Abstract
Naturally generated autoantibodies to tumor-associated antigens such as MUC1 can assist in cancer diagnosis and prognosis. While previous studies have concentrated on the tandem repeat array domain of MUC1, here we focused on MUC1’s signal peptide domain. We used ELISA assays with MUC1-specific epitopes and antibodies to quantify soluble MUC1 antigen and anti-MUC1 autoantibodies against the tandem repeat array and signal peptide domains in 15 naïve donors and 27 multiple myeloma cancer patients. We showed a significant increase in up to 24-fold (P<0.004) only in the levels of anti-MUC1 signal peptide autoantibodies in the sera of multiple myeloma patients vs. naïve donors. This increase stemmed chiefly from the preferred immunogenicity of the signal peptide. Moreover, a significant positive correlation (R2=0.5361, P<0.048, Pearson correlation) was shown between the levels of soluble MUC1 and anti-MUC1 signal peptide autoantibodies in multiple myeloma patients with progressive disease while under therapy. This is an initial report on the existence of autoantibodies to a signal peptide domain in general and to the MUC1 signal peptide domain in particular in cancer patients. The autoantibodies had MUC1 rather than signal peptide specificity. The specific nature of the antigen leading to generation of these autoantibodies is still unclear because it is unlikely that the target antigen is a major histo-compatibility complex-peptide complex and we could not trace soluble MUC1 signal peptide fragments in naïve donors and multiple myeloma patients. Further validation of these findings may improve diagnostic and prognostic capabilities for MUC1-positive multiple myeloma patients and potentially, patients with other MUC1-positive cancers, as well.
Keywords: MUC1, autoantibodies, signal peptide, multiple myeloma, diagnostics
Introduction
Studies investigating the role of tumor-associated antigens (TAAs) in cancer diagnosis and progression have focused on the MUC1 antigen, which is overexpressed in most solid epithelial and non-solid hematological tumors including multiple myeloma (1–3). Soluble MUC1 (sMUC1) levels were reported to be elevated in sera of patients with solid tumors such as breast carcinoma, and non-solid tumors such as multiple myeloma, using FDA approved sMUC1 assays CA15.3 and CA27.29 directed to the extracellular tandem repeat array (TRA) domain (3,4). However, despite the wide tumor distribution of MUC1, use of the CA15.3 and CA27.29 markers is confined to monitoring the prognosis and response to treatment in patients with advanced breast cancer (5) and it is not sensitive enough to be used for early diagnosis.
In contrast, naturally generated autoantibodies to TAAs are detectable even before the tumor is clinically apparent (6), and due to their lower fluctuation and longer half-life in the blood, they may be more appropriate for cancer diagnosis. Anti-MUC1 TRA autoantibody levels were shown to be higher in patients with breast cancer (7) and lower in multiple myeloma (3) patients compared to healthy individuals. One suggested cause for the variations in multiple myeloma patients relates to the presence of immune complexes between sMUC1 and the endogenously generated anti-MUC1 TRA antibodies (3). Bypassing this limitation can potentially be achieved by searching for anti-MUC1 autoantibodies generated to domains flanking the TRA.
Along with others, we have demonstrated the preferred immunogenicity of the signal peptide (SP) domain of MUC1 (8–10). This short domain has promiscuous binding to multiple major histocompatibility complex (MHC) class I and II, a characteristic that supports its process and presentation on the cell surface of various tumor cells and antigen presenting cells (10). In the present study, we questioned whether MUC1’s SP domain preferred MHC class II binding, coupled with in silico prediction of B-cell epitopes, could lead to the induction of a natural anti-SP humoral response in multiple myeloma patients.
Materials and methods
Naïve donors and cancer patients
Blood samples (3 ml) were drawn from 15 naïve healthy volunteers, 18–60 years of age and 27 patients with multiple myeloma, 50–75 years of age. The study patients included 14 with progressive disease under treatment, 7 with active disease under treatment and 6 at best response off therapy. The study was approved by the Institutional Ethics Committees of the participating hospitals.
