Abstract
The cover scales on the wing of the Emerald-patched Cattleheart butterfly, Parides sesostris, contain gyroid-type biological photonic crystals that brightly reflect green light. A pigment, which absorbs maximally at approximately 395 nm, is immersed predominantly throughout the elaborate upper lamina. This pigment acts as a long-pass filter shaping the reflectance spectrum of the underlying photonic crystals. The additional effect of the filtering is that the spatial distribution of the scale reflectance is approximately angle-independent, leading to a stable wing pattern contrast. The spectral tuning of the original reflectance is verified by photonic band structure modelling.
Keywords: photonic crystal, Papilionidae, optical filtering, iridescence, biomaterials
1. Introduction
The brilliant body colours of many animals are due to the specific interaction of incident light with complex structured assemblies of biomaterial that have refractive index variations of the order of the wavelengths of visible light [1–3]. The structural colours are especially diverse in butterflies. Their wing scales have a wide variety of nanostructures with several levels of complexity [4]. The biological function of the structural colorations is mostly for communication and display [5] and also for camouflage [6].
The structural colours of a number of papilionids and lycaenids are produced by three-dimensional biological photonic crystals, which were recently identified as single-network gyroid-type photonic crystals [6–10]. The unit cell defining the crystal is composed of a network of the biomaterial chitin and air. The structure is defined by a minimal surface, the inter-material dividing surface, which divides the two interconnected networks. Gyroids are special in that their chiral structure gives rise to circular dichroism (CD), i.e. the reflectance of incident circular-polarized light depends on the handedness [9,11].
A beautiful representative of the butterflies with brightly coloured scales is the Emerald-patched Cattleheart, Parides sesostris, native to the Americas (figure 1a). Earlier studies have unequivocally identified photonic crystals in the green cover scales. The crystals are assemblies with gyroid-type morphology [7,8] with a chitin filling fraction between 30 and 40 per cent and a cubic unit cell size in the range of 280–330 nm. The photonic crystals are covered by a thick lamellar structure, called a honeycomb [9]. The green scales reflect incident light diffusely and show no specific scale iridescence when illuminated from the side of the honeycomb, which is the case when the green scales are attached to the wing. However, clear iridescence is observed when an isolated green scale is illuminated from the opposite side [3]. Poladian et al. [9] suggested that the honeycomb functions in suppressing the iridescence of the photonic crystal by acting either as a collimating or a diffusing structure.
Figure 1.
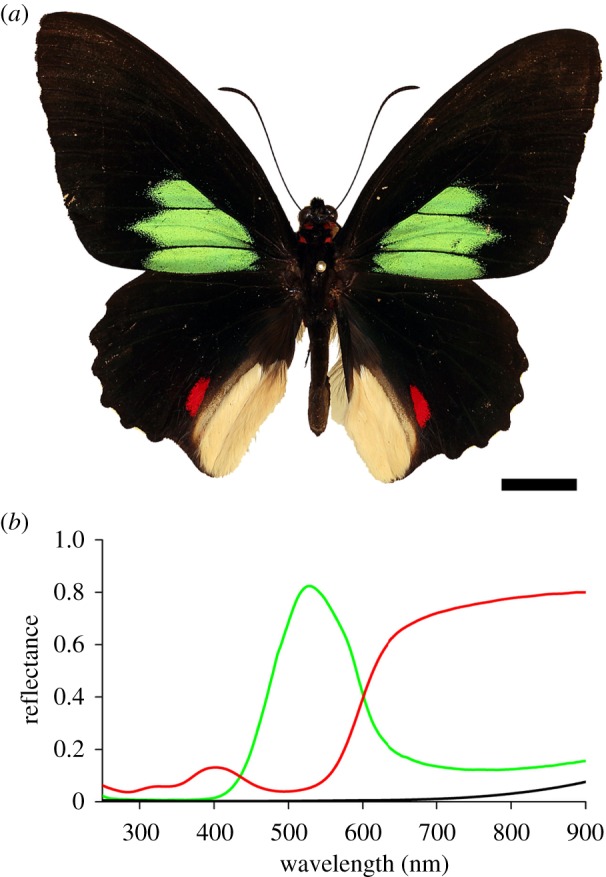
Emerald-patched Cattleheart, Parides sesostris. (a) Photograph showing the upper side of the butterfly with large, green-coloured spots on the forewing and smaller, red spots on the hindwing, framed by black borders. Scale bar, 1 cm. (b) Reflectance spectra of the green- and red-coloured wing patches and the black frame of the upper side of the butterfly wing. Green solid line, green patches; red solid line, red patches; black solid line, black patches.
In the present paper, we revisit the photonic structure of P. sesostris scales and show that the suppression of the photonic crystals' iridescence is mainly achieved owing to a pigment immersed throughout the honeycomb structure which in addition acts as a scattering device, thus randomizing light propagation. By photonic band structure modelling, we show how the pigment narrows the intrinsic reflectance spectrum of the photonic structure.
2. Material and methods
2.1. Animals
The investigated specimens of the Emerald-patched Cattleheart, P. sesostris (Cramer, 1779; Papilionidae), caught in Satipo, Peru, were obtained through Tropical Butterflies and Insects of America (Tampa, FL, USA).
