Abstract
The helicity of DNA and its long-range chiral packing are widespread phenomena; however, the packing mechanism remains poorly understood both in vivo and in vitro. Here, we report the extraordinary DNA chiral self-assembly by silica mineralization, together with circular dichroism measurements and electron microscopy studies on the structure and morphology of the products. Mg2+ ion and diethylenetriamine were found to induce right- and left-handed chiral DNA packing with two-dimensional-square p4mm mesostructures, respectively, to give corresponding enantiomeric impeller-like helical DNA–silica complexes. Moreover, formation of macroscopic impeller-like helical architectures depends on the types of polyamines and co-structure-directing agents and pH values of reaction solution. It has been suggested that interaction strength between negatively charged DNA phosphate strands and positively charged counterions may be the key factor for the induction of DNA packing handedness.
Keywords: chirality; polyamine, DNA–silica complex; biomolecule; self-assemble; helical architecture; liquid crystal
1. Introduction
The biomimetic synthesis strategies using biomolecular building blocks and assemblies as templates have recently attracted much attention for constructing well-defined two- and three-dimensional mesostructures and macroscopic morphologies [1]. DNA, with the ability to store and encode genetic information, is one of the most attractive biomolecular templates with a well-defined double-stranded helical structure that cannot be prepared by artificial polymers or low-molecular weight gels. The DNA molecules can be extremely compacted to a high-ordered packing structure in sperm heads, virus capsids and bacteria nucleoids [2]. In general, linear DNA fragments are able to form multiple liquid crystal phases, including isotropic, blue, cholesteric, columnar hexagonal and crystalline phases, which are sensitive to counterion strength, pH, concentration, temperature and other parameters [3–10]. Furthermore, the phase transformation and coexistence of the long-range chiral cholesteric ordering as well as the two-dimensional columnar packing have been found both in vivo and in vitro [11–14].
In our previous work, we synthesized DNA–silica complexes (DSCs) based on DNA self-assembly with a co-structure-directing agent (CSDA) [15,16], such as quaternary ammonium silane N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride (TMAPS). Interestingly, DSCs with rare two-dimensional-square p4mm symmetry have been formed upon quaternary ammonium-phosphate electrostatic ‘zipping’ effect along the DNA–DNA contacts [17]. Very recently, we found that alkaline earth metal ions can induce the two-dimensional-square p4mm mesostructure into layer-by-layer chiral DNA packing to form impeller-like helical DNA–silica complexes (IHDSCs). The handedness of IHDSCs was tuned by the reaction temperature, pH value and quaternary ammonium group of TMAPS to phosphate group of DNA molar ratio according to the enantiomeric layer-by-layer chiral DNA packing [18].
Polyamines are ubiquitous small polycations with multiple functions in the cell growth and differentiation, which exert a remarkable effect on many metabolic pathways, in particular, those involving nucleic acids [19–21]. The DNA condensation induced by polyamines into compact structures is a common feature of native genomes [10,22–24]. It has been reported that the diethylenetriamine behaves similarly as divalent alkaline earth metal ions such as Mg2+ in linking the phosphate groups of adjacent DNA molecules by electrostatic or hydrogen bonding of coordinating water molecules [25–29]. Here, we describe our efforts to facilitate the IHDSCs with macroscopic helical morphology in the presence of various amines. Meanwhile, the reversibility of chiral DNA packing is also discussed. Owing to the framing of DNA packing structures in a rigid silica wall, the structures and helical morphologies of the IHDSCs have been thoroughly investigated by X-ray diffraction (XRD), solid-state diffuse-reflectance circular dichroism (DRCD), scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM).
2. Methods
2.1. Materials
DNA sodium salt from Herring testes was from Sigma, formally listed as Type XIV. TMAPS (50% in methanol), tetraethyl orthosilicate (TEOS), MgCl2·6H2O, ethylenediamine, diethylenetriamine and spermine were from Sinopharm Chemical Reagent Co. Ltd. 3-Aminopropyltrimethoxysilane (APS), N-(2-aminoethyl)-3-aminopropyl methyltrimethoxysilane (DAPS) and (3-trimethoxysilylpropyl)diethylenetriamine (TAPS) were from TCI. All the regents were used as received without further purification.
2.2. Synthesis of the impeller-like helical DNA–silica complexes
In a typical synthesis, DNA was dissolved in deionized water by stirring under room temperature (approx. 298 K) with the final concentration of 1 mg g−1 water. Then, the solution was ultrasonic treated by an ultrasonic cell crusher at 400 W for 2.5 h. Diethylenetriamine was added to DNA solution, and the pH value was adjusted to 5.4 by HCl or NaOH. Then, 60 μl TMAPS and 100 μl TEOS were added with stirring successively. The mixture was stirred vigorously for 20 min at 273 K, and then allowed to react under static conditions at 273 K for 10 days. White powder was collected by centrifugation and washed with deionized water twice to remove unreacted DNA and silica source and then freeze dried at −60°C.
2.3. Characterization
Powder XRD patterns were recorded on a Rigaku X-ray diffractometer (D/MAX-2200/PC) equipped with Cu Kα1 radiation (wavelength 1.5406 Å) at the scanning rate of 0.1° per minute over the range of 2–7° (2θ). DRCD spectra were taken on a JASCO J-815 spectropolarimeter fitted with DRCD apparatus. The microscopic features of all samples were observed with SEM on a JEOL JSM-7401F scanning electron microscope. To observe genuine external surfaces, the samples were observed without any metal coating. Low-accelerating voltage (1 kV with point resolution of approx. 1.4 nm) was chosen for all samples. For TEM observation, the samples were crushed in an agate mortar, dispersed in ethanol and dropped on a carbon thin film on a Cu grid. HRTEM was performed with a JEOL JEM-2100 microscope equipped with LaB6 gun operating at 200 kV (Cs = 1.0 mm, Point resolution 2.3 Å). Images were recorded with a KeenView CCD camera (resolution 1376 × 1032 pixels, pixel size 6.45 × 6.45 μm) at 50 000–120 000 times magnification under low-dose conditions.
