Abstract
The aim of the present study was to investigate the effect of ZnPcS2P2-meditated sonodynamic therapy (SDT) on U251 human glioma cells and to identify its underlying biological mechanism. The growth inhibition rate was determined by MTT assay. The apoptotic rate was examined by flow cytometry. Fine structures were observed with transmission electron microscopy (TEM). Generation of reactive oxygen species (ROS) was detected spectrophotometrically. Caspase-3, -8 and -9 expression was detected by Western blot analysis. The growth inhibition rate of U251 human glioma cells indicated that ZnPcS2P2-meditated SDT had a better growth inhibition rate of tumor cells at a concentration of 5.0 μg/ml ZnPcS2P2, at a 4-h incubation time with ZnPcS2P2, and at 6 h re-incubation following SDT. At 6 h after SDT, the growth inhibition rate of cells was significantly higher compared to other groups, apoptosis could be detected in SDT by flow cytometry. TEM examination revealed morphological features of apoptosis or necrosis. Furthermore, caspase-3, -8 and -9 expression following SDT was found to be increased by Western blot analysis. Finally, generation of ROS in cells was also elevated. In conclusion, ZnPcS2P2-SDT is capable of inducing U251 cell apoptosis or necrosis and has satisfying antitumor effects. The mechanism of ZnPcS2P2-meditated SDT involves ROS generation in U251 cells, which initiates subsequent apoptosis through the mitochondrial and death receptor pathways.
Keywords: sonodynamic therapy, apoptosis, ZnPcS2P2, human U251 glioma cells, reactive oxygen species
Introduction
Gliomas are the most common types of tumors of the central nervous system and are resistant to numerous treatments, including radiation, chemotherapy or other adjuvant therapies. Although considerable progress has been made in the treatment of glioma, its prognosis remains poor (1). A new modality is urgently required in order to combat gliomas. Sonodynamic therapy (SDT), a combination of sonosensitizers and ultrasound irradiations, has been considered to be an effective method owing to increasing sonosensitizer-derived radicals. SDT is capable of effectively enhancing the cytotoxicity of sonosensitizers, which preferentially accumulate in the tumor site with minimal damage of peripheral healthy tissues. Thus, this would be a valuable cancer therapy modality (2,3).
The sonosensitizer is the key factor of SDT. Photosensitizers have been used in SDT as well as in photodynamic therapy (PDT). Certain drugs, including hematoporphyrin, photofrin II, ATX-70 and ATX-S10, have been demonstrated to induce cell killing when activated by ultrasound exposure. It has previously been indicated that these chemicals, originally generated in PDT, were therefore applicable as sonosensitizers for tumor treatment in combination with ultrasound. In the present preliminary study, we used a new domestic photosensitizer, di-sulfo-di-phthalimidomethyl phthalolcyanine zinc (ZnPcS2P2) (4–6). The new photosensitizer (wavelength 670 nm) has been studied in choroidal neovascularization and bone marrow purging for PDT (7). ZnPcS2P2 has certain advantages over other conventional photosensitizers, such as porphyrin derivatives (8), for example, the exicited triplet state of ZnPcS2P2 has a larger quantum yield, longer lifetime and the amphipathic structure of ZnPcS2P2 results in the increase of selective uptake of sensitizer by tumor cells. Moreover, a repeated-dose toxicity study of ZnPcS2P2-based PDT in beagle dogs demonstrated it to be a safe and promising approach in the clinic, as this drug is eliminated more quickly from the body with the result of decreased skin phototoxicity from natural light. It has been demonstrated that ZnPcS2P2 may be a good sensitizer for use in SDT.
As we know, apoptosis is often initiated by an extrinsic (activated caspase-8) or an intrinsic pathway (activated caspase-9) (9). The extrinsic pathway functions are capable of directly activating caspase-8 via the death receptors on the cell surface; nevertheless, the intrinsic pathway regulates the activation of caspase-9 and, subsequently, the activation of caspase-3. In the present study, we aimed to evaluate the effects of killing glioma cells and indentified the intrinsic or extrinsic apoptosis pathways of the newly established sonosensitizer by SDT in an in vitro model.
Materials and methods
Sonosensitizer
ZnPcS2P2 was a gift from the Department of Chemistry, Institute of Research on Functional Materials, Fuzhou University (China). Its chemical structure is shown in Fig. 1. ZnPcS2P2 is an odorless, cyan liquid that is insoluble in water. The liquid has been identified to have a chemical purity of >95.0% via gas chromatography and infrared spectroscopy. ZnPcS2P2 was dissolved in a solution comprising Cremophor EL 2% (V/V), propylene glycol 20% (V/V), NaCl 0.9% (W/W) and was stored in the dark at 4°C. These dosing solutions were prepared immediately prior to use.
Figure 1.