2.2. Photography
A specimen of P. sesostris of the Lepidoptera collection in the Natural History Museum Naturalis (Leiden, The Netherlands; curator Dr B. van Bekkum-Ansari) was photographed with a Canon EOS-30D camera equipped with an F70 macro-objective and a Nikon SB-800 flash (figure 1a). Details of the scale arrangement on the wings were photographed with a Zeiss Axioskop microscope (Carl Zeiss AG, Oberkochen, Germany), applying epi-illumination and using a Kappa DX-40 (Kappa optronics GmbH, Gleichen, Germany) digital camera. For fluorescence photographs, 450–490 nm or 510–560 nm excitation light was used and the emission was filtered by a 520 or a 590 nm high-pass filter, respectively.
2.3. Spectrophotometry
The absorbance spectrum of the pigment in the green wing scales of P. sesostris was calculated from transmittance spectra obtained from single wing scales, immersed in a fluid with refractive index 1.56 (Cargille Labs, Cedar Grove, NJ, USA), and measured with a microspectrophotometer; a Leitz-Ortholux microscope with a 20/0.46 Olympus objective, connected to an AvaSpec-2048-2 spectrometer (Avantes, Eerbeck, The Netherlands). Reflectance spectra of intact wings were measured with a bifurcated probe (Avantes FCR-7UV200) connected to the AvaSpec-2048-2 spectrometer. The light source was a xenon or a Deuterium-Halogen (Avantes D(H)-S) lamp. For the reflectance measurements, a white diffuse reflectance tile (Avantes WS-2) served as a reference.
2.4. Imaging scatterometry
The far-field spatial distribution of the light scattered from single scales and wing patches, glued to the end of pulled micropipettes, was visualized with an imaging scatterometer (ISM). The scatterometer is built around an ellipsoidal mirror, which collects light from a full hemisphere around its first focal point where the sample is positioned [12]. A small piece of magnesium oxide served as a white diffuse reference object. Images were acquired with an Olympus DP-70 camera and were subsequently corrected for geometrical distortions using a Matlab-routine.
2.5. Scanning electron microscopy
The anatomical structure of the wing scales was investigated, after sputtering with palladium, with a Philips XL30-ESEM scanning electron microscope (SEM).
2.6. Photonic band structure modelling
We modelled the single-network gyroid structure using the MIT photonic band structure solver [13]. The filling function of a single-network gyroid lattice is given by Schwartz' G surface:
where X = 2πx/a, Y = 2πy/a and Z = 2πz/a, with x, y and z being the coordinates of the cubic basic structure with lattice constant a; for the P. sesostris scales, we took a = 310 nm. A threshold parameter t defines the separation between the interconnected cuticle and air networks and thus determines the filling fraction of the material in the unit cell. In the case where the volume is filled with cuticle (f(X,Y,Z) < t), the refractive index of the material was set to n(X,Y,Z) = 1.56; for the air-filled volume areas (f(X,Y,Z) ≥ t), the refractive index was n(X,Y,Z) = 1. The threshold was set to −0.3, resulting in a cuticle filling fraction of approximately 40 per cent [3,7].
3. Results
3.1. Optical appearance and scale fluorescence
The visual appearance of the butterfly P. sesostris is quite striking: while the overall colour of the upper side of the wings is jet-black, owing to highly melanized scales, large green spots dominate the forewings and smaller red spots mark the hindwings (figure 1a). The green areas have a metallic appearance, clearly indicating a structural basis for the coloration, but the red spots display a more diffuse coloration. Spectral measurements with a bifurcated probe yield distinctly different reflectance spectra with shapes, suggesting that the green colour has indeed a structural basis and that the red colour is owing to a pigment that only absorbs in the short and middle wavelength ranges (figure 1b).
Observing the green wing areas with an epi-illumination microscope shows a dense lattice of green-coloured cover scales with black ground scales seen below them (figure 2a). The green colour remains virtually identical upon rotation of the wings, suggesting the presence of a diffusive structure. Very occasionally, aberrant ochre-coloured cover scales can be observed as part of the original scale assembly (white arrowheads in figure 2), indicating the presence of a short-wavelength absorbing pigment. We therefore further investigated the pigment distribution by using the fluorescence attachment of the microscope. Blue- and green-excitation light revealed that the cover scales distinctly fluorescence (figure 2b,c). The colours of the emission of the green and ochre scales observed with blue and green excitation light are very similar, although the fluorescence of the ochre scales is somewhat weaker. This indicates that the different scale types contain the same pigment, and that the amount of pigment is smaller in the ochre scales.
Figure 2.
