Abstract
Accumulation of single-nucleotide polymorphisms (SNPs) in the displacement loop (D-loop) of mitochondrial DNA (mtDNA) may be associated with disease outcome. Our team investigated the prediction power of D-loop SNPs in non-small cell lung cancer (NSCLC) outcome. In an overall multivariate analysis, allele 16390 was identified as an independent predictor for NSCLC outcome. The length of survival of patients with allele 16390A was significantly shorter than that of patients with allele 16390G (relative risk, 0.323; 95% CI, 0.109–0.951; p=0.040). The analysis of genetic polymorphisms in the mitochondrial D-loop can help identify NSCLC patient subgroups at a high risk for a poor disease outcome.
Keywords: displacement loop, non-small cell lung cancer, outcome, single-nucleotide polymorphism, mitochondrial DNA
Introduction
Lung cancer is the most frequent cancer and one of the leading causes of cancer-related deaths worldwide, accounting for 30% of all cancer-related deaths. An epidemiological study estimated that the number of deaths due to lung cancer in 2010 was 1.5 million, rendering lung cancer a major public health challenge (1,2). The annual mortality rate of lung cancer in China is high with approximately 400 thousand deaths (3). Non-small cell lung cancer (NSCLC) accounts for 89% of all lung cancers and approximately one third of NSCLC patients are diagnosed at a locally advanced stage (4,5). Despite aggressive treatment, the prognosis of NSCLC patients is still poor with a 5-year survival rate of approximately 10% and a median survival time of 16–18 months (6,7). Many clinical factors, such as tumor stage, metastasis, gender and weight loss, are predictors of prognosis of NSCLC patients (8), but there are few studies concerning the relationship between oxidative markers and NSCLC prognosis (9).
Lung cancer carcinogenesis is associated with increased oxidative stress which results in DNA damage (10,11). The human mitochondrial genome is a 16-kb closed-circular duplex molecule that contains 37 genes, including two ribosomal RNAs and a complete set of 22 tRNAs (12). Mitochondrial DNA (mtDNA) is believed to be more susceptible to DNA damage and acquires mutations at a higher rate than nuclear DNA because of high levels of reactive oxygen species (ROS), lack of protective histones and the limited capacity for DNA repair in mitochondria (13–15). Somatic mtDNA mutations and polymorphisms are associated with a wide variety of degenerative diseases and cancers (16,17), and can be homoplasmic by clonal expansion (18,19), or heteroplasmic in tumor tissues (20,21). In many cancers, somatic mutations and polymorphisms are located in an mtDNA non-coding region called the displacement loop (D-loop) (22,23), which contains 1122 bp (nucleotides 16024–16569 and 1–576; www.mitomap.org). This region is important for the regulation of both replication and expression of the mitochondrial genome as it contains the leading-strand origin of replication and the main promoter for transcription (24).
Sequence changes have been examined extensively in the D-loop in cancers, but few single-nucleotide polymorphisms (SNPs) have been selected for predicting cancer risk and outcome; their predictive values are still unclear (25–29). In this study, we assessed the prediction power of these SNPs on the outcome of NSCLC patients.
Materials and methods
Tissue specimens and DNA extraction
Blood samples were collected at The Fourth Hospital of Hebei University from NSCLC patients who received treatment at the Department of Respiratory Medicine between 2001 and 2009. The genomic DNA was immediately extracted using the Wizard Genomic DNA extraction kit (Promega, Madison, WI, USA) and stored at −20°C. All procedures were supervised and approved by the Human Tissue Research Committee of our hospital, and an informed consent was obtained from all participants.
PCR amplification and sequence analysis
The forward primer, 5′-CCCCATGCTTACAAGCAAGT-3′ (nucleotide 16190–16209) and reverse, 5′-GCTTTGAGGAGGTAAGC TAC-3′ (nucleotide 602–583) were used for amplification of a 982-bp product from the mtDNA D-loop region. PCR was performed according to the protocol included in the PCR Master Mix kit (Promega) and purified prior to sequencing. Cycle sequencing was carried out with the Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, USA) and the products were then separated on the ABI PRISM Genetic Analyzer 3100 (Applied Biosystems). Polymorphisms were confirmed by repeated analyses from both strands.
Statistical analysis
Survival curves were calculated using the Kaplan-Meier method, and compared with the log-rank test. Multivariate survival analysis was performed using a Cox proportional hazards model. All of the statistical analysis was carried out with the SPSS 13.0 software package (SPSS Co., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
A total of 80 patients were enrolled in this study and a follow-up review was conducted every 3 months for 2 years. One patient was lost to follow-up at the first year and 3 patients were lost at the second year. The remaining 76 patients shared the same performance status (ECOG score, 0). Of these, 20 patients were at stage III, 56 at stage IV and 56 died during follow-up. The data collected during the 2-year follow-up were analyzed for clinical characteristics using the Kaplan-Meier method and were compared by the log-rank test. Gender, age, TNM classification, smoking and histology were not statistically significant predictors of the length of overall survival, however treatment was correlated with survival in these patients (Table I).