In silico prediction software
Binding predictions were performed for the MHC class II (HLA-DRB1) alleles that are most prevalent worldwide. However, to have a defined population, we focused only on the Caucasian population. MHC class II binding prediction was carried out using Propred http://www.imtech.res.in/raghava/propred/ (11) and Immune Epitope www.immuneepitope.org (12). B-cell epitope prediction was evaluated with Kolaskar and Tongaonkar antigenicity score using immune epitope http://tools.immuneepitope.org/tools/bcell/DisplayResultServlet (13). In all prediction methods, only binders >5% were analyzed.
Peptide synthesis
MUC1-SP-L, MUC1-SP-M, TB-Rv0476/4941-SP-L were synthesized by fully automated, solid-phase, peptide synthesis using fluorenylmethyloxycarbonyl (Fmoc)/tBu-strategy and Rink-amide-polystyrene resin at EMC Microcollections, Germany, while MUC1-TRA-L was synthesized using the same methodology at GL Biochem, China. The purity and identity of all peptides was >95%, as determined by HPLC and MS analysis.
ELISA for detecting serum levels of sMUC1 and anti-MUC1 IgG antibodies
Soluble MUC1 levels were evaluated in ELISA plates (F96 Maxisorp, Nunc, Denmark) using commercial anti-MUC1 TRA monoclonal antibodies (mAbs) (clones M4H2 and M2F1 HRP-conjugate) and the ELISA kit (HyTest, Finland) according to the manufacturer’s protocol. MUC1 levels were evaluated using 7 double dilutions of 100 μl of patient sera starting at 1:5. For a MUC1-positive control, we used dilutions (starting at 1:5) of supernatant collected from the DA3-GTRUNK transfected cell line, producing high levels of sMUC1 containing the TRA domain (14). The ELISA plates were developed with TMB/E solution (Southern Biotech, USA) according to the manufacturer’s protocol. The reaction was terminated by adding 50 μl/well of 10% sulfuric acid. Results were measured at 450 nm. For this assay, we used specific titer rather than absolute concentration, as we did not have the appropriate pure antigen as a standard.
Soluble MUC1 SP levels were evaluated in ELISA plates (F96 Maxisorp) coated for 2 h with 5 μg/ml of the anti-SP polyclonal antibodies raised in rabbits to the 17-mer MUC1-SP-M. Next, dilutions of 100 μl of patient sera, (starting at 1:5) were incubated for 2 h. For detection we used 1 μg/ml of biotinconjugated anti-MUC1-SP-M antibodies for 1 h, followed by 1 h incubation at 25°C with streptovidin HRP (BioLegent, USA) diluted 1:10000. At the final step, the ELISA plates were developed with TMB/E solution as described above. As a standard for sMUC1 SP, we used dilutions, starting from 2.5 μg/ml of MUC1’s 21-mer SP domain, MUC1-SP-L (10).
To evaluate the level of anti-MUC1 TRA and SP antibodies in sera, ELISA plates (F96 Maxisorp) were coated with 50 μl of MUC1 peptide at 5 μg/ml in carbonate buffer and incubated overnight at 4°C. Evaluated serum samples were then diluted 1:100, plus 7 additional dilutions in PBS with 0.5% gelatin and incubated for 2 h at 25°C. Next, 50 μl/well of the appropriate secondary anti-IgG antibody HRP-conjugate (Jackson ImmunoResearch, USA) was added at a final dilution of 1:10000 in a blocking buffer and incubated for 1 h at 25°C. Plates were then developed with TMB/E solution as described above. As a positive standard for anti-MUC1 TRA antibodies, we used dilutions starting with 10 μg/ml of the anti-MUC1 TRA mAb H23 (15), raised against the human breast cancer cell line T47D (15) and recognized the TRA epitope APDTRP on the non-glycosylated form of MUC1. As a positive standard for anti-MUC1 SP antibodies, we used dilutions beginning with 10 μg/ml of anti-MUC1-SP-M rabbit polyclonal antibodies. In this assay, serum levels for anti-MUC1 TRA autoantibodies in naïve donors were X≤274 μg/ml and for anti-MUC1 SP auto-antibodies, X≤210.2 μg/ml, based on the average plus standard deviation value determined in 15 naïve healthy individuals.