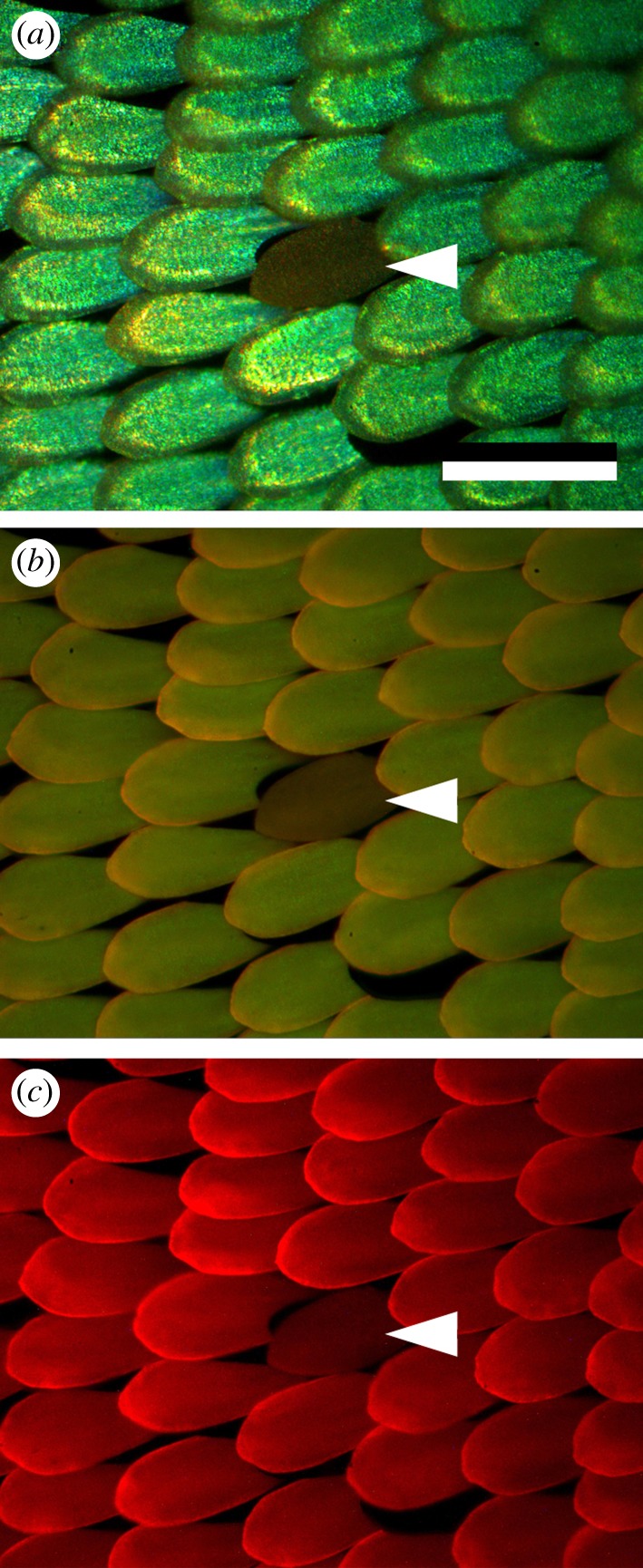
Reflection and fluorescence of the scales in the green wing area. (a) RGB photograph with white light epi-illumination. A dense lattice of green-coloured cover scales is seen above dark brown ground scales. An aberrant ochre scale is seen (white arrowhead). (b) Blue-induced green–yellow fluorescence. (c) Green-induced red fluorescence. The ochre scale fluorescence more weakly than the green scales. Scale bar, 100 µm.
To characterize the pigment, we measured the reflectance and transmittance spectra of single cover scales with a microspectrophotometer (figure 3). The reflectance spectra of individual green cover scales have a peak wavelength λ = 540 ± 5 nm and closely resemble the probe spectra (figure 1b). The reflectance spectra of the ochre scales are virtually flat above λ ∼ 550 nm, but the reflectance decreases with decreasing wavelength, presumably owing to the short-wavelength absorbing pigment [14]. We measured transmittance spectra of isolated green cover scales immersed in a fluid with refractive index n = 1.56; this value is close to that of butterfly scale chitin [15]. The measured spectra indeed revealed a distinct short-wavelength absorbing pigment. The transmittance spectra of the green cover scales and the reflectance spectra of the ochre scales have very similar shapes, confirming the assumption above that the same pigment is present in the green and ochre scales. The transmittance spectra, T(λ), straightforwardly yield absorbance spectra, A(λ), of the pigment via A(λ) = –log10[T(λ)] (figure 3c).
Figure 3.
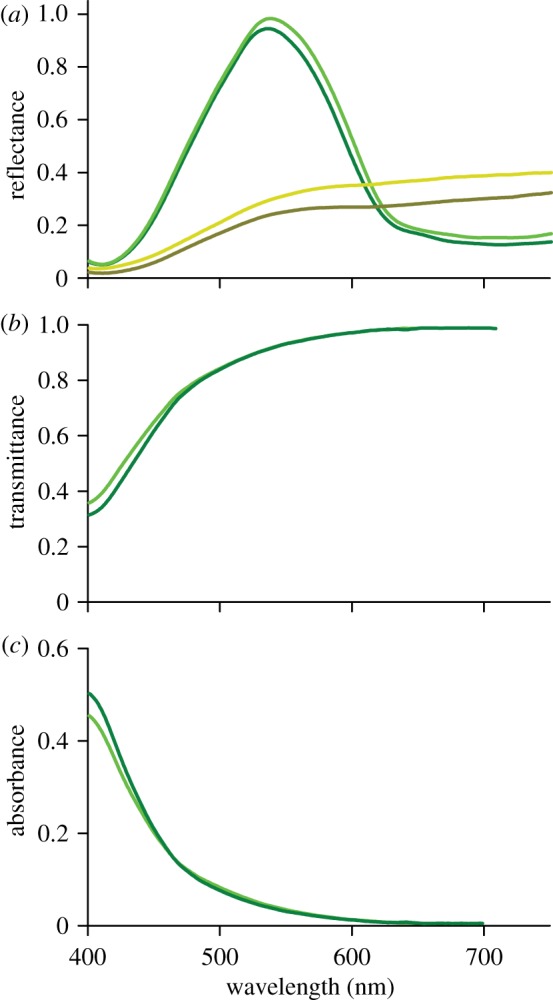
Reflectance and transmittance spectra of cover scales. (a) Reflectance spectra of two green and two ochre scales on an excised wing patch. (b) Transmittance spectra of two isolated green scales immersed in a fluid with refractive index n = 1.56. (c) Absorbance spectra calculated from the transmittance spectra.