Table I.
Univariate analysis of clinical characteristics associated with overall survival in the NSCLC patients.
| Characteristics | No. of cases | 2-year survival rate (%) | p-value |
|---|---|---|---|
| Gender | 0.382 | ||
| Male | 48 | 77.1 | |
| Female | 28 | 67.9 | |
| Age (years) | 0.715 | ||
| ≤45 | 7 | 28.6 | |
| >45 | 69 | 26.1 | |
| TNM classification | 0.520 | ||
| III | 20 | 25.0 | |
| IV | 56 | 26.8 | |
| Smoking | 0.253 | ||
| Yes | 36 | 19.4 | |
| No | 40 | 32.5 | |
| Treatmenta | 0.000 | ||
| Yes | 67 | 29.9 | |
| No | 9 | 0 | |
| Histology | 0.669 | ||
| SQ | 25 | 28.0 | |
| AC | 51 | 25.5 |
NSCLC, non-small cell lung cancer.
Treatment includes chemotherapy, radiotherapy, and chemotherapy plus radiotherapy. SQ, squamous cell carcinoma; AC, adenocarcinoma.
We sequenced the D-loop region in all of the 76 NSCLC patients and 121 SNPs were identified. The relationship between survival and the 121 SNPs was examined. The NSCLC patients were divided into two groups on the basis of their genotype at each SNP site, and overall survival curve was plotted using the Kaplan-Meier method for all NSCLC patients at these sites. A dramatic difference in survival rate was found for nucleotide 16390 (p=0.028) and another nucleotide of 16519 was also identified with borderline level of difference (p=0.075). The minor allele 16390A and 16519C were associated with a shorter length of survival (Fig. 1A and B). We performed multivariate analysis for these predictors including these two SNPs and treatment with the Cox proportional hazards model. As shown in Table II, the 16390 alleles and treatment were identified as independent predictors for NSCLC outcome. The length of survival for patients with the minor allele 16390A genotype was significantly less than that for patients with the common allele 16390G (relative risk, 0.323; 95% CI, 0.109–0.951; p=0.040) at the 16390 site. These data demonstrated the strong prediction power of nucleotide 16390 on outcome for NSCLC patients.
Figure 1.
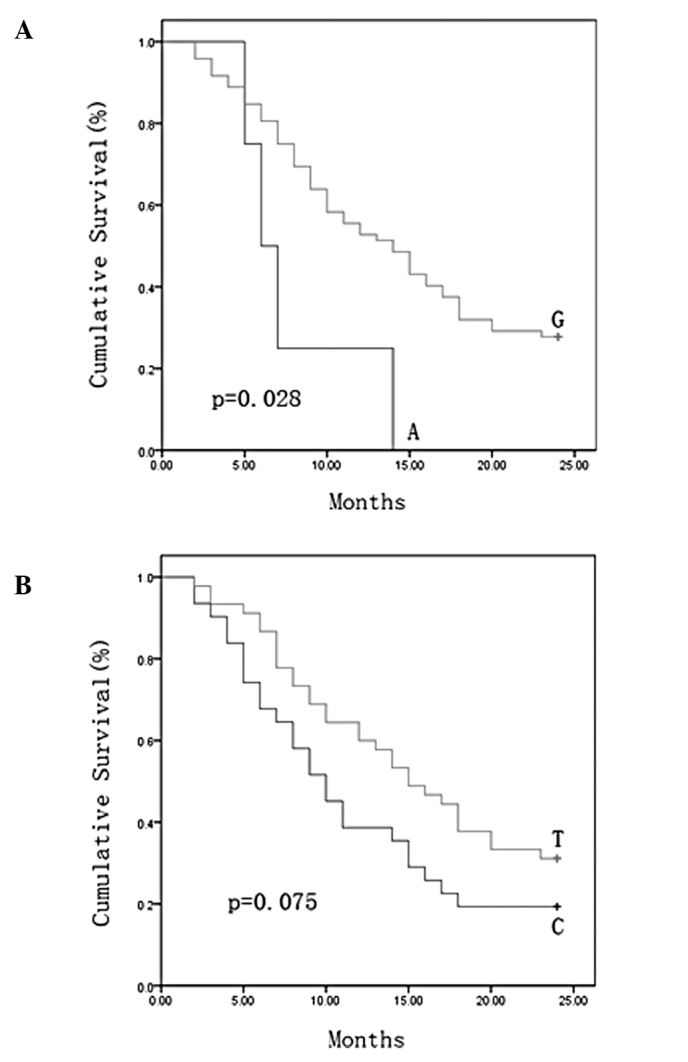
Survival curves according to the nucleotide at position (A) 16390 and (B) 16519 in the displacement loop (D-loop) of NSCLC patients.