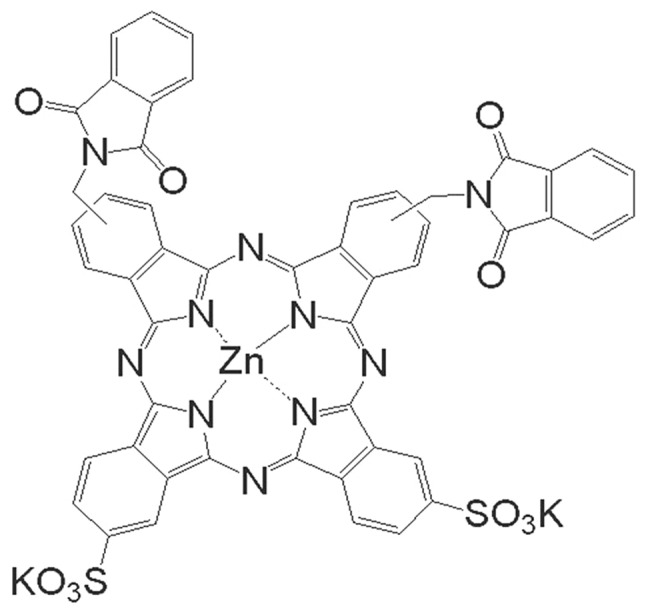
The chemical structure of ZnPcS2P2.
Cell culture
U251 human glioma cells were obtained from the Shanghai institute of Cytobiology (Institute of Chinese Academic Medical Scinence, China) and were continuously cultured in DMEM (Gibco BRL; Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 mmol/l L-glutamine in a humidified incubator at 37°C and 5% CO2. Cells in the exponential growth phase were used for all experiments.
Setup of sonication experiment
A multi-function physical therapy ultrasound device (Tianshi Technologies Ltd. Co., Beijing, China) was used to generate ultrasound at 1 MHz. Ultrasonic intensities (0.5 W/cm2) were measured by a stainless-steel ball radiometer (diameter 0.32 cm) (10). In this study, the ultrasound transducer was placed in a 37°C water bath filled with degassed water and stained with black ink (Fig. 2).
Figure 2.
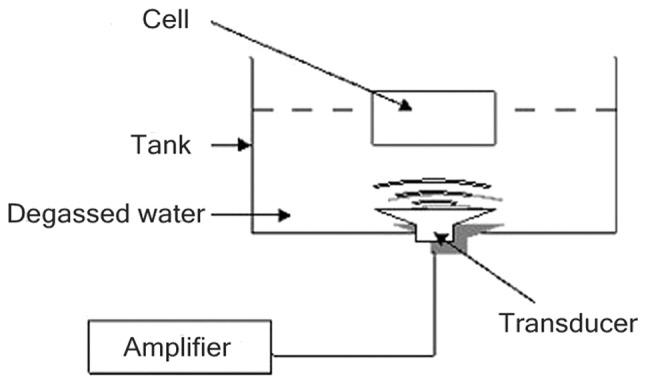
Experimental setup.
Reagents and antibodies
The monoclonal anti-β-actin antibody and anti-caspase-3, -8 and -9 antibodies were obtained from Sigma-Aldrich (St. Louis, MO, USA). Annexin-V-FITC Apoptosis Detection Kit was obtained from BD Biosciences (Franklin Lakes, NJ, USA). 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) was obtained from Calbiochem (La Jolla, CA, USA). All other chemicals and cell-culture reagents were purchased from Laohekou Jing hong Chemical Co. Ltd. (China). All solvents used in chemical reactions were anhydrous and obtained as such from commercial sources. All other reagents were used as supplied, unless otherwise stated.
Selection of drug conditions for SDT
U251 human cells were transferred to 6 wells in the centre of a 96-well plate at a density of 8×103 cells/well and in the same way, transferred to 36 sections of a 96-well plate. The following day, each of the 6 plates were changed by DMEM with various final concentrations of ZnPcS2P2 (0.625, 1.250, 2.500, 5.000, 10.000 and 20.000 μg/ml), cells were continuously cultured in a humidified incubator at 37°C and 5% CO2 for 4 h, then subjected to ultrasound treatment at 1.0 MHz and 0.5 W/cm2 for 2 min. Cells were re-incubated for up to 4 h and subjected to MTT assay.
Similarly, cells were transferred to 36 sections of a 96-well plate, were re-incubated with a final concentration of ZnPcS2P2 5.0 μg/ml for (1, 2, 3, 4, 5 and 6 h) and then underwent ultra-sound treatment. Cells were subsequently evaluated by MTT assay following re-incubation for 4 h.
Cells of 36 sections of a 96-well plate (with a final concentration of ZnPcS2P2 5.0 μg/ml) were incubated for up to 4 h, followed by ultrasound treatment, and subjected to MTT assay at various time-points post-SDT (3, 6, 12, 24, 48 and 96 h).
The growth inhibition rate was calculated as: inhibition rate (%) = (1-OD treatment group/OD control group) x 100%.
