Abstract
Dihydrofolate reductase (DHFR) is the major target of methotrexate, a key component in childhood acute lymphoblastic leukemia (ALL) treatment. Polymorphisms in the gene coding for DHFR have been associated with adverse event treatment. This study evaluated the effect of the -A317G and C829T polymorphisms in the DHFR gene on survival and risk of relapse of ALL. Seventy patients with ALL and 100 healthy individuals were genotyped by the polymerase chain reaction-restriction fragment length polymorphism method. An association between the polymorphisms and the risk of relapse was found (p<0.05); patients with the -317G/G genotype were found to have an 8.55 (95% CI 1.84–39.70) higher chance of relapse and carriers of the 829T/T genotype had a 14.0 (95% CI 1.13–172.63) higher chance of relapse. Other variables, such as age and leukocyte count, were associated (p<0.05) with the risk of relapse of the disease. Individuals with the G/G and T/T genotype of the -A317G and C829T polymorphisms had poorer survival compared to other genotype groups (log-rank test; p<0.05). Although preliminary, these data seem to suggest a role for the DHFR polymorphisms in the risk of relapse of ALL and the mortality risk in these patients.
Keywords: acute lymphoblastic leukemia, dihydrofolate reductase, risk of relapse, -A317G polymorphism, C829T polymorphism, survival in acute lymphoblastic leukemia
Introduction
In Mexico, acute leukemia is considered a public health issue; it represents the fourth leading cause of mortality of all neoplastic malignancies in children under 15 years of age (1). The mortality rate from 1996 to 2000 was 63.7 per 1 million children, one of the highest rates reported in the world (2). In 2005, leukemia was the second cause of mortality in the State of Guerrero in children less than 15 years of age, according to the National Institute of Statistics, Geography and Computing (INEGI) (3).
Methotrexate (MTX) is an antineoplastic agent used in the treatment of patients with acute lymphoblastic leukemia (ALL) and was introduced five decades ago to clinical oncology. It is presently used in the treatment of other neoplastic diseases, including osteosarcoma, breast cancer, head and neck cancer, and non-Hodgkin's lymphoma (4,5). MTX is a folic acid antagonist, and its efficacy as an antineoplastic treatment is largely attributed to its high affinity for dihydrofolate reductase (DHFR) (EC 1.5.1.3), the enzyme which is responsible to catalyze the reduction of dihydrofolate (DHF) to tetrahydrofolate (THF) (6). The major mechanism of MTX action involves competitive inhibition of DHFR, leading to the impaired regeneration of THF from DHF; essential for the biosynthesis of purines and thymidylate, thus it also blocks the novo synthesis of DNA (7,8). A subset of patients develop adverse events of resistance to MTX; however, approximately 80% of ALL children experience good clinical response (5,9,10).
The mechanisms that lead to clinical failure to MTX are DHFR overexpression, impaired intracellular transport and decreased levels of reduced folate carrier at the cell membrane (11,12). Changes in the levels of DHFR expression and consequently in the sensitivity to MTX can also be due to single nucleotide polymorphisms (SNPs), particularly those located in the regulatory elements. The C829T SNP is located at the 223 nucleotide downstream from the stop codon between the first and second polyadenylation sites in the 3′UTR of the DHFR gene, which leads to the stability of mRNA (13). A previous study reported that the -A317G SNP in the DHFR promoter region results in higher transcriptional activity (14). The -A317G and C829T SNPs in the DHFR gene have not been studied as a factor for the risk of relapse to ALL in Mexico. In the present study, our objective was to evaluate the effect of the -A317G and C829T polymorphisms in the DHFR gene on survival and risk of relapse of ALL.
Materials and methods
Study population
A case control study was carried out in the Pediatric Oncology Service of the State Cancer Institute (SCI) from the South of Mexico (Acapulco, Guerrero), between September 1996 and May 2009. The cases consisted of 70 patients diagnosed with ALL through bone marrow aspirate based on French-American-British morphological criteria, cytochemical staining properties and immunophenotyping of blast cells.
The diagnosis of ALL was further subclassified as T-lineage (CD3+, CD7+ plus CD2+ or CD5+, or both) or B-lineage (CD22+, CD19+, HLA-DR+, CD10+). Patients were treated with VDCPM (vincristine 1.4 mg/m2 at days 1, 8, 15 and 22, daunorubicin 45 mg/m2 at days 1–3, cyclophosphamide 0.75 mg/m2 at days 1 and 15, prednisone 40 mg/m2 at days 1–28 and intrathecal methotrexate 8 mg/m2 for 1–2 years, 10 mg/m2 for 2–3 years, 12 mg/m2 for 3–8 years and 15 mg/m2 for >8 years) or VDLPM (vincristine 1.4 mg/m2 at days 1, 8, 15 and 22, daunorubicin 45 mg/m2 at days 1–3, asparaginase 6,000 U/m2 at days 19–28, prednisone 40 mg/m2 at days 1–28 and intrathecal methotrexate 8 mg/m2 for 1–2 years, 10 mg/m2 for 2–3 years, 12 mg/m2 for 3–8 years and 15 mg/m2 for >8 years) regimens (15).