Table II.
Multivariate analysis of prognostic factors associated with overall survival in NSCLC patients with Cox proportional hazards model.
| Factors | Relative risk | 95% CI | p-value |
|---|---|---|---|
| Treatment | 0.143 | 0.065–0.315 | 0.000 |
| Nucleotide | |||
| 16390 | 0.323 | 0.109–0.951 | 0.040 |
| 16519 | 0.628 | 0.361–1.093 | 0.100 |
NSCLC, non-small cell lung cancer.
Discussion
We previously identified cancer risk and outcome-associated SNPs of the D-loop in several types of cancer (25,26,30). In this study, selected SNPs in the mtDNA D-loop were examined for their ability to predict cancer outcome in NSCLC patients. Two SNPs, 16390 (G/A) and 16519 (T/C), were identified by the log-rank test for their association with overall survival. Multivariate survival analysis identified 16390 (G/A) as independent prediction markers for NSCLC outcome.
The mtDNA D-loop is important for regulation of mitochondrial genome replication and expression. SNPs in this region may affect mtDNA replication so as to alter the electron transport chain, which is responsible for the high ROS release and nuclear genome damage as well as cancer initiation and promotion (31–33). Overexpression of protein binding to the D-loop region has been proven to increase ROS and affect tumor progression (34).
In conclusion, SNPs in the mtDNA D-loop were found to be independent prognostic markers for NSCLC outcome. The analysis of genetic polymorphisms in the D-loop may help to identify patient subgroups at a high risk for poor disease outcome, thereby helping to refine therapeutic decisions in NSCLC.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 30801384).
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Cao C, Zhang YM, Wang R, Sun SF, Chen ZB, Ma HY, Yu YM, Ding QL, Shu LH, Deng ZC. Excision repair cross complementation group 1 polymorphisms and lung cancer risk: a meta-analysis. Chin Med J (Engl) 2011;124:2203–2208. [PubMed] [Google Scholar]
- 3.Yang L, Yang G, Zhou M, Smith M, Ge H, Boreham J, Hu Y, Peto R, Wang J, Chen Z. Body mass index and mortality from lung cancer in smokers and nonsmokers: a nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009;125:2136–2143. doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 4.Gandara D, Narayan S, Lara PN, Jr, Goldberg Z, Davies A, Lau DH, Mack P, Gumerlock P, Vijayakumar S. Integration of novel therapeutics into combined modality therapy of locally advanced non-small cell lung cancer. Clin Cancer Res. 2005;11:5057s–5062s. doi: 10.1158/1078-0432.CCR-05-9012. [DOI] [PubMed] [Google Scholar]
- 5.Yin M, Liao Z, Huang YJ, Liu Z, Yuan X, Gomez D, Wang LE, Wei Q. Polymorphisms of homologous recombination genes and clinical outcomes of non-small cell lung cancer patients treated with definitive radiotherapy. PLoS One. 2011;6:e20055. doi: 10.1371/journal.pone.0020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J, Deschamps C. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 7.Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, Joshi R, Ferry DR, Rudd RM, Spiro SG, Cook JE, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999;17:3188–3194. doi: 10.1200/JCO.1999.17.10.3188. [DOI] [PubMed] [Google Scholar]
- 8.Bi N, Yang M, Zhang L, Chen X, Ji W, Ou G, Lin D, Wang L. Cyclooxygenase-2 genetic variants are associated with survival in unresectable locally advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2383–2390. doi: 10.1158/1078-0432.CCR-09-2793. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Srivastava S, Prasad R, Natu SM, Mittal B, Negi MP, Srivastava AN. Oxidative stress in non-small cell lung cancer patients after chemotherapy: association with treatment response. Respirology. 2010;15:349–356. doi: 10.1111/j.1440-1843.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 10.Loft S, Svoboda P, Kawai K, Kasai H, Sørensen M, Tjønneland A, Vogel U, Møller P, Overvad K, Raaschou-Nielsen O. Association between 8-oxo-7,8-dihydroguanine excretion and risk of lung cancer in a prospective study. Free Radic Biol Med. 2011 Oct 20; doi: 10.1016/j.freeradbiomed.2011.10.439. (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11.Lawless MW, O’Byrne KJ, Gray SG. Oxidative stress induced lung cancer and COPD: opportunities for epigenetic therapy. J Cell Mol Med. 2009;13:2800–2821. doi: 10.1111/j.1582-4934.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annu Rev Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- 13.DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001;106:18–26. doi: 10.1002/ajmg.1392. [DOI] [PubMed] [Google Scholar]