3.2. Scale ultrastructure
We investigated the anatomy of the Parides scales with SEM (figure 4a; see also fig. 21 of Ghiradella [4]). Both cover and ground scales are composed of two laminae. The ground scales have a classical shape [4] with a thin, about flat lower lamina and a structured upper lamina with ridges and crossribs (arrowhead in figure 4a and electronic supplementary material, figure S2). The cover scales are very differently and much more elaborately structured. The upper lamina, thickness 3–5 µm, has a ‘honeycomb’ structure [9], which is basically a stretched and elaborated version of the upper lamina of the ground scales. The honeycomb consists of tall ridges connected by numerous crossribs [4]. The lower lamina is thin and about as flat as in the ground scales, but a prominent layer exists in between the upper and lower lamina of the cover scales. This layer, with thickness 4.0 ± 0.3 µm [9], consists of domains of single-network gyroid photonic crystals [7,8]. We determined a typical domain size of approximately 5 × 5 µm2 and for the cubic unit cell size of the gyroid photonic crystals 310 ± 30 nm, in agreement with previous studies [7,8].
Figure 4.
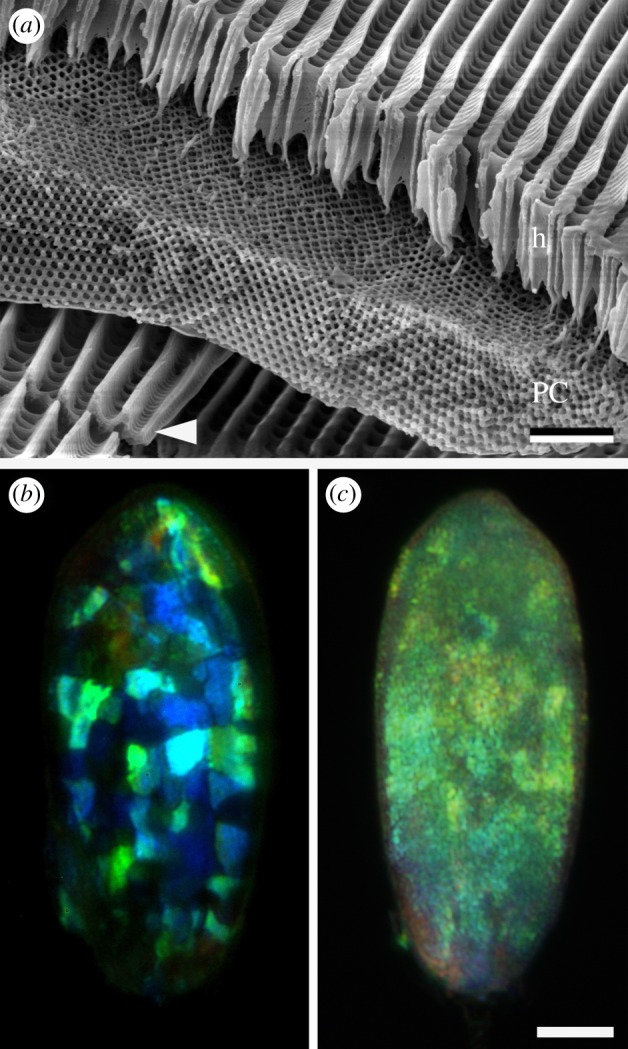
Anatomy of a cover scale and filter effects. (a) SEM of a fractured scale showing the structural composition of the scale. The approximately 5 µm thick single-network gyroid photonic crystal layer (PC) is covered by the approximately 5 µm honeycomb structure (h), acting as a pigment filter. Note the undifferentiated ground scale below the cover scale (white arrowhead). Scale bar, 2 µm. (b) A single scale upside-down observed through crossed polarizers. (c) A single scale upside-up observed through crossed polarizers. Single domains are clearly visible when the scale is upside-down (b), but are indiscernible in the normal scale orientation (c). Scale bar, 20 µm.
The presence of the crystal domains can also be demonstrated by light microscopy. When observing the cover scales from the adwing (under side) through an epi-illumination polarization microscope with crossed analyser and polarizer, differently coloured domains are strikingly visible (figure 4b). The intensity of the domain reflections strongly varies upon rotation of the scale. When scales are observed from the abwing (upper side), the domains are vaguely visible, apparently due to the filtering and diffusing effects of the honeycomb structure in the upper lamina (figure 4c; see also electronic supplementary material, figure S1).