Examination of the SDT effect on the growth of human U251 glioma cells
In the control group, cells were neither treated with ZnPcS2P2 nor with ultrasound. In the ZnPcS2P2 group, cells were treated with ZnPcS2P2 alone (final concentration 5.0 μg/ml, incubation time 4 h). In the ultrasound group, cells were treated with ultrasound alone. In the SDT group, cells were treated with ZnPcS2P2 (5.0 μg/ml, incubation time 4 h) and then subjected to ultrasound treatment at 1.0 MHz and 0.5 W/cm2 for 2 min. Cells were re-incubated for up to 6 h and subjected to MTT assay following treatment.
Examination of SDT-induced apoptosis by flow cytometry
Following treatment, cells were re-incubated for up to 6 h, 4 groups of cells (control, ZnPcS2P2 alone, ultrasound alone and ZnPcS2P2 + ultrasound) were re-suspended in a concentration of 5×106 cells/ml and washed twice with pre-cooling of the phosphate-buffered saline (PBS). Following washing with Annexin-V binding buffer, cells were stained with FITC-conjugated Annexin-V and propidium iodide (PI) reagents for 15 min as per the manufacturer’s instructions and further examined by flow cytometry (FACScalibur; Becton-Dickinson). The percentages of dead cells and those undergoing apoptosis were analyzed using Cell Quest Software.
Transmission electron microscope examination of subcellular structure
For the electron microscope examination, cells from each group were re-incubated for up to 6 h and then washed twice with PBS and fixed in a mixture of 2.5% glutaraldehyde and osmium tetroxide. Cells were then dehydrated in progressive 10-min steps for 3 times in ethanol/water (70, 90 and 96%, respectively), followed by isoamyl acetate. Finally, cells were stained with uranyl acetate (Guangzhou Chemical Reagent Factory, China) and lead citrate (Guangzhou Chemical Reagent Factory). Ultra-thin sections were examined under a transmission electron microscope (JEM-1220, Japan).
Western blot analysis
To examine protein expression, cells of various groups were separately washed, collected and homogenized in a lysis buffer (10 mM Tris-HCl, pH 8.0, 0.32 mM sucrose, 5 mM EDTA, 2 mM DTT, 1 mM phenylmethyl sulfonylfluoride and 1% Triton X-100), followed by centrifugation. Proteins in various groups were separately electrophoresed on sodium dodecyl sulfate (SDS) polyacrylamide gel (12%), the gel-separated proteins were transferred to nitropure nitrocellulose membranes (Santa Cruz Biotechnology, CA, USA), and the membranes were probed overnight at 4°C with primary antibodies. Each of the targeted proteins was immunostained by anti-β-actin and anti-caspases-3, -8 and -9 (each 1:1000) antibodies. Following probing, the membranes were washed 3 times and incubated for 1 h at room temperature with the respective alkaline phosphatase-conjugated secondary antibodies (Sigma) prior to visualization using a chemiluminescence detection kit (Sigma).
Measurement production of intracellular reactive oxygen species (ROS)
The production of intracellular ROS was assayed spectrophotometrically with dichlorofluorescin diacetate (DCFH-DA) as described previously (11). Cells with or without treatment were harvested, washed and re-suspended in 500 μl PBS containing DCFH-DA (final concentration, 5 μmol/l), and incubated at 37°C in the dark for 30 min. the concentration of ROS was monitored spectrophotometrically (F-2000 Hitachi; Hitachi Corp. Tokyo, Japan) at 534 nm following excitation at 488 nm.
Statistical analysis
Statistical evaluation was performed using the least significant difference t-test, Dunnett test or paired t-test using the SPSS 11.0 Software system. Data are presented as the means ± standard error (SE). P<0.05 was considered to indicate a statistically significant difference.
Results
Selection of drug conditions and analysis of growth inhibition rate in SDT by MTT
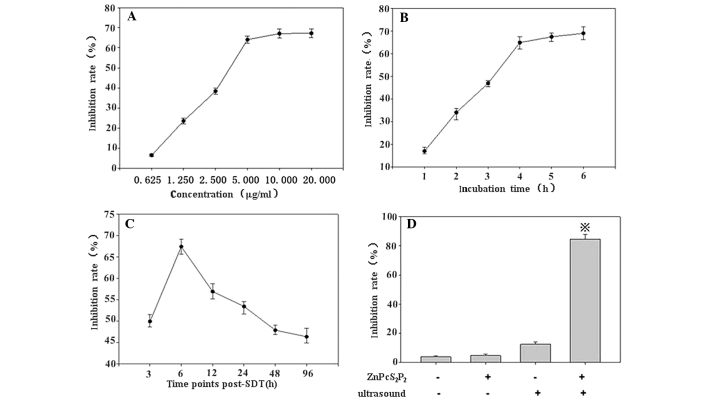
A MTT assay revealed that when the concentration of ZnPcS2P2 was >5.0 μg/ml, the growth inhibition rate was less concentration-dependent (Fig. 3A) and that when the incubation time of ZnPcS2P2 was >4 h the growth inhibition rate was less incubation time-dependent (Fig. 3B). Conversely, the MTT assay revealed that growth inhibition occurred in a concentration- and incubation time-dependent manner when the concentration of ZnPcS2P2 was <5.0 μg/ml or the incubation time was <4 h. In addition, the growth inhibition rate did not significantly increase when re-incubation time was beyond 6 h following SDT. Therefore, when the incubation time was 4 h, and the concentration of ZnPcS2P2 was 5.0 μg/ ml, 6 h following SDT, the inhibition rate was highly notable (Fig. 3A–C). Thus, we selected the 5.0 μg/ml concentration of ZnPcS2P2, the 4-h incubation time and at 6 h post-SDT to study the ZnPcS2P2-mediated SDT effects on U251 glioma cells.
