Abstract
MASPIN, which is also known as Serpin B5, is a novel tumor suppressor. Emerging evidence suggests that MASPIN acts as a multifaceted protein in various types of cancer, including prostate, breast and pancreatic cancer. It interacts with diverse groups of intercellular and extracellular proteins, regulating cell adhesion, motility, apoptosis and angiogenesis, and is involved in mammary gland development. As MASPIN is a multifunctional factor in cancer pathways, its function remains poorly illuminated. In this study, we compared the protein profiles of LC5 cell lines with MASPIN overexpression and knockdown using comparative two-dimensional gel electrophoresis. The differences in protein expression, visualized as differences in spots, were identified by time-of-flight (TOF)/TOF mass spectometry. Significant differences were observed between overexpressing and knocked down cells, including eight spots that were unique and sixteen spots that were up- or down-regulated by more than 4-fold. Six genes, including Sdccag8, Ldoc1, SCAI, SDCCAG3, CT62 and NEDD9 were unique in MASPIN-expressing cell lines, but absent in knock-out cell lines, in which most of them play a significant role in the invasion of cancer cells. Moreover, the Brms1 and CAGE1 genes were identified as being uniquely expressed in knocked down cell lines, which were associated with the development and progression of tumors. The data from this study shed some light on the function, as well as the general network mechanisms of MASPIN in lung cancer.
Keywords: MASPIN, lung cancer, two-dimensional gel electrophoresis
Introduction
MASPIN, a novel serine protease inhibitor (Serpin), inhibits tumor invasion and metastasis of mammary carcinoma. The gene encoding MASPIN has been initially identified in a search of genes that display no or down-regulated expression in human breast carcinoma cells (1). This 42-kDa protein is known also as Serpin B5, protease inhibitor-5 or proteinase inhibitor-5. As a tumor suppressor gene, MASPIN is involved in tumor invasion, metastasis and angiogenesis. However, the mechanisms of MASPIN are little known, particularly its signal networks in lung cells.
MASPIN acts as a tumor suppressor gene by impairing tumor growth, cell motility and metastasis. Shi et al demonstrated that MASPIN reduced tumor growth as well as invasion and metastasis through a combination of reduced angiogenesis and increased apoptosis in a transgenic mouse model that overexpressed MASPIN (2). Abraham et al reported that MASPIN increased cell adhesion to the extracellular matrix in prostate tumor cells and that it was capable of decreasing the tumorigenic and metastatic potential of prostate tumors (3). The decreased expression of MASPIN in prostate cancer has also been reported to inversely correlate with the development of local recurrence or systemic tumor progression (4). Furthermore, it has been reported that MASPIN is involved in the processes of embryonic development, apoptosis and angiogenesis. Schaefer and Zhang demonstrated that MASPIN played a significant role in mammary development in a stage-dependent manner (5). The expression levels of MASPIN are lower in virgin and early pregnancy mammary glands than in late pregnancy and lactating mammary glands. Cher et al demonstrated that MASPIN expression inhibited osteolysis, tumor growth and angiogenesis in a model of prostate cancer bone metastasis (6). Finally, the local delivery of MASPIN to human prostate tumor cells in a mouse model blocked tumor growth and dramatically reduced the density of tumor-associated microvessels (5).
Over the past decade, with the expansion of studies on MASPIN, novel protein-binding partners have been identified and have provided insight into the molecular aspects on regulation and divergent mechanisms. Naturally, the MASPIN gene contains a number of potential transcription factor binding elements, which are related to the regulation of its expression. These include activator protein-1 (Ap-1), E26 transformation specific-1 (Ets), hormonal responsive elements (HREs) and p53 binding sites (5). Recently, it has been shown that MASPIN may be affected by promoter methylation levels and by histone modification (7). These data indicate the complexity of the MASPIN network. In this study, we used comparative proteomics to systemically study the protein profile changes in lung cell lines. The large-scale data and system biology methodology may help us to illustrate the function of MASPIN, its regulation pathways and networks in lung cancer.