Statistical analysis
Results were statistically analyzed with Student’s t-test, Fisher’s exact test or in the case of correlations, with Pearson correlation coefficient. In all methods, the minimum level of significance for a two-tailed test was set at P<0.05.
Results
MUC1-derived SP epitope has high antigenic profile and promiscuous MHC class II binding
Selection of appropriate immunodominant antigens for the induction of a natural humoral response was performed using various in silico prediction methods, as described in Materials and methods. Using these tools, we searched the MUC1 SP domain for sequences with the following properties: superior B-cell antigenicity profile and preferred CD4+ T-cell activation via high binding properties to a defined list of abundant MHC class II alleles. The outcome of our search was the selection for further use of the 17-mer peptide MUC1-SP-M containing amino acids 10–21 in MUC1 SP (Table I). As a control from the known MUC1 TRA domain, we used the 25-mer MUC1 TRA-derived peptide MUC-TRA-L (16,17). As a non-MUC1 SP-derived control, we used a 19-mer SP sequence TB-Rv0476/4941-SP-L, which included the entire SP domain of the Mycobacterium tuberculosis (MTb) antigen Rv0476/4941 (18) (Table I).
Table I.
List of peptides used in this study.
| VXL IDa | Published ID (ref)b | Length (-mer) | Position | Sequence | Target antigen/Domainc |
|---|---|---|---|---|---|
| VXL100 MUC1-SP-L |
MUC1-SP-L (10) | 21 | 1–21 | MTPGTQSPFFLLLLLTVLTVV-NH2 | MUC1 (SP) |
| VXL3A MUC1-SP-M |
- | 17 | 10–21 | KKFLLLLLTVLTVVKKK | MUC1 (SP) |
| VXL25 MUC1-TRA-L |
BLP25 (16,17) | 25 | 130–154 | STAPPAHGVTSAPDTRPAPGSTAPP | MUC1 (TRA) |
| VXL211 TB-Rv0476/4941-SP-L |
VXL211 (18) | 19 | 1–19 | MLVLLVAVLVTAVYAFVHA-NH2 | Uncharacterized protein Rv0476/4941 (SP) |
VXL ID nomenclature: SP, signal peptide; TRA, tandem repeat array; L, long; M, moderate; TB, tuberculosis.
BLP25 vaccine contain in addition to the 25-mer antigenic epitope a liposome and cytokines.
Uncharacterized protein Rv0476/4941 originated from Mycobacterium tuberculosis.
Endogenously generated anti-MUC1 antibodies in naïve donors
Our initial aim was to determine the presence of endogenous anti-MUC1 SP IgG antibodies in naïve donors and multiple myeloma patients. Tables II and III summarize the results in 15 naïve healthy donors and 27 multiple myeloma cancer patients, respectively, at different disease stages. A baseline of 210.2 μg/ ml for anti-MUC1 SP and 274 μg/ml for anti-MUC1 TRA was set for positive autoantibody expression levels based on their expression levels in naïve donors, as described in Materials and methods. Interestingly, anti-MUC1 SP autoantibody serum levels were significantly lower (P<0.008, t-test) than those of anti-MUC1 TRA IgG autoantibodies, suggesting a lower antigen expression profile for SP vs. TRA in the naïve donors.