Complete remission was defined by <5% blast cells in the bone marrow and normalization of peripheral blood counts at 4 weeks after starting induction therapy (16). Relapse was defined as the reappearance of >20% blast cells in the marrow, or the presence of localized leukemic infiltrates at any site after completion of induction chemotherapy (16). Worse outcome was defined as a lack of response to induction therapy, a relapse after achieving complete remission or death due to any cause (16). Risk classification: standard risk: 1–9 years of age and presenting white blood cell (WBC) count of <50,000/mm3; high risk: <1 and >9 years of age and WBC count >50,000/mm3 (17). The controls were 100 healthy individuals (4–10×103 leukocytes/mm3) without a family history of leukemia. Subjects in both groups in the study were 1–18 years of age, included both genders and were residence in the State of Guerrero, Mexico.
Specimen collection
A bone marrow and/or blood sample was taken from the 170 participants and placed in tubes with anticoagulant. Leukocytes were purified from the whole blood sample by a selective osmotic lysis of erythrocytes; the leukocyte genomic DNA and RNA total was extracted by the phenol-chloroform technique (18).
Genotyping of the -A317G and C829T polymorphisms in the DHFR
The -A317G polymorphism (dbSNP; rs408626) was detected using previously reported PCR primers (14): forward primer 5′-GTAGGTTCTGTCTGGGACTGG-3′ and reverse primer 5′-GCAGCTTTCTTCTAGTCACCC-3′; and using previously established protocols (19). The PCR products (400 bp) were digested with 4 units of the HinfI enzyme (Invitrogen Life Technologies, USA). Individuals with the A/A genotype presented two fragments (266 and 134 bp), individuals with the A/G genotype presented four fragments (266, 134, 83 and 51 bp) and those with the G/G genotype presented three fragments (266, 83 and 51 bp).
The C829T polymorphism (ddsSNP; rs34764978) was detected using previously reported PCR primers (20): forward primer 5′-CTTCTCCAAGACCCCAACTG-3′ and reverse primer 5′-CTTCCAGGTTGTTTTCAATTTTT-3′; and using previously established protocols (19), The amplified products (269 bp) were digested with 3 units of the TspRI enzyme (New England Biolabs, Berverly, MA, USA). The C/C genotype presented three fragments (203, 36 and 30 bp), the C/T genotype presented four fragments (239, 203, 36 and 30 bp) and the T/T genotype presented two fragments (239 and 30 bp).
Statistical analysis
Continuous data are presented as the means ± standard deviation or median, 25th and 75th inter-quartiles. Categorical data were compared by Chi-square or Fisher's exact test. Univariate logistic regression analysis for the association with the risk of relapse of ALL was tested first for -A317G and C829T genetic polymorphisms, gender and other clinical characteristics, and those factors were included into a second multivariate logistic analysis. The log-rank test and Kaplan-Meier curves were used to analyze the effect of the -A317G and C829T genetic polymorphisms, gender and relapse of ALL on overall survival (OS). OS was defined as the time between surgery and either death or the time of the last follow-up. The Hardy-Weinberg equilibrium (HWE) was used to determine the genetic equilibrium in the healthy group. p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA) and STATA software, version 9.2 (StataCorp, College Station, TX, USA).
Ethics statement
The bone marrow samples of the patients and blood samples of healthy individuals used in this study were part of the samples taken for clinical diagnostic tests in the hospital. Since no extra amount of samples was collected from the study subjects, informed consent was obtained from all the individuals or their guardians, after a detailed briefing of the study purposes. The study and the informed consent procedure were approved by the Institutional Review Board of the Cancer Institute from Guerrero State, Mexico.
Results
Characteristics of the study population
The 70 ALL patients had ages ranging from 1.0 to 18 years (mean ± SD, 7.65±4.67 years). There were 45 (64.29%) males and 25 (35.71%) females. Eighteen patients (25.71%) were in the age group of 1–9 years (standard risk). Fifty-two patients (72.29%) were <1 year and >9 years of age (high risk) at the time of initial diagnosis. Relapse of ALL occurred in 68.57% of the patients. WBC and characteristics of immunophenotype are depicted in Table I.
Table I.