Figure 3.
Selection of drug conditions. (A) Analysis of the growth inhibition rate of various concentrations of ZnPcS2P2, (B) incubation times and (C) time-points, post-SDT by MTT assay. The results revealed that when the concentration of ZnPcS2P2 was >5.0 μg/ml and incubation time was >4 h, the growth inhibition rate did not markedly increase, and the inhibition rate was significantly higher at 6 h post-SDT. (D) Growth inhibition rates of the 4 groups of cells (control, ZnPcS2P2 alone, ultrasound alone and ZnPcS2P2 + ultrasound), determined by MTT assay. The inhibition rate in the SDT group was significantly higher than the other 3 groups. Data are represented as the means ± standard error (n=6). *P< 0.05 denotes statistically significant differences vs. control. SDT, sonodynamic therapy.
Examination of the SDT effect on the growth of U251 human glioma cells
SDT will be most effective when the ZnPcS2P2 concentration in the glioma cells is at its maximum. As shown in Fig. 3D, when the concentration of ZnPcS2P2 was 5.0 μg/ml and the incubation time was 4 h, the synergistic effect between ZnPcS2P2 and ultrasound exposure on cell growth inhibition was evident. The growth inhibition rate of the 4 groups of the cells were determined by MTT assay post-treatment, the inhibition rate in the SDT group was significantly higher than in the other 3 groups (p<0.05), the inhibition rate was at its peak (87.86±2.45%) 6 h following SDT (Fig. 3D).
Apoptosis assessment by flow cytometry
The percentage of apoptotic cells was calculated by flow cytometry using the Annexin-V/PI double-staining assay (Fig. 4A–D). ZnPcS2P2 treatment alone showed no significant apoptotic effect (0.03±0.01 vs. 0.15±0.01%, p>0.05), ultrasound treatment displayed a slight apoptotic effect (4.61±0.24 vs. 0.03±0.01%, p<0.05) and SDT treatment displayed a clear apoptotic effect (30.93±1.34 vs. 0.03±0.01%, p<0.01).
Figure 4.
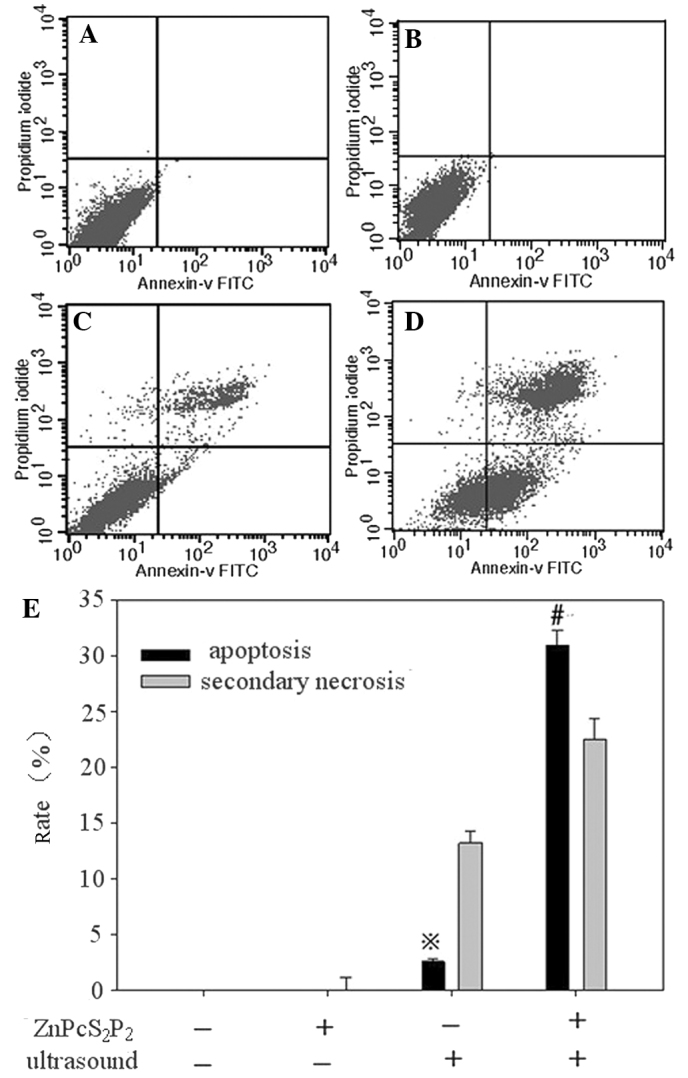
Flow cytometric analysis of apoptosis in U251 cells. (A) Control group, (B) ZnPcS2P2 alone group, (C) ultrasound alone group, (D) ZnPcS2P2 + ultrasound group. In each panel, the upper right quadrants represent the percentage of secondary necrosis cells (Annexin-V binding and for PI uptake). The lower right quadrant contains the apoptotic cells (positive for Annexin-V and negative for PI, demonstrating cytoplasmic membrane integrity) undergoing SDT-induced apoptosis and necrosis in U251 cells. Data are expressed as the means ± standard error (n=6). (E) #P<0.01 denotes statistically significant differences vs. control, *P<0.01 denotes statistically significant differences vs. control. SDT, sonodynamic therapy; PI, propidium iodide.