Table II.
sMUC1 and anti-MUC1 autoantibody levels in naïve donors.
| Sample no. | Sample characterization | sMUC1 TRA (μg/ml)a | sMUC1 SP (μg/ml)a | Anti-MUC1 TRA Ab (μg/ml)b | Anti-MUC1 SP Ab (μg/ml)b |
|---|---|---|---|---|---|
| 1 | Naïve healthy donor | Negative | Negative | 224 | 178 |
| 2 | Naïve healthy donor | Negative | Negative | 265 | 159 |
| 3 | Naïve healthy donor | Negative | Negative | 184 | 145 |
| 4 | Naïve healthy donor | Negative | Negative | 161 | 185 |
| 5 | Naïve healthy donor | Negative | Negative | 205 | 185 |
| 6 | Naïve healthy donor | Negative | Negative | 230 | 200 |
| 7 | Naïve healthy donor | Negative | Negative | 281 | 216 |
| 8 | Naïve healthy donor | Negative | Negative | 318 | 10 |
| 9 | Naïve healthy donor | Negative | Negative | 105 | 110 |
| 10 | Naïve healthy donor | Negative | Negative | 320 | 166 |
| 11 | Naïve healthy donor | Negative | Negative | 203 | 10 |
| 12 | Naïve healthy donor | Negative | Negative | 147 | 200 |
| 13 | Naïve healthy donor | Negative | Negative | 200 | 184 |
| 14 | Naïve healthy donor | Negative | Negative | 134 | 100 |
| 15 | Naïve healthy donor | Negative | Negative | 152 | 10 |
| Average | 208.60 | 137.20 | |||
| Standard deviation | 65.35 | 72.97 |
Serum levels of MUC1 antigen and anti-MUC1 antibodies were measured by ELISA assay as described in Materials and methods. Positive sMUC1 level is set for titer of X>1:5.
Naïve sera for anti-MUC1 TRA antibodies epitope is X≤274 μg/ml and for anti-MUC1 SP autoantibodies is X≤210.2 μg/ml, based on the average plus standard deviation values determined in 15 naïve healthy individuals.
Table III.
sMUC1 and anti-MUC1 autoantibody levels in multiple myeloma patients.
| Patient no. | Patient status | sMUC1 TRA (titer)a | sMUC1 SP (titer) | Anti-MUC1 TRA Ab (μg/ml)b | Anti-MUC1 SP Ab (μg/ml)b | Anti-MUC1 TRA Ab (Positivity) | Anti-MUC1 SP Ab (Positivity) |
|---|---|---|---|---|---|---|---|
| 1 | Active disease; under therapy | Negative | Negative | 440 | 740 | + | + |
| 2 | Progressive disease; under therapy | Negative | Negative | 183 | 150 | - | - |
| 3 | At best response; off therapy | 1:10 | Negative | 382 | 312 | + | + |
| 4 | Active disease; under therapy | 1:5 | Negative | 190 | 350 | - | + |
| 5 | Active disease; under therapy | 1:10 | Negative | 85 | 50 | - | - |
| 6 | Active disease; under therapy | Negative | Negative | 97 | 130 | - | - |
| 7 | At best response; off therapy | 1:10 | Negative | 355 | 456 | + | + |
| 8 | At best response; off therapy | Negative | Negative | 211 | 581 | - | + |
| 9 | Active disease; under therapy | Negative | Negative | 132 | 1036 | - | + |
| 10 | At best response; off therapy | Negative | Negative | 158 | 746 | - | + |
| 11 | Progressive disease; under therapy | Negative | Negative | 238 | 525 | - | + |
| 12 | Progressive disease; under therapy | Negative | Negative | 63 | 500 | - | + |
| 13 | At best response; off therapy | Negative | Negative | 500 | 1000 | + | + |
| 14 | Active disease; under therapy | 1:10 | Negative | 292 | 884 | + | + |
| 15 | Progressive disease; under therapy | 1:40 | Negative | 980 | 3400 | + | + |
| 16 | At best response; off therapy | Negative | Negative | 761 | 1500 | + | + |
| 17 | Progressive disease; under therapy | 1:20 | Negative | 91 | 100 | - | - |
| 18 | Progressive disease; under therapy | Negative | Negative | 728 | 424 | + | + |
| 19 | Progressive disease; under therapy | 1:10 | Negative | 795 | 339 | + | + |
| 20 | Progressive disease; under therapy | Negative | Negative | 120 | 80 | - | - |