3. Results and discussion
The DNA molecules were chosen in this study (ranging from 100 to 300 bp in length; prepared by sonication), because DNA molecules ranging in this length are comparable with the persistence length and behave like rigid rods. These linear DNA fragments in aqueous solution can be easily packed into various liquid-crystalline phases [4]. The formation of DSCs was based on the co-structure-directing effect of TMAPS [15,16]. The positively charged quaternary ammonium group of TMAPS not only acts as a DNA condensing agent but also is able to co-condense with a silica source, TEOS, to achieve the subsequent assembly of a silica framework.
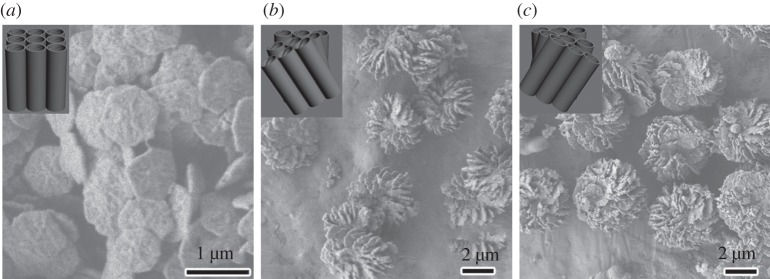
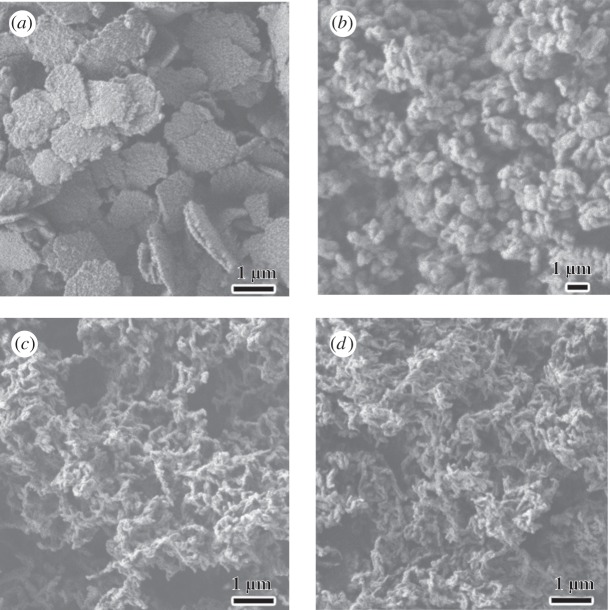
Figure 1 shows the macroscopic morphologies and corresponding DNA packing mesostructures of the samples synthesized without any addition, with addition of Mg2+ and diethylenetriamine, respectively. The molar composition of the reaction gel was DNA (phosphate group) : counterion : TMAPS : TEOS : H2O = 1 : x : 4 : 15 : 18 000, where x = 0 (a) and 0.75 (b,c), respectively. As shown in our previous report, the DSCs synthesized without counterion addition showed hexagonal morphologies with two-dimensional-square p4mm symmetry (figure 1a) [17]. Nevertheless, it was interesting to note that the extraordinary impeller-like helical architectures were synthesized with addition of Mg2+ ions. The handedness of the impellers is defined depending on arranged direction of the blades: IHDSC with blades arranged in a clockwise manner associates to the left-handed and that with a counterclockwise arrangement corresponds to the right-handed. The enantiomeric excess (ee) is defined by [(l − r)/(l + r)] × 100 per cent to express enantiopurity, where l and r are the amount of the left-handed and right-handed IHDSCs. The IHDSCs synthesized in the presence of Mg2+ were found be predominately left-handed, with an absolute ee value of 40 per cent, by counting characteristic morphologies from 500 randomly chosen particles (figure 1b). The IHDSCs particles were uniform in size of approximately 3 µm and blade thickness of approximately 80 nm [18]. However, the IHDCSs synthesized in the presence of diethylenetriamine showed the opposite handedness of impeller-like helical morphology compared with the impellers synthesized with Mg2+ ions (figure 1c). The IHDSCs synthesized with addition of diethylenetriamine had similar diameter of approximately 4 µm and blade thickness of approximately 80 nm. It has been estimated that the blades of the impeller were predominately right-handed, with an absolute ee of −50 per cent. The results unambiguously reveal that the handedness of IHDSCs depends on the types of counterions, which can effect and even reverse the handedness of chiral DNA packing.
Figure 1.
Macroscopic morphologies of the DNA–silica complexes. Scanning electron microscopy (SEM) images the samples synthesized without any addition (a), with addition of Mg2+ (b) and diethylenetriamine (c). Inset of SEM images: the long-range DNA packing ordering.