Fine structure changes
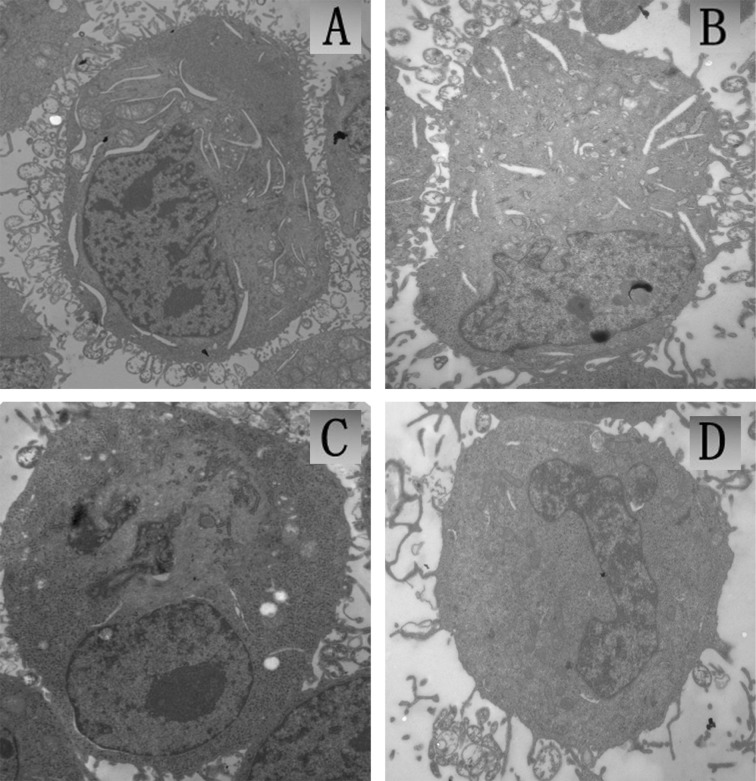
The TEM observation of U251 tumor cells immediately following SDT treatment is shown in Fig. 5. The cells in the control group were intact with a rich cytoplasm, mitochondria were integrated, cell membranes and the nuclear envelope were intact and nuclear materials were dense (Fig. 5A). The morphology of cells in the ZnPcS2P2 group was similar to that in the control group, cell membranes remained intact with a rich cytoplasm (Fig. 5B). In the ultrasound group, a small number of cells displayed limited microvilli, cavitation bubbles, slightly condensed chromatin and presented characteristics of early stage apoptosis (Fig. 5C). In the SDT group, a portion of cell microvilli had vanished, the volume of the cell nucleus had decreased and chromatin was gathered densely, thus presenting characteristics of apoptosis (Fig. 5D).
Figure 5.
TEM images of U251 cells in vitro (x10,000). (A) Control group with untreated intact cells, (B) cells with 5.0 μg/ml ZnPcS2P2 alone, (C) cells irradiated with US alone, (D) cells irradiated with US in the presence of 5.0 μg/ml ZnPcS2P2. TEM, transmission electron microscopy; US, ultrasound.
Analysis of caspase expressions
The SDT-induced apoptotic pathway was investigated by Western blot analysis of caspase expressions. Caspases-8, -9 and -3 are key factors in the extrinsic and intrinsic apoptosis pathway, thus, we measured the activities of these caspases. As shown in Fig. 7, caspases-3, -8 and -9 were significantly activated following ultrasound treatment with ZnPcS2P2. The expression of activated caspases-8, -9 and -3 significantly increased in the SDT group (Fig. 6)
Figure 7.