| 21 | Progressive disease; under therapy | 1:20 | Negative | 3200 | 812 | + | + |
| 22 | Progressive disease; under therapy | Negative | Negative | 291 | 594 | + | + |
| 23 | Progressive disease; under therapy | Negative | Negative | 500 | 732 | + | + |
| 24 | Progressive disease; under therapy | 1:10 | Negative | 500 | 191 | + | - |
| 25 | Progressive disease; under therapy | 1:20 | Negative | 860 | 950 | + | + |
| 26 | Active disease; under therapy | 1:20 | Negative | 195 | 162 | - | - |
| 27 | Progressive disease; under therapy | 1:5 | Negative | 222 | 976 | - | + |
| Percentage of patients with positive anti-MUC1 SP- and MUC1 TRA-specific antibodies levels | 14/27 | 20/27 | |||||
Serum levels of MUC1 antigen and anti-MUC1 antibodies were measured by ELISA assay as described in Materials and methods. Positive sMUC1 level is set for titer of X>1:5.
Naïve sera for anti-MUC1 TRA antibodies epitope is X≤274μg/ml and for anti-MUC1 SP autoantibodies is X≤210.2μg/ml, based on the average plus standard deviation values determined in 15 naïve healthy individuals.
Endogenously generated anti-MUC1 antibodies in patients with multiple myeloma
We next evaluated the levels of anti-MUC1 SP and MUC1 TRA IgG autoantibodies in 27 multiple myeloma patients. Although we observed elevated levels of anti-MUC1 TRA autoantibodies in the entire group of 27 patients vs. the 15 naïve donors, the differences were not significant (P=0.111, t-test). Unexpectedly, we observed an elevation of up to 24-fold in the concentration of autoantibodies to the MUC1 SP domain in most patients (Table III). This elevation was highly significant (P<0.004, t-test) in the entire group of 27 multiple myeloma vs. the 15 naïve donors, in spite of the small group size and disease stage variability. Anti-MUC1 SP autoantibodies had antigen (i.e., MUC1) rather than general SP specificity, since no reactivity to the non-TAA SP TB-Rv0476/4941-SP-L was observed in several patients (data not shown). Importantly, a high concentration of anti-MUC1 SP autoantibodies was also present in patients with minimal disease (characterized as ‘patients at best response off therapy’), four of which had undetectable sMUC1 levels, suggesting a potential role for these autoantibodies in monitoring minimal disease and plausibly disease onset. The preferred immunogenicity of MUC1 SP vs. TRA domains can be further demonstrated in the same group, in which the concentration of the anti-SP autoantibodies was significantly higher (P<0.03, t-test) than that of the anti-MUC1 TRA levels. In these patients, we ruled out any potential influence on sMUC1 levels by anti-MUC1 TRA autoantibodies with a dedicated ELISA that analyzed the amount of sMUC1 in antigen-antibody complexes (data not shown). This supports the immunodominant properties of MUC1 SP regarding antibody production as predicted in silico.
We further analyzed the percentage of patients with positive anti-MUC1 SP- and MUC1 TRA-specific titers. A positive response was set for the average plus one standard deviation as described in Materials and methods. Results in this analysis (Table III) further supported our initial observation on the elevated production of anti-MUC1 SP autoantibodies. In particular, most patients, 20/27 (74%) had positive anti-MUC1 SP-specific autoantibodies, while only 14/27 (51.8%) had positive anti-MUC1 TRA-specific autoantibodies. Although these differences were not significant (P=0.158, Fisher’s exact test), they showed a positive trend for the selectivity of MUC1’s SP vs. TRA domain in multiple myeloma patients.