3.3. Iridescence of the wing scales
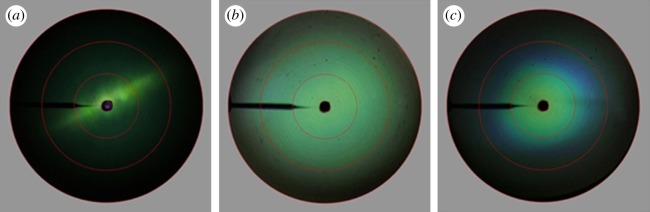
We further investigated the effect of the honeycomb in the upper lamina on the reflection properties of the photonic crystal layer by imaging scatterometry. Single wing scales were glued to the tip of pulled glass micropipettes, and we then measured the spatial scattering profiles applying white-light illumination with variable apertures [12,16]. Figure 5a presents the scatterogram of a single cover scale owing to about normal, narrow aperture (approx. 5°) illumination with white light. The scattering pattern is very directional and marked by a green line. The line is presumably owing to diffraction at the raster of parallel ridges that extends out of the upper lamina, because similar diffraction lines are seen in the scatterograms of other scales with clear ridges, notably in Morpho butterflies [12] and pierids [17,18]. The scatterogram additionally features a diffuse green background. This runs counter to what may be expected for ideal photonic crystals. When the domains are large, illumination with a narrow aperture beam creates a single prominent spot [19], and when the domains are small-grain, a diffuse scatterogram with discernible spots results [6]. Presumably therefore, the honeycomb in the upper layer of the green scales affects the reflection properties of the gyroid-type photonic crystals (figure 4b).
Figure 5.
Scattering patterns of a single cover scale. (a) Narrow-beam white-light illumination. The scattering is primarily a restricted line. (b,c) Wide-aperture white-light illumination of the upper side (b) and the under side (c) of a single scale. The scattering by the scale upper (abwing) side is green and virtually angle-independent, while scattering by the under (adwing) side shows strong iridescence, i.e. a blue shift occurs with an increasing angle of light incidence. The red circles indicate scattering angles of 5°, 30°, 60° and 90°.
To further assess the effect of the upper pigment filter on the photonic crystal reflection, we illuminated the under and the upper side of a single scale with a wide-aperture white-light beam (figure 5b,c). The resulting scatterogram of the upper side is green and approximately angle-independent. Reflectance measurements from various spots in the scatterogram using an optical fibre [18,20] indeed showed no changes in the spectra with changes of the detection angle or polarization of the incident light. The under side of the scale however showed strong iridescence, i.e. the peak wavelength changed with the angle of light incidence and observation (figure 5c). The reflectance in the centre of the scatterogram is mainly green-coloured, identical to the reflected light with normal illumination of the upper side. For larger scattering angles, greater than 30°, the reflected colour is strongly blue-shifted. We hence conclude that the upper honeycomb layer acts as both a diffuser for the reflected light and a suppressor of the iridescence of the gyroid photonic crystals.
3.4. Photonic band structure diagram modelling
To understand the impact of the pigment filter on the ‘original’ photonic response of the photonic crystals, we simulated the photonic response of an ideal gyroid photonic crystal using a block-iterative frequency-domain solver for Maxwell's equations [13]. We calculated the photonic band structure diagram (PBD) for a chitin-filling fraction of 40 per cent (t = −0.3), converted into wavelengths by using a unit cell size of a = 310 nm (figure 6). Constructive interference happens only for paths lying on the outer borders of the first Brillouin zone (FBZ) of the underlying unit cell structure, because Bragg's law is fulfilled for these space points. For the gyroid morphology in the wing scales, the underlying unit cell is base-centred cubic and the FBZ thus has face-centred cubic symmetry [21,22].
Figure 6.
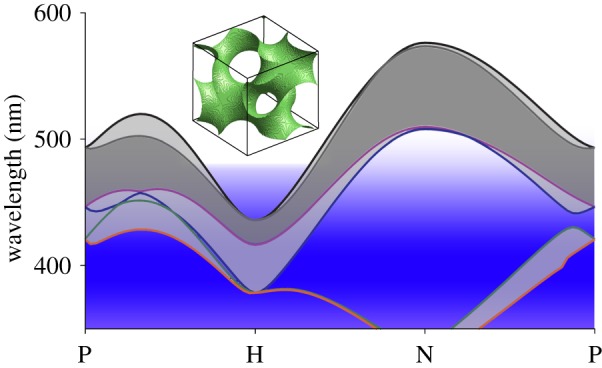
Photonic band structure diagram of the single-network gyroid PC of P. sesostris of which the lowest six bands are shown. The photonic bandgap opens between separated bands, causing reflection of incident light in the regime between the bands (grey shaded areas). The abscissa indicates the high-symmetry points of the first Brillouin zone determining the reflectance from the photonic crystal (see text). Further, the absorption range of the pigment filtering the reflection of the photonic crystal is indicated by the blue area. The inset shows the unit cell of the gyroid nanostructure used for modelling.
The peak reflectance of the photonic crystals in the P. sesostris green scales strongly changes with their orientation. For the investigated crystal structure, the longest peak wavelength (the mid of the bandgap) of approximately 560 nm is predicted for a crystal oriented with point N upwards, while for a H-oriented crystal a multi-peaked reflectance with a minimal peak of approximately 395 nm is predicted. With a peak wavelength ratio of the lowest and highest wavelength of approximately 1.43 [9], this iridescence is quite strong for an unfiltered gyroid structure.