Materials and methods
Cell culture
The human lung carcinoma cell line, LC5, was kindly provided by the Cell Line Center of the Chinese Academy of Science. The LC5 human lung carcinoma cell line was maintained in RPMI-1640 (Life Technologies, Gaithersburg, MD, USA) with 1 mM glutamate, 100 U/ ml penicillin, 100 ng/ml streptomycin and 10% fetal calf serum (FCS). The cells were cultured at 37°C in a humidified atmosphere of 5% CO2. Cell viability was evaluated by trypan blue exclusion assay.
Porcine cytomegalovirus (pCMV)-maspin construction
For construction of the pCMV-MASPIN plasmid, full-length MASPIN complementary DNA (cDNA) was cloned into the pCMV-Taq4C vector (Invitrogen, Carlsbad, CA, USA), using the primers, 5′-atggatgccctgcaactagca-3′ and 5′-ttaaggagaac agaatttg-3′ (Sangon Co., Shanghai, China).
Transfection of the lung cancer cells
The cells were transfected with small interfering RNA (siRNA) or plasmids using the Effectene (Qiagen, Valencia, CA, USA) or Amaxa electroporation system (Amaxa, Gaithersburg, MD, USA), according to the manufacturer’s instructions. To develop cell lines, which stably expressed MASPIN, following transfection, the cells were then incubated in the medium with Geneticin 418 for 48 h, and selected for 2 weeks. We used a targeted SMART pool of siRNAs from Dharmacon (Lafayette, CO, USA) to knockdown MASPIN expression with the siLentFect lipid kit. In parallel, cells were transfected with a fluorescein isothiocyanate-labeled, nonspecific siRNA with a scrambled sequence.
Western blot analysis
Cells were harvested with ice-cold phosphate-buffered saline and then lysed in lysis buffer containing 10 mM Tris (pH 7.5), 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid, 1% SDS, 1 mM sodium orthovanadate and a mixture of protease inhibitors (Roche, Indianapolis, IN, USA). The lysates were sonicated for 10 sec, centrifuged for 20 min at 20,000 × g, and then stored at −70°C. Equal amounts (25 μg) of the cell lysates were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were subjected to Western blot analysis, using an enhanced chemiluminescence system (Amersham Corp., Arlington Heights, IL, USA).
Two-dimensional gel electrophoresis (2DE) and time-of-flight (TOF)/TOF mass spectometry (MS)
Proteins were collected from LC5 cells as described previously. The extraction buffer included: 9 M urea, 0.5% SDS, 4% CHAPS, 65 mM DTT, nucleases mixture (GE Healthcare, Piscataway, NJ, USA) and protease inhibitor mixture (GE Healthcare). The crude extraction was then treated with Clean-Up Kit (GE Healthcare). The protein (100 μg) was loaded independently using 18 cm pH3–10 (L) stripes by the GE Healthcare 2DE system. Second dimensional SDS-PAGE was carried out, using a 12% gel and stained with the PlusOne Silver Staining Kit (GE Healthcare). The gel images were analyzed by the ImageMaster™ 2D Platinum v7.0 (GE Healthcare). The spots were identified by 6200 Series Accurate-Mass TOF LC/MS (Agilent, Santa Clara, CA, USA).
Results
Overexpression and knockdown of MASPIN in LC5 human lung carcinoma cells
Total RNA was isolated from four exponentially selected cell lines with overexpressed and knocked down MASPIN, respectively. The results suggest that all eight cell lines were successful. All of the transfected cells had a high expression of MASPIN, while MASPIN expression was almost eliminated by siRNA-MASPIN (Fig. 1). Furthermore, the expression of MASPIN protein was confirmed by Western blot analysis. The abundance of MASPIN was detected in the overexpressed cell lines by a specific anti-MASPIN antibody, but was undetectable in the knocked down cell line (Fig. 2). The results indicated that the overexpression or knockdown of MASPIN in LC5 cell lines was successful.