Figure 2a shows the XRD patterns of the samples shown in figure 1. Two well-resolved reflections in the range of 2θ = 2–7° were observed with a d-spacing ratio of √2, indicating that all the samples had highly ordered two-dimensional-square lattice with a unit cell parameter of a is approximately 2.5 nm. The structures of the samples were further confirmed by the HRTEM analysis, clearly suggesting the highly ordered two-dimensional-square p4mm mesostructure (figure 2b). Figure 2c shows the DRCD and UV–vis spectra of the DSC and IHDSCs shown in figure 1. As expected, no DRCD signal was observed from the sample synthesized without any counterion addition, clearly indicating the absence of long-range chiral packing of DNA molecules (figure 2c-(a)). However, with Mg2+ addition, two strongly positive DRCD signals were observed at around 230 and 293 nm (figure 2c-(b)). Compared with positive non-conservative DRCD signals, the IHDSCs synthesized in the presence of diethylenetriamine showed exactly opposite negative DRCD signals (figure 2c-(c)). The non-conservative DRCD results suggest that long-range chiral DNA packing is similar to the CD ellipticities exhibited for the cholesteric liquid crystal phase. The signal of non-conservative CD ellipticities reflects the sense of chiral DNA packing: negative CD signal corresponds to a left-handed long-range chiral packing, whereas positive CD signals are associated with a right-handed cholesteric twist [6,30–38]. Therefore, it is easily deduced that macroscopic impeller-like helical architecture is originated from the long-range chiral DNA packing. The two-dimensional-square p4mm DNA arrangement and the chiral DNA packing models were inserted in figure 1a–c, respectively. These results from DRCD signals are accurately consistent with the handedness reversal of macroscopic morphologies from SEM observations: the right-handed chiral DNA packing stabilizes and facilitates the macroscopic left-handed impeller-like helical architectures, whereas the left-handed DNA cholesteric chirality can induce enantiomeric right-handed IHDSCs.
Figure 2.
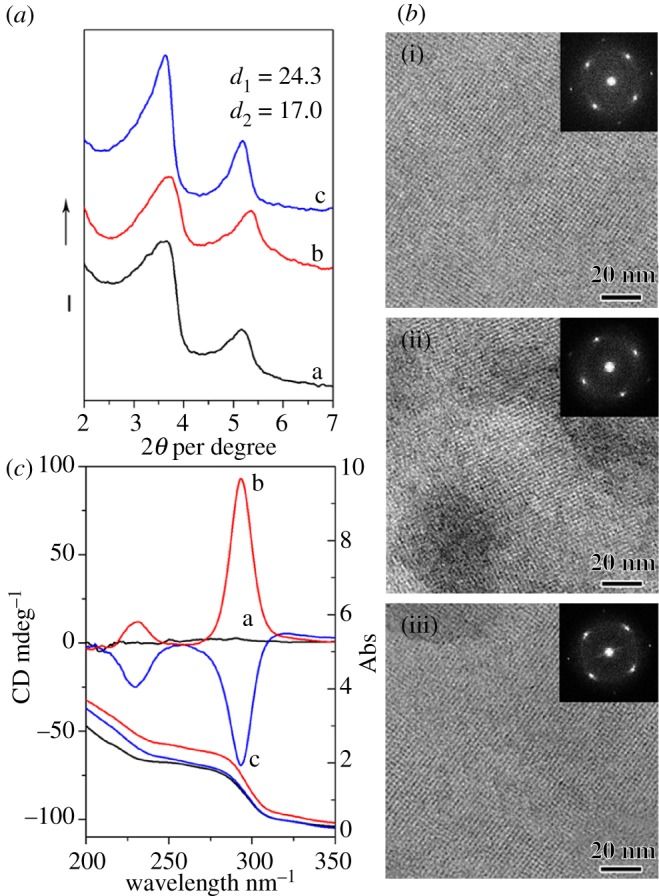
X-ray diffraction patterns (a), transmission electron microscopy images (b) and diffuse-reflectance circular dichroism and UV–vis spectra (c) of the samples shown in figure 1.
The interaction between DNA and polyamines has been extensively studied [22–24]. Diethylenetriamine has the ability to form the ‘ion-bridging’ between DNA molecules based on short-range electrostatic attractions [39,40], which link two negatively charged phosphate sites of adjacent DNA molecules and forms dislocated arrays of DNA, which can self-assemble to long-range chiral DNA liquid crystal phases. When TMAPS was added to the DNA and diethylenetriamine-mixed solution, cationic quaternary ammonium groups bind with phosphate groups of DNA through electrostatic interaction and the alkoxysilane sites of TMAPS co-condensed with TEOS to form the silica framework. It is noteworthy that the two-dimensional-square p4mm symmetry was obtained by an electrostatic ‘zipper’ formed interactions between the negatively charged phosphate strands and positively charged grooves of opposing molecules [41–44]. On the other hand, owing to chiral nature of cholesteric phase induced by the addition of diethylenetriamine, competition was expected to occur between the two-dimensional columnar ordering and the tendency to twist, which finally induced and facilitated the formation of extraordinary impeller-like helical architectures.