The measured reflectance spectra had peak wavelengths at 540 ± 10 nm, while the pigment in the upper layer absorbs below 450 nm (figure 6, blue band). Figure 6 illustrates that the pigment filter strongly suppresses the gyroid photonic crystal's iridescence. The filtering narrows the reflectance spectrum to the wavelength range of 450–600 nm. Consequently, blue- and UV-reflecting crystals (figure 4b) do not contribute to the overall wing colour.
4. Discussion
4.1. Function of the pigment filter
The scales in the brilliant green-coloured wing areas of P. sesostris have a special photonic system consisting of a layer with a honeycomb structure on the top of a layer with differently oriented gyroid-type photonic crystals (figure 4a). The honeycomb structure contains a pigment, absorbing in the UV- and blue-wavelength range, which acts as a dense optical filter in front of the photonic crystals. The diffusive honeycomb causes approximately angle-independent scattering (figure 5 and electronic supplementary material, figure S1), confirming the hypothesis of Poladian et al. [9]. We furthermore demonstrated that the filter curtails the reflectance spectrum of the photonic crystal layer to the green wavelength range (figure 6; see also fig. 15 of Poladian et al. [9]). The filter effectively narrows the reflectance spectrum, and thus creates the brilliant metallic green colour observed on the intact wing (figure 1a). The PBD modelling thus shows that an angle-independent green colour using a multi-domain gyroid photonic crystal layer can be well achieved with selective spectral filtering.
A less obvious function of the absorbing pigment filter is the elimination of CD. For the topologically comparable gyroid-type photonic crystals of the Green Hairstreak, Callophrys rubi, a strong CD has been predicted to exist in the UV- and blue-wavelength ranges, from about 370 to 440 nm (H-orientated crystal, figure 6; [9–11]; see also fig. 2 of Saba et al. [11]). In the green scales of P. sesostris, these wavelength ranges are absorbed (and thus the CD is eliminated there) by the frontal pigment filter. Polarization-dependent spectral measurements from the scatterograms of scales illuminated from the upper side (figure 4b) indeed showed no significant polarization dependency.
4.2. Combination of structural and pigmentary coloration
Optical filtering of structural coloration by a frontal pigment layer very similar to that of P. sesostris was recently discovered in the Papilio nireus group [23,24]. In the butterflies of the nireus group, a frontal UV-absorbing filter tunes the reflectance of a thin film structure and, as in P. sesostris scales, suppresses the angle dependency of the reflectance spectrum. The same tuning principle of selective filtering the spectral reflectance of photonic structures appears to exist also in other butterfly groups, e.g. in Heliconiinae (A. J. M. Vey, B. D. Wilts & D. G. Stavenga 2011, unpublished data) and in birds [25]. The common, unstructured butterfly scales, which are built according to a basic bauplan [1,4], approximate ideal diffusers. When these scales contain a spectrally selective absorbing pigment, their diffusive properties result in a dull, angle-independent coloration, as in the typical papilionid ground scales (figure 4a, see also [4]), and in the red spots on the hindwings of P. sesostris (figure 1a).
4.3. Biological implications
The presented photonic mechanism produces angle-independent, green-coloured wing patches, which together with the intense black surrounding wing parts create a high-contrast signal [26] that provides a high within-pattern colour contrast contributing to the conspicuousness of the animals [27,28]. The green and red wing patches presumably serve as an aposematic signal, because Parides species are known to elicit aversive behaviour from bird predators [29]. Many insect species exhibit green coloration for camouflage [6,7], generally produced by structural coloration except for a few rare cases [30].
4.4. Photonic structures in butterflies
The three-dimensional gyroid-type photonic crystals in the wing scales of P. sesostris are not unique. All three-dimensional photonic crystals in butterflies have been identified to be of the gyroid type [7,8,10]. They occur in different families, e.g. the lycaenids and papilionids. Saranathan et al. [8] recently demonstrated that specific interactions of the cell plasma membrane and the intracellular smooth endoplasmatic reticulum initiate the development of the gyroid networks in the nascent wing scales by cubic membrane folding.
Interestingly, other minimal surfaces, as simple cubic-type (P) or the diamond-type (D) photonic crystals have not been found in butterflies. Diamond-type photonic crystals have been identified in beetles [1,19,31,32], but gyroid-type photonic crystals have not been discovered in beetles, so far. This suggests the hypothesis that the developmental pathways in different insect groups are fundamentally different, but it also poses the question if different crystal types, as gyroid- or diamond-type crystals with different iridescent and polarization characteristics have special advantages for the different insects. As mentioned above, the different crystal types seem to be developmentally constrained to different groups of insects. For butterflies, as C. rubi, it was shown that the gyroid-type photonic crystals are advantageous in foliaceous background for camouflage against predators but they simultaneously can serve as an identification signal to conspecifics [6].