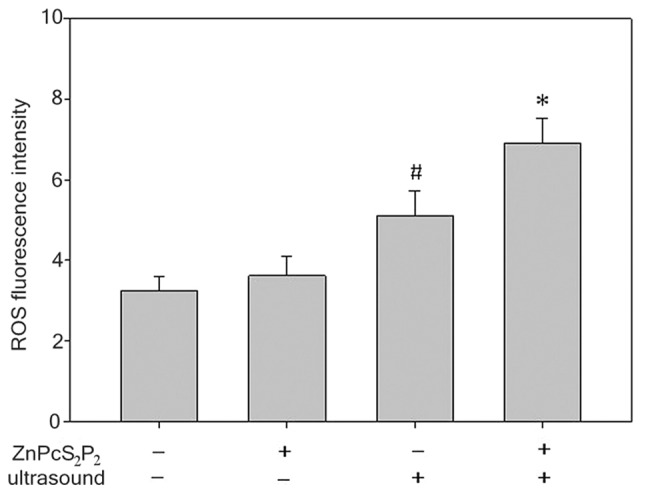
Bar graph showing the changes of the levels of ROS in various groups. Data are expressed as the means ± standard error (n=6). *P<0.05 denotes statistically significant difference vs. the other 3 groups, #P<0.05 denotes statistically significant difference vs. control group and ZnPcS2P2-alone group. ROS, reactive oxygen species.
Figure 6.
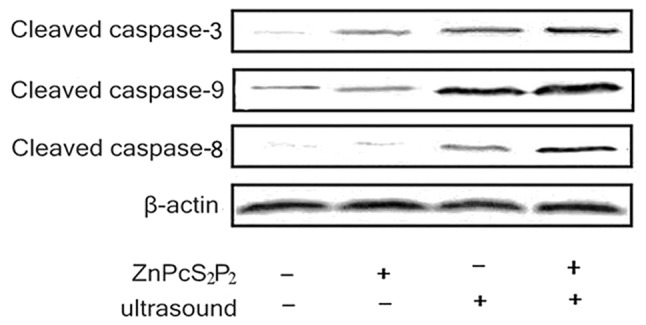
Western blot analysis of cleaved caspases-9,-8 and -3 in various groups at 6 h post-sonication. β-actin was used as the control.
Intracellular ROS
The production of ROS was determined by a fluorescence spectrophotometer with DCFH-DA (Fig. 7). The production of ROS in the SDT group significantly increased compared to the other 3 groups (p<0.05). In the ultrasound group, the production of ROS slightly increased compared to the ZnPcS2P2 group and the control group (p<0.05). However, there was no significant difference in ROS production between the ZnPcS2P2 and the control groups (p>0.05). The results indicate that the synergistic effect of ultrasound and ZnPcS2P2 clearly enhances ROS production.
Discussion
Sonodynamic therapy (SDT) is the foundation on which ultrasound is capable of activating the sonosensitizer in order to generate cytotoxic substances and further kill tumor cells. Owing to the fact that the sonosensitizer has high accumulation in tumor tissue, SDT can not only maximally kill tumor cells, but also protect normal brain tissue from impairment (12).
Until now, the majority of sonosensitizers in SDT are not chemosynthetic compounds. In the present experiment, ZnPcS2P2, a porphyrin compound created by artificial chemosynthesis that has stable physical and chemical properties, was used. In a previous study, ZnPcS2P2-mediated PDT improved the effects of killing leukemia HL60 and K562 cells (7). In this study, we aimed to observe the effects and mechanism of SDT with ZnPcS2P2.
In order to evaluate the effects of ZnPcS2P2-mediated SDT, we first aimed to pinpoint optimal concentrations and incubation times of ZnPcS2P2 in order to ensure the best result of ZnPcS2P2-mediated, SDT-induced U251 glioma cell death by MTT assay. Our experimental results indicated that the inhibition rate of tumor cells increased gradually with the elevation of drug concentration to some extent. When the concentration of the drug was ≥5.0 μg/ml, the inhibition rate of the tumor did not significantly increase (Fig. 3A). A similar phenomenon presented in the screening of the incubation time. The inhibation rate of the tumor had a close correlation with the incubation time to some extent. However, when the incubation time was ≥4 h, the inhibition rate of the tumor also did not significantly increase (Fig. 3B). In addition, the growth inhibition rate did not significantly increase when re-incubation time exceeded 6 h post-SDT. This phenomenon may be explained by the fact that ZnPcS2P2 was slowly absorbed by the U251 glioma cells, and when the glioma cells achieved maximum saturation, the inhibition rate of the glioma cells plateaued. (Fig. 3A and B). We observed various inhibition rates of the tumor cells in the 4 groups (control, ZnPcS2P2 alone, ultrasound alone and SDT). The results revealed that the inhibition rate of the tumor cells in the SDT group was significantly higher than in the other 3 groups at 6 h. This shows that the ultrasound activation of ZnPcS2P2 can have a greater killing effect on tumor cells compared with sonosentizer or ultrasound therapy alone. Therefore, we considered ZnPcS2P2 to be a good sonosensitizer for use in SDT.