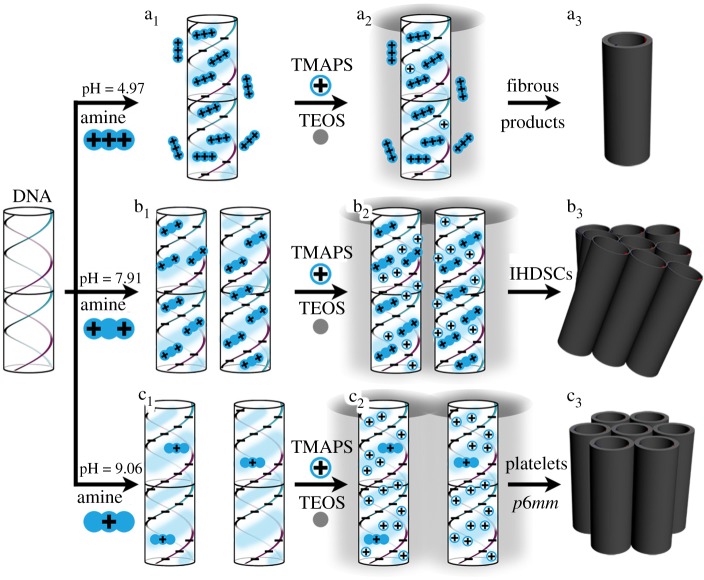
The handedness reversal of IHDSCs may originate from the different interaction strengths among negatively charged phosphate groups of DNA and positively charged (quaternary) ammonium groups of diethylenetriamine and TMAPS. As shown in our previous report, chiral DNA packing can be reversed from right-handed to left-handed attributing to the increase in linking strength between quaternary ammonium groups of DNA and phosphate groups DNA [18]. The interaction linking can be strengthened by increasing the TMAPS–DNA molar ratio, reaction temperature and pH value, which can induce the formation of right-handed impeller-like helical morphologies. The results indicate that weak interaction strength between quaternary ammonium groups of TMAPS and phosphate groups of DNA favours the formation of right-handed chiral DNA packing, whereas strong interaction strength is associated with left-handed long-range chirality. Compared with Mg2+, the interaction strength between DNA and diethylenetriamine is weaker because water molecules surrounding diethylenetriamine by hydrogen bonding will decrease the positive charge of the amines and hinder the linking between diethylenetriamine and DNA. When TMAPS was added to the reaction solution, a stronger interaction between DNA and TMAPS was formed owing to weaker strength between DNA and polyamine. Therefore, in the synthesis system, diethylenetriamine induces the formation of left-handed chiral DNA packing and corresponding macroscopic right-handed impeller-like helical architectures, whereas Mg2+ ion favours left-handed IHDSCs (scheme 1, which will be discussed in detail later). Unfortunately, the mechanism of formation of the chiral DNA packing structure and the corresponding helical architectures, which can be reversed depending on DNA–DNA interaction strength, is still unclear.
Scheme 1.
Schematic of the mechanism of formation of DNA packing synthesized with different pH in the presence of diethylenetriamine [17,18].
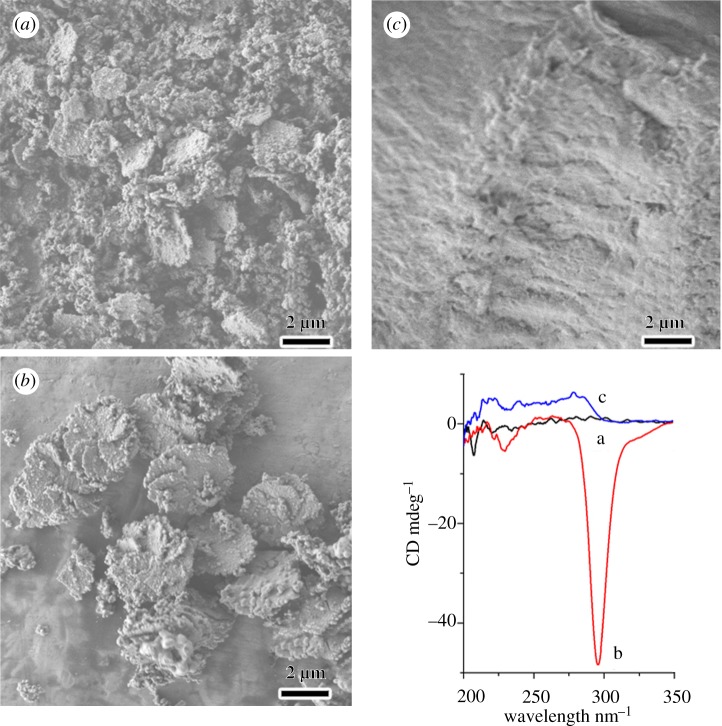
To investigate the linking behaviour of the ‘ion-bridging’ between DNA molecules, various amines were introduced to the synthesis system (figure 3). It was found that the impeller-like helical architecture cannot be obtained with addition of ethylamine, whereas hexagonal platelets and irregular nanoparticles, in this case, have been formed (figure 3a). Owing to the monovalent positive charge, ethylamine is difficult to link two adjacent DNA molecules and form chiral DNA packing structures, thus hexagonal platelets with two-dimensional columnar ordering were formed by the electrostatic ‘zipper’ effect. The non-appearance of DRCD signal further proves the absence of the long-range chirality. Divalent ethylenediamine, however, was found to induce the formation of IHDSCs (figure 3b). Compared with the sample synthesized with addition of diethylenetriamine (figure 1c), the sample synthesized in the presence of ethylenediamine exhibited a smaller inclination angle of the blades and larger core diameter. Impurities and particles with imperfect shapes can be also observed. The weaker non-conservative DRCD signals indicated the smaller twist angles or looser chiral packing ordering. The phenomenon depends on the divalent property of ethylenediamine. On the one hand, ethylenediamine can link two different phosphate groups of adjacent DNA molecules and further assemble to the chiral liquid crystal phase, which leads to the formation of impeller-like helical morphologies. On the other hand, in contrast with diethylenetriamine that has three amino groups, ethylenediamine is less charged, which weakens the linking strength of the DNA molecules and favours a loosely packed chiral DNA mesophase, showing smaller inclination angle of the blades and relatively lower crystallinity. Furthermore, in the presence of spermine, a highly positively charged amine, the IHDSCs were not readily formed. Irregular morphologies (not impeller) were obtained when spermine was used as the counterion (figure 3c). Although it has been reported that spermine can lead to the formation of the chiral cholesteric phase [21,23], the interaction strength between spermine and DNA may be too strong, which hindered the linking between the quaternary ammonium groups of TMAPS and phosphate groups of DNA molecules. Thus, the interaction strength between TMAPS and DNA is too weak to form an electrostatic ‘zipper’ and subsequent two-dimensional columnar ordering. Therefore, spermine did not facilitate impeller-like helical morphology. It is concluded that the formation of IHDSC morphology relies on both the type of ‘ion-bridging’ and the co-assembly of TMAPS and silica condensation along DNA molecules.
Figure 3.