General characteristics of the population and clinical data of patients with childhood acute lymphoblastic leukemia (ALL) and healthy individuals.
| Variable | Patients with ALL (n=70) | Healthy individuals (n=100) |
|---|---|---|
| Age (years; mean ± SD) | 7.65±4.67 | 9.99±5.49 |
| No. of leukocytes/mm3 | 13,000 (5,400–39,000)a | 8,000 (7,000–9,000)a |
| Gender | ||
| Male | 45 (64.29) | 53 (53.00) |
| Female | 25 (35.71) | 47 (47.00) |
| Status of participants | ||
| Alive | 30 (42.86) | 100 (100) |
| Deceased | 40 (57.14) | 0 |
| Immunophenotype | ||
| B-lineage | 66 (94.28) | 0 |
| T-lineage | 4 (5.72) | 0 |
| Risk by age | ||
| Standard (1–9 years) | 18 (25.71) | 0 |
| High (<1 and >9 years) | 52 (74.29) | 0 |
| Relapse during treatment | ||
| No | 22 (31.43) | 0 |
| Yes | 48 (68.57) | 0 |
Data indicate n (%);
median (percentiles 25–75).
We also included 100 control subjets (controls). In this group, the age range was 1–18 years (mean ± SD, 9.99±5.49 years), and the leukocyte count was normal (4–10×103 leucocytes/mm3; median 8,000). In this group, 53 healthy individuals (53%) were male and 47 (47%) were female (Table I).
Associations of -A317G and C829T SNP of DHFR with risk of ALL
The allele and genotype distributions for two SNPs of DHFR in the cases and controls are summarized in Table II. The genotype frequencies of these polymorphisms were in HWE (p>0.05) in the controls. When the genotype frequencies were compared between cases and controls, they showed a statistically significant association with the disease. The distribution of -A317G and C829T SNP genotypes was significant between the cases and controls (p=0.037 and p=0.016, respectively), and the A/G and C/T genotypes were more prevalent among the patients (47.14 and 75.71%, respectively). The homozygous variant G/G [odds ratio (OR)=2.89, 95% CI 1.22–6.86] and heterozygote variant C/T (OR=2.83, 95 %CI 1.03–4.88) were risk factors for ALL (Table II).
Table II.
Genotype and allele frequencies of DHFR SNPs in the childhood acute lymphoblastic leukemia (ALL) cases and controls, and association with risk of ALL.
| Modela | Genotype | ALL cases (n=70) | Controls (n=100) | p-valueb | OR | 95% CI | p-valuec | p-value HWE |
|---|---|---|---|---|---|---|---|---|
| -A317G (rs408626) | ||||||||
| Co | A/A | 14 (20.00) | 36 (35.00) | 0.037e | 1.00 | 0.152d | ||
| A/G | 33 (47.14) | 42 (43.57) | 2.24 | 1.03–4.88 | 0.042e | |||
| G/G | 23 (32.86) | 22 (21.43) | 2.89 | 1.22–6.86 | 0.016e | |||
| Do | A/A | 14 (20.00) | 36 (35.00) | 1.00 | ||||
| A/G + G/G | 56 (80.00) | 64 (65.00) | 2.47 | 1.19–5.11 | 0.015e | |||
| Re | A/A + AG | 47 (67.14) | 78 (78.57) | 1.00 | ||||
| G/G | 23 (32.86) | 22 (21.43) | 1.74 | 0.87–3.45 | 0.116 | |||
| Allele | ||||||||
| A | 61 (43.57) | 114 (57.00) | 0.011e | 1.00 | ||||
| G | 79 (56.43) | 86 (43.00) | 1.73 | 1.12–2.68 | 0.014e | |||
| C829T (rs34764978) | ||||||||
| Co | C/C | 10 (14.29) | 33 (33.00) | 0.016e | 1.00 | 0.077d | ||
| C/T | 53 (75.71) | 56 (56.00) | 2.83 | 1.27–6.33 | 0.011e | |||
| T/T | 7 (10.00) | 11 (11.00) | 1.97 | 0.60–6.46 | 0.261 | |||
| Do | C/C | 10 (14.29) | 33 (33.00) | 1.00 | ||||
| C/T + T/T | 60 (85.71) | 67 (67.00) | 2.69 | 1.22–5.95 | 0.014e | |||
| Re | C/C + C/T | 63 (90.00) | 89 (89.00) | 1.00 | ||||
| T/T | 7 (10.00) | 11 (11.00) | 0.89 | 0.33–2.44 | 0.835 | |||
| Allele | ||||||||
| C | 73 (52.14) | 122 (61.00) | 0.119 | 1.00 | ||||
| T | 67 (47.86) | 78 (39.00) | 1.38 | 0.89–2.12 | 0.151 |
Data indicate n (%).
Genetic model: Co, codominant, Do, dominant, Re, recessive.
Obtained by the Chi-square test.