Apoptosis is one of the major modes of death of neurospongioma. Li et al first discovered that SDT with hematoporphyrin monomethyl ether was capable of inducing apoptosis of C6 tumor cells through the mitochondrial pathway (13). The apoptotic rate in the 4 groups was evaluated by flow cytometry. The early apoptotic rate was 30.93%, significantly higher than the other 3 groups. These results indicate that ZnPcS2P2, similar to 5-AlA or porphyrins, could be used in SDT. It is suggested that the ZnPcS2P2-mediated SDT killing of U251 cells involves the induction of apoptosis.
To further clarify the apoptosis phenomena by morphological change, we observed the subcellular structure in U251 cells following SDT. In the SDT group, it was revealed that a section of the cell microvilli had vanished, the volume of the cellular nucleus became small and chromatin gathered densely, thus displaying the characteristics of apoptosis or necrosis (13). The result of TEM examination also suggested that ZnPcS2P2-mediated SDT destroyed not only the biological membrane but also the formation of the genetic system of tumor cells (Fig. 5). The damage of membranous organelles greatly influenced the stabilization of the microsurroundings and inward, as well as outward, energy exchange. The condensation of nuclear chromatin would result in an interruption of gene expression and regulation. These changes could destroy metabolism, affect functions and eventually lead to cell death (14).
Apoptosis is characterized by a number of well-defined features, including phosphatidyserine exposure, membrane blabbing, activation of caspase, chromatin condensation and DNA fragmentation (15). Classical apoptosis may proceed by the intrinsic and/or extrinsic pathway (16–18). In the intrinsic pathway, mitochondria are induced to release a number of factors, leading to the formation of the apoptosome, which is comprised of the adapter protein Apaf-1, cytochrome c and caspase-9 (19,20). The extrinsic pathway, is believed to be based on death receptor-dependent recruitment of the adaptor protein, Fas-associated death domain (FADD), which, in turn, promotes dimerization and subsequent activation of caspase-8 (21). Liu et al first discovered that SDT was capable of inducing apoptosis through a mitochondrial pathway (22,23). Two apoptotic pathways activate certain members of the caspase family, respectively, but the activation of caspase-3 finally commences apoptosis. Some investigators report that the death acceptor pathway is not included in HMME-meditated SDT induction of C6 cells apoptois (24), which indicates that the ultrasound activation of various sonosensitizers to kill different cell lines involves various apoptotic pathways. Western blot analysis in the 4 groups revealed that caspases-3, -8 and -9 were significantly activated following ultrasound treatment with ZnPcS2P2. It was indicated that both the death acceptor and mitochondrial pathways occured in process of ultrasound-activing ZnPcS2P2 to kill U251 cells.
To further study the mechanisms of the ultrasound activationof ZnPcS2P2 to kill U251 cells, we detected the level of cellular ROS. ROS, which include hydroxyl radicals, superoxide anions, singlet oxygens and hydrogen peroxides, are by-products of cellular metabolism. Excessive intracellular ROS is capable of damaging critical biomolecules and eventually results in several biological effect disorders, including alterations in signal transduction and gene expression for mitogenesis, mutagenesis and cell death (25,26). Analysis of data revealed that the level of ROS was markedly elevated in SDT compared with the other groups. This indicates that ROS plays a crucial role in the ZnPcS2P2-mediated SDT killing of U251 cells.
In conclusion, ZnPcS2P2-SDT is capable of inducing cell apoptosis and necrosis in U251 cells. Ultrasound therapy, combined with ZnPcS2P2, has satisfying antitumor effects. ROS generation in U251 cells plays a crucial role in ZnPcS2P2-SDT-induced cell death and initiates subsequent apoptosis through mitochondrial and death receptor pathways following ZnPcS2P2-SDT.
Acknowledgments
This study was supported by the National Natural Science Foundation (30970834; 81072079), Technological Key Research Projects of Heilongjiang province (GC10C304-1).