- 14.Beal MF. Mitochondia, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 15.Lightowlers RN, Chinnery PF, Turnbull DM, Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet. 1997;13:450–455. doi: 10.1016/s0168-9525(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DC. Mouse models for mitochondrial disease. Am J Med Genet. 2001;106:71–93. doi: 10.1002/ajmg.1393. [DOI] [PubMed] [Google Scholar]
- 17.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 18.Nomoto S, Yamashita K, Koshikawa K, Nakao A, Sidransky D. Mitochondrial D-loop mutation as clonal markers in multicentric hepatocellular carcimona and plasma. Clin Cancer Res. 2002;8:481–487. [PubMed] [Google Scholar]
- 19.Mambo E, Gao X, Cohen Y, Guo Z, Talalay P, Sidransky D. Electrophile and oxidant damage of mitochondrial DNA leading to rapid evolution of homoplasmic mutations. Proc Natl Acad Sci USA. 2003;100:1838–1843. doi: 10.1073/pnas.0437910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneyama H, Hara T, Kato Y, Yamori T, Matsuura ET, Koike K. Nucleotide sequence variation is frequent in the mitochondrial DNA displacement loop region of individual human tumor cells. Mol Cancer Res. 2005;3:14–20. [PubMed] [Google Scholar]
- 21.Jakupciak JP, Maragh S, Markowitz ME, Greenberg AK, Hoque MO, Maitra A, Barker PE, Wagner PD, Rom WN, Srivastava S, Sidransky D, O’Connell CD. Performance of mitochondrial DNA mutations detecting early stage cancer. BMC Cancer. 2008;8:285. doi: 10.1186/1471-2407-8-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nashikawa M, Nishiguchi S, Shiomi S, Tamori A, Koh N, Takeda T, Kubo S, Hirohashi K, Kinoshita H, Sato E, Inoue M. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Res. 2001;61:1843–1845. [PubMed] [Google Scholar]
- 23.Sanchez-Cespedes M, Parrella P, Nomoto S, Cohen D, Xiao Y, Esteller M, Jeronimo C, Jordan RC, Nicol T, Koch WM. Identification of a mononucleotide repeat as a major target for mitochondrial DNA alterations in human tumors. Cancer Res. 2001;61:7015–7019. [PubMed] [Google Scholar]
- 24.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Zhang F, Wang C, Wang S, Shiao YH, Guo Z. Identification of sequence polymorphism in the D-loop region of mitochondrial DNA as a risk factor for hepatocellular carcinoma with distinct etiology. J Exp Clin Cancer Res. 2010;29:130. doi: 10.1186/1756-9966-29-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Zhang F, Fan H, Peng L, Zhang R, Liu S, Guo Z. Sequence polymorphisms of mitochondrial D-loop and hepatocellular carcinoma outcome. Biochem Biophys Res Commun. 2011;406:493–496. doi: 10.1016/j.bbrc.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 27.Navaglia F, Basso D, Fogar P, Sperti C, Greco E, Zambon CF, Stranges A, Falda A, Pizzi S, Parenti A, Pedrazzoli S, Plebani M. Mitochondrial DNA D-loop in pancreatic cancer: somatic mutations are epiphenomena while the germline 16519 T variant worsens metabolism and outcome. Am J Clin Pathol. 2006;126:593–601. doi: 10.1309/GQFCCJMH5KHNVX73. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Bamlet WR, de Andrade M, Boardman LA, Cunningham JM, Thibodeau SN, Petersen GM. Mitochondrial genetic polymorphisms and pancreatic cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1455–1459. doi: 10.1158/1055-9965.EPI-07-0119. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, McDonnell SK, Hebbring SJ, Cunningham JM, St Sauver J, Cerhan JR, Isaya G, Schaid DJ, Thibodeau SN. Polymorphisms in mitochondrial genes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3558–3566. doi: 10.1158/1055-9965.EPI-08-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang R, Wang R, Zhang F, Wu C, Fan H, Li Y, Wang C, Guo Z. Single-nucleotide polymorphisms in the mitochondrial displacement loop and outcome of esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:155. doi: 10.1186/1756-9966-29-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandy B, Davison AJ. Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic Biol Med. 1990;8:523–539. doi: 10.1016/0891-5849(90)90152-9. [DOI] [PubMed] [Google Scholar]
- 32.Gille JJ, Joenje H. Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res. 1992;275:405–414. doi: 10.1016/0921-8734(92)90043-o. [DOI] [PubMed] [Google Scholar]
- 33.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dement GA, Maloney SC, Reeves R. Nuclear HMGA1 nonhistone chromatin proteins directly influence mitochondrial transcription, maintenance, and function. Exp Cell Res. 2007;313:77–87. doi: 10.1016/j.yexcr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