Obtained by the logistic regression analysis, taking reference to AA and CC genotypes;
HWE (Hardy-Weinberg equilibrium) to controls. OR, odds ratio; 95% CI, 95% confidence interval.
Significant p<0.05.
Genotype distribution and allele frequency of SNPs in individuals with and without relapse of ALL
The A/A genotype was present in 10.42% of the patients with relapse, compared to 40.91% of those who did not relapse, whereas 50% of the patients with relapse were carriers of the A/G genotype compared to 40.91% of those who did not relapse, and 39.58% of the patients with relapse were carriers of the G/G genotype, compared to 18.18% of those who did not relapse. Regarding the distribution of the C829T polymorphism in patients with relapse, 81.25% were carriers of the C/T genotype, 12.50% had the T/T genotype and only 6.25% carried the C/C genotype. In contrast to those who did not relapse, 31.82% were carriers of the C/C genotype, 63.64% of the C/T genotype and 4.54% carriers of the T/T genotype. Genotypic and allelic frequencies of both polymorphisms were statistically significant between patients with and without relapse of ALL (p<0.05) (Table III).
Table III.
Genotype distribution and allele frequency of the -A317G and C829T polymorphisms of DHFR in individuals with and without relapse of childhood acute lymphoblastic leukemia.
| Without relapse n (%) | With relapse n (%) | p-valuea | |
|---|---|---|---|
| -A317G (rs408626) | |||
| Genotypes | |||
| A/A | 9 (40.91) | 4 (8.33) | |
| A/G | 9 (40.91) | 25 (52.08) | |
| G/G | 4 (18.18) | 19 (39.58) | 0.014b |
| Allele | |||
| A | 27 (61.36) | 34 (35.42) | |
| G | 17 (38.64) | 62 (64.58) | 0.004b |
| C829T (rs34764978) | |||
| Genotypes | |||
| C/C | 7 (31.82) | 3 (6.25) | |
| C/T | 14 (63.64) | 39 (81.25) | |
| T/T | 1 (4.54) | 6 (12.50) | 0.015b |
| Allele | |||
| C | 28 (63.64) | 45 (46.88) | |
| T | 16 (36.36) | 51 (53.12) | 0.048b |
Obtained by the Chi-square test;
Significant p<0.05.
Risk of relapse based on the -A317G and C829T SNP genotypes and other risk factors in ALL
In a logistic regression analysis, an association was found between the -A317G, C829T polymorphisms and the risk of relapse of disease (p<0.05). Those patients carrying the G/G genotype of the -A317G polymorphism, showed a significant increase in the risk of relapse of ALL (OR=8.55, 95% CI 1.84–39.70) compared to carriers of the A/A genotype (p=0.006) (Table IV). Carriers of the T/T genotype of the C829T polymorphism had a 14.00 greater chance of a relapse of the disease (OR=14.00, 95% CI 1.13–172.63) compared to carriers of the C/C genotype (p=0.039) (Table IV). Other variables, such as age, leukocytes, but not gender, were associated with the risk of relapse of disease (p<0.05) (Table IV).
Table IV.
Association of the -A317G and C829T polymorphisms in the DHFR gene and other clinical features with the risk of relapse of childhood acute lymphoblastic leukemia.
| Modela | Genotype | No. (%) | OR | 95% CI | p-valueb |
|---|---|---|---|---|---|
| -A317G (rs408626) | |||||
| Co | A/A | 14 (20.00) | 1.00 | ||
| A/G | 33 (47.14) | 4.80 | 1.26–18.24 | 0.021c | |
| G/G | 23 (32.86) | 8.55 | 1.84–39.70 | 0.006c | |
| Do | A/A | 14 (20.00) | 1.00 | ||
| A/G + G/G | 56 (80.00) | 7.62 | 2.01–28.81 | 0.003c | |
| Re | A/A + AG | 47 (67.14) | 1.00 | ||
| G/G | 23 (32.86) | 2.94 | 0.86–10.06 | 0.084 | |
| C829T (rs34764978) | |||||
| Co | C/C | 10 (14.29) | 1.00 | ||
| C/T | 53 (75.71) | 6.50 | 1.47–28.67 | 0.013c | |
| T/T | 7 (10.00) | 14.00 | 1.13–172.63 | 0.039c | |
| Do | C/C | 10 (14.29) | 1.00 | ||
| C/T + T/T | 60 (85.71) | 7.00 | 1.60–30.54 | 0.010c | |
| Re | C/C + C/T | 63 (90.00) | 1.00 | ||
| T/T | 7 (10.00) | 3.00 | 0.348–26.55 | 0.323 | |
| Gender | |||||
| Female | 25 (35.71) | 1.00 | |||
| Male | 45 (64.29) | 1.38 | 0.49–3.92 | 0.540 | |
| Risk by age | |||||
| 1–9 years (low risk) | 18 (25.71) | 1.00 | |||
| <1 and >9 years (high risk) | 52 (74.29) | 4.54 | 1.14–18.09 | 0.032c | |
| Leukocytes at diagnosis | |||||
| <50,000/mm3 | 18 (25.71) | 1.00 | |||
| >50,000/mm3 | 52 (74.29) | 7.64 | 1.90–30.73 | 0.004c |
Genetic model: Co, codominant; Do, dominant; Re, recessive.