Figure 1.
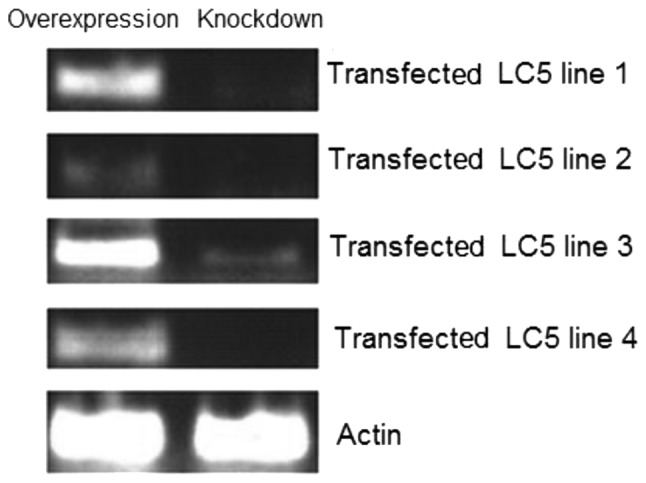
Northern (RNA) blot analysis of MASPIN mRNA in four overexpressed and knock-out cell lines.
Figure 2.
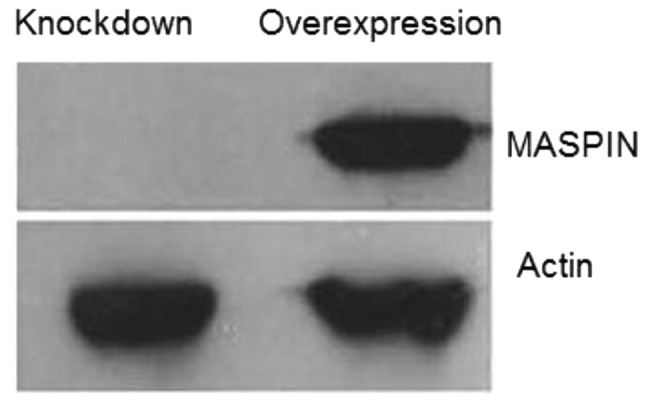
Western blot analysis of MASPIN protein in overexpressed and knock-out cell lines.
Spot identification by TOF/TOF MS
The global view of a representative two-dimensional gel is shown in Fig. 3. The MASPIN overexpression and knockdown samples were loaded three times. Over 1,400 spots were represented in three duplicates. The excellent reproduction in protein pattern and protein density between all cell lines is evident in the comparison of four 2D gels to each other. All gels were matched to the LC5 cells reference 2D map and the relative density of each protein spot was quantified using ImageMaster software. The data are shown in Table I.
Figure 3.
Two-dimensional polyacrylamide gel electrophoresis. Proteins from a lysate of pCMV-maspin-transfected LC5 cell line 3 were subjected to 18 cm pH3–10 (L) stripes by the GE Healthcare two-dimensional gel electrophoresis system. pCMV, porcine cytomegalovirus.
Table I.
Spots identification by TOF/TOF MS.