Expression levels of soluble MUC1 SP in naïve donors and multiple myeloma patients
Previous studies have presented sMUC1 expression in multiple myeloma patients, but none focused on the SP domain of MUC1. This is a significant point, especially in light of the knowledge about immune complexes between the sMUC1 containing the TRA domain and anti-MUC1 TRA autoantibodies and their effect on sMUC1 levels. We therefore questioned whether the high anti-MUC1 SP autoantibody levels were merely an outcome of the immuno-dominant properties of MUC1’s SP and/or a consequence of the minimal interference by plausible novel sMUC1 variants containing the SP domain. Our results (Tables II and III) confirmed a negative expression of sMUC1 containing the SP domain in all serum samples of both healthy naïve donors and multiple myeloma cancer patients and ruled out the presence of immune complexes around MUC1 SP autoantibodies.
Correlation between serum levels of sMUC1 and anti-MUC1 autoantibodies
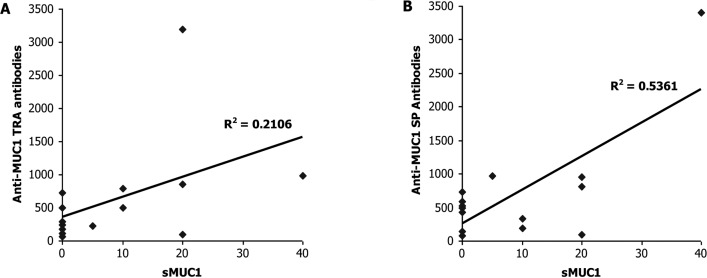
After finding elevated levels of anti-MUC1 TRA and SP antibodies in the majority of the multiple myeloma patients, we sought to explore any interactions between sMUC1 and the anti-MUC1 SP autoantibody levels in multiple myeloma patients at different disease stages. We also wanted to reconfirm past observations in multiple myeloma patients vis-à-vis an inverse correlation between the levels of sMUC1 and anti-MUC1 TRA autoantibodies. In line with previous publications, we found no significant correlation (R2=0.2106, P= 0.469, Pearson correlation) between sMUC1 levels and autoantibodies to MUC1 TRA among the group of 14 multiple myeloma patients characterized with ‘progressive disease under therapy’ (Fig. 1A). In contrast, a significant positive correlation (R2=0.5361, P<0.048, Pearson correlation) was found between sMUC1 serum levels and anti-MUC1 SP autoantibodies among this group of 14 multiple myeloma patients (Fig. 1B). These results were unexpected, especially since they were obtained from a relatively small patient population without selecting for key parameters such as age and gender, which can influence the expression levels of MUC1 and anti-MUC1 antibodies. No significant correlation was found in a similar analysis performed on the other patients with multiple myeloma classified as ‘active disease under therapy’ or ‘patients at best response off therapy’ with both anti-MUC1 SP and anti-MUC1 TRA autoantibodies (data not shown).
Figure 1.
Correlation between serum levels of soluble MUC1 and anti-MUC1 TRA or SP autoantibodies. Soluble MUC1 levels in 14 multiple myeloma patients with progressive disease under therapy, were correlated with the serum levels of anti-MUC1 TRA autoantibodies (A) and anti-MUC1 SP autoantibodies (B). A significant positive correlation (R2=0.5361, P<0.048, Pearson correlation coefficient) was found only between the serum levels of sMUC1 and anti-MUC1 SP autoantibodies.
Discussion
Despite the wide distribution and high immunogenicity of MUC1 TAA and anti-MUC1 autoantibodies, their utilization in cancer diagnosis and prognosis is still limited (5). Whereas over the last three decades, studies with sMUC1 have concentrated on the TRA domain (19–22), here we suggest for the first time a novel strategy focused on non-TRA, anti-MUC1 SP IgG autoantibodies.