Obtained by logistic regression analysis;
Significant p<0.05; OR, odds ratio; 95% CI, 95% confidence interval.
The following variables were included in a multivariate analysis: leukocytes count at diagnosis, age, -A317G and C829T polymorphism genotypes, to determine whether either or both the SNPs predict the risk of relapse independently. It was observed that A/G or G/G genotype carriers (p=0.041 and p=0.017, respectively), together with the C/T or T/T genotype carriers (p=0.015 and p=0.049, respectively), were two independent prognostic markers for the risk of relapse of ALL compared to the other variables (Table V).
Table V.
Factors influencing the risk of relapse of ALL in a multivariate regression analysis.
| OR | 95% CI | p-valuea | |
|---|---|---|---|
| -A317G (rs408626) | |||
| A/G | 4.06 | 0.01–0.58 | 0.041b |
| G/G | 6.75 | 0.07–0.67 | 0.017b |
| C829T (rs34764978) | |||
| C/T | 7.49 | 0.08–0.71 | 0.015b |
| T/T | 12.38 | 0.002–0.93 | 0.049b |
| Risk by age | |||
| <1 and >9 years (high risk) | 0.86 | 0.25–2.96 | 0.814 |
| Leukocytes at diagnosis | |||
| >50,000/mm3 | 0.52 | 0.11–2.36 | 0.395 |
OR, odds ratio; 95% CI, 95% confidence interval.
Obtained by multivariate logistic regression analysis.
Significant p<0.05.
Association between DHFR -A317G and C829T SNP and overall survival in the ALL patients
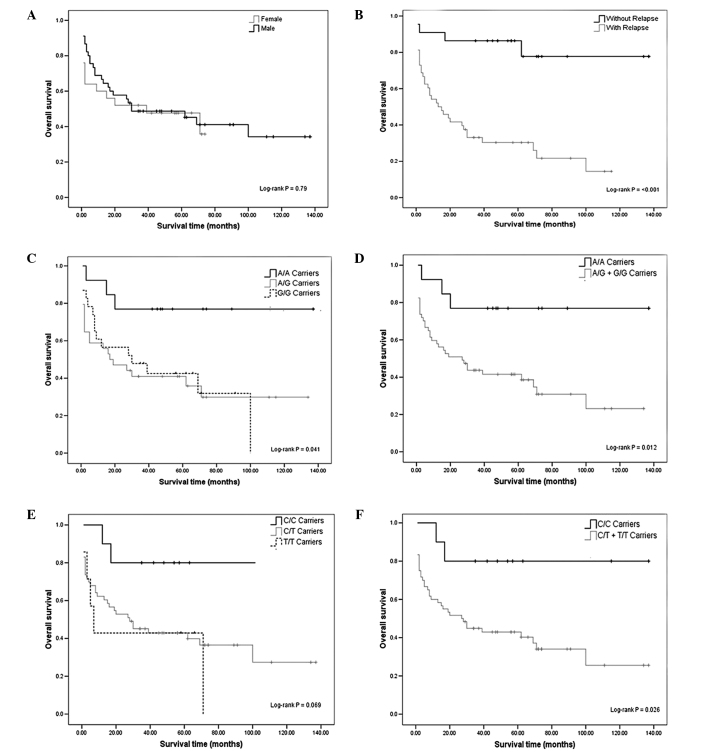
Kaplan-Meier analysis of OS curves showed significant results between the relapse of ALL and DHFR -A317G, C829T polymorphisms. We found no significant associations between gender and OS (log-rank test; p=0.79) (Fig. 1A). A different rate of OS was evident between the individuals with and without relapse of ALL (log-rank test; p<0.001) (Fig. 1B). In fact, 81.82% of the patients without relapse were alive, while only 25% of patients with relapse were alive (Table I). Those patients with relapse had a 13.5 greater chance of death (OR=13.5, 95% CI 3.81–47.84) compared to those without relapse (p<0.001) (data not shown).
Figure 1.
Kaplan-Meier curves considering the influence of gender, relapse, and dihydrofolate reductase (DHFR) -A317G and C829T polymorphisms on overall survival (OS) of acute lymphoblastic leukemia (ALL) patients. (A) OS in females and males with ALL. (B) OS between patients with and without relapse. (C) Association between OS and the DHFR -A317G polymorphism. (D) Combined genotypes A/G + G/G vs. A/A in 70 children with ALL. (E) Association between OS and the DHFR C829T polymorphism. (F) Combined genotypes C/T + T/T vs. C/C in 70 children with ALL. Ticks indicate censoring.
