, Ohta T, Fujimura T, Ninomiya I, Fushida S, Hattori T, Miwa K. Duodenal juice stimulates oesophageal stem cells to induce Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol Rep. 2006;15:1469–1475. [PubMed] [Google Scholar]