Morphologies and structures of the DNA–silica complexes synthesized in the presence of various polyamines. Scanning electron microscopy images and corresponding diffuse-reflectance circular dichroism spectra of the samples synthesized with addition of (a) ethylamine, (b) ethylenediamine and (c) spermine.
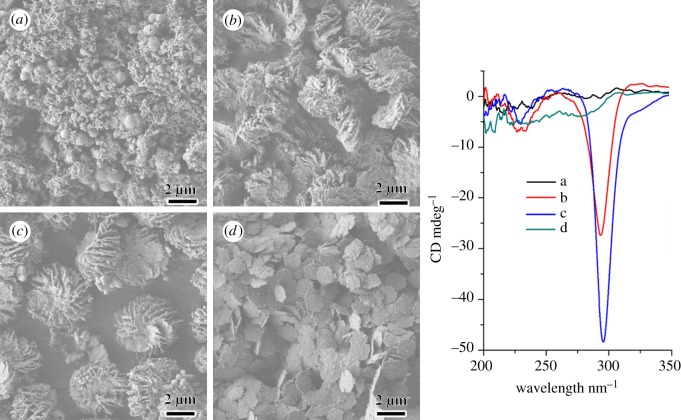
The ionization degree of DNA and polyamines may also affect the formation of IHDSCs, thus the pH value of the reaction solution was tuned by changing the acidity or basicity. Figure 4 shows macroscopic morphological transformation of the DCSs synthesized with addition of diethylenetriamine under different pH of 4.97, 5.80, 7.91 and 9.06, respectively. The SEM image of the sample synthesized at a pH value of 4.97 showed fibrous and spherical morphologies (figure 4a and electronic supplementary material, figure S1-a). When the pH value was raised to 5.80, IHDSCs with an average diameter of approximately 3 µm can be observed (figure 4b). The particle size became larger (average diameter of approx. 4 µm) with the increase of pH value to 7.91 (figure 4c). When the reaction was carried out at a pH value of 9.06, however, the macroscopic helicity disappeared. Hexagonal platelets with a uniform diameter of approximately 1.3 µm were obtained (figure 4d). It is interesting to note that TEM images of the samples synthesized at a pH value of 9.06 show a two-dimensional-hexagonal p6mm structure (electronic supplementary material, figures S1-d and S2-d), which is different from the typical IHDSCs with two-dimensional-square p4mm symmetry (electronic supplementary material, figures S1-b,c and S2-b,c). Corresponding DRCD signals of the DSCs synthesized with different pH values were also shown in figure 4. The sample synthesized at pH values higher than 10.89 showed irregular spherical and fibrous morphologies, similar to the samples synthesized at pH value of 4.97.
Figure 4.
Scanning electron microscopy images and corresponding diffuse-reflectance circular dichroism spectra of the DNA–silica complexes synthesized under different pH values. The pH values of reaction solutions were (a) 4.97, (b) 5.80, (c) 7.91 and (d) 9.06, respectively.
The formation of different macroscopic architectures can be explained in terms of different ionization degrees of phosphate groups of DNA and ammonium groups of diethylenetriamine (scheme 1). It is well-known that phosphate acid is a weak acid and the DNA molecule is a polyanion with an isoelectric point of approximately 4–4.5, whereas diethylenetriamine is a weak polycation. At the very early stage of the reaction, DNA molecules are dispersed in the reaction solution. When the pH value is low, DNA is less charged, whereas the ethylenetriamine is highly charged and interacts strongly with phosphate of DNA. An excess of ethylenetriamine hinders the interaction between DNA and TMAPS; DNA molecules can not form high-ordered packing and subsequently are transcribed into diverse fibrous products in the lower pH (scheme 1a). When the pH of reaction solution is neutral (in the pH range of 5.80–7.91), ethylenetriamine aggregates DNA into chiral packing and TMAPS acts as an electrostatic ‘zipper’ between quaternary ammonium phosphate groups, which finally lead to the formation of the left-handed-twisted p4mm-structured DNA packing structure. A certain twist angle for each layer, would be attributed to the linkage of two different DNA sites by ethylenetriamine (similar to Mg2+), and the dislocated array of DNA molecules possesses an intrinsic tendency to self-organize into cholesteric-like mesophases (scheme 1b). However, at the higher pH, the diethylenetriamne has low ionization and DNA is highly charged. The neutral ethylenetriamine can hardly interact with DNA, whereas TMAPS interact strongly with the phosphate group of DNA and subsequently assemble with TEOS into the silica framework. However, the repulsing force between negatively charged DNA and TEOS increases the distance between DNA molecules, which inhibits the formation of quaternary ammonium-phosphate electrostatic ‘zipper’ DNA–DNA contacts. Therefore, the usually observed columnar hexagonal close packing of DNA is formed [4] with a d-spacing of 3.0 nm (much larger than 2.5 nm in DSC with two-dimensional p4mm structure; scheme 1c). Therefore, the level of ionization of both the phosphate strands in DNA and the amino groups in diethylenetriamine can be easily changed by varying the pH value, which results in different repulsing forces between DNA molecules and further leads to different condensation states and DNA packing ordering.
It was also found that the handedness of IHDSCs (right-handed macroscopic IHDSC morphology) could not be reversed with the change of pH value owing to the strong interaction strength between DNA and TMAPS. Besides, the handedness of IHDSCs cannot be reversed by increasing the TMAPS–DNA molar ratio and reaction temperature. The results may originate from the interaction strength between negatively charged phosphate groups of DNA and positively charged amino groups of diethylenetriamine. Diethylenetriamine is a weak polycation, the ‘ion-bridging’ between DNA and ethylenetriamine cannot be obviously strengthened by increasing the TMAPS–DNA ratio, pH value and temperature. The weak interaction strength between DNA and diethylenetriamine facilitated the linking between DNA and TMAPS, and finally resulted in the formation of right-handed IHDSC architectures.