The relationship between OS and the polymorphisms was calculated. Individuals with the G/G genotype had poorer OS compared to the A/A genotype (log-rank test; p<0.05) (Fig. 1C and D). We found no significant association for the DHFR C829T polymorphism, although we observed a reduction in survival at 11 years of follow-up among T/T-carriers with respect to that of patients with the wild-type genotype (log-rank test; p=0.069) (Fig. 1E). However, we carried out log-rank test for combined genotypes, C/T + T/T vs. C/C (Fig. 1F), showing significant genotype-dependent effects for OS (log-rank test; p=0.026).
Discussion
There have been attempts to explain the mechanisms by which patients show different response to the same drug used to treat ALL. However, there are few studies addressing the association of SNPs with response to treatment in patients with ALL. There are no studies in Mexico regarding the -317A/G and 829C/T polymorphisms of DHFR; therefore, it is important to determine their distribution in the Mexican population, its association and impact on the risk of relapse and survival in patients with ALL.
Patients with ALL predominantly showed the heterozygous A/G genotype (47.14%) of the -A317G polymorphism (Table II), a result similar to that reported by Dulucq et al, in a Canadian population where the A/G genotype was reported as the most frequent in individuals with ALL (48.4%) (14). However, the genotypic frequencies A/A (31.4%), A/G (48.4%) and G/G (20.2%) reported by Dulucq et al are statistically significant to those found in this study (p=0.040).
The genotypic frequencies of the C829T polymorphism reported in this study differ (p<0.001) from those reported by Goto et al, in a Japanese population with ALL, where the C/C genotype (83.8%) was the most frequently reported, followed by the C/T genotype (10.8%) and the T/T genotype (5.4%) (13). The C829T polymorphism has been studied in other disorders that involve the metabolic pathway of folate. However, it was not identified in non-Japanese American, Caucasian or Israeli populations (21–23), suggesting that the C829T polymorphism was found more frequently in patients with ALL than in other disorders in which the activity enzymatic of DHFR is involved.
In the present study, 93.75% of the patients with relapse were carriers of the T allele (Table III); this agrees with previous experimental studies, which identified the T allele as a risk for relapse to ALL (28). This suggests that the presence of the -A317G and C829T polymorphisms, and the strong association with the risk of relapse (p<0.05) (Table IV) may be a factor that led to more than 50% of deaths in the patients with ALL included in this study.
In many studies, age has been found to be a prognostic factor in childhood ALL (24); this feature also retained its significance in our study. Patients in the age group of 1–9 years (low risk) had the best prognosis, whereas patients <1 and >9 years of age (high risk) showed the worst prognosis (OR=4.54, 95% CI 1.14–18.09) (Table IV), which agrees with other studies (25).
Regarding gender, it has been reported in various studies that females have better survival than males of the same age with ALL (24,26). However, we did not observe such a gender-based outcome (p>0.05) (Table IV), similarly to Dulucq et al, Kim et al and Dervieux et al, (14,27,28). This could be due to the small sample size of the female group (n=25/70) in our study. The WBC has also been reported as a prognostic factor in many studies on pediatric ALL. We observed that a WBC count more than 50×109 leukocytes/mm3 at diagnosis was associated with poor outcome (OR=7.64, 95% CI 1.90–30.73) (Table IV), similar to other studies (24,29). Immunophenotype has been considered as the most important prognostic factor impacting the therapeutic strategy. The pre-B ALL and the immature T-cell precursor are generally associated with a poorer prognosis (30). Two studies have shown that the Hispanic population has a high frequency of B-cell precursor immunophenotype (31,32). Our findings are consistent with these studies; we found that the 68.18% of cases with relapse of leukemia were classified within this immunological lineage (data not shown).
On separate analyses of the -A317G and C829T polymorphisms in the DHFR gene, analyses of the G/G and T/T genotypes alone appear to be worse prognostic markers than leukocytes at diagnosis and age (OR=8.55, 95% CI 1.84–39.70; OR=14.00, 95% CI 1.13–172.63; OR=7.64, 95% CI 1.90–30.73; OR=4.54, 95% CI 1.14–18.09, respectively). However, in a multivariate analysis we observed that the -A317G and C829T polymorphisms were the worst independent prognostic factors. The analysis showed that G/G and T/T genotypes were independent prognostic markers, excluding leukocytes at diagnosis and age (Table V). A second goal of this study was to investigate the impact of the polymorphisms on survival. The OS rate of G and T allele carriers of the -A317G and C829T polymorphisms, respectively, was lower than that of patients carrying A and C alleles. Our results are in line with those reported by Dulucq et al (14), showing an association between the G/G variant and reduced survival in ALL patients. We also evaluated the relationship between OS and the C829T polymorphism. During follow-up, we observed a reduction in OS among T-carriers and patients with the wild-type genotype (Fig. 1).