While naïve donors had significantly lower IgG autoantibody levels to MUC1 SP compared to MUC1 TRA epitopes, the levels in multiple myeloma patients were significantly higher than that of MUC1 TRA. This outcome was related to the immunodominant properties of SP also described in previous publications (9,10). Baty and Lazdunski reported the induction of preferential IgG titer to the small (Mr 2600) alkaline phosphatase SP domain (23) following vaccination with the much larger (Mr 45600) precursor protein. In a different study, Harboe et al reported the production of high polyclonal antibodies in rabbits to a 13-mer fragment from the SP domain of the MTb antigen, MPT83 (24).
The specific nature of the antigen leading to the generation of anti-MUC1 SP autoantibodies is still unclear. In this study, we showed the absence of the sMUC1 SP domain in serum samples of naïve donors and multiple myeloma patients. Yet, it is still possible that in multiple myeloma patients, undetectable levels of SP fragments from debris of tumor cells could reach the blood stream. An alternative source for MUC1 SP is cell surface expression. We and others demonstrated the expression of MUC1 SP in association with MHC class I and II in the context of cancer cells (8–10). Since antibody production to MHC-peptide complexes is not trivial due to the various binding restrictions of different MHC alleles, a third, still unknown mechanism could be the primary resource for the MUC1 SP antigen.
Results in the current study suggested a MUC1-specific rather than SP-specific autoantibody titer. Previous reports showed either cross reactivity between different SP sequences when a large precursor was used as an antigen (23) or antigen specificity when a linear SP fragment was used (24). In our opinion, these differences stem from the nature of the sequence used for vaccination and its ability to form conformation structures. A larger sequence, such as the one used by Baty and Lazdunski (23), forms conformational structures that lead to cross-reactivity between different SPs. We plausibly overcame this conformation structure by using only amino acids 10–21 from the total 21-mer of SP domain of MUC1 and by adding several lysine residues at the N and C terminal ends of this peptide, which form a linear structure. In summary, we hypothesize that in addition to the antigen-specific antibodies recognizing a linear sequence, there are potentially SP-specific autoantibodies that recognize a SP conformation motif.
Past studies on multiple myeloma reported a negative correlation between the levels of sMUC1 and anti-MUC1 TRA autoantibodies (3). We confirmed these findings, but also presented a statistically significant, positive correlation between sMUC1 and anti-MUC1 SP autoantibody levels in multiple myeloma patients with progressive disease under therapy.
This is the first report on the presence of autoantibodies to the MUC1 SP domain in cancer patients. Additional analysis needs to be performed on a larger, homogeneous patient population, especially at disease onset, before firm conclusions can be made. One such analysis should involve the existence of IgM autoantibodies to the MUC1 SP domain and the switch from IgM to IgG. This would assist us to better understand the entire process of anti-MUC1 SP antibody formation. Moreover, in-depth analysis needs to be performed in breast cancer patients where the CA15.3 marker is still in use.