In order to further investigate the interaction between DNA and CSDA, APS has been employed as CSDA. Figure 5a,b shows SEM images of the DSCs synthesized without any counterion addition and with addition of diethylenetriamine, respectively. Thin platelets with irregular shapes were synthesized in the absence of diethylenetriamine under mild conditions (pH value of 7.6 and synthesis temperature of 25°C). The platelets are uniform in size with an average diameter of approximately 1.5 µm and a uniform thickness of approximately 100 nm. The HRTEM observations showed the two-dimensional-square p4mm structure (electronic supplementary material, figure S3-a), suggesting the electrostatic ‘zipper’ with interactions between the phosphate group of DNA and amino group of APS, similar to the sample shown in figure 1a. Other morphologies including spheres and fibres can be facilitated by varying the pH value of the reaction system (not shown). With addition of diethylenetriamine for inducing chiral DNA packing, it was found that both helical morphology and platelet morphology cannot be obtained (electronic supplementary material, figure S3-b). It is well-known that APS contains an amino group in the hydrophilic site whose ionization degree can be tuned by changing the pH. Considering that the ionization degree of both DNA and diethylenetriamine can be changed, the proper interaction among DNA, diethylenetriamine and APS may be difficult to be formed. Therefore, only nanoparticles with irregular morphology were obtained. The formation of IHDSCs was also investigated by various amines tethered to CSDA. DAPS and TAPS, with two and three amino groups in the hydrophilic sites, respectively, have been introduced into the DSC synthesis system. However, the amino groups of CSDAs were tethered to the silicate surface, which may block the formation of ‘ion-bridging’ between adjacent DNA molecules, and the strong electrostatic interaction between DAPS–TAPS and DNA is incongruent for the electrostatic ‘zipper’ formation. Therefore, only DAPS and TAPS induced irregular fibrous morphologies (figure 5c,d and electronic supplementary material, figure S3-c,d).
Figure 5.
Morphologies of the DNA–silica complexes synthesized with various co-structure-directing agents (CSDAs). With aminopropyltrimethoxysilane as CSDAs, the samples were synthesized without any counterions addition (DNA–APS) (a) and with addition of diethylenetriamine (DNA–diethylenetriamine–APS) (b), respectively. Without the addition of any counterions, the samples were synthesized with N-(2-aminoethyl)-3-aminopropyl methyltrimethoxysilane (DNA–DAPS) (c) and (3-trimethoxysilylpropyl)diethylenetriamine (DNA–TAPS) (d) as a CSDA, respectively. The pH of synthesis solutions was 7.60.
4. Conclusion
Chiral DNA liquid crystal phase as well as the corresponding helical silica replica were induced and facilitated in the presence of counterions. The handedness of IHDSCs can be tuned and even reversed by changing the counterions. It was found that the formation of impeller-like helical morphology depends on the types of CDSAs, polyamines and pH values of the synthetic mixture. Moreover, ionization degrees of DNA, CSDAs and polyamines exerted a key effect on the formation of IHDSCs. However, the reason for the enantiomeric DNA packing induced in the presence of Mg2+ and polyamine remains unclear. Further work is in progress to find the formation and evolution mechanism of impeller-like helical architectures. We suggest that the research can provide a possible route to condense DNA in fluid structures using polyamines and create a new class of ordered macroscopic helical materials.
Acknowledgements
We acknowledge the support of the National Natural Science Foundation of China (grant no. 20890121) and the 973 project (2009CB930403).
References
- 1.Cölfen H., Mann S. 2003. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 42, 2350–2365 10.1002/anie.200200562 (doi:10.1002/anie.200200562) [DOI] [PubMed] [Google Scholar]
- 2.Strzelecka T. E., Davidson M. W., Rill R. L. 1988. Multiple liquid crystal phases of DNA at high concentrations. Nature 331, 457–460 10.1038/331457a0 (doi:10.1038/331457a0) [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield V. A. 1996. DNA condensation. Curr. Opin. Struc. Biol. 6, 334–341 10.1016/S0959-440X(96)80052-2 (doi:10.1016/S0959-440X(96)80052-2) [DOI] [PubMed] [Google Scholar]
- 4.Livolant F., Leforestier A. 1996. Condensed phases of DNA: structures and phase transitions. Prog. Polym. Sci. 21, 1115–1164 10.1016/S0079-6700(96)00016-0 (doi:10.1016/S0079-6700(96)00016-0) [DOI] [Google Scholar]
- 5.Livolant F., Levelut A., Doucet J., Benoit J. 1989. The highly concentrated liquid-crystalline phase of DNA is columnar hexagonal. Nature 339, 724–726 10.1038/339724a0 (doi:10.1038/339724a0) [DOI] [PubMed] [Google Scholar]