In conclusion, the -A317G and C829T polymorphisms are strongly associated with the risk of relapse to ALL, presenting a higher risk of relapse in ALL carriers of G/G and T/T genotypes than in carriers of the A/A and C/C genotypes, respectively. Our data showed that the polymorphisms of DHFR C829T and -A317G had an impact on survival of ALL Mexican patients. These data seem to suggest a role for the DHFR polymorphisms in the relapse of ALL and overall survival. This is the first study which evaluated the effect of the C829T polymorphism on overall survival. However, this analysis was based only on 70 patients and needs to be confirmed in a larger population.
Acknowledgments
The authors would like to thank the National Council of Science and Technology (CONACYT, Mexico) for a fellowship awarded to Yazmín Gómez-Gómez (August 2007 to July 2009). They also thank the technicians of the Laboratorio de Biomedicina Molecular for their laboratory assistance, and Dinorah Leyva-Illades (Texas A&M Health Science Center) for revising the English style of the manuscript.
References
- 1.Coronel-Morán QR. Importancia del laboratorio en el diagnóstico y pronóstico de leucemia aguda linfoblástica de la infancia. Acta Pediátrica de México. 2005;26:129–136. [Google Scholar]
- 2.Mejia-Arangure JM, Bonilla M, Lorenzana R, et al. Incidence of leukemias in children from El Salvador and Mexico City between 1996 and 2000: population-based data. BMC Cancer. 2005;5:33. doi: 10.1186/1471-2407-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salud/INEGI, S.d., Fuente: base de datos de defunciones INEGI/Secretaria de Salud, Dirección General de Información en Salud, México. doi: 10.1590/s0036-36342005000200013. Información disponible en URL www.inegi.org.mx/ (Accessed April, 2008). [DOI] [PubMed] [Google Scholar]
- 4.Cheok MH, Evans WE. Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nat Rev Cancer. 2006;6:117–129. doi: 10.1038/nrc1800. [DOI] [PubMed] [Google Scholar]
- 5.Hider SL, Bruce IN, Thomson W. The pharmacogenetics of methotrexate. Rheumatology (Oxford) 2007;46:1520–1524. doi: 10.1093/rheumatology/kem147. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Goodey NM, Benkovic SJ, Kohen A. Coordinated effects of distal mutations on environmentally coupled tunneling in dihydrofolate reductase. Proc Natl Acad Sci USA. 2006;103:15753–15758. doi: 10.1073/pnas.0606976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpato JP, Fossati E, Pelletier JN. Increasing methotrexate resistance by combination of active-site mutations in human dihydrofolate reductase. J Mol Biol. 2007;373:599–611. doi: 10.1016/j.jmb.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 8.Allemann RK, Evans RM, Tey LH, et al. Protein motions during catalysis by dihydrofolate reductases. Philos Trans R Soc Lond B Biol Sci. 2006;361:1317–1321. doi: 10.1098/rstb.2006.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fotoohi AK. Resistance of human leukaemia cells to the antimetabolites. Oncology-Pathology, Suecia. 2007;1:1–84. [Google Scholar]
- 10.Serra M, Reverter-Branchat G, Maurici D, et al. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann Oncol. 2004;5:151–160. doi: 10.1093/annonc/mdh004. [DOI] [PubMed] [Google Scholar]
- 11.De Jonge R, Hooijberg JH, van Zelst BD, et al. Effect of polymorphisms in folate-related genes on in vitro methotrexate sensitivity in pediatric acute lymphoblastic leukemia. Blood. 2005;106:717–720. doi: 10.1182/blood-2004-12-4941. [DOI] [PubMed] [Google Scholar]
- 12.Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26:153–181. doi: 10.1007/s10555-007-9049-z. [DOI] [PubMed] [Google Scholar]
- 13.Goto Y, Yue L, Yokoi A, et al. A novel single-nucleotide polymorphism in the 3′-untranslated region of the human dihydrofolate reductase gene with enhanced expression. Clin Cancer Res. 2001;7:1952–1956. [PubMed] [Google Scholar]
- 14.Dulucq S, St-Onge G, Gagne V, et al. DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood. 2008;111:3692–3700. doi: 10.1182/blood-2007-09-110593. [DOI] [PubMed] [Google Scholar]