| Protein name | Quantity in MASPIN overexpression sample (V %) | Quantity in MASPIN knockdown sample (V %) | Ratio overexpression/knockdown |
|---|---|---|---|
| Sdccag8 | Unique | - | - |
| Ldoc1 | Unique | - | - |
| SCAI | Unique | - | - |
| SDCCAG3 | Unique | - | - |
| CT62 | Unique | - | - |
| Brms1 | Unique | - | - |
| CAGE1 | - | Unique | - |
| NEDD9 | - | Unique | - |
| TSP1 | 0.288 | 0.012 | 24.0 |
| RUNX | 0.294 | 0.017 | 17.3 |
| ORAOV1 | 0.335 | 0.022 | 15.2 |
| PTK2 | 0.396 | 0.024 | 16.5 |
| CASC5 | 0.415 | 0.065 | 6.38 |
| BCAR1 | 0.583 | 0.088 | 6.23 |
| GREB1L | 0.676 | 0.096 | 7.04 |
| BASE | 0.688 | 0.152 | 4.54 |
| CTAG2 | 0.707 | 0.169 | 4.18 |
| Blcap, | 0.077 | 0.835 | 0.09 |
| Casc5 | 0.098 | 0.799 | 0.12 |
| Casc3 | 0.104 | 0.761 | 0.13 |
| Hic2 | 0.112 | 0.733 | 0.15 |
| SNCG | 0.127 | 0.704 | 0.18 |
| HIC1 | 0.144 | 0.698 | 0.21 |
| DLC1 | 0.151 | 0.668 | 0.23 |
TOF MS, time-of-flight mass spectrometry.
The results indicate that several tumor suppressor genes are related to the expression of MASPIN. In this study, we have found that at least six genes were unique in MASPIN-overexpressed cell lines, in which most of them play crucial roles in the invasion of cancer cells. For example, the suppressor of cancer cell invasion (SCAI) acts as an inhibitor of cancer cell invasion through the transcriptional control of β1-integrin (8). The serologically defined colon cancer antigen 3 (SDCCAG3) is necessary for the presentation of TNF receptor 1 on the cell surface (9). Leucine zipper, down-regulated in cancer 1 (LDOC1) inhibits NF-κB activation and sensitizes pancreatic cancer cells to apoptosis (10). In addition, breast cancer metastasis suppressor 1 (Brms1) functions as a co-repressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis, and inhibits gene expression by targeting NF-κB activity (11).
However, two genes were discovered to be unique in knockdown cell lines that are associated with the development and progression of tumors. The expression of cancer-associated gene 1 (CAGE1) protein is associated with the progression of tumors and has been used as a response criteria in several tumors during chemotherapy (12). The neural precursor cell expressed developmentally down-regulated protein 9 (NEDD9), as a melanoma metastasis gene, is regulated in human neuroblastoma cells and in the embryonic hindbrain by all-trans retinoic acid (13,14).
Other findings from this study were that nine genes were extremely highly expressed in the MASPIN-overexpressed cell lines. Most of these highly expressed genes are associated with anti-thrombosis, inhibition of tumor cell mobility, metastasis and angiogenesis. For example, soluble or local platelet-released thrombospondin-1 (TSP-1) may protect unfolded endothelium-bound and subendothelial von Willebrand factor (VWF) from degradation by plasma ADAMTS13, thus securing platelet tethering and thrombus adherence to the inflamed and injured endothelium, respectively (15). Furthermore, runt-related transcription factor 1 (RUNX1) plays a crucial role in the transition of endothelial cells to hematopoietic cells, and in the down-regulation of fetal liver kinase-1. By contrast, Runx1 is weakly expressed in early erythroid cells, and its expression is rapidly extinguished during later stages of erythropoiesis (16,17). In addition, protein tyrosine kinase 2 (PTK2), or Focal adhesion kinase 1, plays a crucial role in generating cell survival signals, as well as the cleavage of FAK during caspase-mediated apoptosis (18). FAK catalytic activities are crucial in promoting VEGF-associated tumor angiogenesis and protease-associated tumor metastasis. Support is growing for the theory that FAK and Src may be therapeutically relevant targets in the inhibition of tumor progression (19).
Discussion
The findings of this study illustrate the importance of the MASPIN gene and protein in regulating the signaling pathway of tumor initiation, promotion and progression, which includes a very complicated and intricate control system with multiple layers that are connected to the expression of the MASPIN gene, including transcription factors, transcription factor binding elements and DNA methylation coalescence.