- 6.Reich Z., Wachtel E. J., Minsky A. 1994. Liquid-crystalline mesophases of plasmid DNA in bacteria. Science 264, 1460–1463 10.1126/science.8197460 (doi:10.1126/science.8197460) [DOI] [PubMed] [Google Scholar]
- 7.Reich Z., Levin-Zaidman S., Gutman S. B., Arad T., Minsky A. 1994. Supercoiling-regulated liquid-crystalline packaging of topologically-constrained, nucleosome-free DNA molecules. Biochemistry 33, 14 177–14 184 10.1021/bi00251a029 (doi:10.1021/bi00251a029) [DOI] [PubMed] [Google Scholar]
- 8.Koltover I., Salditt T., Rädler J. O., Safinya C. R. 1998. An inverted hexagonal phase of cationic liposome–DNA complexes related to DNA release and delivery. Science 281, 78–81 10.1126/science.281.5373.78 (doi:10.1126/science.281.5373.78) [DOI] [PubMed] [Google Scholar]
- 9.Evans H. M., Ahmad A., Ewert K., Pfohl T., Martin-Herranz A., Bruinsma R., Safinya C. 2003. Structural polymorphism of DNA–dendrimer complexes. Phys. Rev. Lett. 91, 075501. 10.1103/PhysRevLett.91.075501 (doi:10.1103/PhysRevLett.91.075501) [DOI] [PubMed] [Google Scholar]
- 10.Pelta J., Livolant F., Sikorav J. L. 1996. DNA aggregation induced by polyamines and cobalthexamine. J. Biol. Chem. 271, 5656. 10.1074/jbc.271.10.5656 (doi:10.1074/jbc.271.10.5656) [DOI] [PubMed] [Google Scholar]
- 11.Kamien R. D., Nelson D. R. 1995. Iterated moiré maps and braiding of chiral polymer crystals. Phys. Rev. Lett. 74, 2499–2502 10.1103/PhysRevLett.74.2499 (doi:10.1103/PhysRevLett.74.2499) [DOI] [PubMed] [Google Scholar]
- 12.Kamien R. D., Nelson D. R. 1996. Defects in chiral columnar phases: tilt-grain boundaries and iterated moiré maps. Phys. Rev. E 53, 650–666 10.1103/PhysRevE.53.650 (doi:10.1103/PhysRevE.53.650) [DOI] [PubMed] [Google Scholar]
- 13.Livolant F., Leforestier A. 2000. Chiral discotic columnar germs of nucleosome core particles. Biophys. J. 78, 2716–2729 10.1016/S0006-3495(00)76816-0 (doi:10.1016/S0006-3495(00)76816-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leforestier A., Bertin A., Dubochet J., Richter K., Sartori Blanc N., Livolant F. 2008. Expression of chirality in columnar hexagonal phases or DNA and nucleosomes. Comptes Rendus Chim. 11, 229–244 10.1016/j.crci.2007.09.008 (doi:10.1016/j.crci.2007.09.008) [DOI] [Google Scholar]
- 15.Che S., Garcia-Bennett A. E., Yokoi T., Sakamoto K., Kunieda H., Terasaki O., Tatsumi T. 2003. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat. Mater. 2, 801–805 10.1038/nmat1022 (doi:10.1038/nmat1022) [DOI] [PubMed] [Google Scholar]
- 16.Che S., Liu Z., Ohsuna T., Sakamoto K., Terasaki O., Tatsumi T. 2004. Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 10.1038/nature02529 (doi:10.1038/nature02529) [DOI] [PubMed] [Google Scholar]
- 17.Jin C., Han L., Che S. 2009. Synthesis of a DNA–silica complex with rare two-dimensional square p4mm symmetry. Angew. Chem. Int. Ed. 48, 9268–9272 10.1002/anie.200904494 (doi:10.1002/anie.200904494) [DOI] [PubMed] [Google Scholar]
- 18.Liu B., Han L., Che S. 2012. Formation of enantiomeric impeller-like helical architectures by DNA self-assembly and silica mineralization. Angew. Chem. Int. Ed. 51, 923–927 10.1002/anie.201105445 (doi:10.1002/anie.201105445) [DOI] [PubMed] [Google Scholar]
- 19.Tabor C. W., Tabor H. 1984. Polyamines. Annu. Rev. Biochem. 53, 749–790 10.1146/annurev.bi.53.070184.003533 (doi:10.1146/annurev.bi.53.070184.003533) [DOI] [PubMed] [Google Scholar]
- 20.Thomas T., Thomas T. 2001. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 58, 244–258 10.1007/PL00000852 (doi:10.1007/PL00000852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas T., Balabhadrapathruni S., Gallo M. A., Thomas T. 2002. Development of polyamine analogs as cancer therapeutic agents. Oncol. Res.: Preclin. Clin. Cancer Ther. 13, 123–135 10.1007/PL00000852 (doi:10.1007/PL00000852) [DOI] [PubMed] [Google Scholar]
- 22.Sikorav J., Pelta J., Livolant F. 1994. A liquid crystalline phase in spermidine-condensed DNA. Biophys. J. 67, 1387–1392 10.1016/S0006-3495(94)80640-X (doi:10.1016/S0006-3495(94)80640-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelta J., Jr, Durand D., Doucet J., Livolant F. 1996. DNA mesophases induced by spermidine: structural properties and biological implications. Biophys. J. 71, 48–63 10.1016/S0006-3495(96)79232-9 (doi:10.1016/S0006-3495(96)79232-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raspaud E., Durand D., Livolant F. 2005. Interhelical spacing in liquid crystalline spermine and spermidine-DNA precipitates. Biophys. J. 88, 392–403 10.1529/biophysj.104.040113 (doi:10.1529/biophysj.104.040113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izatt R. M., Christensen J. J., Rytting J. H. 1971. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem. Rev. 71, 439–481 10.1021/cr60273a002 (doi:10.1021/cr60273a002) [DOI] [PubMed] [Google Scholar]
- 26.Bloomfield V. A. 1991. Condensation of DNA by multivalent cations: considerations on mechanism. Biopolymers 31, 1471–1481 10.1002/bip.360311305 (doi:10.1002/bip.360311305) [DOI] [PubMed] [Google Scholar]