- 15. Seguro-popular: Secretaria de Salud/Seguro Popular, Información disponible en URL http://www.seguro-popular.salud.gob.mx/ (Accessed May, 2009). [Google Scholar]
- 16.Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122–3133. [PubMed] [Google Scholar]
- 17.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Merante F, Raha S, Reed JK, Proteau G. The simultaneous isolation of RNA and DNA from tissues and cultured cells. Methods Mol Biol. 1996;58:3–9. doi: 10.1385/0-89603-402-X:3. [DOI] [PubMed] [Google Scholar]
- 19.Organista-Nava J, Gómez-Gómez Y, Saavedra-Herrera MV, et al. Polymorphisms of the γ-glutamyl hydrolase gene and risk of relapse to acute lymphoblastic leukemia in Mexico. Leukemia Res. 2010;34:728–732. doi: 10.1016/j.leukres.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Kooloos WM, Wessels JAM, van der Straaten T, Allaart CF, Huizinga TWJ, Guchelaar HJ. Functional polymorphisms and methotrexate treatment outcome in recent-onset rheumatoid arthritis. Pharmacogenomics. 2010;11:163–175. doi: 10.2217/pgs.09.139. [DOI] [PubMed] [Google Scholar]
- 21.Gellekink H, Blom HJ, van der Linden IJ, den Heijer M. Molecular genetic analysis of the human dihydrofolate reductase gene: relation with plasma total homocysteine, serum and red blood cell folate levels. Eur J Hum Genet. 2007;15:103–109. doi: 10.1038/sj.ejhg.5201713. [DOI] [PubMed] [Google Scholar]
- 22.Mishra PJ, Longo GSA, Menon LG, Abali EE, Humeniuk R, Cole PD, Kamen BA, Banerjee D, Bertino JR. The 829C-T single nucleotide polymorphism in the 30 UTR of the dihydrofolate reductase gene results in methotrexate resistance and is rare among non-Japanese American patients. Proc Amer Assoc Cancer Res. 2006;301:1274. [Google Scholar]
- 23.Parle-McDermott A, Pangilinan F, Mills JL, et al. The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population. Am J Med Genet Part A. 2007;143:1174–1180. doi: 10.1002/ajmg.a.31725. [DOI] [PubMed] [Google Scholar]
- 24.Ng SM, Lin HP, Ariffin WA, Zainab AK, Lam SK, Chan LL. Age, sex, haemoglobin level, and white cell count at diagnosis are important prognostic factors in children with acute lymphoblastic leukemia treated with BFM-type protocol. J Trop Pediatr. 2000;46:338–343. doi: 10.1093/tropej/46.6.338. [DOI] [PubMed] [Google Scholar]
- 25.Frankel LS, Ochs J, Shuster JJ, et al. Therapeutic trial for infant acute lymphoblastic leukemia: the Pediatric Oncology Group experience (POG 8493) J Pediatr Hematol Oncol. 1997;19:35. doi: 10.1097/00043426-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Shuster JJ, Wacker P, Pullen J, et al. Prognostic significance of sex in childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16:2854–2863. doi: 10.1200/JCO.1998.16.8.2854. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Kang SB, Chung HH, Kim JW, Park NH, Song YS. XRCC1 Arginine 194 Tryptophan and GGH-401 Cytosine/Thymine polymorphisms are associated with response to platinum-based neoadjuvant chemotherapy in cervical cancer. Gynecol Oncol. 2008;111:509–515. doi: 10.1016/j.ygyno.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Dervieux T, Greenstein N, Kremer J. Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis Rheum. 2006;54:3095–3103. doi: 10.1002/art.22129. [DOI] [PubMed] [Google Scholar]
- 29.Chessells JM, Veys P, Kempski H, et al. Long term follow up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen SH, Yang CP, Hung IJ, Jaing TH, Shih LY, Tsai MH. Clinical features, molecular diagnosis, and treatment outcome of infants with leukemia in Taiwan. Pediatr Blood Cancer. 2010;55:1264–1271. doi: 10.1002/pbc.22731. [DOI] [PubMed] [Google Scholar]
- 31.Daniel-Cravioto A, Gonzalez-Bonilla CR, Mejia-Arangure JM, et al. Genetic rearrangement MLL/AF4 is most frequent in children with acute lymphoblastic leukemias in Mexico City. Leuk Lymphoma. 2009;50:1352–1360. doi: 10.1080/10428190903015636. [DOI] [PubMed] [Google Scholar]
- 32.Bernaldez-Rios R, Ortega-Alvarez MC, Perez-Saldivar ML, Alatoma-Medina NE, del Campo-Martinez M. The age incidence of childhood B-cell precursor acute lymphoblastic leukemia in Mexico City. J Pediatr Hematol/Oncol. 2008;30:199–203. doi: 10.1097/MPH.0b013e318162bcdc. [DOI] [PubMed] [Google Scholar]