The expression level of MASPIN was used as an indicator for tumor aggressiveness and metastatic potential. Breast cancer progression from ductal carcinoma in situ, to locally invasive cancer, and finally to lymph node metastasis has been shown to correlate with a stepwise decrease in MASPIN expression (20). Umekita et al reported that the expression of MASPIN predicted poor prognosis in breast cancer patients (21). The down-regulation of the tumor suppressor gene, MASPIN, in breast carcinoma was associated with a higher risk of distant metastasis (20). In this study, we also demonstrate that MASPIN may be an indicator of aggressiveness and metastatic potential for lung cancer cells owing to its dual role in the inhibition of tumor cells.
Previous studies on the regulatory mechanisms of MASPIN have shown that hypermethylation and histone deacetylation lead to silencing of the MASPIN gene in human breast cancer (22,23). Bass et al demonstrated that MASPIN was a non-inhibitory Serpin, which inhibited the migration of tumor and vascular smooth muscle cells (24). Recombinant MASPIN specifically inhibited activators of the cell surface-associated urokinase-type plasminogen and fibrinogen-bound tissue-type plasminogen. Endogenous MASPIN was also a potent inhibitor of the pericellular urokinase-type plasminogen activator and may block tumor invasion and motility by inhibiting localized pericellular proteolysis (25).
Jiang et al reported that MASPIN sensitizes breast carcinoma cells to induced apoptosis (26). Systemic delivery of the MASPIN gene in a syngeneic tumor model inhibited breast tumor progression (27). In this study, comparative proteomic analysis was used to systemically study the protein profile change in a lung cell line.
This study may help to illustrate the function of MASPIN, as well as the regulatory pathway and functional network of MASPIN in lung cancer. However, further studies are required to investigate the functions of MASPIN in further detail.
References
- 1.Zou Z, Gao C, Nagaich AK, et al. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000;275:6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- 2.Shi HY, Zhang W, Liang R, et al. Modeling human breast cancer metastasis in mice: maspin as a paradigm. Histol Histopathol. 2003;18:201–206. doi: 10.14670/HH-18.201. [DOI] [PubMed] [Google Scholar]
- 3.Abraham S, Zhang W, Greenberg N, Zhang M. Maspin functions as tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells. J Urol. 2003;169:1157–1161. doi: 10.1097/01.ju.0000040245.70349.37. [DOI] [PubMed] [Google Scholar]
- 4.Machtens S, Serth J, Bokemeyer C, et al. Expression of the p53 and Maspin protein in primary prostate cancer: correlation with clinical features. Int J Cancer. 2001;95:337–342. doi: 10.1002/1097-0215(20010920)95:5<337::aid-ijc1059>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer JS, Zhang M. Role of maspin in tumor metastasis and angiogenesis. Curr Mol Med. 2003;3:653–658. doi: 10.2174/1566524033479519. [DOI] [PubMed] [Google Scholar]
- 6.Cher ML, Biliran HR, Jr, Bhagat S, et al. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci USA. 2003;100:7847–7852. doi: 10.1073/pnas.1331360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokras A, Coffin J, Field L, et al. 2006:611–617. doi: 10.1093/molehr/gal074. [DOI] [PubMed] [Google Scholar]
- 8.Brandt DT, Baarlink C, Kitzing TM, et al. SCAI acts as a suppressor of cancer cell invasion through the transcriptional control of beta1-integrin. Nat Cell Biol. 2009;11:557–568. doi: 10.1038/ncb1862. [DOI] [PubMed] [Google Scholar]