- 27.Duguid J. G., Bloomfield V. A. 1995. Aggregation of melted DNA by divalent metal ion-mediated cross-linking. Biophys. J. 69, 2642–2648 10.1016/S0006-3495(95)80134-7 (doi:10.1016/S0006-3495(95)80134-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berti L., Burley G. A. 2008. Nucleic acid and nucleotide-mediated synthesis of inorganic nanoparticles. Nat. Nanotechnol. 3, 81–87 10.1038/nnano.2007.460 (doi:10.1038/nnano.2007.460) [DOI] [PubMed] [Google Scholar]
- 29.Sundaresan N., Suresh C. H., Thomas T., Thomas T., Pillai C. 2008. Liquid crystalline phase behavior of high molecular weight DNA: a comparative study of the influence of metal ions of different size, charge and binding mode. Biomacromolecules 9, 1860–1869 10.1021/bm800101x (doi:10.1021/bm800101x) [DOI] [PubMed] [Google Scholar]
- 30.Jordan C., Lerman L., Venable J. 1972. Structure and circular dichroism of DNA in concentrated polymer solutions. Nature 236, 67–70 10.1038/newbio236067a0 (doi:10.1038/newbio236067a0) [DOI] [PubMed] [Google Scholar]
- 31.Livolant F., Maestre M. F. 1988. Circular dichroism microscopy of compact forms of DNA and chromatin in vivo and in vitro: cholesteric liquid-crystalline phases of DNA and single dinoflagellate nuclei. Biochemistry 27, 3056–3068 10.1021/bi00408a058 (doi:10.1021/bi00408a058) [DOI] [PubMed] [Google Scholar]
- 32.Bustamante C., Samori B., Builes E. 1991. Daunomycin inverts the long-range chirality of DNA condensed states. Biochemistry 30, 5661–5666 10.1021/bi00237a004 (doi:10.1021/bi00237a004) [DOI] [PubMed] [Google Scholar]
- 33.Minsky A. 1998. The chiral code: from DNA primary structures to quaternary assemblies. Chirality 10, 405–414 10.1002/(SICI)1520-636X(1998)10:5%3C405::AID-CHIR6%3E3.0.CO;2-4 (doi:10.1002/(SICI)1520-636X(1998)10:5<405::AID-CHIR6>3.0.CO;2-4) [DOI] [Google Scholar]
- 34.Kypr J., Kejnovská I., Renčiuk D., Vorlíčková M. 2009. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 37, 1713–1725 10.1093/nar/gkp026 (doi:10.1093/nar/gkp026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spada G. P., Brigidi P., Gottarelli G. 1988. The determination of the handedness of cholesteric superhelices formed by DNA fragments. J. Chem. Soc. Chem. Commun. 953–954 10.039/C39880000953 (doi:10.039/C39880000953) [DOI] [Google Scholar]
- 36.Gottarelli G., Spada G. P., Mariani P., De Morais M. M. 1991. The effect of ethidium bromide on the liquid crystalline phases of aqueous DNA. Chirality 3, 227–232 10.1002/chir.530030404 (doi:10.1002/chir.530030404) [DOI] [PubMed] [Google Scholar]
- 37.Bonazzi S., Capobianco M., De Morais M. M., Garbesi A., Gottarelli G., Mariani P., Ponzi Bossi M. G., Spada G. P., Tondelli L. 1991. Four-stranded aggregates of oligodeoxyguanylates forming lyotropic liquid crystals: a study by circular dichroism, optical microscopy, and X-ray diffraction. J. Am. Chem. Soc. 113, 5809–5816 10.1021/ja00015a040 (doi:10.1021/ja00015a040) [DOI] [Google Scholar]
- 38.Proni G., Gottarelli G., Mariani P., Spada G. P. 2000. The chirality of the cholesteric phases of DNA and G-wires: its connection to their molecular structures. Chem. Eur. J. 6, 3249–3253 10.1002/1521-3765(20000901)6:17%3C3249::AID-CHEM3249%3E3.0.CO;2-F (doi:10.1002/1521-3765(20000901)6:17<3249::AID-CHEM3249>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 39.Raspaud E., Olvera De La Cruz M., Sikorav J. L., Livolant F. 1998. Precipitation of DNA by polyamines: a polyelectrolyte behavior. Biophys. J. 74, 381–393 10.1016/S0006-3495(98)77795-1 (doi:10.1016/S0006-3495(98)77795-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saminathan M., Antony T., Shirahata A., Sigal L. H., Thomas T., Thomas T. 1999. Ionic and structural specificity effects of natural and synthetic polyamines on the aggregation and resolubilization of single-, double-, and triple-stranded DNA. Biochemistry 38, 3821–3830 10.1021/bi9825753 (doi:10.1021/bi9825753) [DOI] [PubMed] [Google Scholar]
- 41.Kornyshev A., Leikin S. 1999. Electrostatic zipper motif for DNA aggregation. Phys. Rev. Lett. 82, 4138–4141 10.1103/PhysRevLett.82.4138 (doi:10.1103/PhysRevLett.82.4138) [DOI] [Google Scholar]
- 42.Chesnoy S., Huang L. 2000. Structure and function of lipid–DNA complexes for gene delivery. Annu. Rev. Biophys. Biomol. Struct. 29, 27–47 10.1146/annurev.biophys.29.1.27 (doi:10.1146/annurev.biophys.29.1.27) [DOI] [PubMed] [Google Scholar]
- 43.Evans H. M., Ahmad A., Ewert K., Pfohl T., Martin-Herranz A., Bruinsma R., Safinya C. 2003. Structural polymorphism of DNA–dendrimer complexes. Phys. Rev. Lett. 91, 755 011–755 014 10.1103/PhysRevLett.91.075501 (doi:10.1103/PhysRevLett.91.075501) [DOI] [PubMed] [Google Scholar]
- 44.Harreis H. M., Likos C. N., Löwen H. 2003. Azimuthal frustration and bundling in columnar DNA aggregates. Biophys. J. 84, 3607–3623 10.1016/S0006-3495(03)75092-9 (doi:10.1016/S0006-3495(03)75092-9) [DOI] [PMC free article] [PubMed] [Google Scholar]