The green-coloured wing areas of papilionids are realized with various optical mechanisms. While the Emerald-patched Cattleheart, P. sesostris, and the Kaiser-i-Hind butterfly, Teinopalpus imperalis, apply three-dimensional photonic crystals [7–9,33] to the Common Bluebottle, Graphium sarpedon, a stable green coloration is achieved by the sole use of a combination of different pigments in the wing substrate [30], and in the Peacock, Papilio blumei, the wing scales' lumen is filled with a stack of tilted multilayers, which result in additive mixing of yellow and blue colours to achieve a bright green coloration [34]. The diversity of optimized photonic structures present in butterflies and other animals [1,2,7,33] may provide a valuable source for bioinspired applications [31,35,36].
Acknowledgements
We thank H. L. Leertouwer and A. J. M. Vey for cooperation, three anonymous reviewers for constructive comments and the Materials Science Group (Prof. J. Th. De Hosson, University of Groningen) for providing SEM facilities. This study was financially supported by AFOSR/EOARD (grant FA8655-08-1-3012) and partially supported by NCF, The Netherlands.
References
- 1.Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific [Google Scholar]
- 2.Vukusic P., Sambles J. R. 2003. Photonic structures in biology. Nature 424, 852–855 10.1038/nature01941 (doi:10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 3.Vukusic P. 2009. Advanced photonic systems on the wing-scales of Lepidoptera. In Functional surfaces in biology: little structures with big effects (ed. Gorb S. N.), pp. 237–258 New York, NY: Springer [Google Scholar]
- 4.Ghiradella H. 2010. Insect cuticular surface modifications: scales and other structural formations. Adv. Insect Physiol. 38, 135–180 10.1016/S0065-2806(10)38006-4 (doi:10.1016/S0065-2806(10)38006-4) [DOI] [Google Scholar]
- 5.Doucet S. M., Meadows M. G. 2009. Iridescence: a functional perspective. J. R. Soc. Interface 6(Suppl. 2), S115–S132 10.1098/rsif.2008.0395.focus (doi:10.1098/rsif.2008.0395.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michielsen K., De Raedt H., Stavenga D. G. 2010. Reflectivity of the gyroid biophotonic crystals in the ventral wing scales of the Green Hairstreak butterfly, Callophrys rubi. J. R. Soc. Interface 7, 765–771 10.1098/rsif.2009.0352 (doi:10.1098/rsif.2009.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michielsen K., Stavenga D. G. 2008. Gyroid cuticular structures in butterfly wing scales: biological photonic crystals. J. R. Soc. Interface 5, 85–94 10.1098/rsif.2007.1065 (doi:10.1098/rsif.2007.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saranathan V., Osuji C. O., Mochrie S. G., Noh H., Narayanan S., Sandy A., Dufresne E. R., Prum R. O. 2010. Structure, function, and self-assembly of single network gyroid (I4132) photonic crystals in butterfly wing scales. Proc. Natl Acad. Sci. USA 107, 11 676–11 681 10.1073/pnas.0909616107 (doi:10.1073/pnas.0909616107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poladian L., Wickham S., Lee K., Large M. C. 2009. Iridescence from photonic crystals and its suppression in butterfly scales. J. R. Soc. Interface 6(Suppl. 2), S233–S242 10.1098/rsif.2008.0353.focus (doi:10.1098/rsif.2008.0353.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder-Turk G. E., Wickham S., Averdunk H., Brink F., Fitz Gerald J. D., Poladian L., Large M. C., Hyde S. T. 2011. The chiral structure of porous chitin within the wing-scales of Callophrys rubi. J. Struct. Biol. 174, 290–295 10.1016/j.jsb.2011.01.004 (doi:10.1016/j.jsb.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 11.Saba M., Thiel M., Turner M. D., Hyde S. T., Gu M., Grosse-Brauckmann K., Neshev D. N., Mecke K., Schröder-Turk G. E. 2011. Circular dichroism in biological photonic crystals and cubic chiral nets. Phys. Rev. Lett. 106, 103902. 10.1103/PhysRevLett.106.103902 (doi:10.1103/PhysRevLett.106.103902) [DOI] [PubMed] [Google Scholar]
- 12.Stavenga D. G., Leertouwer H. L., Pirih P., Wehling M. F. 2009. Imaging scatterometry of butterfly wing scales. Opt. Express 17, 193–202 10.1364/OE.17.000193 (doi:10.1364/OE.17.000193) [DOI] [PubMed] [Google Scholar]
- 13.Johnson S., Joannopoulos J. 2001. Block-iterative frequency-domain methods for Maxwell's equations in a planewave basis. Opt. Express 8, 173–190 10.1364/OE.8.000173 (doi:10.1364/OE.8.000173) [DOI] [PubMed] [Google Scholar]
- 14.Wilts B. D., Pirih P., Stavenga D. G. 2011. Spectral reflectance properties of iridescent pierid butterfly wings. J. Comp. Physiol. A 197, 693–702 10.1007/s00359-011-0632-y (doi:10.1007/s00359-011-0632-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vukusic P., Sambles J. R., Lawrence C. R., Wootton R. J. 1999. Quantified interference diffraction in single Morpho butterfly scales. Proc. R. Soc. Lond. B 266, 1403–1411 10.1098/rspb.1999.0794 (doi:10.1098/rspb.1999.0794) [DOI] [Google Scholar]