- 9.Neznanov N, Neznanova L, Angres B, Gudkov AV. Serologically defined colon cancer antigen 3 is necessary for the presentation of TNF receptor 1 on cell surface. DNA Cell Biol. 2005;24:777–785. doi: 10.1089/dna.2005.24.777. [DOI] [PubMed] [Google Scholar]
- 10.Nagasaki K, Schem C, von Kaisenberg C, et al. Leucine-zipper protein, LDOC1, inhibits NF-kappaB activation and sensitizes pancreatic cancer cells to apoptosis. Int J Cancer. 2003;105:454–458. doi: 10.1002/ijc.11122. [DOI] [PubMed] [Google Scholar]
- 11.Cicek M, Fukuyama R, Welch DR, Sizemore N, Casey G. Breast cancer metastasis suppressor 1 inhibits gene expression by targeting nuclear factor-kappaB activity. Cancer Res. 2005;65:3586–3595. doi: 10.1158/0008-5472.CAN-04-3139. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Lim Y, Lee D, et al. Identification and characterization of a novel cancer/testis antigen gene CAGE-1. Biochim Biophys Acta. 2003;1625:173–182. doi: 10.1016/s0167-4781(02)00620-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Gans JD, Nogueira C, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Merrill RA, See AW, Wertheim ML, Clagett-Dame M. Crk-associated substrate (Cas) family member, NEDD9, is regulated in human neuroblastoma cells and in the embryonic hindbrain by all-trans retinoic acid. Dev Dyn. 2004;231:564–575. doi: 10.1002/dvdy.20159. [DOI] [PubMed] [Google Scholar]
- 15.Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorsbach RB, Moore J, Ang SO, Sun W, Lenny N, Downing JR. Role of RUNX1 in adult hematopoiesis: analysis of RUNX1-IRES-GFP knock-in mice reveals differential lineage expression. Blood. 2004;103:2522–2529. doi: 10.1182/blood-2003-07-2439. [DOI] [PubMed] [Google Scholar]
- 17.Hirai H, Samokhvalov IM, Fujimoto T, Nishikawa S, Imanishi J, Nishikawa S. Involvement of Runx1 in the down-regulation of fetal liver kinase-1 expression during transition of endothelial cells to hematopoietic cells. Blood. 2005;106:1948–1955. doi: 10.1182/blood-2004-12-4872. [DOI] [PubMed] [Google Scholar]
- 18.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 19.Bernard-Trifilo JA, Lim ST, Hou S, Schlaepfer DD, Ilic D. Analyzing FAK and Pyk2 in early integrin signaling events. Curr Protoc Cell Biol Chapter 14: Unit 14. 7:2006. doi: 10.1002/0471143030.cb1407s30. [DOI] [PubMed] [Google Scholar]
- 20.Maass N, Teffner M, Rosel F, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol. 2001;195:321–326. doi: 10.1002/path.948. [DOI] [PubMed] [Google Scholar]
- 21.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Expression of maspin predicts poor prognosis in breast-cancer patients. Int J Cancer. 2002;100:452–455. doi: 10.1002/ijc.10500. [DOI] [PubMed] [Google Scholar]
- 22.Futscher BW, Oshiro MM, Wozniak RJ, et al. Role for DNA methylation in the control of cell type specific maspin expression. Nat Genet. 2002;31:175–179. doi: 10.1038/ng886. [DOI] [PubMed] [Google Scholar]
- 23.Maass N, Biallek M, Rosel F, et al. Hypermethylation and histone deacetylation lead to silencing of the maspin gene in human breast cancer. Biochem Biophys Res Commun. 2002;297:125–128. doi: 10.1016/s0006-291x(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 24.Bass R, Fernandez AM, Ellis V. Maspin inhibits cell migration in the absence of protease inhibitory activity. J Biol Chem. 2002;277:46845–46848. doi: 10.1074/jbc.C200532200. [DOI] [PubMed] [Google Scholar]
- 25.Biliran H, Jr, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res. 2001;61:8676–8682. [PubMed] [Google Scholar]
- 26.Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- 27.Shi HY, Liang R, Templeton NS, Zhang M. Inhibition of breast tumor progression by systemic delivery of the maspin gene in a syngeneic tumor model. Mol Ther. 2002;5:755–761. doi: 10.1006/mthe.2002.0602. [DOI] [PubMed] [Google Scholar]