References
- 1.Perilongo G. Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol. 2005;75:301–307. doi: 10.1007/s11060-005-6754-8. [DOI] [PubMed] [Google Scholar]
- 2.Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219–222. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessel D, Lo J, Jeffers R, Fowlkes JB, Cain C. Modes of photodynamic vs. sonodynamic cytotoxicity. J Photochem Photobiol B. 1995;28:219–221. doi: 10.1016/1011-1344(94)07111-z. [DOI] [PubMed] [Google Scholar]
- 4.Huang JL, Chen NS, Huang JD, et al. Metal phthalocyanine as photosensitizer for photodynamic therapy(PDT) - preparation, characterization and anticancer activities of an amphiphilic phthalocyanine ZnPcS2P2. Sci China Ser B. 2001;44:113–122. [Google Scholar]
- 5.Liu W, Chen N, Jin H, et al. Intravenous repeated-dose toxicity study of ZnPcS2P2-based-photodynamic therapy in beagle dogs. Regul Toxicol Pharmaco. 2007;l47:221–231. doi: 10.1016/j.yrtph.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Huang HF, Chen YZ, Wu Y, Chen P. Purging of murine erythroblastic leukemia by ZnPcS2P2-based-photodynamic therapy. Bone Marrow Transplant. 2006;37:213–217. doi: 10.1038/sj.bmt.1705216. [DOI] [PubMed] [Google Scholar]
- 7.Huang HF, Chen YZ, Wu Y. Experimental studies of the effects of ZnPcS2P2-based-photodynamic therapy on bone marrow purging. Chin Med J (Engl) 2005;118:105–110. [PubMed] [Google Scholar]
- 8.Huang HF, Chen YZ, Wu Y. Mitochondria-dependent apoptosis induced by a novel amphipathic photochemotherapeutic agent ZnPcS2P2 in HL60 cells. Acta Pharmacol Sin. 2005;26:1138–1144. doi: 10.1111/j.1745-7254.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn F, Averbuch AJ, O’Brien WD., Jr A primary method for the determination of ultrasonic intensity with the elastic sphere radiometer. Acoustica. 1977;38:58–61. [Google Scholar]
- 11.Orie NN, Zidek W, Tepel M. Chemoattractant- and mitogen-induced generation of reactive oxygen species in human lymphocytes: the role of calcium. Exp Physiol. 1999;84:515–520. [PubMed] [Google Scholar]
- 12.Kuroki M, Hachimine K, Abe H, et al. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007;27:3673–3677. [PubMed] [Google Scholar]
- 13.Li JH, Song DY, Xu YG, Huang Z, Yue W. In vitro study of haematoporphyrin monomethyl ether-mediated sonodynamic effects on C6 glioma cells. Neurol Sci. 2008;29:229–235. doi: 10.1007/s10072-008-0972-8. [DOI] [PubMed] [Google Scholar]
- 14.Barati AH, Mokhtari-Dizaji M. Ultrasound dose fractionation in sonodynamic therapy. Ultrasound Med Biol. 2010;36:880–887. doi: 10.1016/j.ultrasmedbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Wang XB, Liu QH, Wang P, Zhang K, Tang W, Wang BL. Enhancement of apoptosis by sonodynamic therapy with protoporphyrin IX in isolate sarcoma 180 cells. Cancer Biother Radiopharm. 2008;23:238–246. doi: 10.1089/cbr.2007.0436. [DOI] [PubMed] [Google Scholar]
- 16.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 17.Aziz MH, Sundling KE, Dreckschmidt NE, Verma AK. Protein kinase C epsilon inhibits UVR-induced expression of FADD, an adaptor protein, linked to both Fas- and TNFR1-mediated apoptosis. J Invest Dermatol. 2009;129:2011–2021. doi: 10.1038/jid.2008.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SG, Jong HS, Kim TY, et al. Transforming growth factor-beta 1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol Biol Cell. 2004;15:420–434. doi: 10.1091/mbc.E03-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SA, Kim SJ, Chung KC. Huntingtin-interacting protein 1-mediated neuronal cell death occurs through intrinsic apoptotic pathways and mitochondrial alterations. FEBS Lett. 2006;580:5275–5282. doi: 10.1016/j.febslet.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 20.Felderhoff-Mueser U, Sifringer M, Pesditschek S, et al. Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis. 2002;11:231–245. doi: 10.1006/nbdi.2002.0521. [DOI] [PubMed] [Google Scholar]
- 21.Milner AE, Palmer DH, Hodgkin EA, et al. Induction of apoptosis by chemotherapeutic drugs: the role of FADD in activation of caspase-8 and synergy with death receptor ligands in ovarian carcinoma cells. Cell Death Differ. 2002;9:287–300. doi: 10.1038/sj.cdd.4400945. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Wang X, Wang P, Qi H, Zhang K, Xiao L. Sonodynamic effects of protoporphyrin IX disodium salt on isolated sarcoma 180 cells. Ultrasonics. 2006;45:56–60. doi: 10.1016/j.ultras.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 23.Quan-hong L, Shi-hui S, Ya-ping X, et al. Synergistic anti-tumor effect of ultrasound and hematoporphyrin on sarcoma 180 cells with special reference to the changes of morphology and cytochrome oxidase activity of tumor cells. J Exp Clin Cancer Res. 2004;23:333–341. [PubMed] [Google Scholar]
- 24.Dai S, Hu S, Wu C. Apoptotic effect of sonodynamic therapy mediated by hematoporphyrin monomethyl ether on C6 glioma cells in vitro. Acta Neurochir. 2009;151:1655–1661. doi: 10.1007/s00701-009-0456-5. [DOI] [PubMed] [Google Scholar]
- 25.Reinecke F, Levanets O, Olivier Y, et al. Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells. Biochemistry. 2006;395:405–415. doi: 10.1042/BJ20051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su CC, Lin JG, Li TM, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4389. [PubMed] [Google Scholar]