- 16.Vukusic P., Stavenga D. G. 2009. Physical methods for investigating structural colours in biological systems. J. R. Soc. Interface 6(Suppl. 2), S133–S148 10.1098/rsif.2008.0386.focus (doi:10.1098/rsif.2008.0386.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giraldo M. A., Yoshioka S., Stavenga D. G. 2008. Far field scattering pattern of differently structured butterfly scales. J. Comp. Physiol. A 194, 201–207 10.1007/s00359-007-0297-8 (doi:10.1007/s00359-007-0297-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirih P., Wilts B. D., Stavenga D. G. 2011. Spatial reflection patterns of iridescent wings of male pierid butterflies: curved scales reflect at a wider angle than flat scales. J. Comp. Physiol. A 197, 987–997 10.1007/s00359-011-0661-6 (doi:10.1007/s00359-011-0661-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilts B. D., Michielsen K., De Raedt H., Stavenga D. G. 2012. Hemispherical Brillouin zone imaging of a diamond-type biological photonic crystal. J. R. Soc. Interface 9, 1609–1614 10.1098/rsif.2011.0730 (doi:10.1098/rsif.2011.0730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavenga D. G., Wilts B. D., Leertouwer H. L., Hariyama T. 2011. Polarized iridescence of the multilayered elytra of the Japanese jewel beetle, Chrysochroa fulgidissima. Phil. Trans. R. Soc. B 366, 709–723 10.1098/rstb.2010.0197 (doi:10.1098/rstb.2010.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashcroft N. W., Mermin N. D. 1976. Solid state physics. New York, NY: Holt, Rinehart and Winston [Google Scholar]
- 22.Joannopoulos J. D. 2008. Photonic crystals: molding the flow of light, 2nd edn. Princeton, NJ: Princeton University Press [Google Scholar]
- 23.Wilts B. D., Trzeciak T. M., Vukusic P., Stavenga D. G. In press. Papiliochrome II pigment reduces angle-dependency of structural wing colouration in nireus group papilionids. J. Exp. Biol. [DOI] [PubMed] [Google Scholar]
- 24.Vukusic P., Hooper I. 2005. Directionally controlled fluorescence emission in butterflies. Science 310, 1151. 10.1126/science.1116612 (doi:10.1126/science.1116612) [DOI] [PubMed] [Google Scholar]
- 25.Berg M. L., Bennett A. T. D. 2010. The evolution of plumage colouration in parrots: a review. Emu 110, 10–20 10.1071/MU09076 (doi:10.1071/MU09076) [DOI] [Google Scholar]
- 26.Kelber A., Osorio D. 2010. From spectral information to animal colour vision: experiments and concepts. Proc. R. Soc. B 277, 1617–1625 10.1098/rspb.2009.2118 (doi:10.1098/rspb.2009.2118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mappes J., Marples N., Endler J. A. 2005. The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603 10.1016/j.tree.2005.07.011 (doi:10.1016/j.tree.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 28.Endler J. A., Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547 10.1086/382662 (doi:10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro C. E. G. 1996. Palatability and escaping ability in Neotropical butterflies: Tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 59, 351–365 10.1111/j.1095-8312.1996.tb01471.x (doi:10.1111/j.1095-8312.1996.tb01471.x) [DOI] [Google Scholar]
- 30.Stavenga D. G., Giraldo M. A., Leertouwer H. L. 2010. Butterfly wing colors: glass scales of Graphium sarpedon cause polarized iridescence and enhance blue/green pigment coloration of the wing membrane. J. Exp. Biol. 213, 1731–1739 10.1242/jeb.041434 (doi:10.1242/jeb.041434) [DOI] [PubMed] [Google Scholar]
- 31.Biró L. P., Vigneron J. 2011. Photonic nanoarchitectures in butterflies and beetles: valuable sources for bioinspiration. Laser Photon. Rev. 5, 27–51 10.1002/lpor.200900018 (doi:10.1002/lpor.200900018) [DOI] [Google Scholar]
- 32.Galusha J. W., Richey L. R., Gardner J. S., Cha J. N., Bartl M. H. 2008. Discovery of a diamond-based photonic crystal structure in beetle scales. Phys. Rev. E 77, 050904. 10.1103/PhysRevE.77.050904 (doi:10.1103/PhysRevE.77.050904) [DOI] [PubMed] [Google Scholar]
- 33.Ghiradella H. 1991. Light and color on the wing: structural colors in butterflies and moths. Appl. Opt. 30, 3492–3500 10.1364/AO.30.003492 (doi:10.1364/AO.30.003492) [DOI] [PubMed] [Google Scholar]
- 34.Vukusic P., Sambles J. R., Lawrence C. R. 2000. Colour mixing in wing scales of a butterfly. Nature 404, 457. 10.1038/35006561 (doi:10.1038/35006561) [DOI] [PubMed] [Google Scholar]
- 35.Kolle M., Salgard-Cunha P. M., Scherer M. R., Huang F., Vukusic P., Mahajan S., Baumberg J. J., Steiner U. 2010. Mimicking the colourful wing scale structure of the Papilio blumei butterfly. Nat. Nanotechnol. 5, 511–515 10.1038/nnano.2010.101 (doi:10.1038/nnano.2010.101) [DOI] [PubMed] [Google Scholar]
- 36.Parker A. R., Townley H. E. 2007. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2, 347–353 10.1038/nnano.2007.152 (doi:10.1038/nnano.2007.152) [DOI] [PubMed] [Google Scholar]