Abbreviations:
- MHC,
major histocompatibility complex; mAb, monoclonal antibody;
- SP,
signal peptide;
- sMUC1,
soluble MUC1;
- TRA,
tandem repeat array;
- TAA,
tumor-associated antigen
References
- 1.Croce MV, Isla-Larrain MT, Demichelis SO, Gori JR, Price MR, Segal-Eiras A. Tissue and serum MUC1 mucin detection in breast cancer patients. Breast Cancer Res Treat. 2003;81:195–207. doi: 10.1023/A:1026110417294. [DOI] [PubMed] [Google Scholar]
- 2.Luminari S, Goldaniga M, Ceccherelli F, Guffanti A, Bombardieri E, Marcheselli R, et al. Prevalence and prognostic significance of sMUC-1 levels in plasma cell dyscrasias. Br J Haematol. 2003;121:772–774. doi: 10.1046/j.1365-2141.2003.04353.x. [DOI] [PubMed] [Google Scholar]
- 3.Treon SP, Maimonis P, Bua D, Young G, Raje N, Mollick J, et al. Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood. 2000;96:3147–3153. [PubMed] [Google Scholar]
- 4.Reddish MA, MacLean GD, Poppema S, Berg A, Longenecker BM. Pre-immunotherapy serum CA27.29 (MUC-1) mucin level and CD69+ lymphocytes correlate with effects of Theratope sialyl-Tn-KLH cancer vaccine in active specific immuno therapy. Cancer Immunol Immunother. 1996;42:303–309. doi: 10.1007/s002620050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gion M, Mione R, Leon AE, Dittadi R. Comparison of the diagnostic accuracy of CA27.29 and CA15.3 in primary breast cancer. Clin Chem. 1999;45:630–637. [PubMed] [Google Scholar]
- 6.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–1394. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 7.MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin-associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emphasis Tumor Immunol. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 9.Carmon L, El-Shami KM, Paz A, Pascolo S, Tzehoval E, Tirosh B, et al. Novel breast-tumor-associated MUC1-derived peptides: characterization in Db-/- x beta2 microglobulin (beta2m) null mice transgenic for a chimeric HLA-A2.1/Db-beta2 micro-globulin single chain. Int J Cancer. 2000;85:391–397. [PubMed] [Google Scholar]
- 10.Kovjazin R, Volovitz I, Kundel Y, Rosenbaum E, Medalia G, Horn G, et al. ImMucin: a novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine. 2011;29:4676–4686. doi: 10.1016/j.vaccine.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 12.Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, et al. The immune epitope database 2.0. Nucleic Acids Res. 2010;38:D854–D862. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- 14.Horn G, Gaziel A, Wreschner DH, Smorodinsky NI, Ehrlich M. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Exp Cell Res. 2009;315:1490–1504. doi: 10.1016/j.yexcr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Keydar I, Chou CS, Hareuveni M, Tsarfaty I, Sahar E, Selzer G, et al. Production and characterization of monoclonal antibodies identifying breast tumor-associated antigens. Proc Natl Acad Sci USA. 1989;86:1362–1366. doi: 10.1073/pnas.86.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small cell lung cancer. Clin Lung Cancer. 2001;3:49–58. doi: 10.3816/clc.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 17.Sangha R, North S. L-BLP25: a MUC1-targeted peptide vaccine therapy in prostate cancer. Expert Opin Biol Ther. 2007;7:1723–1730. doi: 10.1517/14712598.7.11.1723. [DOI] [PubMed] [Google Scholar]
- 18.Kovjazin R, Volovitz I, Daon Y, Vider-Shalit T, Azran R, Tsaban L, et al. Signal peptides and trans-membrane regions are broadly immunogenic and have high CD8+ T cell epitope densities: implications for vaccine development. Mol Immunol. 2011;48:1009–1018. doi: 10.1016/j.molimm.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolopoulos V, McKenzie IF. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. doi: 10.1615/critrevimmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 20.Graham RA, Burchell JM, Taylor-Papadimitriou J. The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother. 1996;42:71–80. doi: 10.1007/s002620050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- 22.Tang CK, Katsara M, Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Rev Vaccines. 2008;7:963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- 23.Baty D, Lazdunski C. An anti-(signal peptide) antibody: purification, properties and use as a conformational probe. Eur J Biochem. 1979;102:503–507. doi: 10.1111/j.1432-1033.1979.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 24.Harboe M, Whelan AO, Ulvund G, McNair J, Pollock JM, Hewinson RG, et al. Generation of antibodies to the signal peptide of the MPT83 lipoprotein of Mycobacterium tuberculosis. Scand J Immunol. 2002;55:82–87. doi: 10.1046/j.1365-3083.2002.01030.x. [DOI] [PubMed] [Google Scholar]